3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(10):1890-1902. doi:10.7150/ijms.95952 This issue Cite
Research Paper
ICOS-ICOSL pathway enhances NKT-like cell antiviral function in pregnant women with COVID-19
1. Department of Clinical Laboratory, First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China.
2. Anhui Medical University, Hefei, Anhui, China.
3. Department of Clinical Laboratory, Anhui Provincial Maternity and Child Health Hospital, Hefei, China.
† Lu Zong and Yuanling Zheng contributed equally to this work and share first authorship.
Received 2024-3-5; Accepted 2024-7-12; Published 2024-7-16
Abstract
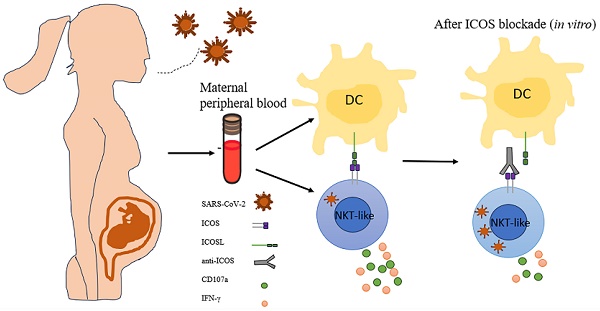
Objective: The immune response initiated by SARS-CoV-2 infection in pregnancy is poorly elucidated. We aimed to access and compare the antiviral cellular responses and lymphocytes activation between healthy pregnancies and pregnant women infected with SARS-CoV-2.
Methods: We detected the immunological changes of lymphocytes in peripheral blood of healthy non-pregnant women, non-pregnant women with COVID-19, healthy pregnant women, pregnant women with COVID-19 and convalescent group by flow cytometry. In vitro blockade was used to identify NKT-like cell activation through ICOS-ICOSL pathway.
Results: We found that CD3+CD56+ NKT-like cells decreased significantly in COVID-19 positive pregnant women compared to healthy pregnant women. NKT-like cells of pregnant women expressed higher level of activating receptors CD69 and NKp46 after SARS-CoV-2 infection. Particularly, they also increased the expression of the co-stimulatory molecule ICOS. NKT-like cells of pregnant women with COVID-19 up-regulated the expression of IFN-γ, CD107a and Ki67. Meanwhile, we found that ICOSL expression was significantly increased on pDCs in pregnant women with COVID-19. Blocking ICOS in vitro significantly decreased the antiviral activity of NKT-like cells in COVID-19 positive pregnant women, suggesting that ICOS-ICOSL may play an important role in the virus clearance by NKT-like cells.
Conclusions: During SARS-CoV-2 infection, NKT-like cells of pregnant women activated through ICOS-ICOSL pathway and played an important role in the antiviral response.
Keywords: COVID-19, CD3+CD56+ NKT-like cells, Pregnancy, ICOS
Introduction
As a highly pathogenic virus, SARS-CoV-2 infection can lead to acute immune activation and long-term clinical sequelae [1]. During infection, SARS-CoV-2 can cause changes in the autophagolysosomal pathway, in which ORF7a can evade the degradation of host autophagic lysosomes [2, 3]. While pregnancy develops a special immunological challenge because a genetically nonself fetus must be supported within the female [4]. Immune tolerance at the maternal-fetal interface during pregnancy is important for maintaining systemic immune homeostasis [5]. The immune response of pregnant women to SARS-CoV-2 infection remains to be explained. Studies showed that symptomatic pregnant women had higher IgG, IgM, and IgA titers than the asymptomatic ones [6, 7]. For cellular immune responses, the level of NK cell and γδ T cell activation is comparable between SARS-CoV-2 infected pregnant women and healthy pregnancies [8]. Previous data show that pregnant women with COVID-19 could induce virus-specific T cell responses and tolerogenic myeloid dendritic cells activation in pregnant women [9]. It is urgent to study the antiviral cellular responses and lymphocytes activation between healthy pregnancies and SARS-CoV-2 infected pregnant women.
Human CD3+CD56+ NKT-like cells are a distinct population of T cells which exhibit both innate and adaptive immunity characters. NKT-like cells participate in antiviral immune responses and show high killing potential against tumor cells [10, 11]. In HIV infections, activated NKT-like cells could effectively reduce the viral load in serum by secreting IFN-γ and CD107a [12]. Moreover, recovered hepatitis E virus (HEV) patients exhibited more activated phenotype of CD16+ NKT-like cells in their peripheral blood, indicating NKT-like cells may also contribute to controlling of HEV infection [13]. In COVID-19, previous reports have showed that there was a reduced percentage of CD3+CD56+ NKT like cells in the peripheral blood of severe group compared to those of mild group, and circulating NKT-like cell frequency could be identified as a predictive biomarker for clinical outcome [14, 15]. The phenotypic and functional changes of NKT-like cells in COVID-19 have not been elucidated, nor have they been reported in pregnant women infected with COVID-19.
Inducible costimulatory (ICOS), an important member of CD28 family, is usually expressed on activated T cells. ICOS ligand (ICOSL), which belongs to B7 family, is expressed on B cells, professional antigen-presenting cells and nonlymphoid cells. ICOS signaling is particularly important for complete germinal center development, T cell-dependent B cell responses and stimulating effector T cell responses. ICOS signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation [16]. ICOS pathway in CD8+ T cells contribute to the formation of tissue-resident memory T cells [17]. Besides, ICOS pathway helps to improve the persistence of CD8+ T cells in CAR-T therapy of tumor [18]. In our study, we found ICOS of NKT-like cells engaged with ICOSL on plasmacytoid DCs, and promote the antiviral function of NKT-like cells in pregnant women with COVID-19.
Methods
Sample collection and treatment
Blood samples of pregnant women and non-pregnant women with COVID-19 were collected from the First Affiliated Hospital of Anhui Medical University from December 2022 to May 2024. Non-pregnant women with COVID-19 (COVID-19 group) and pregnant women with COVID-19 (PCOVID-19 group) were diagnosed according to SARS-CoV-2 RNA positive by reverse transcriptase polymerase chain reaction (Daan, China). All enrolled COVID-19 positive women were symptomatic. The main symptoms were high fever (>37.5°C), respiratory infections and muscle soreness. Blood samples from COVID-19 group and PCOVID-19 group were collected 2-3 days after the onset of the disease. Convalescent pregnant women (PConvalescent group) were chosen at least 2 months after the SARS-CoV-2 RNA test turned negative. PConvalescent group didn't have infection symptoms nor fever. The healthy pregnant women (PHC group) samples were collected from women who underwent antenatal examination and tested negative for SARS-CoV-2 and other infectious diseases in the First Affiliated Hospital of Anhui Medical University and Anhui Provincial Maternity and Child Health Hospital. Healthy non-pregnant women (HC group) and COVID-19 group were all women of reproductive age.
After 2ml of peripheral blood was mixed in an EDTA anticoagulant tube, neutrophils, white blood cells, lymphocytes, monocytes, platelets and hemoglobin were detected by XN9000 automatic detector (Sysmex, Japan) and its corresponding reagents (all from Sysmex, Japan). Peripheral blood mononuclear cells were obtained by ficoll density gradient centrifugation or hemolysin treatment. Then the cells were labeled with antibodies for flow cytometry detection and, otherwise, were mixed with frozen solution and preserved at -80°C until the day of the experiment.
Study approval
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of The First Affiliated Hospital of Anhui Medical University. The patients/participants provided their written informed consent to participate in this study (Quick-PJ 2023-13-39).
Flow cytometry
For surface marker detection, the obtained PBMCs were labeled with flow cytometric antibodies, washed and detected on flow cytometer (BD FACSCanto plus, BD Biosciences). For intracellular staining, PBMCs were stimulated by PMA (50 ng/ml, Multisciences, China) and ionomycin (1 μg/ml, Sigma, USA) at 37 °C for 3.5 hours. Monensin (2.5 μg/ml; Multisciences, China) was added at the last hour. Then cells were washed, labeled with surface markers, fixed, permeabilized, stained with cytokine antibodies, and finally detected by flow cytometer. The data were analyzed by FlowJo VX analysis. The antibodies used in the study were as follow: Alexa Fluor 488-anti-NKG2A, PerCP-Cy5.5-anti-CD69, BV510-anti-Tim-3, PE-Cy7-anti-CD107a, BV605-anti-TIGIT, FITC-anti-ICOS, PerCP-Cy5.5-anti-HLA-DR, PE-anti-ICOSL and PerCP-Cy5.5-anti-CD3, APC-R700-anti-CD123 were from Biolegend. BV421-anti-PD-1, BV510-anti-CD3, PE-Cy7-anti-CD3, APC-R700-anti-CD56, BV605-anti-CD3, APC-H7-anti-CD8, BV510-anti-Granzyme B, BB700-anti-IFN-γ, BV510-anti-CD4, PE-anti-NKp46, BV605-anti-CD14, PE-CY7-anti-CD16, BV421-anti-CD80, BV421-anti-CD19, PE-anti-Annexin V, PerCP-Cy5.5-anti-7AAD, BV421-anti-Granzyme B, APC-anti-Ki67, PerCP-Cy5.5-anti-IFN-γ, APC-anti-CD107a, BV421-anti-CD56, APC-R700-anti-CD8, BV421-anti-CD275, PE-anti-CD123, APC-anti-CD56, APC-anti-CD20, APC-anti-CD19, APC-anti-CD3 and APC-anti-CD66b were from BD Biosciences. FITC-anti-NKG2A was from Miltenyi Biotec. FITC-anti-CD11c was from eBioscience.
Cytometric bead array (CBA) assay
The soluble cytokines were detected with the detection kit of IFN-γ/ IL-1β/ IL-2/ IL-4/ IL-5/ IL-6/ IL-8/ IL-10/ IL-12p70/ IL-17A/ IL-17F/ IL-22/ TNF-α/ TNF-β (QuantoBio, China). The samples are processed in accordance with the instructions and then detected on BEAMDIAG (China).
In vitro blocking assay
First, mouse anti-human CD3(5μg/ml, Clone: OKT3, Biolegend) was added in 96-well plates and incubated overnight at 4°C. Then the liquid was discarded on the second day. The isolated PBMCs were suspended in 1640 culture medium (1.5% HEPES, 1% streptomycin/ penicillin, 10% fetal bovine serum). Anti-ICOS (2.5μg/ml, Clone: 669214, biotechne) or mouse IgG was added in the corresponding wells. The cells were cultured at 37°C, 5% CO2 for 72 hours. Then the cells were harvested and washed by PBS. Later, the cells underwent conventional cytokine antibodies labeling, and were finally tested by flow cytometer. Considering the internalization of TCR/CD3 during T cell activation with anti-CD3, we chose to label the fluorescent antibody (PerCP-Cy5.5-anti-CD3, Clone: HIT3a, Biolegend) differently from the stimulated anti-CD3 clone.
Data analysis
All data were represented as mean ± standard error of the mean (SEM) and analyzed by GraphPad Prism 9.4.1 software. According to the distribution of data, it was determined that one-way ANOVA or the Kruskal-Walli's test was used for data analysis, and paired t-tests were used in the blocking assay. P value< 0.05 was considered statistically significant.
Results
Pregnant women with COVID-19 did not get cytokine storms
A total of 18 healthy non-pregnant women, 13 non-pregnant women with COVID-19, 37 healthy pregnant women, 32 pregnant women with COVID-19 and 30 convalescent pregnant women were enrolled in this study. The clinical characteristics of healthy non-pregnant women (HC group), non-pregnant women with COVID-19 (COVID-19 group), healthy pregnant women (PHC group), pregnant women with COVID-19 (PCOVID-19 group), and convalescent pregnancies (PConvalescent group) are shown in Table 1. The minimum gestational age of the selected samples was 6w+4 and the maximum gestational age was 38w+5. Pregnant women in the second and third trimester were the majority of the samples selected in the experiment. The level of alanine aminotransferase and aspartate aminotransferase didn't increase in pregnant women with COVID-19, suggesting the liver function was not impaired. At the same time, we found that the D-dimer of pregnant women was significantly higher than that of healthy women, but there was no difference between infected pregnant women and healthy pregnant women. The immune status of pregnant women changes significantly. A pro-inflammatory state predominates during the first trimester mainly to protect the mother, followed by the second trimester which is predominated by the anti-inflammatory state, and lastly, the inflammatory environment returns to induce childbirth in the third trimesters [19-21]. Although the anti-inflammatory state can promote the development of the fetus, it also leaves the mother in an immunosuppressive state and more susceptible to infection [22, 23]. Most of the previous literature has reported the cytokine storm in COVID-19 patients, and it is more obvious in severe patients [24, 25]. We detected the level of serum cytokines during the three trimesters and found that the cytokine storms did not occur in pregnant women with COVID-19 (Figure S1A-N). Except for IL-8 decreased in the second trimester after infected with SARS-CoV-2, IL-2, TNF-α, TNF-β, IFN-γ, IL-4, IL-5, IL-6, IL-10, IL-17A, IL-17F, IL-22, IL-1β and IL-12p70 didn't change in the three trimesters between healthy pregnancies and pregnant women with COVID-19.
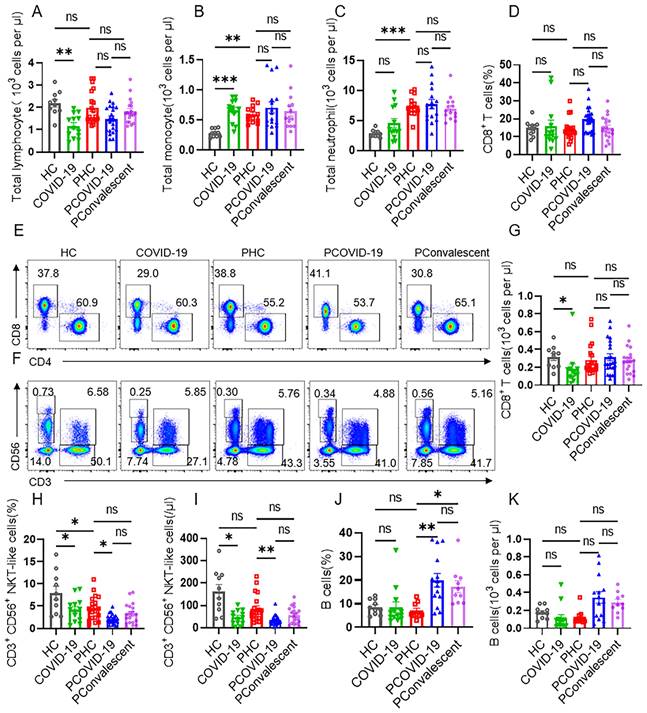
CD3+CD56+ NKT-like cells decrease dramatically in pregnant women with COVID-19 compared with healthy pregnant women
Despite the fact that lymphocytes decreased seriously in non-pregnant people during COVID-19, we found there was no statistical difference in the cellularity of total lymphocytes among PHC group, PCOVID-19 group and PConvalescent group (Figure 1A). After pregnancy, there is a physiological increase in monocytes and neutrophils (Figure 1B-C), which is consistent with other reports [26]. Compared to PHC group, the numbers of monocytes and neutrophils didn't change in pregnant women after SARS-CoV-2 infection (Figure 1B-C). T cells are important cells against virus infection in adaptive immunity. We found no changes in the proportion and cellularity of T cells, CD4+ T cells and CD8+ T cells between PHC group and HC group. The proportion and absolute count of total T cells and CD4+ T cells in COVID-19 group showed a significant decrease compared to HC group. But there were not statistically significant among the three pregnant groups (Figure S2A-D). Despite an increased absolute count of CD8+ T cells was observed, but the proportion count of CD8+ T cells did not differ significantly in the non-pregnant groups, possibly because of the downward trend in the total lymphocytes number (Figure 1D-E and 1G). COVID-19 is an acute disease of rapid onset. Therefore, we detected the cells of innate immunity next. We found that the proportion and cellularity of the CD56bri natural killer (NK) cells and CD56dim NK cells were comparable among the five groups (Figure S2E-H). However, the proportion and absolute count of NKT-like cells of the COVID-19 group were significantly lower than those of HC group (Figure 1F and 1H-I). The proportion of NKT-like cells in PHC group also showed a slight decrease compared to HC group, but there was no difference in absolute counts. Combined with these two effects, the proportion and absolute count of NKT-like cells in infected COVID-19 positive pregnant women showed a more obvious downward trend compared with the PHC group (Figure 1F and 1H-I). In addition, we found that the number and proportion of B cells in PCOVID-19 group were higher than those in PHC group, indicating an activated humoral immune response after SARS-CoV-2 infection (Figure 1J-K).
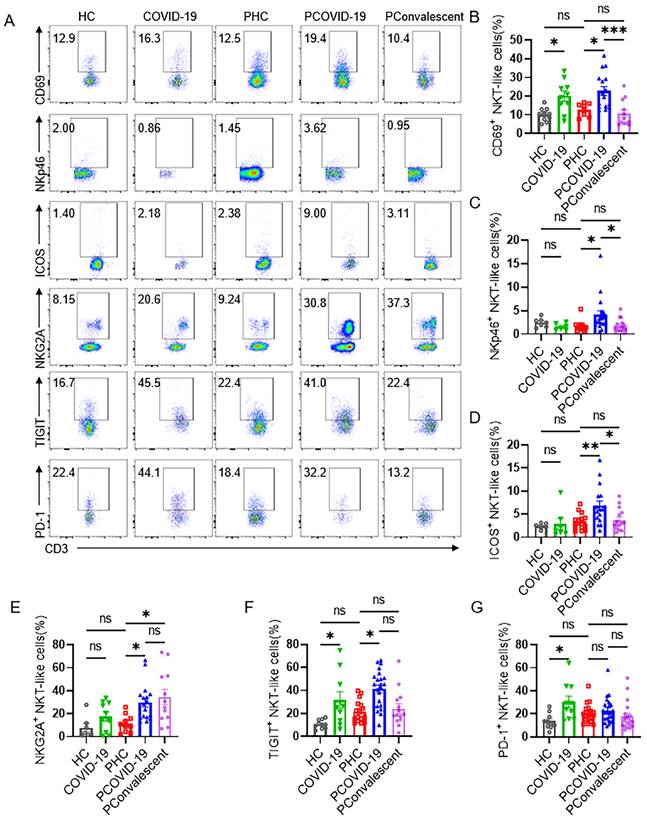
NKT-like cells in pregnant women with COVID-19 express high level of co-stimulating receptor ICOS
Further, we detected the phenotype of CD3+CD56+ NKT-like cells and found that the expression of activating receptor CD69, increased remarkably on NKT-like cells of pregnant women with COVID-19 compared with PHC group, non-pregnant women infected was also significantly higher than that in HC group (Figure 2A-B). NKp46, one of the important NK cell receptors, was also expressed higher on NKT-like cells in PCOVID-19 group, showing an activated state (Figure 2A and 2C). Co-stimulatory molecule ICOS augments T cell differentiation and cytokine secretion, and provides critical signals for antibody production [27], so we evaluated ICOS and found NKT-like cells of pregnant women increased the expression of the ICOS during COVID-19 (Figure 2A and 2D). In addition, we also found that there was no difference in the expression of ICOS on CD56bri NK, CD56dim NK cells, CD4+ T and CD8+ T cells between PCOVID-19 group and PHC group (Figure S3A-D). Lymphocytes increased their co-inhibitory receptor expression to avoid excessive activation and subsequent apoptosis in COVID-19 and other viral infections [28-30]. In our study, we also observed that the expression of inhibitory receptors NKG2A and TIGIT on NKT-like cells increased significantly in pregnant women with COVID-19 compared with PHC group (Figure 2A, 2E-F). There was no significant difference in the expression of PD-1 among the three pregnant groups (Figure 2G). Moreover, we found that the immunophenotype of PHC group was comparable with that of HC group, indicating the physiology of pregnancy did not significantly change the immune status of NKT-like cells.
Clinical characteristics of healthy non-pregnant women, non-pregnant women with COVID-19, healthy pregnant women, pregnant women with COVID-19 and convalescent group.
| HC n=18 | COVID-19 n=13 | PHC n=37 | PCOVID-19 n=32 | PConvalescent n=30 | P value | |
|---|---|---|---|---|---|---|
| Age | 34.61 (34.5) | 42.92 (41) | 30.89 (31) | 29.58 (30) | 30.53 (30.5) | 0.033 |
| Gestational weeks | ||||||
| First trimester (<13+6 w) | 6/37 (16.21%) | 7/32 (21.88%) | 1/30 (3.33%) | |||
| Second trimester (14+0 w-27+6 w) | 18/37 (48.65%) | 11/32 (34.37%) | 24/30 (80%) | |||
| Third trimester (28+0 w-40+6 w) | 13/37 (35.14%) | 14/32 (43.75%) | 5/30 (16.67%) | |||
| Comorbidities | ||||||
| Diabetes | 0 | 0 | 2/37 (5.41%) | 1/32 (3.13%) | 1/30 (3.33%) | |
| Hypertension | 0 | 0 | 1/37 (2.7%) | 1/32 (3.13%) | 0 | |
| laboratory index | ||||||
| Alanine aminotransferase, U/L | 16.67 (15) | 39.83 (17) | 14.17 (10.5) | 26.82 (15) a | 19.1 (17) | 0.0078 |
| Aspartate aminotransferase, U/L | 23.5 (18.5) | 33.58 (31.8) | 19.63 (18) | 27.35 (20.5) | 20.9 (19) | 0.1309 |
| Alkaline phosphatase, U/L | 61.79 (62) | 83.72 (76) | 98.79 (76) | 104.41 (97.5) | 64.6 (63.5) | 0.0110 |
| C-reactive protein, mg/L | 2.7 (2.62) b | 14.41 (9.9) | 4.01 (4.55) | 21.59 (6.16) | 4.65 (4.51) | 0.0012 |
| D-dimer, mg/L | 0.28 (0.28) c | 0.88 (1.03) | 1.23 (1) | 1.94 (1.57) | 1.01 (0.92) | <0.0001 |
Skewed distribution: Median (IQR).
a: Comparison between PCOVID-19 group and PConvalescent group: P < 0.05
b: Comparison between HC group and COVID-19 group: P < 0.05.
c: Comparison between HC group and PHC group: P < 0.05
CD3+CD56+ NKT-like cells decrease dramatically in pregnant women with COVID-19 compared with healthy pregnant women. (A-C) The cellularity of lymphocytes, monocytes and neutrophils in peripheral blood of healthy control (HC group), non-pregnant women with COVID-19 (COVID-19 group), healthy pregnant women control (PHC group), pregnant women with COVID-19 (PCOVID-19 group) and convalescent patients (PConvalescent group). (E-F) Representative flow cytometry graphs of CD4+ T cells, CD8+ T cells, CD56bri NK cells, CD56dim NK cells, CD3+CD56+ NKT-like cells and T cells. (D, G-K) Flow cytometry detected the percentages and absolute number of (D and G) CD8+ T cells, (H-I) CD3+CD56+ NKT-like T cells and (J-K) CD19+ B cells among HC group, COVID-19 group, PHC group, PCOVID-19 group, and PConvalescent group. Data are shown as mean ± SEM. One-way ANOVA was used to determine statistical significance. *P< 0.05; **P <0.01; ***P <0.001; ns, not significant.
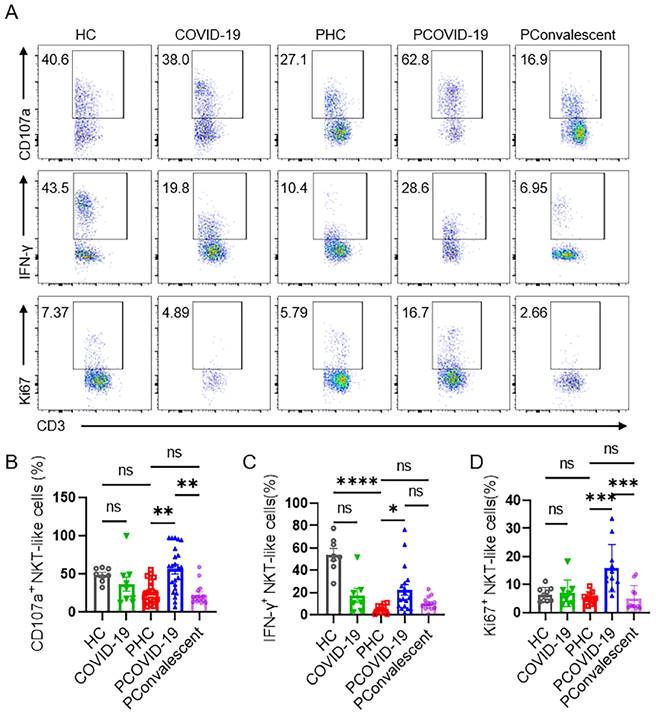
Escalating antiviral function and proliferation ability of NKT-like cells in pregnant women with COVID-19
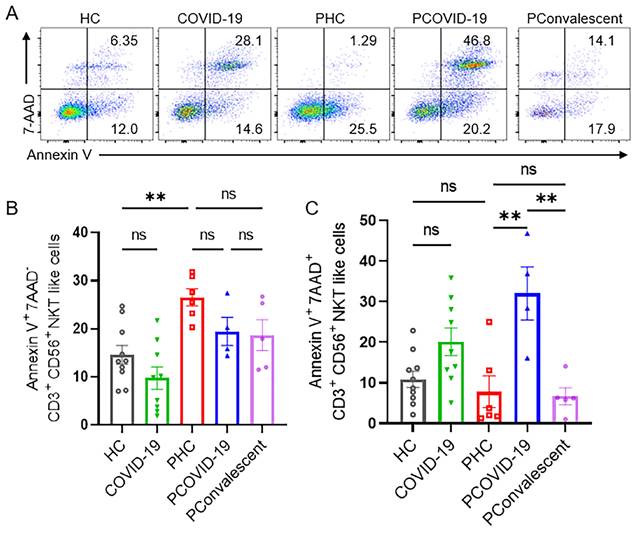
We monitored the degranulation ability and IFN-γ secretion of NKT-like cells in pregnant patients during SARS-CoV-2 infection. As an important marker of cytolysis, we found that CD107a expressed on NKT-like cells of PCOVID-19 group significantly increased compared with healthy pregnant women, and recovered in convalescence (Figure 3A-B). IFN-γ expressed by NKT-like cells was significantly decreased in women after pregnancy (Figure 3A and 3C). Besides, compared with the PHC group, IFN-γ secreted by NKT-like cells also showed an increased trend in PCOVID-19 group, and recovered in convalescence (Figure 3A and 3C). In addition, proliferation level is an important marker for lymphocytes activation, so we measured the proliferation marker Ki67. We found NKT-like cells of pregnant women showed high proliferation ability after SARS-CoV-2 infection and recovered in convalescent stage (Figure 3A and 3D). At the same time, we detected changes of apoptosis levels in COVID-19 positive pregnant women. Annexin V and 7-AAD staining showed NKT-like cells of pregnant women with COVID-19 got an early apoptotic level comparable with PHC group. However, we also found that the level of early apoptosis in healthy pregnant women was significantly higher than that in healthy non-pregnant women (Figure 4A-B). Moreover, we observed a significant elevation on late apoptosis levels in PCOVID-19 group compared to PHC group, which subsequently returned to normal levels in the convalescence (Figure 4A and 4C).
The phenotype of CD3+CD56+ NKT-like cells in COVID-19 positive pregnant women. (A) The representative flow cytometry graphs of CD69+, NKp46+, ICOS+, NKG2A+, TIGIT+, PD-1+ NKT-like cells among the HC group, COVID-19 group, PHC group, PCOVID-19 group and PConvalescent group. (B-G) The expression of (B) CD69, (C) NKp46, (D) ICOS, (E) NKG2A, (F) TIGIT and (G) PD-1 on NKT-like cells among the HC group, COVID-19 group, PHC group, PCOVID-19 group and PConvalescent group. Results are shown as mean ± SEM. One-way ANOVA was used to determine statistical significance. *P< 0.05; **P <0.01; ***P <0.001; ns, not significant.
CD56bri NK cells are abundant cytokine producers after activation. However, we didn't observe significant differences in the expression of CD107a and Ki67 of this subpopulation among the five groups (Figure S4A, S4C). The expression of IFN-γ by CD56bri NK cells decreased after pregnancy in a physiological manner (Figure S4B), the same as CD56dim NK cells, CD4+ T and CD8+ T cells (Figure S4E, S5B, S5E). CD56dim NK cells, the main subgroup of NK cells, have robust cytotoxicity [31]. We found that the level of CD107a were higher on CD56dim NK cells of COVID-19 positive pregnant women than those of PHC group, and decreased in convalescent group (Figure S4D). CD4+ T and CD8+ T cells showed the similar trend in the expression of CD107a as CD56dim NK cells (Figure S5A, S5D). Ki67 expressed by CD56dim NK cells, CD4+ T and CD8+ T cells didn't change after COVID-19 (Figure S4F, S5C, S5F). Above data demonstrated that NKT-like cells in pregnant women escalate their antiviral function and proliferation ability, and may play an important role in defending against SARS-CoV-2 infection.
NKT-like cells of pregnant women enhanced their antiviral ability and proliferation rate after SARS-CoV-2 infection. (A) The representative flow cytometry graphs of CD107a+ NKT-like cells, IFN-γ+ NKT-like cells and Ki67+ NKT-like cells among the HC group, COVID-19 group, PHC group, PCOVID-19 group, PConvalescent group. (B-D) The expression of (B) CD107a, (C) IFN-γ and (D) Ki67 on NKT-like cells among the HC group, COVID-19 group, PHC group, PCOVID-19 group, PConvalescent group. Results are shown as mean ± SEM. One-way ANOVA was used to determine statistical significance. *P< 0.05; **P <0.01; ***P <0.001; ****P <0.001; ns, not significant.
ICOSL on plasmacytoid DCs engaged with NKT-like cells in COVID-19 positive pregnant women
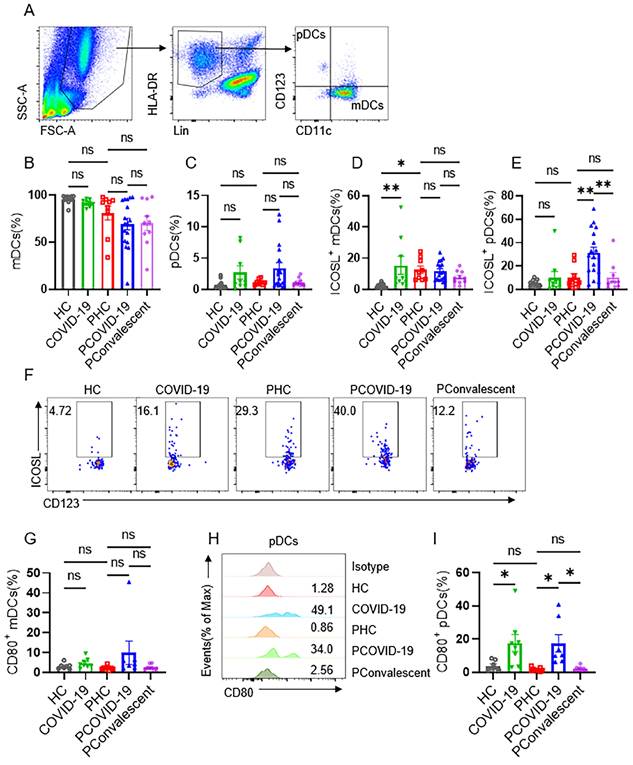
ICOS-ICOSL pathway is important for stimulating effector immune response. We further examined the expression of ICOSL in COVID-19 positive pregnant women. We first detected monocytes and found the proportion of CD14+CD16- (classical), CD14+CD16+ (intermediate) and CD14-CD16+ (non-classical) monocytes group didn't change among the five groups (Figure S6A-D). Nor did the expression of ICOSL on monocytes (Figure S6E-G). Then, we measured dendritic cells (DCs), which contains two main subsets: myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). We found that the ratio of mDCs and pDCs did not differ among the five groups (Figure 5A-C). The expression of ICOSL in mDCs in COVID-19 group was significantly higher than that in HC group, and ICOSL expression in mDCs in PHC group was significantly higher than that in HC group (Figure 5D). But there was no difference in the expression of ICOSL on mDCs among the three pregnant groups (Figure 5D). While ICOSL expression on pDCs of PCOVID-19 group increased significantly compared with PHC group, and returned to normal level in convalescent group (Figure 5E-F). At the same time, we also detected the expression of CD80 on DCs, and found that CD80 showed a significant increase on pDCs of pregnant and non-pregnant patients infected with COVID-19 (Figure 5H-I). This indicates that pDCs activate during COVID-19 invasion, but this activation is not specific to pregnant women. These data suggest that pDCs may activate NKT-like cells through ICOS-ICOSL pathway in pregnant women with COVID-19.
ICOS blockade reduce the effector function of NKT-like cells in COVID-19 positive pregnant women
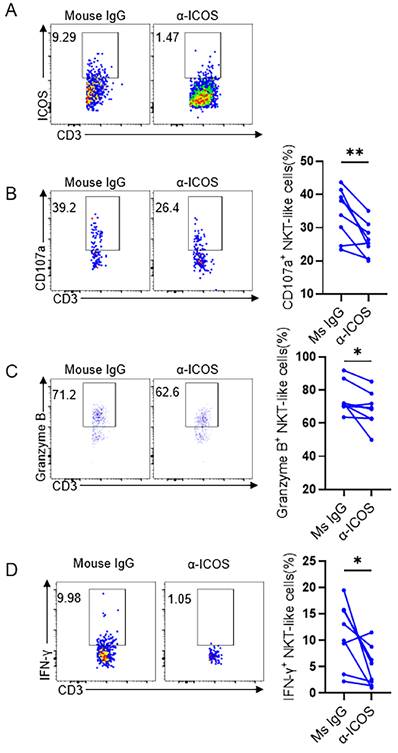
The up-regulation of ICOS on NKT-like cells and the increased expression of the ligand ICOSL on pDCs suggest that ICOS-ICOSL pathway may be a potential way of NKT-like cell activation. To assess this hypothesis, ICOS blockade were taken on isolated PBMCs which were then stimulated by anti-CD3 in vitro for 72 hours in pregnant women with COVID-19 (Figure 6A). We found that the expression of CD107a, granzyme B and IFN-γ on NKT-like cells was reduced in the presence of anti-ICOS blocking antibodies (Figure 6B-D), suggesting that ICOS-ICOSL pathway indeed play an important role in stimulating effector response of NKT-like cells for pregnant women defending against SARS-CoV-2 infection.
Discussion
We found NKT-like cells of pregnant women exhibited a more activated phenotype and stronger cytotoxic ability after SARS-CoV-2 infection. ICOS is well known for its involvement in B cell differentiation and providing critical T cell help to B cells. We found NKT-like cells may engage with pDCs through ICOS-ICOSL pathway in pregnant women with COVID-19. Our study provides insights in clinical treatment and vaccine design strategies for pregnant women.
The apoptotic levels of NKT-like cells among the HC group, COVID-19 group, PHC group, PCOVID-19 group and PConvalescent group. (A) Representative flow graphs of Annexin V and 7-AAD staining of NKT-like cells. (B-C) The statistical diagram of (B) Annexin V+7-AAD- NKT-like cells and (C) Annexin V+7-AAD+ NKT-like cells among the HC group, COVID-19 group, PHC group, PCOVID-19 group and PConvalescent group. Results are shown as mean ± SEM. One-way ANOVA was used to determine statistical significance **P <0.01; ns, not significant.
Expression of ICOSL on plasmacytoid DCs and myeloid DCs in COVID-19 positive pregnant women. (A) Gating strategy for myeloid DCs (mDCs): Lin- (CD3, CD19, CD20, CD56, CD66b) HLA-DR+CD123-CD11c+ and plasmacytoid DCs (pDCs): Lin- HLA-DR+CD123+CD11c-. (B-C) The proportion of (B) mDCs and (C) pDCs among the HC group, COVID-19 group, PHC group, PCOVID-19 group, PConvalescent group. (D-E) The expression of ICOSL on (D) mDCs and (E) pDCs among the HC group, COVID-19 group, PHC group, PCOVID-19 group, PConvalescent group. (F)The representative flow cytometry graphs of ICOSL on pDCs among the HC group, COVID-19 group, PHC group, PCOVID-19 group and PConvalescent group. Expression of CD80 on (G) mDCs and (I) pDCs among the HC group, COVID-19 group, PHC group, PCOVID-19 group and PConvalescent group. (H) Representative histogram graphs for the expression of CD80 on pDCs. Results are shown as mean ± SEM. One-way ANOVA was used to determine statistical significance. *P <0.05; **P <0.01; ns, not significant.
Previous reports show that the body develops severe cytokine storms after SARS-CoV-2 infection and manifests multiple organ damage in fatal cases [24, 32]. While pregnant women, show a more complicated humoral immune response during COVID-19. As the time progressed from the first trimester to the second trimester of pregnancy, Th1 cell-mediated immunity changes to Th2 cell dominated environment. Both pro-inflammatory and anti-inflammatory immunity were activated in parallel during SARS-CoV-2 infection, thus avoiding a potential pro-inflammatory cytokine storm. This coincidence of immunomodulation during pregnancy may protect pregnant women with COVID-19 from cytokine storms and the development of acute respiratory distress syndrome [33]. We didn't find any Th1/Th2/Th17 cytokine level changes in our study. But Valeria Garcia-Flores et al. showed that SARS-CoV-2-positive pregnant women had a mild inflammatory response, possibly due to the presence of severely ill patients [34].
B cells play an important role in the defense against viral infections such as SARS-CoV-2 [35]. As disease severity progress, pregnant women infected with COVID-19 got an increase in naïve, immature B-cells and a decrease in memory B-cells, whose maturation capacity to form plasma blast cells remains fine [36]. For regulatory B cells, on one hand, the frequency of CD24hiCD38hi regulatory B cells is reduced in critically ill COVID-19 patients and their ability to secrete IL-10 was also impaired [37]. On the other hand, Breg is important for the maintenance of immune tolerance in pregnant women during early pregnancy [38]. The combination of these two aspects may develop a compromised anti-SARS-CoV-2 immunity in SARS-CoV-2-positive pregnant women. The function of Breg cells in COVID-19 during pregnancy need to be further explored.
As a bridge between innate and adaptive immunity, NKT-like cells express a high-density TCR-CD3 complex and a low level of co-stimulating molecule CD28, and could mediate cytotoxicity restricted in a non-MHC dependent pathway [39, 40]. There is also viewpoint suggesting CD28 appears to play a predominant role in priming T cells during Th2 immune response, whereas ICOS is more likely to regulate effector T cell responses [41]. In our study, ICOS-ICOSL pathway can activate NKT-like cells in antiviral response. Another co-stimulatory molecule, CD137, can also induce the expansion and activation of NKT-like cells [42]. In the design of CAR-T co-stimulatory domains, the incorporation of ICOS significantly increased the persistence of T cells than the presence of CD28 or CD137 alone [18, 43, 44]. The role of other costimulatory signals for NKT cells to exert effector response needs to be further explored.
ICOS blockade reduced the cytokine secretion level of NKT-like cells in COVID-19 positive pregnant women. (A) In vitro blocking effect of anti-ICOS on NKT-like cells. (B-D) The frequency of (B) CD107a+ NKT-like cells, (C) Granzyme B+ NKT-like cells and (D) IFN-γ+ NKT-like cells of COVID-19 positive pregnant women in the presence of anti-ICOS mAb or isotype mAb. Data are shown as mean ± SEM. Paired Student's t-tests were performed. *P< 0.05; **P <0.01; ns, not significant.
We found that the cellularity and absolute count of NKT-like cells of COVID-19 positive pregnant women were lowest among the five groups. Although the proliferation of NKT-like cells was increased, pregnancy is physiologically associated with increased levels of early apoptosis, and SARS-CoV-2 infection increased the late apoptosis of NKT-like cells (Annexin V+7AAD+). The combination of high levels of late apoptosis and physiological changes during pregnancy may ultimately lead to the reduction of NKT-like cells in the peripheral blood of COVID-19 positive pregnant women. Besides, in PConvalescent group, we found that the phenotype and antiviral ability of NKT-like cells did not recover to the level of healthy pregnant women. It has been reported that T cells still showed a slightly enhanced activation and proliferation rate after recovering from COVID-19 for months, indicating that these individuals were in a phase of ongoing restoration of immune homeostasis [45], so the function of NKT-like cells requires long-term dynamic monitoring to find when they return to normal.
Because the large blood volume required to obtain pure pDCs and NKT-like cells by flow sorting is limited by ethics, we cannot obtain sufficient blood volume from pregnant women who have babies. Previous studies have shown that compared with mDCs, pDCs preferentially up-regulate the expression of ICOSL upon virus antigen stimulation [46]. Our data also showed ICOSL expression on pDCs increased dramatically in PCOVID-19 group. For mDCs, we found that the expression of ICOSL on mDCs was significantly higher in PHC group compared to HC group, indicating the process of pregnancy can lead to the increased expression of ICOSL on mDCs. While the ICOSL expression between PCOVID-19 group and PHC group showed no difference. We also found that the expression of ICOSL also showed an increased trend in monocytes during COVID-19, although there was no statistical difference. It has been previously reported that circulating monocytes can migrate to tissues and differentiate into dendritic cells and macrophages [20, 47]. So we speculate that monocytes may also play an indispensable role in activating lymphocytes through ICOS-ICOSL pathway.
Plasmacytoid dendritic cells are a unique group of cells in the immune system that produce large amounts of type I interferon α to resist viruses [48, 49]. pDCs are the dominant IFN-α-producing cells in response to SARS-CoV-2 and can lead to macrophage activation severe COVID-19 patients [50, 51]. Studies have also shown that IFN-α is significantly elevated in COVID-19 positive mothers and in cord blood of their neonates [36]. It has also been reported that IFN-α released by pDCs can enhance the cytolytic activity of CD56+ T cells [52]. Therefore, we speculate that pDCs may also enhance the antiviral activity of NKT-like cells by secreting IFN-α in SARS-CoV-2-infected pregnant women, which needs to be further studied.
SARS-CoV-2 infection in pregnant women has also been associated with a mild cytokine response in cord blood [34]. Previous studies have shown that in pregnant women infected with COVID-19, IgG levels in peripheral blood and umbilical cord blood are high, and the levels in symptomatic patients are higher than those in asymptomatic patients [6, 8]. TNF-α, IFN-α, IFN-γ, and IL-6 were significantly elevated in both the peripheral blood of COVID-19 positive pregnant women and cord blood, which indicate both threat to mother and fetus [36]. In addition, unique inflammatory responses against the SARS-CoV-2 are induced at the maternal-fetal interface, and these inflammatory responses are mainly controlled by maternal T cells and fetal stromal cells [34]. The immune status changes of cord blood in COVID-19 positive pregnant women are hoped to be further explored in the future.
In conclusion, we found that NKT-like cells activated and enhanced their antiviral activity obviously in SARS-CoV-2 infection during pregnancy. NKT-like cells may play an important role in the resistance to SARS-CoV-2 virus by engaging with pDCs through ICOS-ICOSL pathway. ICOS blockade significantly relieved the antiviral activity of NKT-like cells. Because of the physiological change in pregnant women, pregnant women infected SARS-CoV-2 showed complicated immune statuses. Our study has important clinical guidance for the treatment of COVID-19 in pregnant women.
Abbreviations
HC group: healthy non-pregnant women; COVID-19 group: non-pregnant women with COVID-19; PHC group: healthy pregnant women control; PCOVID-19 group: pregnant women with COVID-19; PConvalescent group: convalescent patients; Inducible costimulatory: ICOS; ICOS ligand: ICOSL; myeloid dendritic cells: mDCs; plasmacytoid dendritic cells: pDCs.
Supplementary Material
Supplementary figures.
Acknowledgements
Funding
This work was supported by the Key project of the Scientific Research Program of the Education Department of Anhui Province [grant number 2023AH053296], the Major Research Program of Natural Science Foundation of China [grant number 92269109], the Natural Science Foundation of Anhui Province [grant number 2208085Y29], the Outstanding Youth Project of the Scientific Research Foundation of the Education Department of Anhui Province of China [grant number 2023AH020048], and the Youth Project of Natural Science Foundation of Anhui Province [grant number 2008085QH373].
Data availability
The values of all statistical charts in the article are available in the supporting data in the attached table.
Author contributions
Lu Zong and Meijuan Zheng carried out project administration, visualization, methodology and formal analysis. Lu Zong, Yuanling Zheng and Xiaojing Yu performed the experiments and carried out the data analyses. Xiaojing Yu, Ruoyu Huang, Xiaoran Dai collected specimens and analyzed the data. Lu Zong, Yuanling Zheng and Meijuan Zheng wrote the manuscript. Guoxiu Yan and Yuanhong Xu revised the manuscript. Lu Zong and Yuanling Zheng have contributed equally to this work and share first authorship.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Liang WZ, Wang SQ, Wang H, Li XS, Meng QX, Zhao Y. et al. When 3D genome technology meets viral infection, including SARS-CoV-2. Journal of Medical Virology. 2022;94:5627-39
2. Li S, Li XB, Liang HW, Yu KK, Zhai JB, Xue MZ. et al. SARS-CoV-2 ORF7a blocked autophagy flux by intervening in the fusion between autophagosome and lysosome to promote viral infection and pathogenesis. Journal of Medical Virology. 2023;95:e29200
3. Li S, Zhang H, Li WC, Zhai JB, Li XB, Zheng CF. The role of SARS-CoV-2 ORF7a in autophagy flux disruption: implications for viral infection and pathogenesis. Autophagy. 2024;20:1449-1451
4. Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019;4:eaat6114
5. Xi CX, Yan ZH, Bai DD, Zhang YL, Wang BY, Han XX. et al. Immune rebalancing at the maternal-fetal interface of maternal SARS-CoV-2 infection during early pregnancy. Protein Cell. 2024;15:460-73
6. Martin-Vicente M, Carrasco I, Munoz-Gomez MJ, Lobo AH, Mas V, Vigil-Vazquez S. et al. Antibody levels to SARS-CoV-2 spike protein in mothers and children from delivery to six months later. Birth. 2023;50:418-27
7. Kubiak JM, Murphy EA, Yee J, Cagino KA, Friedlander RL, Glynn SM. et al. Severe acute respiratory syndrome coronavirus 2 serology levels in pregnant women and their neonates. Am J Obstet Gynecol. 2021;225:73 e1- e7
8. Habel JR, Chua BY, Kedzierski L, Selva KJ, Damelang T, Haycroft ER. et al. Immune profiling of SARS-CoV-2 infection during pregnancy reveals NK cell and gammadelta T cell perturbations. JCI Insight. 2023;8:e167157
9. Hsieh LE, Grifoni A, Dave H, Wang J, Johnson D, Zellner J. et al. SARS-CoV-2-specific T cell responses and immune regulation in infected pregnant women. J Reprod Immunol. 2022;149:103464
10. Tao L, Wang S, Kang G, Jiang S, Yin W, Zong L. et al. PD-1 blockade improves the anti-tumor potency of exhausted CD3(+)CD56(+) NKT-like cells in patients with primary hepatocellular carcinoma. Oncoimmunology. 2021;10:2002068
11. Li Z, Wu Y, Wang C, Zhang M. Mouse CD8(+)NKT-like cells exert dual cytotoxicity against mouse tumor cells and myeloid-derived suppressor cells. Cancer Immunol Immunother. 2019;68:1303-15
12. Jiang Y, Cui X, Cui C, Zhang J, Zhou F, Zhang Z. et al. The function of CD3+CD56+ NKT-like cells in HIV-infected individuals. Biomed Res Int. 2014;2014:863625
13. Das R, Tripathy A. Increased expressions of NKp44, NKp46 on NK/NKT-like cells are associated with impaired cytolytic function in self-limiting hepatitis E infection. Med Microbiol Immunol. 2014;203:303-14
14. Zingaropoli MA, Perri V, Pasculli P, Cogliati Dezza F, Nijhawan P, Savelloni G. et al. Major reduction of NKT cells in patients with severe COVID-19 pneumonia. Clin Immunol. 2021;222:108630
15. Kreutmair S, Unger S, Nunez NG, Ingelfinger F, Alberti C, De Feo D. et al. Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia. Immunity. 2022;55:366-75
16. Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E. et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity. 2015;42:239-51
17. Peng C, Huggins MA, Wanhainen KM, Knutson TP, Lu H, Georgiev H. et al. Engagement of the costimulatory molecule ICOS in tissues promotes establishment of CD8(+) tissue-resident memory T cells. Immunity. 2022;55:98-114 e5
18. Guedan S, Posey AD, Shaw C, Wing A, Da T, Patel PR. et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. Jci Insight. 2018;3:e96976
19. Forger F, Villiger PM. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat Rev Rheumatol. 2020;16:113-22
20. True H, Blanton M, Sureshchandra S, Messaoudi I. Monocytes and macrophages in pregnancy: The good, the bad, and the ugly. Immunol Rev. 2022;308:77-92
21. Cornish EF, Filipovic I, Asenius F, Williams DJ, McDonnell T. Innate Immune Responses to Acute Viral Infection During Pregnancy. Front Immunol. 2020;11:572567
22. Stanczyk P, Jachymski T, Sieroszewski P. COVID-19 during pregnancy, delivery and postpartum period based on EBM. Ginekol Pol. 2020;91:417-23
23. Obuchowska A, Standylo A, Obuchowska K, Kimber-Trojnar Z, Leszczynska-Gorzelak B. Cytokine Storms in the Course of COVID-19 and Haemophagocytic Lymphohistiocytosis in Pregnant and Postpartum Women. Biomolecules. 2021;11:1202
24. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250-6
25. Hoque MN, Sarkar MMH, Khan MA, Hossain MA, Hasan MI, Rahman MH. et al. Differential gene expression profiling reveals potential biomarkers and pharmacological compounds against SARS-CoV-2: Insights from machine learning and bioinformatics approaches. Front Immunol. 2022;13:918692
26. Dockree S, Shine B, Pavord S, Impey L, Vatish M. White blood cells in pregnancy: reference intervals for before and after delivery. EBioMedicine. 2021;74:103715
27. Sperling AI, Bluestone JA. ICOS costimulation: It's not just for TH2 cells anymore. Nat Immunol. 2001;2:573-4
28. Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J. et al. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A. 2018;115:4749-54
29. Rha MS, Jeong HW, Ko JH, Choi SJ, Seo IH, Lee JS. et al. PD-1-Expressing SARS-CoV-2-Specific CD8(+) T Cells Are Not Exhausted, but Functional in Patients with COVID-19. Immunity. 2021;54:44-52 e3
30. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D. et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-5
31. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633-40
32. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463-9
33. Chen G, Liao Q, Ai J, Yang B, Bai H, Chen J. et al. Immune Response to COVID-19 During Pregnancy. Front Immunol. 2021;12:675476
34. Garcia-Flores V, Romero R, Xu Y, Theis KR, Arenas-Hernandez M, Miller D. et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. 2022;13:320
35. Ripa M, Galli L, Poli A, Oltolini C, Spagnuolo V, Mastrangelo A. et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infec. 2021;27:451-7
36. Hora S, Pahwa P, Siddiqui H, Saxena A, Kashyap M, Sevak JK. et al. Metabolic alterations unravel the maternofetal immune responses with disease severity in pregnant women infected with SARS-CoV-2. J Med Virol. 2023;95:e29257
37. Saito S, Bozorgmehr N, Sligl W, Osman M, Elahi S. The Role of Coinhibitory Receptors in B Cell Dysregulation in SARS-CoV-2-Infected Individuals with Severe Disease. J Immunol. 2024;212:1540-52
38. Esteve-Sole A, Luo Y, Vlagea A, Deya-Martinez A, Yague J, Plaza-Martin AM. et al. B Regulatory Cells: Players in Pregnancy and Early Life. Int J Mol Sci. 2018;19:2099
39. Michel JJ, Turesson C, Lemster B, Atkins SR, Iclozan C, Bongartz T. et al. CD56-expressing T cells that have features of senescence are expanded in rheumatoid arthritis. Arthritis Rheum. 2007;56:43-57
40. Pita-Lopez ML, Pera A, Solana R. Adaptive Memory of Human NK-like CD8(+) T-Cells to Aging, and Viral and Tumor Antigens. Front Immunol. 2016;7:616
41. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515-48
42. Zhu BQ, Ju SW, Shu YQ. CD137 enhances cytotoxicity of CD3(+)CD56(+) cells and their capacities to induce CD4(+) Th1 responses. Biomed Pharmacother. 2009;63:509-16
43. Abreu TR, Fonseca NA, Goncalves N, Moreira JN. Current challenges and emerging opportunities of CAR-T cell therapies. J Control Release. 2020;319:246-61
44. Wang Y, Zhong K, Ke J, Chen X, Chen Y, Shu W. et al. Combined 4-1BB and ICOS co-stimulation improves anti-tumor efficacy and persistence of dual anti-CD19/CD20 chimeric antigen receptor T cells. Cytotherapy. 2021;23:715-23
45. Liu J, Yang X, Wang H, Li Z, Deng H, Liu J. et al. Analysis of the Long-Term Impact on Cellular Immunity in COVID-19-Recovered Individuals Reveals a Profound NKT Cell Impairment. mBio. 2021;12:e00085-21
46. Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA. et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105-15
47. Coillard A, Segura E. In vivo Differentiation of Human Monocytes. Front Immunol. 2019;10:1907
48. Cai C, Tang YD, Xu G, Zheng C. The crosstalk between viral RNA- and DNA-sensing mechanisms. Cell Mol Life Sci. 2021;78:7427-34
49. Van der Sluis RM, Holm CK, Jakobsen MR. Plasmacytoid dendritic cells during COVID-19: Ally or adversary? Cell Rep. 2022;40:111148
50. Laurent P, Yang C, Rendeiro AF, Nilsson-Payant BE, Carrau L, Chandar V. et al. Sensing of SARS-CoV-2 by pDCs and their subsequent production of IFN-I contribute to macrophage-induced cytokine storm during COVID-19. Sci Immunol. 2022;7:eadd4906
51. Venet M, Ribeiro MS, Décembre E, Bellomo A, Joshi G, Nuovo C. et al. Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Nature Communications. 2023;14:694
52. van Beek JJP, Florez-Grau G, Gorris MAJ, Mathan TSM, Schreibelt G, Bol KF. et al. Human pDCs Are Superior to cDC2s in Attracting Cytolytic Lymphocytes in Melanoma Patients Receiving DC Vaccination. Cell Rep. 2020;30:1027-38 e4
Author contact
![]() Corresponding authors: Lu Zong: Ph.D., Department of Clinical Laboratory, First Affiliated Hospital of Anhui Medical University, #218 Jixi Road, Hefei 230032, Anhui, China. Email: zongluustc.edu.cn. Meijuan Zheng: M.D., Ph.D., Department of Clinical Laboratory, First Affiliated Hospital of Anhui Medical University, #218 Jixi Road, Hefei 230022, China. E-mail: mjzhengustc.edu.cn.
Corresponding authors: Lu Zong: Ph.D., Department of Clinical Laboratory, First Affiliated Hospital of Anhui Medical University, #218 Jixi Road, Hefei 230032, Anhui, China. Email: zongluustc.edu.cn. Meijuan Zheng: M.D., Ph.D., Department of Clinical Laboratory, First Affiliated Hospital of Anhui Medical University, #218 Jixi Road, Hefei 230022, China. E-mail: mjzhengustc.edu.cn.

 Global reach, higher impact
Global reach, higher impact