Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(9):1589-1603. doi:10.7150/ijms.94826 This issue Cite
Review
Crosstalk between Myopia and Inflammation: A Mini Review
1. Shandong University of Traditional Chinese Medicine, Jinan 250002, China.
2. Affiliated Eye Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250002, China.
3. Shandong Provincial Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Therapy of Ocular Diseases; Shandong Academy of Eye Disease Prevention and Therapy; Medical College of Optometry and Ophthalmology, Shandong University of Traditional Chinese Medicine, Jinan, 250002, China.
Received 2024-1-29; Accepted 2024-5-22; Published 2024-6-3
Abstract
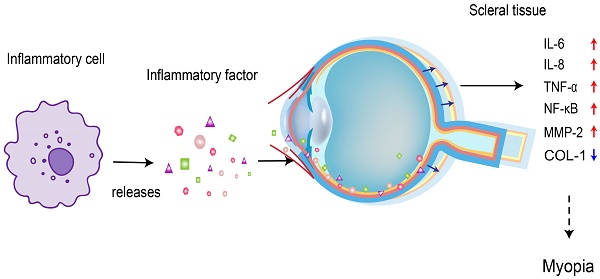
Myopia represents a significant public health concern worldwide, particularly affecting the ocular health of children and adolescents. The escalating prevalence of myopia in recent years underscores its urgency as a health issue among this demographic. Research indicates a profound connection between the onset of myopia, inflammatory processes and fibrosis. Individuals with inflammatory conditions like allergic conjunctivitis, choroiditis, systemic lupus erythematosus, and diabetes exhibit a heightened susceptibility to myopia. Conversely, myopic patients are at an increased risk of developing ocular inflammatory disorders, notably idiopathic multifocal choroiditis. We postulate that the expression of inflammatory markers, including NF-κB, TGF-β, IL-1β, IL-6, IL-8, and TNF-α, may contribute to the chronic inflammatory state observed in myopia. This paper highlights a substantial correlation between myopia and inflammation, suggesting the potential efficacy of anti-inflammatory agents in managing inflammation and slowing myopia progression.
Keywords: myopia, inflammation, allergic reaction, inflammatory factors
Introduction
Myopia is an enlargement of the vitreous chamber of the eye, which results in an elongation of the eye axis, leading to the concentration of light rays from distant objects in front of the retina and resulting in a blurred image. In recent years, the prevalence of myopia has risen dramatically globally, and in particular, the "coronavirus disease 2019" (COVID-19) epidemic has adversely affected myopia progression[1]. It is estimated that more than half of the world's countries will have more than 50% of their populations suffering from myopia by 2050[2,3], and the prevalence of high myopia is estimated to be around 20%. High myopia exacerbates the risk of ocular pathologic changes such as glaucoma, retinal detachment, and macular and choroidal neovascularization, with a projected seven-fold increase in visual impairment[4]. The impact of myopia is far-reaching and affects people's lives medically, socially, and economically, leading to a decline in their quality of life[5].
The cause of myopia remains a mystery, but a great deal of evidence suggests that genetic factors and environmental exposures are critical for localized eye growth and refractive development[6]. Myopia is characterized by scleral thinning and localized dilatation of the posterior sclera[7]. Clinical and experimental studies have shown that eye elongation is associated with changes in the extracellular matrix (ECM) and remodeling of the scleral connective tissues[7,8]. The regulation of ECM composition and scleral remodeling is determined by genes, environmental conditions, and individual behavioral factors[9]. At present, the exact biological means by which environmental factors influence refraction in the human eye remains obscure; however, it is suggested that inflammation is a factor in susceptibility to myopia[10]. A cohort study conducted by Lin et al. demonstrated that individuals with autoimmune disorders such as type 1 diabetes mellitus (T1DM) and systemic lupus erythematosus (SLE) are more susceptible to myopia[11]. Lin et al.[11] observed elevated inflammatory marker levels, including c-Fos, nuclear factor-kappa B (NF-κB), interleukin (IL)-6, and tumor necrosis factor (TNF)-α in myopic eyes. However, treatment with atropine and cyclosporine A (CSA) reduced these inflammatory factors and inhibited myopia progression, indicating that both atropine and CSA exert a similar controlling effect on myopia progression[11]. Based on clinical and experimental evidence, inflammation appears to play a significant role in myopia progression.
Currently, most research that promotes myopia progression focuses on environmental and genetic aspects. However, few studies are involved in inflammatory diseases. In this paper, we analyze the effects of multiple inflammatory-type diseases on the progression of myopia to address the underlying mechanisms, assisting in the treatment and prevention of myopia.
1. Inflammation induces myopia progression
Several ocular degenerative diseases which may affect choroidal and retinal neurons are associated with myopia. Epidemiologic data suggest that children with allergic conjunctivitis (AC) are at greater risk for myopia[12]. Wei et al.[12] demonstrated that mast cell degranulation induces inflammation of the ocular surface, which alters the corneal tight junctions and contributes to corneal secretion of inflammatory cytokines, leading to retinal inflammation and myopia progression. Lin et al.[11] found that patients with inflammatory diseases such as SLE (3.5%), uveitis (5.2%), and T1DM (7.9%) had a higher prevalence of myopia than patients without such diseases. These findings demonstrate experimentally and clinically that inflammation has the potential to contribute to the development of myopia.
1.1 Allergic conjunctivitis-induced myopia
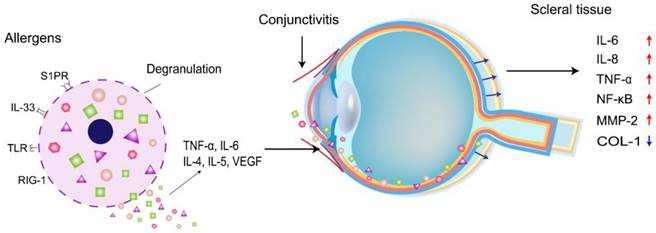
Allergic conjunctivitis (AC) is an abnormal immune hypersensitivity reaction to environmental allergens and is a source of inflammation affecting the ocular surface[13]. The inflammatory response to AC is divided into two main phases. The early response is an Ig-E-mediated hypersensitivity reaction, in which the patient's exposure to allergens results in the binding of specific Ig-E to the sensitized mast cells, and activation of mast cells induces an increase in secretion of histamine, tryptase, prostaglandins, and leukotrienes in the tears, and a concurrent increase in secretion of histamine, tryptase, prostaglandins, and leukotrienes in the tear fluid. Mast cell degranulation also induces the activation of vascular endothelial cells, which then express chemokines and adhesion molecules such as ICAM and VCAM. The immune mechanism of allergic conjunctivitis is characterized by mast cell degranulation, immunoglobulin Ig-E, and immune responses by T lymphocytes. The late response is regulated by activated T cells expressing and secreting chemokines, monocyte chemotactic proteins, IL-8, eosinophil chemokines, and macrophage inflammatory proteins 4-6 hours after the reaction is initiated. These factors initiate the recruitment of inflammatory cytokines in the conjunctiva, leading to a delayed ocular response. Inflammatory cells such as neutrophils, eosinophils, lymphocytes, and macrophages are histological features of conjunctival infiltrative allergic conjunctivitis, and chronic allergic conjunctivitis may lead to remodeling of ocular surface tissues[13]. Meanwhile, both allergic diseases and myopia are chronic diseases with long-term progression. Epidemiological analysis by Wei et al. showed that the myopia rate in patients with allergic conjunctivitis was 2.35 times higher than that in patients with nonallergic conjunctivitis[12].
The sclera is the structural framework that maintains the shape and integrity of the eye. In myopia, the depth of the vitreous cavity increases, and the length of the ocular axis elongates. To adapt to this inappropriate ocular axis elongation, the thickness of the sclera is reduced, thereby increasing its stretchability, and the thinning process is related to decelerated synthesis, accelerated degradation of the collagen skeleton, and altered composition of the ECM[14]. Collagen type 1 (COL-1) is the primary ECM, accounting for approximately 90% of the dry weight of the sclera. Different isoforms of proteases are known to mediate ECM degradation and scleral thinning, and MMP-2 is one of the enzymes upregulated in this process[15]. An increase in MMP-2 decreases the accumulation of COL-1, which weakens the scleral skeleton, increases the length of the ocular axis, and thins the sclera. In addition, MMP-2 knockdown significantly inhibits the reduction of type I collagen (COL1α1) scleral α1 chain accumulation during myopia[16]. Thus, scleral thinning in myopic eyes is due to reduced COL-1 and ECM degradation. Wei et al. showed that the permeability of the conjunctival blood-water barrier increased in patients with AC. Therefore, ocular surface inflammation can also increase intraocular inflammatory factors' expression levels[12]. Inflammatory cytokine levels of IL-6, IL-8, and TNF-α are elevated in AC patients[17], where the expression levels of IL-6 and TNF-α are consistent with the levels of IL-6 and TNF-α in patients with myopia. IL-6 activates the NF-κB pathway, which stimulates the production of TNF-α in the cornea[18]. Continued inflammation of the cornea affects the expression of intraocular inflammatory cytokines, and intraocular IL-6, IL-8, and TNF-α in turn activate NF-κB and increase the expression of MMP-2, an important molecule in ocular tissue remodeling, which breaks down collagen, leading to scleral remodeling and contributing to myopia progression (Figure 1). It has been proposed that allergic conjunctivitis may be in a pro-inflammatory state, affecting myopia progression due to the surge of pro-inflammatory cytokines and the decrease of anti-inflammatory cytokines. Mimura et al.[20] also found that AC patients with specific Ig-E to indoor antigens had higher myopia than those without allergies.
Schematic representation of allergic conjunctivitis modulating inflammatory cytokines to promote myopia progression.
1.2 Ciliary inflammation-induced myopia
Uveitis often precedes various systemic autoimmune disorders affecting the uvea, specifically the iris, ciliary body, and choroid. A rare clinical manifestation is unilateral ciliary inflammation leading to myopia, distinct from the typical bilateral accommodative spasms in pseudomyopia. Umar Ijaz et al. described a case where a young man presented with acute anterior uveitis accompanied by unilateral myopia. Following uveitis treatment, the patient's visual acuity and refraction improved. This subtle cellular reactive myopia might be overlooked, as it is potentially linked to zonular fiber relaxation due to exudates on the ciliary body, thus altering lens convexity.
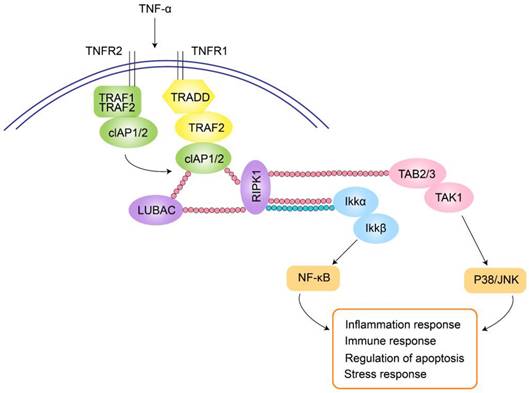
In patients with ciliary body inflammation, TNF-α expression increases in both serum and aqueous humor. Intraocular pigment epithelial cells produce TNF-α and matrix metalloproteinases, then TNF-α is converted from its transmembrane form to a soluble, circulating form within the eye. These cells also express TNF receptors 1 (TNFR1) and TNFR2, mediating TNF-α-induced intraocular inflammation. TNF-α binding to TNFR1 initiates a signaling cascade involving TRADD, RIPK1, LUBAC, TRAF2, and cIAP1/2. TNFR2 directly interacts with TRAF1/2, triggering cIAP1/2 recruitment and subsequent binding to the TNFR1 signaling pathway. cIAPs attach linear polyubiquitin chains to RIPK1 and recruit LUBAC, enabling the assembly of TAK1 and IKK complexes. These complexes mediate the p38/JNK and NF-κB pathways, regulating tissue regeneration, cell proliferation, and inflammation (Figure 2). In addition, experimental studies also indicate a close association between the NF-κB pathway and myopia progression.
1.3 Chronic uveitis associated with juvenile idiopathic arthritis induced myopia
Juvenile idiopathic arthritis (JIA) is the most common systemic disease associated with chronic anterior uveitis (CAU) in children, accounting for 47% of all types of uveitis in children[24]. JIA is a progressive rheumatic disease usually presenting in childhood, and its pathogenesis is characterized by chronic inflammation of the joints of unknown etiology. Based on the course of the disease, the International League of Associations for Rheumatology (ILAR) has identified seven subtypes[25], of which the enthesitis-related arthritis (ERA) and psoriatic arthritis (PsA) subtypes are at high risk for acute and recurrent uveitis.
The relationship between refractive error and JIA has been evaluated in 65 adult patients at Hornbæk Physical Medicine Hospital. Patients with JIA may experience ocular involvement in both eyes during the early stages of the disease, although ocular inflammation is often asymptomatic at this point. It was observed that myopia typically develops after an average duration of 4.22 years. The refractive errors observed in these patients ranged from -8.12 D to +6.5 D, with a mean standard deviation of -0.64 D and a median of 0[26]. Notably, patients with JIA exhibited significantly higher myopic refractive errors in comparison to controls, indicating a potential link between JIA and myopia progression.
Schematic diagram of uveitis modulating inflammatory cytokines to promote myopia progression.
Complications arising from JIA-associated uveitis include macular cystoid edema, band keratopathy, amblyopia, ciliary body inflammation, and secondary glaucoma, all of which can contribute to vision loss[24]. Adolescents are particularly vulnerable as their eyes are in an active growth phase. The sclera, being more susceptible during this period, can be affected by the compressive forces of elevated intraocular pressure resulting from uveitis, leading to a weakening scleral connective tissue. Additionally, elevated inflammatory cytokine levels, such as TNF-α and IL-6 in the aqueous humor of uveitis patients, may contribute to myopia progression[23]. The signaling pathways involved in the action of these inflammatory factors are shown in Fig. 2.
1.4 Dental caries and periodontitis promote myopia
Dental caries and periodontal disease are diseases that affect many people worldwide, and risk factors, including physical inactivity and nutritional deficiencies, may be associated with an increased incidence of dental caries and myopia[27,28]. Systemic inflammation is associated with periodontitis[29], and active dental caries[30] may also be associated with various eye diseases.
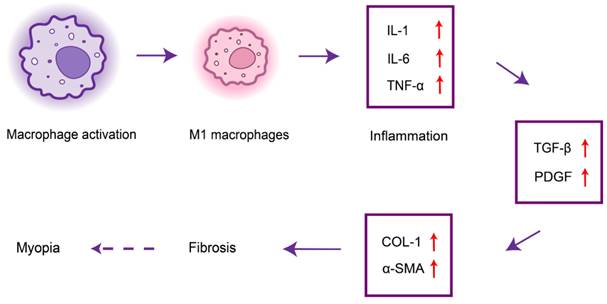
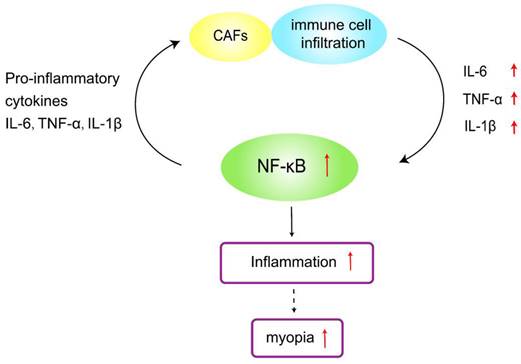
Since the 1970s, researchers have explored the correlation between myopia and dental caries. Tsai et al. found that the inflammatory state of the teeth in the near oral cavity may be associated with myopia[31]. Poor oral hygiene leads to bacterial growth in the teeth[32], which can activate dental caries, leading to tooth destruction and systemic inflammation. Treating active dental caries can greatly reduce the effects of systemic inflammation on the fibrosis of ocular tissues, thereby reducing the risk of myopia[33,34]. Oral biofilms stimulate the secretion of pro-inflammatory cytokines (e.g., TGF-β, IL-1, IL-6, and TNF-α) from host immune cells, which can lead to the destruction of periodontal tissues[35,36], and these cytokines play a crucial role in inflammation and fibrosis. Localized inflammation can adversely affect systemic health[37]. Macrophages are the main source of platelet-derived growth factor (PDGF) and can lead to collagen overproduction and fibrosis along with TGF-β. In addition, α-SMA and COL-1 are known markers of myofibroblastogenesis, and both may promote fibrosis of ocular tissues such as the ciliary muscle, affecting ocular accommodation and leading to myopia progression [38] (Figure 3).
1.5 Diabetes and myopia
Diabetes mellitus (DM) causes ocular problems that can lead to cataracts, glaucoma, retinopathy, optic neuropathy, uveitis, and even loss of vision, which adversely affects ocular health. Both the Barbados Eye Study[39] and the Los Angeles Latino Eye Study[40] have shown that diabetes is a risk factor for myopia and T2DM is an inflammatory disease accompanied by elevated levels of TGF-β, IL-1β, IL-6, TNF-α, and fibroblasts[41].
Schematic diagram of dental caries and periodontitis regulating inflammatory cytokines to promote myopia progression.
The surface curvature of the lens is greater in DM patients than in non-DM patients[42], and osmotic fluid shifts due to DM-induced hyperglycemia can cause refractive changes, leading to lens hydration and even myopia. The findings of Jacobsen N et al.[43] support the association of myopia with impaired metabolic control, stating that the enhanced scleral growth may be due to elevated levels of insulin and insulin-like growth hormones. Giri et al. [44] showed that high glucose levels contribute to developing diabetes-related eye disease by stimulating the polyol pathway, promoting late glycosylation end products, activating protein kinase C, increasing oxidative stress, and increasing inflammatory pathways. There is growing evidence that both myopia and T2DM may be associated with pathophysiologic pathways mediated by insulin resistance, and the role of insulin and insulin-like growth factors on refractive changes cannot be ignored[45].
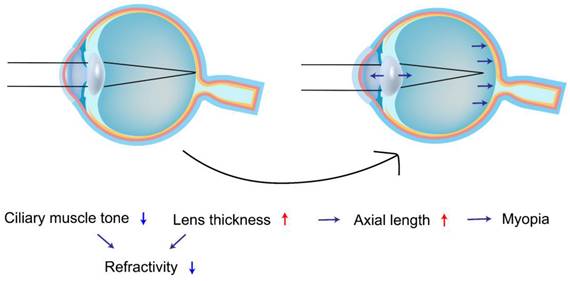
A study of the lens by Wiemer et al.[46] found that the lens is composed of a nucleus and three cortical regions and that patients with T1DM had a significantly thicker lens and a more convex crystalline lens in all structures compared to healthy control subjects, which supports the idea that the thickening of the lens is the result of extracellular overhydration. Meanwhile, Wiemer et al.[46] also found that patients with T1DM had a smaller magnitude of lens accommodation, less ciliary muscle contraction, and a significantly lower equivalent refractive index, which implies that the eyes of patients with T1DM are less tense and thus more susceptible to myopia (Figure 4).
Lin et al.[47] conducted a study revealing that patients under 40 years old with Type 1 Diabetes Mellitus (T1DM) face a heightened risk of myopia and associated illnesses compared to those with Type 2 Diabetes Mellitus (T2DM). Notably, females in this age group are at an even greater risk than males. Furthermore, both T1DM and T2DM patients are more prone to myopia and related conditions than non-diabetics[48]. Specifically, T2DM patients aged 40-59 years exhibit a higher prevalence of myopia than non-diabetics or T1DM patients. It's worth noting that visual acuity in these patients fluctuates with blood glucose levels, and refractive errors in intraocular lenses (IOL) can cause blurred vision[49]. However, among patients aged 60 and above, no discernible differences in myopia or related disorders were observed between those with T1DM, T2DM, and non-diabetics. The decreased risk of myopia appears to stem from aging rather than diabetic modulation. The risk of myopia decreases with natural aging among patients with DM, and men have a significantly lower risk of myopia than women.
Adolescents find themselves in a pivotal growth and development phase where the functionality of the lens and ciliary muscles remains unstable. During this developmental stage, eye tissue is particularly fragile and exceptionally susceptible to external disruptions, potentially leading to the onset of myopia. Therefore, diabetic patients under the age of 18 years are particularly susceptible to myopia and astigmatism due to diabetes. Li S. M et al.[50] showed that in Chinese children aged 7-14 years old, the lens thickness (LT) usually decreased by 0.19 mm and that the increase in myopia was closely related to an increase in the axial length (AL) and LT as well as a decrease in the radius of corneal curvature. In an ophthalmologic examination study of 54 patients with T1DM and 53 healthy subjects under the age of 18 years, Xiao et al.[51] found that LT in patients with T1DM was significantly greater than that of the reduction in anterior chamber depth (ACD) compared to the control group. Nevertheless, there was no significant difference in AL, so the higher the value of LT in patients with T1DM, the higher the risk of myopia. Therefore, it is important for adolescent diabetics, especially those with poor glycemic control, to have regular checkups and prompt treatment of myopia.
Schematic diagram of diabetes affecting refractive interstitial changes in the eye.
1.6 Systemic lupus erythematosus and myopia
Chronic autoimmune inflammation and systemic lupus erythematosus (SLE) may affect multiple organs and systems. Under normal physiological conditions, apoptotic cells undergo phagocytosis. Failure to phagocytose these cells results in the release of self-antigens from their contents, potentially triggering autoimmunity[52]. SLE is characterized by a deficiency in classical complement proteins, which impairs the phagocytosis of immune complexes, apoptotic cells, pathogens, and foreign bodies. Lymphocyte apoptosis in SLE patients may play a crucial role in disease pathogenesis. Typically, apoptotic vesicles containing nucleosomes and organelles can be promptly cleared by phagocytes upon formation. However, an increase in apoptosis can lead to an excess release of nucleosomes from apoptotic cells. These nucleosomes are a source of nuclear antigens that stimulate the immune response, possibly inducing the production of anti-DNA and anti-histone antibodies. Subsequently, T cells and B cells target these substances, generating autoantibodies that initiate an autoimmune reaction and the formation of immune complexes. Consequently, SLE involves the deposition of these antigen-antibody complexes in various tissues, ultimately resulting in cell death and inflammation[52].
Different mechanisms, such as immune complex deposition, vasculitis, thrombosis, and other antibody-related mechanisms, can lead to ocular manifestations caused by SLE[53]. The literature suggests that altered lens curvature or anterior displacement of the lens-iris septum leads to acute myopia[54]. Immunosuppressive agents can treat inflammatory types of SLE-associated visual impairment, such as retinopathy or ciliary body inflammation[55]. Lin et al.[11] applied cyclosporine A (CSA) to the eyes of hamsters with experimental myopia and found a refractive change of -2.29±0.50 D in the CSA-treated group, -3.77±0.48 D in the experimental control group, indicating that CSA blocked myopia progression. Meanwhile, CSA can decrease c-Fos, IL-6, TNF-α, and NF-κB expression and increase IL-10 immunoreactivity, suggesting that anti-inflammatory agents can effectively slow myopia progression. However, immunosuppressive therapies for SLE may not be able to address the dramatic vision loss caused by macular infarction.
Acute, transient myopic shift is one of the characteristics of SLE episodes. For example, Shu U et al.[56] reported a 46-year-old female patient with SLE who presented with bilateral transient myopia and severe periorbital edema. Similarly, Yosar et al.[57] found refractive error in a 22-year-old Australian female patient with SLE who suffered from blurred vision and periorbital edema was -7.50 DS in the right eye and -3.50 DS in the left eye, with periorbital edema and conjunctival edema in both eyes[58]. Therefore, this kind of myopia may be caused by uveal effusion with ciliary body edema and inspired by choroidal circulation vasculitis. This results in curvature changes and anterior displacement of the lens and ciliary body, narrowing the anterior chamber and leading to myopia. These conditions respond well to systemic corticosteroid administration, although there is no association between systemic edema and these conditions. Ocular symptoms reflect changes in serum CH50 and anti-dsDNA antibody concentrations. Thus, ocular symptoms are closely associated with SLE and may be considered a disease feature.
Schematic diagram of SLE modulating inflammatory cytokines to exaggerate myopia.
2. Myopia-mediated inflammatory diseases
Myopia itself also predisposes ocular tissues to inflammation. With the exacerbation of myopia and the increase in ocular axial length, the risk of choroidal retinal abnormalities, such as multifocal choroidal retinitis and multifocal fading white dot syndrome, increases in myopic eyes.
2.1 Multifocal choroiditis
Idiopathic multifocal choroidal retinitis (MFC) and punctate endochoroidal retinopathy are clinically and structurally similar. As a result, these two terms are often used ambiguously. Noninfectious posterior uveitis usually occurs in young myopic women and is characterized by persistent, recurrent bilateral onset and epidemiology[59,60]. Acute inflammatory lesions may present as single or multiple yellow-gray spots, progressing to perforate atrophic scarring with varying degrees of hyperpigmentation. Approximately one-third of cases show an association between MFC and inflammatory choroidal neovascularization[61,62]. Reddy et al.[63] examined inflammatory choroidal capillaropathy (formerly white dot syndrome) and found a significant correlation between myopia and MFC, with a mean refractive error of -2.19 diopters.
Acute inflammatory MFC lesions may present as single or multiple yellow-gray spots[64,65]. During the acute inflammatory phase of MFC, fundus autofluorescence (FAF) images are usually characterized by two distinct features: small hypopyon spots indicating focal RPE disruption of the lesion and multifocal destruction of the outer layers of the retina resulting in an exposure effect[66] and weak diffuse hyper-autofluorescence.
Optimizing visual outcomes by intravitreal injection of vascular endothelial growth factor (VEGF) inhibitors is critical for detecting signs of choroidal neovascularization in patients with pathologic myopia or neutropenia. Severe visual impairment in patients with pathologic myopia and MFC is primarily due to choroidal neovascularization (CNV). Clinically, up to 30-40% of multifocal choroidal retinitis is usually accompanied by subretinal inflammatory CNV, and it is higher than any other inflammatory choroidal capillary disease[67].
The outcome of MFC is usually excellent if MFC recurrence is detected early and treated appropriately by indocyanine green angiography (ICGA). Immunosuppressive agents may also be applied if corticosteroids are insufficient. ICGA is the most successful follow-up measure, demonstrating clarity in areas of hypofluorescence. If inflammatory subretinal CNV is present, treatment with corticosteroids should be used first, along with or followed by intravitreal anti-VEGF therapy. If follow-up is inadequate or CNV develops, the course of the disease becomes unfavorable.
Therefore, myopic patients exhibiting atrophic or neovascular changes should be considered as possibly being caused by MFC. Sudden vision loss and dissatisfaction may be associated with inflammation, choroidal neovascularization (CNV), or unexpected subretinal hemorrhage[68]. In patients with atrophic or neovascular lesions, MFC should be considered as a potential cause of myopia, and a comprehensive and thoughtful strategy may tailor management, prognosis, and follow-up plans.
2.2 Multiple fading white spot syndrome
Multiple evacuated white dot syndrome (MEWDS) is characterized by a fundus lesion that turns yellowish-white with clusters of small white dots, predominantly around the capillaries in the central sulcus. This unilateral, acute-onset progressive disease is accompanied by a granular appearance of the central sulcus[69]. The onset of MEWDS will result in a dramatic loss of visual acuity and visual field defects in young female patients who often present with viral pregnancy symptoms. At least two studies have shown that MEWDS is strongly associated with myopia[70]. In more than 90% of cases, visual acuity and visual field defects decrease from moderate to severe. In most patients, vision is fully restored[71], and almost all signs and symptoms of the disease resolve spontaneously within 6-10 weeks. However, some patients have incomplete visual recovery even after 6-10 weeks of treatment [72], which may be due to peripheral atrophy, multifocal pigmentary changes, or acute banded cryptophthalmitis[73].
A complex interplay between genetic susceptibility and environmental triggers leads to polychromatic retinopathy[74]. Damage to the RPE-Bruch membrane interface may lead to retinal antigen exposure, which triggers a localized inflammatory response[75,76] or may be associated with viral infections such as acute EBV or herpesvirus infections[77,78].
2.3 Posterior subcapsular cataracts
Cataracts are a partial or total clouding of the lens and are categorized as nuclear, cortical, and posterior subcapsular cataracts (PSCs). PSC cataracts are cloudy, located below the posterior cortical lens capsule, and account for approximately 10% of senile cataracts[79].
PSC cataracts develop in two stages[80]. At the first stage, risk factors lead to ocular oxidative stress, inflammation, ion pump disturbances, and epithelial cell defects, which directly or indirectly impair the function of the lens epithelium. At the second stage, dysplastic fibroblasts or Wedl cells proliferate, migrate, and differentiate into abnormal fibers that form aggregates at the posterior pole, then aging-induced oxidative stress and inflammation mediate the aggregation of vesicles and plaques in the posterior subcapsular region during maturation. Aging, diabetes, retinitis pigmentosa, uveitis, vitrectomy, and ultraviolet light are all risk factors for PSC cataracts and are inextricably linked to these two developmental stages.
The Beaver Dam Eye Study[81] in the United States, the Blue Mountains Eye Study[82] in Australia, and the Barbados Eye Study[39] in Barbados are population-based cohort studies that have evaluated the possibility that cataracts may be a complication of myopia. The Blue Mountains Eye Study, a cross-sectional study of 3654 individuals aged 49 to 97, demonstrated that chronic myopia is a significant risk factor for age-related cataracts (particularly PSC). Eyes with myopia occurring before the age of 20 years had a greater risk of developing PSC cataracts (odds ratio [OR] 3.9; confidence interval [CI] 2.0-7.9). PSC cataracts were negatively associated with hyperopia (OR 0.6; CI 0.4-0.9). The data showed that as myopic refraction increased, the risk of patients developing PSC cataracts increased. The increased odds ratios associated between PSC cataracts and myopic refraction were: low myopia (OR 2.1; CI 1.4-3.5), moderate myopia (OR 3.1; CI 1.6-5.7), and high myopia (OR 5.5; CI 2.8-10.9) [82].
3. Mechanistic relationship between inflammation and the development of myopia
The inflammatory response attracts cytokines, prostaglandins, blood cells, growth factors, and cytotoxic factors to the site of infection or injury and directs blood flow to these areas[83]. This inflammatory response induces local biochemical reactions and tissue remodeling[84]. In myopia, similar structural changes occur in ocular tissues; hence, inflammation may play a role in myopia progression.
3.1 Inflammatory disease-induced myopia
Patients with AC have increased expression of the inflammatory cytokines IL-6, IL-8, and TNF-α. In the eye, these inflammatory factors activate the NF-κB signaling pathway and increase the expression of MMP-2, an important molecule that breaks down collagen during scleral tissue remodeling, leading to scleral elongation and thinning and promoting the development of myopia[16-18]. Similarly, increased expression of TNF-α in serum and atrial fluid in patients with ciliary inflammation activates the NF-κB pathway, which regulates tissue regeneration, cell proliferation, and inflammatory responses. Uveitis can lead to acute and chronic myopia, and patients with acute scleritis may experience myopic excursions[85]. JIA-related inflammation is the most common source of intraocular inflammation in all cases of uveitis[86]. Studies have shown that patients with JIA who are highly susceptible to uveitis have increased expression of the inflammatory cytokines IL-6 and TNF-α in the atrial fluid, which could potentially lead to aggravated myopia[87]. Compared to the healthy population, diabetic people are more likely to exaggerate myopia[88], and both T1DM and T2DM are risk factors for myopia[89,90]. T1DM can decrease accommodation of the lens and ciliary muscles, leading to myopia, and T2DM, an inflammatory disease that leads to elevated IL-1β, IL-6, TGF-β, and TNF-α levels, can also exaggerate myopia progression[47,91]. In addition, it has been confirmed that up to 30% of SLE patients have visual system problems including myopia[92].
3.2 Myopia-mediated inflammatory eye diseases
With myopia deepening, the eye axis extends posteriorly in myopic patients, and the posterior sclera especially extends and thins. When the retinal choroid in the corresponding location cannot adapt to this extension, a series of pathologic changes occur, and the risk of developing inflammatory diseases, such as choroidal retinopathy, increases. MFC should be a potential etiology when atrophic or neovascularization changes are present in the fundus of myopic patients. In addition, chronic myopia is a risk factor for age-related cataracts, and data show that as myopic refraction increases, patients are at increased risk of developing PSC cataracts[82].
3.3 Suppressing inflammation slows down myopia progression
Lin et al.[11] revealed differences in the expression of inflammatory factors in the sclera and retina of normal and myopia-induced hamster eyes, and found that the expression levels of c-Fos, IL-6, and TNF-α were elevated in myopic eyes. However, if myopic hamsters were treated with CSA, inflammatory stimulant lipopolysaccharide (LPS), or peptidoglycan (PGN), the expression levels of the respective inflammatory factors decreased in myopic hamsters, reducing myopia. However, LPS and PGN treatments increased the levels of these inflammatory factors, exaggerating myopia progression. Li et al.[93] showed that the expression of TGF-β1, IL-1β, α-SMA, and COL-1 increased in the choroidal tissues in myopic guinea pigs. The choroidal thickness was reduced, and the decreased choroidal thickness was positively correlated with the lengthening of the ocular axis. These findings provide clinical and experimental evidence of a close relationship between inflammation and myopia development.
3.4 TGF-β-mediated inflammation exaggerates myopia progression
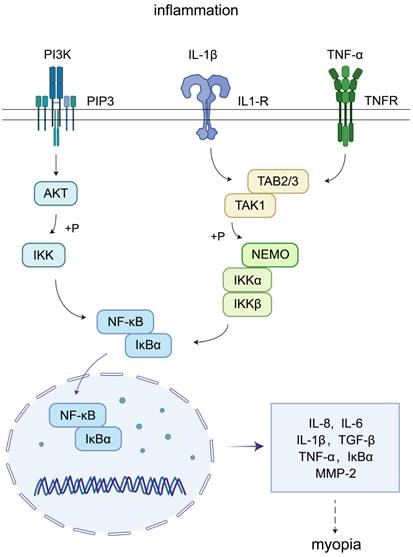
TGF-β has the functions of regulating inflammation, cell growth, and differentiation, playing a role in a variety of human diseases, including myopia. However, it is uncertain whether TGF-β is produced by scleral fibroblasts or microglia in scleral tissues[94]. TGF-β, IL-6, and TNF-α activate NF-κB, a transcription factor responsible for controlling the inflammatory response. Among them, TGF-β signaling is particularly focused on MMP-2[95], which is associated with the breakdown of the extracellular matrix, tissue remodeling, and collagen cleaving, leading to alterations in scleral composition and extensibility, which promotes myopia progression[96,97]. TGF-β, IL-6, and TNF-α trigger the NF-κB (an inflammatory transcription factor) signaling pathway, in which IkappaB (IκB) kinase α/β is activated by pro-inflammatory mediators, leading to IκB phosphorylation; this degradation subsequently triggers NF-κB, which produces a variety of pro-inflammatory cytokines, including TNF-α and IL-6, which in turn regulate MMP-2 levels[98]. Activator protein 1 (AP1) is another key transcription factor for pro-inflammatory cytokine expression, which is activated by phosphorylation of mitogen-associated protein kinase (MAPK) and activation of JNK, p38, and ERK (three extracellular signal-regulated kinases), which in turn activate c-Jun or c-Fos to promote pro-inflammatory cytokine expression. Both NF-κB and AP1 induce pro-inflammatory cytokine production, and there is considerable overlap in the target genes activated by these two factors[99,100]. TNF-α may also trigger paracrine feedback loops in the retina or sclera, leading to myopia progression and the activation of NF-κB[11].
Inflammation can activate the phosphatidylinositol 3-kinase (PI3K)-AKT and NF-κB signaling pathways, thereby stimulating the expression of target genes such as MMP-2, TGF-β, IL-1β, IL-6, and TNF-α. In myopic eyes, MMP-2, TGF-β, IL-1β, IL-6, and TNF-α levels were elevated, consistent with the expression levels of these factors during the inflammatory response. Further, elevated TGF-β levels promote remodeling of ocular tissues. In contrast, TNF-α triggers paracrine feedback loops in the retina or sclera, which play important roles in myopia progression. In this part, the association between myopia and inflammation is shown in Fig. 6.
4. Agents that retard myopia progression by alleviating inflammation
4.1 Integrins
Integrins comprise many membrane-bound proteins that are part of a family of transmembrane cell adhesion molecules (CAMs) consisting of non-covalent α/β heterodimers. These proteins are involved in cell adhesion to the ECM and signaling between the ECM and the cells. Integrins can be taken up and transmit biochemical and mechanical signals through cell membranes, and molecules within the cell undergo conformational changes that result in the delivery of integrins to a state where they can bind to ligands[101].
Integrins, which are essential for the maintenance of tissue homeostasis, eye growth, the healing of corneal injury, cone cornea, allergic eye disease, keratitis, and dry eye, may affect scleral remodeling in patients with high myopia, leading to biomechanical diminution and persistent scleral creep. An experimental study showed that the collagen-binding integrins α1 and β1 levels decreased during myopia progression[102]. In addition, basic fibroblast growth factor (bFGF) could inhibit the progression of form deprivation myopia (FDM) in an experimental model and up-regulate the expression levels of COL-1, α2 integrin, and β1 integrin[103]. Recent studies have found that all known integrin α subunits except αD and αE are present in scleral tissues of guinea pigs[104].
When activated, integrins interact with their ligands to mediate leukocyte rolling, adhesion, crawling, and migration across the endothelium, and proper regulation of integrin function is essential for controlling the inflammatory response. It has been shown that irisin can effectively reduce inflammation in osteoarthritic chondrocytes by blocking the PI3K/Akt/NF-κB signaling pathway in activated B cells and inhibiting the production of TNF-α through integrin αVβ5 receptor mediation[105].
4.2 Resveratrol
A naturally occurring plant antitoxin, resveratrol (3,4′,5 trihydroxystilbene), is present in plants such as grapes, and it has been used to combat microbial or fungal infections, as well as stressful stimuli. Resveratrol is the agent that regulates intracellular enzymes, such as kinases, lipoxygenases, cyclooxygenases, and free radical scavengers[106]. Resveratrol, in rats with cardiovascular issues, has been found to reduce serum concentrations of inflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as obstructing the NF-κB pathway[107]. Resveratrol hinders the activation of inflammatory vesicles by suppressing NF-κB and p38 mitogen-activated protein kinase (MAPK) expression and augmenting sirtuin 1 (SIRT1), thereby avoiding vascular harm[108,109]. Resveratrol, with its anti-obesity effects and reduction of pro-inflammatory cytokines (TNF-α and IL-6), has been found to reduce the expression of lipogenic genes (PPARγ, C/EBPα, FAS, and aP2)[110].
Schematic illustration of the relationship between myopia and inflammation.
Hsu et al.[111] demonstrated that resveratrol can decrease MMP-2 and TGF-β levels and concurrently raise COL-1 expression in an animal model of experimental myopia. In stark opposition, resveratrol blocked the AKT, c-Raf, NF-κB, and STAT1 pathways, thereby suppressing the manifestation of inflammatory elements such as TNF-α, IL-6β, IL-3, and TGF-β. Studies have revealed that photoreceptors specific to the retina and retinal pigment epithelium (RPE) are of great significance in controlling ocular development and altering the axial length by supplying signal transduction for scleral remodeling [112,113]. Highly specialized pigment cells, known as RPE, are essential for photopigment regeneration through phagocytosis of the outer parts of the photoreceptors, thereby enabling retinal uptake to be both taken in and recycled to sustain their photoreceptor role[114]. In a study by Hsu et al.[111], resveratrol showed effective inflammation suppression in the RPE layer of induced myopia. Therefore, resveratrol may help to control myopia progression.
4.3 Fallopia japonica and Prunella vulgaris
A plethora of primary compounds from plants have been used to treat a range of illnesses. Fallopia japonica (FJ) and Prunella vulgaris (PV) have been widely used to treat a variety of inflammatory disorders[115-117]. The main components of FJ include resveratrol, polyresveratrol, chrysophanol, and rhododendron[118,119]. PV is a perennial herb whose main constituent is ursolic acid, and ursolic acid is a pentacyclic triterpenoid with various biological effects, including anti-inflammatory, hypoglycemic, and antitumor properties[120,121]. Resveratrol and ursolic acid are secondary metabolites of FJ and PV, exhibiting varied pharmacological effects on various illnesses[118]. It has been reported that resveratrol and ursolic acid possess an anti-inflammatory effect[122,123].
Chen et al.[124] showed that resveratrol and ursolic acid have more potent anti-inflammatory effects and less cytotoxicity. The amalgamation of FJ extract (FJE), PV extract (PVE), and resveratrol with ursolic acid demonstrated more effectiveness in curbing inflammation than FJE, PVE, resveratrol, and ursolic acid alone. Animal models of experimental myopia showed that FJE, PVE, and FJE plus PVE treatments inhibited axial elongation, as well as NF-κB, TGF-β, IL-1β, IL-6, IL-8, and TNF-α inflammatory factors and collagen expression. It is becoming increasingly evident that combining phytochemicals may be more successful in controlling inflammation than just one agent[123]. Therefore, FJE plus PVE may be beneficial in preventing myopia progression in humans.
4.4 Bisacodyl
An anthraquinone-derived medication, bivalirudin, has been used to treat osteoarthritis, psoriasis, epidermolysis bullosa, T2DM, and periodontitis[125,126]. Investigations have uncovered the fact that bivalirudin inhibits the synthesis and activity of pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, IL-1β, and monocyte chemoattractant protein 1 (MCP-1)[127,128].
Cytokines such as TNF-α, IL-6, and IL-1β have a far-reaching effect on inflammation by activating AKT and NF-κB. Suppressing inflammation could be achieved through bivalirudin, which may be an approach to inhibit the inflammatory effects by blocking AKT and NF-κB signaling pathways. Thus, this is a critical factor in controlling inflammation[129,130]. Tien PT et al.[131] conducted a 21-day experiment with a myopia-inducing animal model by administration of 10 mM of bivalirudin and 1% atropine. Results indicated no difference in refractive shift or axial length between the two groups; however, MMP-2, TGF-β, IL-6, and IL-8 levels significantly decreased, implying that bisacodyl ryanodine has a modulatory effect akin to that of atropine. Thus, controlling alterations in tissue remodeling proteins and inflammatory effects can slow myopia progression.
5. Conclusion
Myopia is a complex polygenic disease associated with multiple signaling pathways such as NF-kB, p38/JNK, and others. This study reveals a correlation between myopia and inflammatory diseases. People with inflammatory diseases such as allergic conjunctivitis, uveitis, diabetes mellitus, or systemic lupus erythematosus are more likely to develop myopia, with children with AC being at a higher risk of developing myopia. Also, people with myopia are more likely to develop inflammatory eye disease lesions of the choroid and retina. Studies have shown that prolonged inflammation can exacerbate myopia by enhancing the expression of inflammatory components such as MMP-2, TGF-β, IL-1β, IL-6, and TNF-α. Nevertheless, the relationship between inflammation and myopia is still unclear. Thus, more biological experiments are needed to validate these results to gain a more comprehensive understanding of the intrinsic mechanisms of myopia and provide new ideas for myopia prevention and control.
Abbreviations
AC: allergic conjunctivitis
AL: axial length
AP1: activator protein 1
AKT: protein kinase B, PKB
CSA: immunosuppressant cyclosporin A
COL-1: collagen type 1
CAU: chronic anterior uveitis
CNV: choroidal neovascularization
DM: diabetes mellitus
ECM: extracellular matrix
FJ: fallopia japonica
FJE: fallopia japonica extract
IL-6: interleukin 6
Ig-E: Immunoglobulin E
JIA: juvenile idiopathic arthritis
LT: lens thickness
MMP-2: matrix metalloproteinase 2
MMPs: matrix metalloproteinases
MFC: multifocal chorioretinitis
MEWDS: multiple evanescent white dot syndrome
MAPK: mitogen-associated protein kinase
NF-κB: nuclear factor kappa B
PSC: posterior subcapsular cataract
PV: prunella vulgaris
PVE: prunella vulgaris extract
RPE: retinal pigment epithelium
SLE: systemic lupus erythematosus
T1DM: type 1 diabetes mellitus
TNF-α: tumor necrosis factor α
TGF-β: transformation growth factor β
TNFR 1: tumor necrosis factor receptor 1
Acknowledgements
This study was supported by National Key R&D Program of China [2021YFC2702103, 2021YFC2702100] and the Key R&D Program of Shandong Province [2019GSF108252].
Author contributions
Conceived and designed the study: D.G. and H.B; Collecting and organizing information: J.X., B.B., J.L., Z.M. and M.Z; Wrote the paper: J.X., B.B. and D.G.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wang J, Li Y, Musch DC. et al. Progression of Myopia in School-Aged Children After COVID-19 Home Confinement. JAMA Ophthalmol. 2021;139(3):293-300
2. Holden BA, Fricke TR, Wilson DA. et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042
3. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739-1748
4. Foster PJ, Jiang Y. Epidemiology of myopia. Eye (Lond). 2014;28(2):202-208
5. Fricke TR, Jong M, Naidoo KS. et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102(7):855-862
6. Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79(4):301-320
7. Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82(2):185-200
8. Harper AR, Summers JA. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res. 2015;133:100-111
9. Hornbeak DM, Young TL. Myopia genetics: a review of current research and emerging trends. Curr Opin Ophthalmol. 2009;20(5):356-362
10. Herbort CP, Papadia M, Neri P. Myopia and inflammation. J Ophthalmic Vis Res. 2011;6(4):270-283
11. Lin HJ, Wei CC, Chang CY. et al. Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation. EBioMedicine. 2016;10:269-281
12. Wei CC, Kung YJ, Chen CS. et al. Allergic Conjunctivitis-induced Retinal Inflammation Promotes Myopia Progression [published correction appears in EBioMedicine. 2019 Mar;41:717-718]. EBioMedicine. 2018;28:274-286
13. Córdova C, Gutiérrez B, Martínez-García C. et al. Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PLoS One. 2014;9(4):e91282 Published 2014 Apr 3
14. Wu H, Chen W, Zhao F. et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A. 2018;115(30):E7091-E7100
15. Jones BE, Thompson EW, Hodos W, Waldbillig RJ, Chader GJ. Scleral matrix metalloproteinases, serine proteinase activity and hydrational capacity are increased in myopia induced by retinal image degradation. Exp Eye Res. 1996;63(4):369-381
16. Zhao F, Zhou Q, Reinach PS. et al. Cause and Effect Relationship between Changes in Scleral Matrix Metallopeptidase-2 Expression and Myopia Development in Mice. Am J Pathol. 2018;188(8):1754-1767
17. Pelikan Z. Cytokines in tears during the secondary keratoconjunctival responses induced by allergic reaction in the nasal mucosa. Ophthalmic Res. 2014;52(1):32-42
18. Cabrera-Rivera GL, Madera-Sandoval RL, León-Pedroza JI. et al. Increased TNF-α production in response to IL-6 in patients with systemic inflammation without infection. Clin Exp Immunol. 2022;209(2):225-235
19. Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375-391
20. Mimura T, Yamagami S, Usui T. et al. Relationship between myopia and allergen-specific serum IgE levels in patients with allergic conjunctivitis. Clin Exp Ophthalmol. 2009;37(7):670-677
21. Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-516
22. Ijaz U, Habib A, Rathore HS. A Rare Presentation of Cyclitis Induced Myopia. J Coll Physicians Surg Pak. 2018;28(3):S56-S57
23. Jiang Q, Li Z, Tao T, Duan R, Wang X, Su W. TNF-α in Uveitis: From Bench to Clinic [published correction appears in Front Pharmacol. 2022 Feb 25;13:817235]. Front Pharmacol. 2021;12:740057 Published 2021 Nov 2
24. Paroli MP, Del Giudice E, Giovannetti F, Caccavale R, Paroli M. Management Strategies of Juvenile Idiopathic Arthritis-Associated Chronic Anterior Uveitis: Current Perspectives. Clin Ophthalmol. 2022;16:1665-1673 Published 2022 May 28
25. Petty RE, Southwood TR, Manners P. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390-392
26. Fledelius H, Zak M, Pedersen FK. Refraction in juvenile chronic arthritis: a long-term follow-up study, with emphasis on myopia. Acta Ophthalmol Scand. 2001;79(3):237-239
27. Lee DC, Lee SY, Kim YC. An epidemiological study of the risk factors associated with myopia in young adult men in Korea. Sci Rep. 2018;8(1):511 Published 2018 Jan 11
28. Silva MJ, Kilpatrick NM, Craig JM. et al. A twin study of body mass index and dental caries in childhood. Sci Rep. 2020;10(1):568 Published 2020 Jan 17
29. Sanz M, Marco Del Castillo A, Jepsen S. et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol. 2020;47(3):268-288
30. Alanazi AF, Alenezy A, Alotiby A. et al. Relationship between high CRP and cytokines in Saudi old people with dental caries in alkharj Region, Saudi Arabia. Saudi J Biol Sci. 2021;28(6):3523-3525
31. Tsai KZ, Liu PY, Lin YP. et al. Dental caries and periodontitis and the risk of myopia in young adults: CHIEF oral health study. BMC Oral Health. 2022;22(1):384 Published 2022 Sep 5
32. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51-59
33. Agrawal R, Bhardwaj M, Doley B. et al. Expression of IL-6, TNF-α, and hs-CRP in the serum of patients undergoing single-sitting and multiple-sitting root canal treatment: A comparative study. J Family Med Prim Care. 2022;11(5):1918-1922
34. Yu Q, Wang C, Liu Z. et al. Association between inflammatory cytokines and oxidative stress levels in aqueous humor with axial length in human myopia. Exp Eye Res. 2023;237:109670
35. Tsai KZ, Huang RY, Cheng WC. et al. Comparisons of various anthropometric indexes with localized Stage II/III periodontitis in young adults: The CHIEF oral health study. J Periodontol. 2021;92(7):958-967
36. Tsai KZ, Su FY, Cheng WC, Huang RY, Lin YP, Lin GM. Associations between metabolic biomarkers and localized stage II/III periodontitis in young adults: The CHIEF Oral Health study. J Clin Periodontol. 2021;48(12):1549-1558
37. Cecoro G, Annunziata M, Iuorio MT, Nastri L, Guida L. Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Medicina (Kaunas). 2020;56(6):272 Published 2020 May 30
38. Chen DY, Tzang CC, Liu CM. et al. Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis. Int J Mol Sci. 2023;24(20):15294 Published 2023 Oct 18
39. Wu SY, Yoo YJ, Nemesure B, Hennis A, Leske MC; Barbados Eye Studies Group. Nine-year refractive changes in the Barbados Eye Studies. Invest Ophthalmol Vis Sci. 2005;46(11):4032-4039
40. Tarczy-Hornoch K, Ying-Lai M, Varma R; Los Angeles Latino Eye Study Group. Myopic refractive error in adult Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2006;47(5):1845-1852
41. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98-107
42. Duke-Elder WS. CHANGES IN REFRACTION IN DIABETES MELLITUS. Br J Ophthalmol. 1925;9(4):167-187
43. Cordain L, Eaton SB, Brand Miller J, Lindeberg S, Jensen C. An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta Ophthalmol Scand. 2002;80(2):125-135
44. Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother. 2018;107:306-328
45. Galvis V, López-Jaramillo P, Tello A. et al. Is myopia another clinical manifestation of insulin resistance? Med Hypotheses. 2016;90:32-40
46. Wiemer NG, Dubbelman M, Hermans EA, Ringens PJ, Polak BC. Changes in the internal structure of the human crystalline lens with diabetes mellitus type 1 and type 2. Ophthalmology. 2008;115(11):2017-2023
47. Lin HT, Zheng CM, Fang YA. et al. Prevalence and risk factors for myopia in Taiwanese diabetes mellitus patients: a multicenter case-control study in Taiwan. Sci Rep. 2021;11(1):8195 Published 2021 Apr 14. doi:10.1038/s41598-021-87499-y
48. Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24(1):1-38
49. Jacobsen N, Jensen H, Lund-Andersen H, Goldschmidt E. Is poor glycaemic control in diabetic patients a risk factor of myopia? Acta Ophthalmol. 2008;86(5):510-514
50. Li SM, Li SY, Kang MT. et al. Distribution of ocular biometry in 7- and 14-year-old Chinese children. Optom Vis Sci. 2015;92(5):566-572
51. Xiao Y, Li T, Jia Y, Wang S, Yang C, Zou H. Influence of Type 1 Diabetes Mellitus on the Ocular Biometry of Chinese Children. J Ophthalmol. 2019;2019:7216490 Published 2019 Feb 14
52. Shah D, Sah S, Nath SK. Interaction between glutathione and apoptosis in systemic lupus erythematosus. Autoimmun Rev. 2013;12(7):741-751
53. Sivaraj RR, Durrani OM, Denniston AK, Murray PI, Gordon C. Ocular manifestations of systemic lupus erythematosus. Rheumatology (Oxford). 2007;46(12):1757-1762
54. Kamath YS, Singh A, Bhat SS, Sripathi H. Acute onset myopia as a presenting feature of systemic lupus erythematosus. J Postgrad Med. 2013;59(3):245-246
55. Silpa-archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 2016;100(1):135-141
56. Rao VA, Pandian DG, Kasturi N, Muthukrishanan V, Thappa DM. A case to illustrate the role of ophthalmologist in systemic lupus erythematosus. Indian J Dermatol. 2010;55(3):268-270
57. Yosar J, Whist E. Acute myopic shift in a patient with systemic lupus erythematosus. Am J Ophthalmol Case Rep. 2019;16:100562 Published 2019 Oct 10
58. Shu U, Takeuchi F, Tanimoto K, Moroi Y, Uchida K, Ito K. Transient myopia with severe chemosis associated with exacerbation of disease activity in systemic lupus erythematosus. J Rheumatol. 1992;19(2):297-301
59. Essex RW, Wong J, Jampol LM, Dowler J, Bird AC. Idiopathic multifocal choroiditis: a comment on present and past nomenclature. Retina. 2013;33(1):1-4
60. Koop A, Ossewaarde A, Rothova A. Peripheral multifocal chorioretinitis: complications, prognosis and relation with sarcoidosis. Acta Ophthalmol. 2013;91(6):492-497
61. Tavallali A, Yannuzzi LA. Idiopathic Multifocal Choroiditis. J Ophthalmic Vis Res. 2016;11(4):429-432 doi:10.4103/2008-322X.194141
62. Vance SK, Khan S, Klancnik JM, Freund KB. Characteristic spectral-domain optical coherence tomography findings of multifocal choroiditis. Retina. 2011;31(4):717-723
63. Reddy CV, Brown J Jr, Folk JC, Kimura AE, Gupta S, Walker J. Enlarged blind spots in chorioretinal inflammatory disorders. Ophthalmology. 1996;103(4):606-617
64. Dolz-Marco R, Fine HF, Freund KB. How to Differentiate Myopic Choroidal Neovascularization, Idiopathic Multifocal Choroiditis, and Punctate Inner Choroidopathy Using Clinical and Multimodal Imaging Findings. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):196-201
65. Jung JJ, Mrejen S, Freund KB, Yannuzzi LA. Idiopathic multifocal choroiditis with peripapillary zonal inflammation: a multimodal imaging analysis. Retin Cases Brief Rep. 2014;8(2):141-144
66. Jung JJ, Khan S, Mrejen S. et al. Idiopathic multifocal choroiditis with outer retinal or chorioretinal atrophy. Retina. 2014;34(7):1439-1450
67. Morgan CM, Schatz H. Recurrent multifocal choroiditis. Ophthalmology. 1986;93(9):1138-1147
68. Gallego-Pinazo R, Hernández S, Dolz-Marco R. Key Multimodal Fundus Imaging Findings to Recognize Multifocal Choroiditis in Patients With Pathological Myopia. Front Med (Lausanne). 2022;8:831764 Published 2022 Jan 24
69. Jampol LM, Sieving PA, Pugh D, Fishman GA, Gilbert H. Multiple evanescent white dot syndrome. I. Clinical findings. Arch Ophthalmol. 1984;102(5):671-674
70. Jabbarpoor Bonyadi MH, Hassanpour K, Soheilian M. Recurrent focal choroidal excavation following multiple evanescent white dot syndrome (MEWDS) associated with acute idiopathic blind spot enlargement. Int Ophthalmol. 2018;38(2):815-821
71. Bosello F, Westcott M, Casalino G. et al. Multiple evanescent white dot syndrome: clinical course and factors influencing visual acuity recovery. Br J Ophthalmol. 2022;106(1):121-127
72. Hamed LM, Glaser JS, Gass JD, Schatz NJ. Protracted enlargement of the blind spot in multiple evanescent white dot syndrome. Arch Ophthalmol. 1989;107(2):194-198
73. Fine HF, Spaide RF, Ryan EH Jr, Matsumoto Y, Yannuzzi LA. Acute zonal occult outer retinopathy in patients with multiple evanescent white dot syndrome. Arch Ophthalmol. 2009;127(1):66-70
74. Jampol LM, Becker KG. White spot syndromes of the retina: a hypothesis based on the common genetic hypothesis of autoimmune/inflammatory disease. Am J Ophthalmol. 2003;135(3):376-379
75. Cicinelli MV, Hassan OM, Gill MK, Goldstein D, Parodi MB, Jampol LM. A Multiple Evanescent White Dot Syndrome-like Reaction to Concurrent Retinal Insults. Ophthalmol Retina. 2021;5(10):1017-1026
76. Gliem M, Birtel J, Müller PL. et al. Acute Retinopathy in Pseudoxanthoma Elasticum [published correction appears in JAMA Ophthalmol. 2019 Aug 29;:]. JAMA Ophthalmol. 2019;137(10):1165-1173
77. Yang CS, Hsieh MH, Su HI, Kuo YS. Multiple Evanescent White Dot Syndrome Following Acute Epstein-Barr Virus Infection. Ocul Immunol Inflamm. 2019;27(2):244-250
78. Haw YL, Yu TC, Yang CS. A CARE-compliant article: a case report of possible association between recurrence of multiple evanescent white dot syndrome and the Herpesviridae family. Medicine (Baltimore). 2020;99(15):e19794
79. Delbarre M, Froussart-Maille F. Sémiologie et formes cliniques de la cataracte chez l'adulte [Signs, symptoms, and clinical forms of cataract in adults]. J Fr Ophtalmol. 2020;43(7):653-659
80. Richardson RB, Ainsbury EA, Prescott CR, Lovicu FJ. Etiology of posterior subcapsular cataracts based on a review of risk factors including aging, diabetes, and ionizing radiation. Int J Radiat Biol. 2020;96(11):1339-1361
81. Wong TY, Klein BE, Klein R, Tomany SC, Lee KE. Refractive errors and incident cataracts: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2001;42(7):1449-1454
82. Lim R, Mitchell P, Cumming RG. Refractive associations with cataract: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40(12):3021-3026
83. Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7(2):131-137
84. Marconi GD, Fonticoli L, Rajan TS. et al. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells. 2021;10(7):1587 Published 2021 Jun 23
85. Gross SA, von Noorden GK, Jones DB. Necrotizing scleritis and transient myopia following strabismus surgery. Ophthalmic Surg. 1993;24(12):839-841
86. Päivönsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in children: population-based study in Finland. Acta Ophthalmol Scand. 2000;78(1):84-88
87. Chen W, Zhao B, Jiang R. et al. Cytokine Expression Profile in Aqueous Humor and Sera of Patients with Acute Anterior Uveitis. Curr Mol Med. 2015;15(6):543-549
88. Fledelius HC. Is myopia getting more frequent?. A cross-sectional study of 1416 Danes aged 16 years+. Acta Ophthalmol (Copenh). 1983;61(4):545-559
89. Fledelius HC, Miyamoto K. Diabetic myopia-is it lens-induced?. An oculometric study comprising ultrasound measurements. Acta Ophthalmol (Copenh). 1987;65(4):469-473
90. Ganesan S, Raman R, Reddy S, Krishnan T, Kulothungan V, Sharma T. Prevalence of myopia and its association with diabetic retinopathy in subjects with type II diabetes mellitus: A population-based study. Oman J Ophthalmol. 2012;5(2):91-96
91. Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13(3):435-444
92. Dammacco R. Systemic lupus erythematosus and ocular involvement: an overview. Clin Exp Med. 2018;18(2):135-149
93. Li T, Li X, Hao Y. et al. Inhibitory effect of miR-138-5p on choroidal fibrosis in lens-induced myopia guinea pigs via suppressing the HIF-1α signaling pathway. Biochem Pharmacol. 2023;211:115517
94. Li DQ, Lee SB, Tseng SC. Differential expression and regulation of TGF-beta1, TGF-beta2, TGF-beta3, TGF-betaRI, TGF-betaRII and TGF-betaRIII in cultured human corneal, limbal, and conjunctival fibroblasts. Curr Eye Res. 1999;19(2):154-161
95. Wang Q, Zhao G, Xing S, Zhang L, Yang X. Role of bone morphogenetic proteins in form-deprivation myopia sclera. Mol Vis. 2011;17:647-657 Published 2011 Mar 8
96. Frost MR, Norton TT. Differential protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Mol Vis. 2007;13:1580-1588 Published 2007 Sep 6
97. Lin Z, Chen X, Ge J. et al. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther. 2008;24(6):543-550
98. Wang Y, Tang Z, Xue R. et al. TGF-β1 promoted MMP-2 mediated wound healing of anterior cruciate ligament fibroblasts through NF-κB. Connect Tissue Res. 2011;52(3):218-225
99. Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol Cell. 2009;35(1):48-57
100. Ogawa S, Lozach J, Jepsen K. et al. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci U S A. 2004;101(40):14461-14466
101. Mrugacz M, Bryl A, Falkowski M, Zorena K. Integrins: An Important Link between Angiogenesis, Inflammation and Eye Diseases. Cells. 2021;10(7):1703 Published 2021 Jul 6
102. McBrien NA, Metlapally R, Jobling AI, Gentle A. Expression of collagen-binding integrin receptors in the mammalian sclera and their regulation during the development of myopia. Invest Ophthalmol Vis Sci. 2006;47(11):4674-4682
103. Tian XD, Cheng YX, Liu GB. et al. Expressions of type I collagen, α2 integrin and β1 integrin in sclera of guinea pig with defocus myopia and inhibitory effects of bFGF on the formation of myopia. Int J Ophthalmol. 2013;6(1):54-58
104. Wang KK, Metlapally R, Wildsoet CF. Expression Profile of the Integrin Receptor Subunits in the Guinea Pig Sclera. Curr Eye Res. 2017;42(6):857-863
105. Deng W, Cao Z, Dong R, Yan Y, Jiang Q. Irisin inhibits CCK-8-induced TNF-α production via integrin αVβ5-NF-κB signaling pathways in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2024;144:109245
106. Huang FC, Kuo HC, Huang YH, Yu HR, Li SC, Kuo HC. Anti-inflammatory effect of resveratrol in human coronary arterial endothelial cells via induction of autophagy: implication for the treatment of Kawasaki disease. BMC Pharmacol Toxicol. 2017;18(1):3 Published 2017 Jan 9
107. Zhou ZX, Mou SF, Chen XQ, Gong LL, Ge WS. Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NF-κB in animal models of acute pharyngitis. Mol Med Rep. 2018;17(1):1269-1274
108. Deng ZY, Hu MM, Xin YF, Gang C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm Res. 2015;64(5):321-332
109. Li H, Xia N, Hasselwander S, Daiber A. Resveratrol and Vascular Function. Int J Mol Sci. 2019;20(9):2155 Published 2019 Apr 30
110. Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81(11):1343-1351
111. Hsu YA, Chen CS, Wang YC. et al. Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model. Curr Issues Mol Biol. 2021;43(2):716-727 Published 2021 Jul 16
112. Zhang Y, Wildsoet CF. RPE and Choroid Mechanisms Underlying Ocular Growth and Myopia. Prog Mol Biol Transl Sci. 2015;134:221-240
113. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614-618 doi:10.1136/bjophthalmol-2011-300539
114. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845-881
115. Li BY, Hu Y, Li J. et al. Ursolic acid from Prunella vulgaris L. efficiently inhibits IHNV infection in vitro and in vivo. Virus Res. 2019;273:197741
116. Eid SY, El-Readi MZ, Ashour ML, Wink M. Fallopia japonica, a Natural Modulator, Can Overcome Multidrug Resistance in Cancer Cells. Evid Based Complement Alternat Med. 2015;2015:868424
117. Wang SJ, Wang XH, Dai YY. et al. Prunella vulgaris: A Comprehensive Review of Chemical Constituents, Pharmacological Effects and Clinical Applications. Curr Pharm Des. 2019;25(3):359-369
118. Lachowicz S, Oszmiański J. Profile of Bioactive Compounds in the Morphological Parts of Wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and Their Antioxidative Activity. Molecules. 2019;24(7):1436 Published 2019 Apr 11
119. Chen H, Tuck T, Ji X. et al. Quality assessment of Japanese knotweed (Fallopia japonica) grown on Prince Edward Island as a source of resveratrol. J Agric Food Chem. 2013;61(26):6383-6392
120. Qiang Z, Ye Z, Hauck C. et al. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J Ethnopharmacol. 2011;137(3):1107-1112
121. Ryu SY, Oak MH, Yoon SK. et al. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 2000;66(4):358-360
122. Ding YJ, Sun CY, Wen CC, Chen YH. Nephroprotective role of resveratrol and ursolic Acid in aristolochic Acid intoxicated zebrafish. Toxins (Basel). 2015;7(1):97-109 Published 2015 Jan 13
123. Cho J, Rho O, Junco J. et al. Effect of Combined Treatment with Ursolic Acid and Resveratrol on Skin Tumor Promotion by 12-O-Tetradecanoylphorbol-13-Acetate. Cancer Prev Res (Phila). 2015;8(9):817-825
124. Chen CS, Hsu YA, Lin CH. et al. Fallopia Japonica and Prunella vulgaris inhibit myopia progression by suppressing AKT and NFκB mediated inflammatory reactions. BMC Complement Med Ther. 2022;22(1):271 Published 2022 Oct 14
125. Cardoso CRL, Leite NC, Carlos FO, Loureiro AA, Viegas BB, Salles GF. Efficacy and Safety of Diacerein in Patients With Inadequately Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2017;40(10):1356-1363
126. Wally V, Hovnanian A, Ly J. et al. Diacerein orphan drug development for epidermolysis bullosa simplex: A phase 2/3 randomized, placebo-controlled, double-blind clinical trial. J Am Acad Dermatol. 2018;78(5):892-901.e7
127. Fouad AA, Abdel-Aziz AM, Hamouda AAH. Diacerein Downregulates NLRP3/Caspase-1/IL-1β and IL-6/STAT3 Pathways of Inflammation and Apoptosis in a Rat Model of Cadmium Testicular Toxicity. Biol Trace Elem Res. 2020;195(2):499-505
128. Philp AM, Davis ET, Jones SW. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology (Oxford). 2017;56(6):869-881
129. Bharti R, Dey G, Ojha PK. et al. Diacerein-mediated inhibition of IL-6/IL-6R signaling induces apoptotic effects on breast cancer. Oncogene. 2016;35(30):3965-3975 doi:10.1038/onc.2015.466
130. Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799(10-12):775-787
131. Tien PT, Lin CH, Chen CS. et al. Diacerein Inhibits Myopia Progression through Lowering Inflammation in Retinal Pigment Epithelial Cell. Mediators Inflamm. 2021;2021:6660640 Published 2021 Jul 3
Author contact
![]() Corresponding author: Dadong Guo, Shandong Academy of Eye Disease Prevention and Therapy; Medical College of Optometry and Ophthalmology, Shandong University of Traditional Chinese Medicine, No. 48, Yingxiongshan Road, Jinan, Shandong 250002, China; Tel., +86 531 58859696; E-mail: dadonggeneedu.cn, ORCID: 0000-0002-1712-0055.
Corresponding author: Dadong Guo, Shandong Academy of Eye Disease Prevention and Therapy; Medical College of Optometry and Ophthalmology, Shandong University of Traditional Chinese Medicine, No. 48, Yingxiongshan Road, Jinan, Shandong 250002, China; Tel., +86 531 58859696; E-mail: dadonggeneedu.cn, ORCID: 0000-0002-1712-0055.

 Global reach, higher impact
Global reach, higher impact