Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(9):1790-1798. doi:10.7150/ijms.96326 This issue Cite
Research Paper
Association of maternal constipation and risk of atopic dermatitis in offspring
1. Children's Medical Center, Taichung Veterans General Hospital, Taichung, Taiwan.
2. Division of Pediatric Gastroenterology, Children's Medical Center, Taichung Veterans General Hospital, Taichung, Taiwan.
3. Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan.
4. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
5. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
6. Department of Allergy, Immunology & Rheumatology, Chung Shan Medical University Hospital, Taichung, Taiwan.
7. Institute of Medicine, College of Medicine, Chung Shan Medical University, Taichung, Taiwan.
8. Department of Nursing, Chung Shan Medical University, Taichung, Taiwan.
9. Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan.
Received 2024-3-16; Accepted 2024-6-12; Published 2024-7-8
Abstract
Objectives: Atopic dermatitis (AD) is a chronic and relapsing dermatologic disease that can affect individuals of all ages, including children and adults. The prevalence of AD has increased dramatically over the past few decades. AD may affect children's daily activities, increase their parents' stress, and increase health expenditure. Constipation is a worldwide issue and may affect the gut microbiome. Some research has indicated that constipation might be associated with risk of atopic disease. The primary objective of this retrospective cohort study was to extend and to explore the link between maternal constipation and risk of atopic dermatitis in offspring.
Methods: Using the Longitudinal Health Insurance Database, a subset of Taiwan's National Health Insurance Research Database, we identified 138,553 mothers with constipation and 138,553 matched controls between 2005 and 2016. Propensity score analysis was used matching birth year, child's sex, birth weight, gestational weeks, mode of delivery, maternal comorbidities, and antibiotics usage, with a ratio of 1:1. Multiple Cox regression and subgroup analyses were used to estimate the adjusted hazard ratio of child AD.
Results: The incidence of childhood AD was 66.17 per 1,000 person-years in constipated mothers. By adjusting child's sex, birth weight, gestational weeks, mode of delivery, maternal comorbidities, and received antibiotics, it was found that in children whose mother had constipation, there was a 1.26-fold risk of AD compared to the children of mothers without constipation (adjusted hazard ratio [aHR]: 1.26; 95% CI, 1.25-1.28). According to subgroup analyses, children in the maternal constipation group had a higher likelihood of AD irrespective of child's sex, birth weight, gestational weeks, mode of delivery, and with or without comorbidities, as well as usage of antibiotics during pregnancy. Compared to the non-constipated mothers, the aHR for the constipated mothers with laxative prescriptions <12 and ≥12 times within one year before the index date were 1.26; 95% CI, 1.24 -1.28 and 1.40; 95% CI, 1.29-1.52, respectively.
Conclusion: Maternal constipation was associated with an elevated risk of AD in offspring. Clinicians should be aware of the potential link to atopic dermatitis in the children of constipation in pregnant women and should treat gut patency issues during pregnancy. More study is needed to investigate the mechanisms of maternal constipation and atopic diseases in offspring.
Introduction
Atopic dermatitis (AD) is a prevalent chronic and recurrent inflammatory skin condition that affects a substantial number of infants and children. The prevalence of AD has increased during the 21st century in both developing and developed countries [1]. Atopic dermatitis (AD) imposes a substantial burden on patients throughout their lifespan, affecting their social, financial, and psychological well-being, and negatively impacting their overall quality of life [2].
AD is a multifactorial disorder associated with potential genes, including genetic predisposition, environmental risk factors, dysregulated innate and acquired immune responses, as well as impaired skin barrier function [3]. Clinical studies have provided evidence supporting the association between the composition of gut microbiota in early life and the development of the immune system, among other factors, in the context of AD [4]. The hygiene hypothesis proposes that decreased exposure to microorganisms in early life may impact the development of allergic diseases [5]. Maintaining a balanced immune response is considered a critical factor in protecting individuals who suffer from AD [6]. The majority of microorganisms in the human microbiome reside in the gastrointestinal tract [7,8]. The gut microbiome is believed to contribute to the development, persistence, and severity of AD through the gut-skin axis [9]. The dysbiosis of the gut microbiome, along with immune system imbalance, may contribute to the development of AD [4]. Microbial colonization in the body begins in earnest during the postpartum period, and this process becomes more established over time [10]. Human microbial colonization begins during fetal development and continues at birth, with infancy and early childhood being recognized as crucial and sensitive periods in the establishment of the gut microbiome [9, 11]. It has been shown that there are relationships among diet, nutrition, intestinal metabolism, and immune-related disease [12]. The gut microbiome has been found to influence the production of short-chain fatty acids (SCFAs), which have been identified as a potential link with AD [9, 13]. Interestingly, some researchers have suggested constipation might be linked to allergic disease [14, 15].
According to a systematic review, the prevalence of constipation was found to be approximately 12% in childhood and 16% in the general population worldwide, based on median estimates [16]. Constipation is a common issue during pregnancy. While only a minority of individuals with constipation seek healthcare for their condition, studies have shown that people with constipation often experience impaired general health, mental health, and social functioning compared to healthy individuals [17-19]. Constipation is highly associated with changes in the colonic flora, such as a decreased abundance of beneficial bacteria, such as Bifidobacteria and Lactobacilli [20]. The establishment of the microbiome begins early in life and is influenced by various maternal and pregnancy-related factors, including maternal diet, genetics, mode of delivery, breastfeeding, and other environmental factors [21]. Cellular leakage, including the passage of maternal IgG and microbial metabolites across the placenta, is important in the regulation of antimaternal immunity from T-cells. This mechanism is believed to be important in modulating immune responses during pregnancy [22]. Mothers share their microbes and metabolites with the fetus, during delivery and lactation, through various routes [23, 24]. In one study it was observed that the maternal gut microbiome serves as the primary source of transmitted strains, which have been found to persist more significantly in the infant gut [10]. The relationship between maternal constipation and the risk of AD in offspring remains unclear. In this study, we hypothesized that maternal constipation may impact the risk of AD in offspring. To investigate this, we analyzed a real-world, population-based retrospective cohort from Taiwan's National Health Insurance Research Database (NHIRD).
Methods
Data source
The retrospective cohort study was conducted using data from Taiwan's National Health Insurance Research Database (NHIRD), a database covering more than 99% of the population of Taiwan. Claims data from 1/1/2003 to 12/31/2017 were included in the study.
The database is an invaluable resource for epidemiological research and has been used extensively [25-29]. In addition, data related to basic parameters, such as patient's record, admission days, treatment, and diagnosis at discharge, can be obtained from the NHIRD. The birth certificate applications contained gestational weeks, delivery type, birth weight, single/multiple birth, stillbirth, and nationality of mother. The Maternal and Child Health Database includes parents and children. Using these databases, we were able to obtain data on the mothers' comorbidities and medications during pregnancy. The study was approved by the ethical review board of Chung Shan Medical University Hospital (approval No. CS2-21006).
Study group and outcome measurement
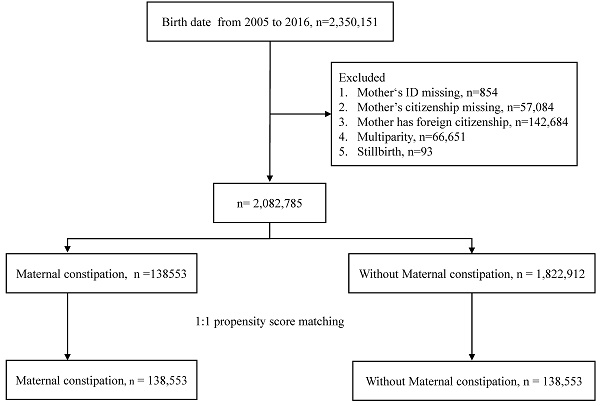
This was a retrospective cohort study that enrolled 2350151 individuals from a birth certificate applications database from 2005 to 2016. After excluding children with missing data on mother's identification and nationality, as well as those with foreign nationality, there were 2082785 children in this study. The exposure group was maternal constipation. The definition of maternal constipation was a diagnosis of constipation (ICD-CM= 564.0, K59.0) with at least 3 outpatient visits or one or more admissions and use of laxatives (ATC code: A06A) one year before birth. The comparison group comprised mothers who had never been diagnosed with constipation during pregnancy. The index date was set as the birth date.
The outcome variable was defined as a diagnosis of atopic dermatitis (ICD-CM=691, L20, L22) and at least three outpatient visits times or at least one hospitalization. Both groups were followed up until the onset of atopic dermatitis, death, or 31 December 2017, whichever occurred first.
Covariates and matching
The baseline characteristics were birth year, child's sex, birth weight (<2500; 2500-3499; ≥3500 gram), gestational weeks (<36; 36-40; ≥41 weeks), delivery (normal spontaneous delivery, NSD; cesarean section, C/S), and maternal comorbidities, including hyperlipidemia (ICD-CM= 272, E78), gestational diabetes (ICD-CM= 648.8, O99.81, O24.41, O24.42, O24.43), gestational hypertension (ICD-CM= 642.3, O13, O16.1, O16.2, O16.3), preeclampsia or eclampsia (ICD-CM= 642.4, 642.5, 642.6, 642.7, O11, O14,O15), rheumatoid arthritis (ICD-CM= 714.0, M05, M06), systemic lupus erythematosus (ICD-CM= 710.0, M32), Sjögren's syndrome (ICD-CM= 710.2, M35.0), ankylosing spondylitis (ICD-CM= 720.0, M45,M46), psoriasis (ICD- 9-CM: 6960, 6961, L40), asthma (ICD-CM= 493, J44, J45), atopic dermatitis (ICD-CM= 691, L20, L22), allergic rhinitis (ICD-CM= 477, J30), anxiety (ICD-CM= 300.0, F41), and depressive disorders (ICD-CM= 293.83, 296.2, 296.3,300.4, 311, F06.3, F32.0, F32.1, F32.2, F32.3, F32.4, F32.5, F32.9, F33.0, F33.1, F33.2, F33.3, F33.4, F33.9, F34.1). We also included pre-existing diabetes (ICD-CM= 250, E10, E11, E13, E14), hypertension (ICD-CM= 401-405, I10-I15) prior to pregnancy as covariates in the baseline characteristics. These comorbidities were defined as occurring two years before the index date and with at least three outpatient visits or one hospitalization. In addition, use of antibiotics in children during the study period was included.
Then, propensity score matching was performed by birth year, child's sex, birth weight, gestational weeks, delivery, mother's comorbidities, and antibiotic between the two groups. The propensity score was a probability that was estimated through logistic regression. The binary variable was the maternal constipation and non-maternal constipation group. By matching the propensity score, it was possible to balance the heterogeneity of the two groups.
Study flow chart.
Statistics analysis
To compare the maternal constipation group and non-maternal constipation group, we utilized the absolute standardized differences (ASD). If the absolute standardized difference was less than 0.1, the characteristics of both groups were deemed to be similar [30]. We calculated the relative risk (RR) and the 95% confidence intervals (CI) via the Poisson regression model. Kaplan-Meier analysis was conducted to determine the cumulative incidence of atopic dermatitis between the two groups. The log-rank test was used to test the significance. To assess the independent risk of the maternal constipation group, we employed the multivariate Cox proportional hazard model to estimate the hazard ratios. The statistical software was SAS version 9.4 (SAS Institute Inc., NC, USA).
Results
The study flow chart is shown in Fig.1. A total of 2,350,151 births were extracted from the database. The excluded data consisted of mother's ID missing, mother's citizenship missing, foreign citizenship of mother, multiparity, and stillbirth. Following exclusion of these individuals, there were 2,082,785 children left. Propensity score matching at a 1:1 ratio was used to match the maternal constipation group and the without maternal constipation group. The remaining children totaled 138,553 in each group. Table 1 shows the demographic characteristics of the constipation group and non-constipation group. There were no statistically significant differences in any of the characteristics of the children after propensity score matching. The incidence of offspring AD was 66.17 per 1,000 person-years in constipated mothers, which was higher than the rate of 53.99 per 1,000 person-years observed in the non-constipated group, as shown in Table 2. Using Poisson regression to analyze the incidence of AD, the relative risk of the maternal constipation group is 1.23-fold higher than that of the group without maternal constipation (relative risk: 1.23; 95% CI, 1.21-1.24).
Demographic characteristics of maternal constipation group and non-constipation group
| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Without maternal constipation N = 1822912 | Maternal constipationN = 138553 | p value | ASD | Without maternal constipation N = 138553 | Maternal constipation N = 138553 | p value | ASD | |
| Birth year | <0.001 | 0.3398 | 1.0000 | 0.0000 | ||||
| 2005 | 161441 (8.86) | 6197 (4.47) | 6162 (4.45) | 6197 (4.47) | ||||
| 2006 | 159067 (8.73) | 7574 (5.47) | 7569 (5.46) | 7574 (5.47) | ||||
| 2007 | 159516 (8.75) | 8388 (6.05) | 8371 (6.04) | 8388 (6.05) | ||||
| 2008 | 153314 (8.41) | 9337 (6.74) | 9313 (6.72) | 9337 (6.74) | ||||
| 2009 | 149087 (8.18) | 9986 (7.21) | 9937 (7.17) | 9986 (7.21) | ||||
| 2010 | 118483 (6.50) | 8989 (6.49) | 8982 (6.48) | 8989 (6.49) | ||||
| 2011 | 142542 (7.82) | 11811 (8.52) | 11785 (8.51) | 11811 (8.52) | ||||
| 2012 | 159044 (8.72) | 14857 (10.72) | 14864 (10.73) | 14857 (10.72) | ||||
| 2013 | 147398 (8.09) | 14391 (10.39) | 14401 (10.39) | 14391 (10.39) | ||||
| 2014 | 152136 (8.35) | 14691 (10.60) | 14711 (10.62) | 14691 (10.60) | ||||
| 2015 | 163163 (8.95) | 16752 (12.09) | 16836 (12.15) | 16752 (12.09) | ||||
| 2016 | 157721 (8.65) | 15580 (11.24) | 15622 (11.28) | 15580 (11.24) | ||||
| Child's sex | 0.0093 | 0.0072 | 0.9879 | 0.0001 | ||||
| Female | 873645 (47.93) | 66904 (48.29) | 66908 (48.29) | 66904 (48.29) | ||||
| Male | 949267 (52.07) | 71649 (51.71) | 71645 (51.71) | 71649 (51.71) | ||||
| Birth weight (gram) | <0.001 | 0.0564 | 0.9902 | 0.0000 | ||||
| <2500 | 115992 (6.36) | 8964 (6.47) | 8958 (6.47) | 8964 (6.47) | ||||
| 2500-3499 | 1425360 (78.19) | 110432 (79.70) | 110461 (79.72) | 110432 (79.70) | ||||
| ≥3500 | 281560 (15.45) | 19157 (13.83) | 19134 (13.81) | 19157 (13.83) | ||||
| Gestational weeks | <0.001 | 0.0000 | 0.5660 | 0.0641 | ||||
| <36 weeks | 59530 (3.27) | 4395 (3.17) | 4299 (3.10) | 4395 (3.17) | ||||
| 36-40 weeks | 1709304 (93.77) | 130920 (94.49) | 130997 (94.55) | 130920 (94.49) | ||||
| ≥41 weeks | 54078 (2.97) | 3238 (2.34) | 3257 (2.35) | 3238 (2.34) | ||||
| Delivery | <0.001 | 0.0644 | 0.8785 | 0.0006 | ||||
| NSD | 1191831 (65.38) | 86301 (62.29) | 86340 (62.32) | 86301 (62.29) | ||||
| C/S | 631081 (34.62) | 52252 (37.71) | 52213 (37.68) | 52252 (37.71) | ||||
| Maternal comorbidity | ||||||||
| Diabetes | 17061 (0.94) | 1590 (1.15) | <0.001 | 0.0208 | 1521 (1.10) | 1590 (1.15) | 0.2135 | 0.0047 |
| Hypertension | 16409 (0.90) | 1163 (0.84) | 0.0207 | 0.0065 | 1077 (0.78) | 1163 (0.84) | 0.0681 | 0.0069 |
| Hyperlipidemia | 10309 (0.57) | 1237 (0.89) | <0.001 | 0.0385 | 1166 (0.84) | 1237 (0.89) | 0.1458 | 0.0055 |
| Gestational diabetes | 30617 (1.68) | 2373 (1.71) | 0.3551 | 0.0026 | 2305 (1.66) | 2373 (1.71) | 0.3160 | 0.0038 |
| Gestational hypertension | 6004 (0.33) | 478 (0.34) | 0.3284 | 0.0027 | 391 (0.28) | 478 (0.34) | 0.0031 | 0.0112 |
| Preeclampsia or eclampsia | 13301 (0.73) | 943 (0.68) | 0.0382 | 0.0059 | 893 (0.64) | 943 (0.68) | 0.2417 | 0.0044 |
| Rheumatoid arthritis | 1646 (0.09) | 159 (0.11) | 0.0038 | 0.0076 | 152 (0.11) | 159 (0.11) | 0.6913 | 0.0015 |
| Systemic lupus erythematosus | 3060 (0.17) | 276 (0.20) | 0.0064 | 0.0073 | 251 (0.18) | 276 (0.20) | 0.2757 | 0.0041 |
| Sjögren syndrome | 3753 (0.21) | 497 (0.36) | <0.001 | 0.0288 | 463 (0.33) | 497 (0.36) | 0.2717 | 0.0042 |
| Ankylosing spondylitis | 1247 (0.07) | 149 (0.11) | <0.001 | 0.0132 | 136 (0.10) | 149 (0.11) | 0.4410 | 0.0029 |
| Psoriasis | 2757 (0.15) | 271 (0.20) | <0.001 | 0.0107 | 258 (0.19) | 271 (0.20) | 0.5716 | 0.0021 |
| Asthma | 21341 (1.17) | 2562 (1.85) | <0.001 | 0.0557 | 2492 (1.80) | 2562 (1.85) | 0.3203 | 0.0038 |
| Atopic dermatitis | 16815 (0.92) | 2095 (1.51) | <0.001 | 0.0538 | 2059 (1.49) | 2095 (1.51) | 0.5736 | 0.0021 |
| Allergic rhinitis | 126586 (6.94) | 15269 (11.02) | <0.001 | 0.1429 | 15307 (11.05) | 15269 (11.02) | 0.8178 | 0.0009 |
| Anxiety | 22916 (1.26) | 4889 (3.53) | <0.001 | 0.1490 | 4888 (3.53) | 4889 (3.53) | 0.9918 | 0.0000 |
| Depressive disorders | 18024 (0.99) | 4142 (2.99) | <0.001 | 0.1437 | 4075 (2.94) | 4142 (2.99) | 0.4531 | 0.0029 |
| Antibiotic | 1255616 (68.880) | 90055 (64.997) | <0.001 | 0.0826 | 90069 (65.01) | 90055 (65.00) | 0.9555 | 0.0002 |
NSD: Normal spontaneous delivery.
C/S: Caesarean section.
Poisson regression in incidence of atopic dermatitis
| Without maternal constipation | Maternal constipation | |
|---|---|---|
| Follow-up duration (years) | 635872 | 605540 |
| Number of atopic dermatitis | 34330 | 40071 |
| Incidence (95% C.I.)† | 53.99 (53.42-54.56) | 66.17 (65.53-66.82) |
| Relative risk (95% C.I.) | Reference | 1.23 (1.21-1.24) |
† per 1000 years
Table 3 shows the Cox proportional hazard model analysis, which demonstrates that maternal constipation was 1.20-fold (aHR: 1.26; 95% CI, 1.25-1.28) higher than that of the non-constipated mothers with a p value < 0.001. In terms of other characteristics, male gender, delivery via cesarean section, mothers with Sjögren syndrome, asthma, atopic dermatitis, allergic rhinitis, anxiety, depressive disorders, and receiving antibiotics during pregnancy had greater risk of developing AD in their offspring. Subgroup analyses were performed and most of the characteristics related to maternal constipation showed a higher adjusted hazard ratio, except the rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, and psoriasis subgroup (rheumatoid arthritis 1.19 95%CI 0.97-1.45, P=.0899, systemic lupus erythematosus 1.22; 95%CI 1.05-1.42, P=0.0114, ankylosing spondylitis 1.00; 95%CI 0.80-1.25, P=1, psoriasis 1.21; 95%CI 1.04-1.41, P=0.0119). In the subgroup analysis, a higher adjusted hazard ratio was noted in most of the characters along with maternal constipation (Table 4).
Comparisons with the non-constipated mothers revealed that the aHR for the constipated mothers with laxatives prescription <12 and ≥12 times within one year before the index date were 1.26; 95% CI, 1.24-1.28 and 1.40; 95% CI, 1.29-1.52, respectively (Table 5). The more severe the maternal constipation, the higher the risk of offspring AD.
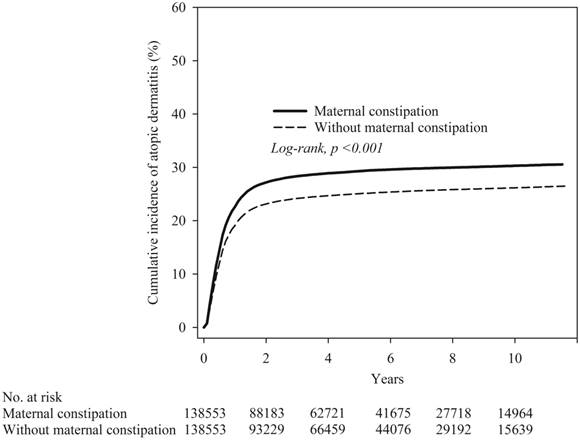
The Kaplan-Meier curves are shown in Figure 2. The cumulative incidence proportion of atopic dermatitis was significantly higher in the maternal constipation than in the maternal non-constipation group, and the log-rank test for the comparison of cumulative incidence curves resulted in a P value of < 0.001.
Discussion
This longitudinal nationwide population cohort study demonstrated a significant association between maternal constipation and risk of atopic dermatitis. In this study, regardless of sex, birth weight, gestational weeks, delivery, comorbidities, and antibiotics use, maternal constipated patients had a 1.26-fold higher risk for AD than non-maternal constipated patients. This finding highlights the significance of promoting healthy bowel habits and intestinal patency to prevent constipation in pregnant mothers, which may potentially reduce the risk of AD in their children.
Our results revealed that certain characteristics of the offspring, including male gender, pre-term under 36 weeks, and birth via cesarean section, were associated with a significantly higher hazard ratio for AD. Similar findings related to sex hormones [31], delivery mode [32, 33], gestational ages have also been associated with greater risk of AD in the literature. Maternal comorbidities, including hypertension, Sjögren syndrome, anxiety, depressive disorder, asthma, allergic rhinitis, and AD have also been associated with a higher risk of AD in offspring. Several previous studies support the current findings showing that there is a correlation between maternal asthma, allergic rhinitis, pregnancy complications, and mental health conditions with their child's likelihood of developing atopic diseases [34-38]. Specifically, the study showed that there is a greater risk of offspring developing atopic dermatitis when mothers use laxatives more frequently. This result implies that the greater the severity of maternal constipation, the more severe is the mother's dysbiosis, which in turn may affect the child's gut microbiome indirectly. The gut dysbiosis might not only be a causative risk factor, but also an accelerative factor in early life.
Kaplan-Meier curve of cumulative incidence proportion of atopic dermatitis in maternal constipation and maternal non-constipation groups
Cox proportional hazard model analysis for risk of atopic dermatitis in offspring
| Crude HR | p value | Adjusted HR† | p value | |
|---|---|---|---|---|
| Maternal constipation | ||||
| No | Reference | Reference | ||
| Yes | 1.20 (1.18-1.22) | <0.001 | 1.26 (1.25-1.28) | <0.001 |
| Child's sex | ||||
| Female | Reference | Reference | ||
| Male | 1.05 (1.03-1.06) | <0.001 | 1.12 (1.10-1.13) | <0.001 |
| Birth weight | ||||
| 2500-3499 | Reference | Reference | ||
| <2500 | 0.97 (0.94-1.00) | 0.0352 | 0.94 (0.91-0.97) | 0.0002 |
| ≥3500 | 0.99 (0.97-1.02) | 0.5841 | 1.03 (1.01-1.05) | 0.0152 |
| Gestational weeks | ||||
| 36-40 weeks | Reference | Reference | ||
| <36 weeks | 0.97 (0.93-1.02) | 0.2033 | 1.01 (0.96-1.06) | 0.8014 |
| Post-term (≥41 weeks) | 0.97 (0.92-1.01) | 0.1571 | 1.05 (1.00-1.10) | 0.0713 |
| Delivery | ||||
| NSD | Reference | Reference | ||
| C/S | 1.07 (1.06-1.09) | <0.001 | 1.10 (1.08-1.11) | <0.001 |
| Maternal comorbidity | ||||
| Diabetes | 1.08 (1.01-1.15) | 0.0249 | 1.05 (0.98-1.13) | 0.1518 |
| Hypertension | 0.95 (0.88-1.03) | 0.2388 | 0.90 (0.82-0.98) | 0.0114 |
| Hyperlipidemia | 1.14 (1.06-1.23) | 0.0004 | 1.05 (0.97-1.14) | 0.1944 |
| Gestational diabetes | 1.03 (0.98-1.09) | 0.2304 | 0.97 (0.92-1.02) | 0.2489 |
| Gestational hypertension | 1.04 (0.91-1.18) | 0.5925 | 1.05 (0.93-1.20) | 0.4337 |
| Preeclampsia or eclampsia | 1.09 (1.00-1.19) | 0.0412 | 1.02 (0.93-1.11) | 0.7206 |
| Rheumatoid arthritis | 1.19 (0.97-1.45) | 0.0899 | 1.17 (0.95-1.43) | 0.1369 |
| Systemic lupus erythematosus | 1.22 (1.05-1.42) | 0.0114 | 1.04 (0.89-1.22) | 0.616 |
| Sjögren syndrome | 1.28 (1.15-1.43) | <0.001 | 1.15 (1.02-1.28) | 0.0181 |
| Ankylosing spondylitis | 1.00 (0.80-1.25) | 1 | 0.97 (0.77-1.22) | 0.7878 |
| Psoriasis | 1.21 (1.04-1.41) | 0.0119 | 1.05 (0.91-1.23) | 0.4941 |
| Asthma | 1.26 (1.20-1.32) | <0.001 | 1.20 (1.14-1.26) | <0.001 |
| Atopic dermatitis | 1.53 (1.46-1.61) | <0.001 | 1.44 (1.37-1.51) | <0.001 |
| Allergic rhinitis | 1.25 (1.23-1.28) | <0.001 | 1.25 (1.22-1.27) | <0.001 |
| Anxiety | 1.18 (1.14-1.23) | <0.001 | 1.10 (1.05-1.14) | <0.001 |
| Depressive disorders | 1.14 (1.10-1.19) | <0.001 | 1.09 (1.05-1.14) | <0.001 |
| Antibiotic | 0.11 (0.11-0.11) | <0.001 | 0.11 (0.11-0.11) | <0.001 |
†Adjusted for sex, birth weight, gestational weeks, delivery, comorbidities, and antibiotics.
NSD: Normal spontaneous delivery.
C/S: Caesarean section.
Since most studies have predominantly investigated the postpartum period in relation to the development of allergic diseases [34, 36, 39], we sought to explore whether this phenomenon could also manifest during earlier phases, such as pregnancy. The alignment between maternal and infant immunity was considered as a potential mechanism linking the maternal microbiome to the acquisition of allergic diseases in offspring [22]. The composition of the perinatal gut microbiota is influenced by various factors, such as gestational age, mode of delivery, genetics, maternal microbiota, and environmental factors. During pregnancy, there is evidence of bacterial translocation, which involves the potential transfer of bacteria or bacterial products from the mother's gut to sites outside the digestive system [11, 40, 41]. Additionally, studies have indicated that the maternal vaginal microbiota plays a vital role in influencing the early establishment of the infant's gut microbiota [42]. Furthermore, maternal exposure to allergens can trigger an inflammatory response via cord blood IgE, which may lead to the early onset of allergic diseases in children [43-45]. Signaling pathways associated with TH1 and TH17 have been demonstrated to be involved in atopic dermatitis, as well as certain autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis [8, 46, 47].
Subgroup analysis of the association between maternal constipation and development of atopic dermatitis in offspring
| Number of atopic dermatitis | ||||
|---|---|---|---|---|
| Without maternal constipation | Maternal constipation | Adjusted HR | p value | |
| Child's sex | ||||
| Female | 16172 (24.17) | 19122 (28.58) | 1.28 (1.26-1.31) | <0.001 |
| Male | 18158 (25.34) | 20949 (29.24) | 1.25 (1.22-1.27) | <0.001 |
| p for interaction = 0.0798 | ||||
| Birth weight (gram) | ||||
| <2500 | 2118 (23.64) | 2551 (28.46) | 1.29 (1.22-1.37) | <0.001 |
| 2500-3499 | 27427 (24.83) | 32004 (28.98) | 1.26 (1.24-1.28) | <0.001 |
| ≥3500 | 4785 (25.01) | 5516 (28.79) | 1.25 (1.20-1.30) | <0.001 |
| p for interaction = 0.6082 | ||||
| Gestational weeks | ||||
| <36 weeks | 1008 (23.45) | 1259 (28.65) | 1.31 (1.21-1.42) | <0.001 |
| 36-40 weeks | 32513 (24.82) | 37893 (28.94) | 1.26 (1.24-1.28) | <0.001 |
| ≥41 weeks | 809 (24.84) | 919 (28.38) | 1.26 (1.15-1.39) | <0.001 |
| p for interaction = 0.7458 | ||||
| Delivery | ||||
| NSD | 20897 (24.2) | 24448 (28.33) | 1.27 (1.24-1.29) | <0.001 |
| C/S | 13433 (25.73) | 15623 (29.9) | 1.26 (1.23-1.29) | <0.001 |
| p for interaction = 0.5414 | ||||
| Maternal comorbidity | ||||
| Diabetes | ||||
| No | 33931 (24.76) | 39588 (28.9) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 399 (26.23) | 483 (30.38) | 1.21 (1.06-1.38) | 0.0057 |
| p for interaction = 0.4391 | ||||
| Hypertension | ||||
| No | 34089 (24.8) | 39737 (28.92) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 241 (22.38) | 334 (28.72) | 1.35 (1.14-1.59) | 0.0006 |
| p for interaction = 0.4348 | ||||
| Hyperlipidemia | ||||
| No | 34018 (24.76) | 39667 (28.89) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 312 (26.76) | 404 (32.66) | 1.26 (1.08-1.46) | 0.0029 |
| p for interaction = 0.8110 | ||||
| Gestational diabetes | ||||
| No | 33740 (24.76) | 39379 (28.92) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 590 (25.6) | 692 (29.16) | 1.22 (1.09-1.36) | 0.0005 |
| p for interaction = 0.5634 | ||||
| Gestational hypertension | ||||
| No | 34237 (24.78) | 39925 (28.92) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 93 (23.79) | 146 (30.54) | 1.39 (1.07-1.82) | 0.015 |
| p for interaction = 0.6134 | ||||
| Preeclampsia or eclampsia | ||||
| No | 34098 (24.77) | 39780 (28.91) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 232 (25.98) | 291 (30.86) | 1.30 (1.10-1.55) | 0.0029 |
| p for interaction = 0.8520 | ||||
| Rheumatoid arthritis | ||||
| No | 34285 (24.77) | 40020 (28.92) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 45 (29.61) | 51 (32.08) | 1.36 (0.87-2.11) | 0.1738 |
| p for interaction = 0.7114 | ||||
| Systemic lupus erythematosus | ||||
| No | 34252 (24.77) | 39983 (28.92) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 78 (31.08) | 88 (31.88) | 0.95 (0.69-1.30) | 0.7274 |
| p for interaction = 0.0451 | ||||
| Sjögren syndrome | ||||
| No | 34199 (24.77) | 39888 (28.89) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 131 (28.29) | 183 (36.82) | 1.40 (1.11-1.77) | 0.0042 |
| p for interaction = 0.4430 | ||||
| Ankylosing spondylitis | ||||
| No | 34300 (24.78) | 40025 (28.92) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 30 (22.06) | 46 (30.87) | 1.55 (0.95-2.52) | 0.0809 |
| p for interaction = 0.2999 | ||||
| Psoriasis | ||||
| No | 34248 (24.76) | 39985 (28.92) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 82 (31.78) | 86 (31.73) | 1.11 (0.81-1.52) | 0.5235 |
| p for interaction = 0.4494 | ||||
| Asthma | ||||
| No | 33563 (24.67) | 39187 (28.82) | 1.27 (1.25-1.28) | <0.001 |
| Yes | 767 (30.78) | 884 (34.5) | 1.20 (1.09-1.32) | 0.0002 |
| p for interaction = 0.2413 | ||||
| Atopic dermatitis | ||||
| No | 33585 (24.61) | 39244 (28.76) | 1.27 (1.25-1.28) | <0.001 |
| Yes | 745 (36.18) | 827 (39.47) | 1.18 (1.06-1.30) | 0.0015 |
| p for interaction = 0.1257 | ||||
| Allergic rhinitis | ||||
| No | 29732 (24.12) | 34961 (28.36) | 1.28 (1.26-1.30) | <0.001 |
| Yes | 4598 (30.04) | 5110 (33.47) | 1.18 (1.14-1.23) | <0.001 |
| p for interaction = 0.0005 | ||||
| Anxiety | ||||
| No | 32935 (24.64) | 38474 (28.78) | 1.27 (1.25-1.28) | <0.001 |
| Yes | 1395 (28.54) | 1597 (32.67) | 1.24 (1.15-1.33) | <0.001 |
| p for interaction = 0.5211 | ||||
| Depressive disorders | ||||
| No | 33212 (24.7) | 38724 (28.81) | 1.26 (1.25-1.28) | <0.001 |
| Yes | 1118 (27.44) | 1347 (32.52) | 1.26 (1.17-1.37) | <0.001 |
| p for interaction = 0.9411 | ||||
| Antibiotics | ||||
| No | 25530 (52.66) | 29335 (60.49) | 1.25 (1.23-1.27) | <0.001 |
| Yes | 8800 (9.77) | 10736 (11.92) | 1.24 (1.20-1.27) | <0.001 |
| p for interaction = 0.0405 | ||||
Adjusted for sex, birth weight, gestational weeks, delivery, comorbidities, and antibiotics
NSD: Normal spontaneous delivery.
C/S: Caesarean section.
In atopic dermatitis, there is often an imbalance in the immune system, with an overactive TH2 response, leading to inflammation and allergic symptoms on the skin [48]. SCFAs actively participate in the maintenance of the intestinal barrier and contribute to the regulation of intestinal motility [49-51]. They have been shown to actively participate in promoting the expansion of regulatory T cells (Tregs) and increasing the production of IL-10. As key drivers, SCFAs contribute to the maintenance of gut homeostasis [52-55]. Patients with constipation have been found to have reduced levels of some organisms (e.g., Bifidobacterium, Lactobacillus, and Bacteroides) compared to the healthy group. Constipation has also been linked to alterations in the structure of the intestinal flora as well as the metabolites of SCFAs [56]. Gestational manipulations in the fecal microbiota are likely to promote the health of the fetus and provide the newborn with a specific microbiome [51, 57]. While it is still unknown how maternal constipation changes the gut microbiome and affects the risk of developing atopic dermatitis in offspring, it might be considered a predisposing factor. Research on the microbiome of constipated mothers during pregnancy may be beneficial in preventing atopic dermatitis in children.
This study had a number of strengths. First, we utilized data from a nationwide database, which provided a larger, population-based sample than previous studies. Additionally, the longitudinal design allowed for causal inference regarding the link between maternal constipation and the development of atopic dermatitis in children. Furthermore, the study had minimal selection bias, information bias, and recall bias. Despite these advantages, there were some limitations.
Cox proportional hazard model analysis for risk of atopic dermatitis in offspring
| N | No of atopic dermatitis | Crude HR | p value | Adjusted HR | p value | |
|---|---|---|---|---|---|---|
| Laxatives frequency | ||||||
| Non-constipation | 138553 | 34330 | Reference | Reference | ||
| Maternal constipation with laxatives prescription <12 times | 136967 | 39528 | 1.20 (1.18-1.22) | <0.001 | 1.26 (1.24-1.28) | <0.001 |
| Maternal constipation with laxatives prescription ≥12 times | 1586 | 543 | 1.51 (1.38-1.64) | <0.001 | 1.40 (1.29-1.52) | <0.001 |
Adjusted for sex, birth weight, gestational weeks, delivery, comorbidities, and antibiotics.
Reliance on ICD-9 and ICD-10 codes may not have fully captured the complexity of the conditions being studied. Additionally, misclassification of exposure and outcome could have occurred due to the use of administrative databases. It would have been beneficial to have additional clinical data or laboratory tests to confirm the diagnosis of constipation and AD. The diagnoses of medical doctors may not have been consistent as individual expertise varies, which might have influenced the validity of the data. However, it is worth noting that any misclassifications in diagnoses were likely to be random, which could have led to an underestimation rather than an overestimation of associations. Additionally, Taiwan's NHI administration has established an ad hoc committee to monitor the accuracy of the claims data to prevent violations. Secondary, the NHIRD's lack of information on covariates such as maternal diet, personal lifestyle, family history, probiotics/prebiotics supplement or over-the-counter medication, laboratory data, or genetic survey, could have limited the ability to fully control for confounding factors in the analyses. Although we adjusted for a variety of comorbidities and medications, and used matched propensity scores to minimize potential confounders, residual unmeasured factors may have introduced bias in our results. Finally, as the majority of our patients were Taiwanese, it is unclear whether the conclusions of our study are generalizable to other ethnic groups.
Conclusions
In conclusion, maternal constipation was associated with a 1.26-fold greater risk of AD in offspring compared with those without maternal constipation. Constipation in pregnant women or mothers should alert the physician to the increased risk of AD in their children. Gut patency issues should not be ignored in constipated mothers. Further studies are needed to determine the precise pathophysiological mechanisms linking maternal constipation and pediatric AD.
Acknowledgements
This work was supported by Taichung Veterans General Hospital Research Foundation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bylund S, Kobyletzki LB, Svalstedt M, Svensson A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm Venereol. 2020;100:adv00160
2. Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-10
3. Wang IJ, Wang JY, Yeh KW. Childhood Atopic Dermatitis in Taiwan. Pediatr Neonatol. 2016;57:89-96
4. Gensollen T, Blumberg RS. Correlation between early-life regulation of the immune system by microbiota and allergy development. J Allergy Clin Immunol. 2017;139:1084-91
5. Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. 2015;136:860-5
6. Fang Z, Li L, Zhang H, Zhao J, Lu W, Chen W. Gut Microbiota, Probiotics, and Their Interactions in Prevention and Treatment of Atopic Dermatitis: A Review. Front Immunol. 2021;12:720393
7. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-10
8. Juarez-Chairez MF, Cid-Gallegos MS, Jimenez-Martinez C, Prieto-Contreras LF, Bollain YGd-l-RJJ. The role of microbiota on rheumatoid arthritis onset. Int J Rheum Dis. 2024;27:e15122
9. Lee SY, Lee E, Park YM, Hong SJ. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol Res. 2018;10:354-62
10. Ferretti P, Pasolli E, Tett A. et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe. 2018;24:133-145.e5
11. Senn V, Bassler D, Choudhury R, Scholkmann F, Righini-Grunder F, Vuille-Dit-Bile RN. et al. Microbial Colonization From the Fetus to Early Childhood-A Comprehensive Review. Front Cell Infect Microbiol. 2020;10:573735
12. Tan J K, McKenzie C, Marino E. et al. Metabolite-Sensing G Protein-Coupled Receptors—Facilitators of Diet-Related Immune Regulation. Annu Rev Immunol. 2017;35:371-402
13. Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-60
14. Huang YC, Wu MC, Wang YH, Wei JC. The influence of constipation on asthma: A real-world, population-based cohort study. Int J Clin Pract. 2021;75:e14540
15. Wu MC, Jan MS, Chiou JY, Wang YH, Wei JC. Constipation might be associated with risk of allergic rhinitis: A nationwide population-based cohort study. PLoS One. 2020;15:e0239723
16. Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011;25:3-18
17. Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218-38
18. Lee Y-F, Wu M-C, Ma K S-K. et al. Association of early childhood constipation with the risk of autism spectrum disorder in Taiwan: Real-world evidence from a nationwide population-based cohort study. Frontiers in Psychiatry. 2023: 14:1116239.
19. Wu MC, Ma KS, Wang YH, Wei JC. Impact of tonsillectomy on irritable bowel syndrome: A nationwide population-based cohort study. PLoS One. 2020;15:e0238242
20. Khalif I, Quigley E, Konovitch E, Maximova I. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Digestive and Liver Disease. 2005;37:838-49
21. Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J Clin Med. 2021;10:459
22. Vuillermin PJ, Macia L, Nanan R, Tang ML, Collier F, Brix S. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin Immunopathol. 2017;39:669-75
23. Kumbhare SV, Patangia DV, Patil RH, Shouche YS, Patil NP. Factors influencing the gut microbiome in children: from infancy to childhood. Journal of Biosciences. 2019;44:49
24. Meital Nuriel-Ohayon † HNaOK. Microbial Changes during Pregnancy, Birth, and Infancy. Frontiers in Microbiology. 2016;7:1031
25. Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health. 2018;40:e2018062
26. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236-42
27. Ma KS, Illescas Ralda MM, Veeravalli JJ, Wang LT, Thota E, Huang JY. et al. Patients with juvenile idiopathic arthritis are at increased risk for obstructive sleep apnoea: a population-based cohort study. Eur J Orthod. 2022;44:226-31
28. Ma KS, Thota E, Huang JY, Wei JC, Resnick CM. Increased risk of temporomandibular joint disorders and craniofacial deformities in patients with juvenile idiopathic arthritis: a population-based cohort study. Int J Oral Maxillofac Surg. 2022;51:1482-7
29. Wu MC, Ma KS, Chen HH, Huang JY, Wei JC. Relationship between Helicobacter pylori infection and psoriasis: a nationwide population-based longitudinal cohort study. Medicine (Baltimore). 2020;99:e20632
30. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH. et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349-58
31. Kanda N, Hoashi T, Saeki H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int J Mol Sci. 2019;20:4660
32. Richards M, Ferber J, Chen H, Swor E, Quesenberry CP, Li DK. et al. Caesarean delivery and the risk of atopic dermatitis in children. Clin Exp Allergy. 2020;50:805-14
33. Stinson LF, Payne MS, Keelan JA. A Critical Review of the Bacterial Baptism Hypothesis and the Impact of Cesarean Delivery on the Infant Microbiome. Front Med (Lausanne). 2018;5:135
34. Stokholm J, Sevelsted A, Anderson UD, Bisgaard H. Preeclampsia Associates with Asthma, Allergy, and Eczema in Childhood. Am J Respir Crit Care Med. 2017;195:614-21
35. Chang HY, Suh DI, Yang SI, Kang MJ, Lee SY, Lee E. et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol. 2016;138:468-75.e5
36. Xu B, Jarvelin MR, Pekkanen J. Prenatal factors and occurrence of rhinitis and eczema among offspring. Allergy. 1999;54:829-36
37. Wang QP, Wu KM, Li ZQ, Xue F, Chen W, Ji H. et al. Association between maternal allergic rhinitis and asthma on the prevalence of atopic disease in offspring. Int Arch Allergy Immunol. 2012;157:379-86
38. Lu Z, Zeng N, Cheng Y, Chen Y, Li Y, Lu Q. et al. Atopic dermatitis and risk of autoimmune diseases: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2021;17:96
39. Rigato P O, Fusaro A E, Victor J R. et al. Maternal immunization to modulate the development of allergic response. Immunotherapy. 2009;1:141-56
40. Jenmalm MC. The mother-offspring dyad: microbial transmission, immune interactions and allergy development. J Intern Med. 2017;282:484-95
41. Miko E, Csaszar A, Bodis J, Kovacs K. The Maternal-Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities. Life (Basel). 2022;12:424
42. Nyangahu DD, Jaspan HB. Influence of maternal microbiota during pregnancy on infant immunity. Clin Exp Immunol. 2019;198:47-56
43. Su KW, Chen PC, Wang IJ. Cord blood soluble Fas ligand and pediatric atopic dermatitis. Allergy Asthma Proc. 2011;32:366-71
44. Hsieh VC, Liu CC, Hsiao YC, Wu TN. Risk of Allergic Rhinitis, Allergic Conjunctivitis, and Eczema in Children Born to Mothers with Gum Inflammation during Pregnancy. PLoS One. 2016;11:e0156185
45. Scirica CV, Gold DR, Ryan L, Abulkerim H, Celedon JC, Platts-Mills TA. et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007;119:81-8
46. Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625-30
47. Luo F, Lee YH, Ma WK, Yong SB, Yao XM, Wei JC. Tumor necrosis factor-alpha inhibitor-associated psoriasis: Facts and hypotheses. Int J Rheum Dis. 2023;26:1011-4
48. Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011;2:110
49. Chu JR, Kang SY, Kim SE, Lee SJ, Lee YC, Sung MK. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomized placebo-controlled intervention study. World J Gastroenterol. 2019;25:6129-44
50. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200
51. Konig J, Wells J, Cani PD, Garcia-Rodenas CL, MacDonald T, Mercenier A. et al. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7:e196
52. Lynch SV. Gut Microbiota and Allergic Disease. New Insights. Ann Am Thorac Soc. 2016;13(Suppl 1):S51-4
53. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159-66
54. Egawa G, Honda T, Kabashima K. SCFAs Control Skin Immune Responses via Increasing Tregs. J Invest Dermatol. 2017;137:800-1
55. Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol. 2015;6:61
56. Tian Y, Zuo L, Guo Q, Li J, Hu Z, Zhao K. et al. Potential role of fecal microbiota in patients with constipation. Therap Adv Gastroenterol. 2020;13:1756284820968423
57. Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, Faas M. et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57:1-14
Author contact
![]() Corresponding author: James Cheng-Chung Wei, Institute of Medicine, Chung Shan Medical University, No. 110, Sec 1, Jianguo N. Road, Taichung City, 40201, Taiwan. Email: jccweicom.
Corresponding author: James Cheng-Chung Wei, Institute of Medicine, Chung Shan Medical University, No. 110, Sec 1, Jianguo N. Road, Taichung City, 40201, Taiwan. Email: jccweicom.

 Global reach, higher impact
Global reach, higher impact