3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(8):1491-1499. doi:10.7150/ijms.96007 This issue Cite
Research Paper
Renal protective effects of Alpinate Oxyphyllae Fructus and mesenchymal stem cells co-treatment against D- galactose induced renal deterioration
1. Department of Chinese Medicine, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan.
2. Integration Center of Traditional Chinese and Modern Medicine, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan.
3. School of Post-Baccalaureate Chinese Medicine, College of Medicine, Tzu Chi University, Hualien, Taiwan.
4. Cardiovascular and Mitochondrial Related Disease Research Center, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan.
5. Department of Psychiatry, Tzu Chi General Hospital, 707, Section 3, Chung-Yang Road, Hualien 97004, Taiwan.
6. School of Medicine Tzu Chi University, 701, Section 3, Chung-Yang Road, Hualien 97004, Taiwan.
7. Department of Emergency Medicine, China Medical University Hospital, Taichung, Taiwan.
8. Division of Cardiovascular Medicine, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
9. Laboratory of Exercise Biochemistry, University of Taipei, Taiwan.
10. Department of Biological Science and Technology, College of Life Sciences, China Medical University, Taichung, Taiwan.
11. Ph.D. Program for Biotechnology Industry, China Medical University, Taichung, Taiwan.
12. School of pharmacy, China Medical University, Taichung, Taiwan.
13. Department of Biological Science and Technology, Asia University, Taichung, Taiwan.
14. Center of General Education, Buddhist Tzu Chi Medical Foundation, Tzu Chi University of Science and Technology, Hualien 970, Taiwan.
15. Graduate Institute of Biomedical Sciences, China Medical University, Taichung 404, Taiwan.
16. Department of Medical Research, China Medical University Hospital, China Medical University, Taichung 404, Taiwan.
* Share equal contribution.
Received 2024-3-6; Accepted 2024-5-14; Published 2024-5-27
Abstract
Age-related structural and functional changes in the kidney can eventually lead to development of chronic kidney disease, which is one of the leading causes of mortality among elderly people. For effective management of age-related kidney complications, it is important to identify new therapeutic interventions with minimal side-effects. The present study was designed to evaluate the synergistic effect of a traditional Chinese herb, Alpinate Oxyphyllae Fructus (AOF), and adipose-derived mesenchymal stem cells (ADMSCs) in ameliorating D-galactose (D-gal)-induced renal aging phenotypes in WKY rats. The study findings showed that D-gal-induced alteration in the kidney morphology was partly recovered by the AOF and ADMSC co-treatment. Moreover, the AOF and ADMSC co-treatment reduced the expression of proinflammatory mediators (NFkB, IL-6, and Cox2) and increased the expression of redox regulators (Nrf2 and HO-1) in the kidney, which were otherwise augmented by the D-gal treatment. Regarding kidney cell death, the AOF and ADMSC co-treatment was found to abolish the proapoptotic effects of D-gal by downregulating Bax and Bad expressions and inhibiting caspase 3 activation. Taken together, the study findings indicate that the AOF and ADMSC co-treatment protect the kidney from D-gal-induced aging by reducing cellular inflammation and oxidative stress and inhibiting renal cell death. This study can open up a new path toward developing novel therapeutic interventions using both AOF and ADMSC to effectively manage age-related renal deterioration.
Introduction
In the past decade, the number of elderly population who are over the age of 65 years has increased significantly due to advanced medical facilities and improved lifestyle [1]. Despite having an improved life expectancy, the elderly population is particularly susceptible to several age-related diseases, including cardiovascular and pulmonary disorders, renal problems, diabetes, gastrointestinal problems, osteoarthritis, neurodegenerative diseases, and even cancer [2]. Thus, it is of prime importance to search for aging interventions that can delay the onset of age-related diseases, as well as subside the consequences of normal aging process, such as physical disabilities and functional limitations [3].
The application of mesenchymal stem cells (MSCs) as regenerative medicine has been increased tremendously in biomedical sciences. MSCs are potent candidates for cell-based therapies due to their unique properties of escaping immune surveillance and inhibiting inflammation after transplantation. Moreover, MSCs can be easily extracted from the bone marrow and adipose tissue, and differentiated into multiple organ-specific cell types [4]. Given the ability of MSCs to repair damaged tissues and organs, several preclinical and clinical studies have been performed to evaluate the potency of MSC-based therapies in age-related diseases, because tissue degeneration is one of the major hallmarks of aging [5-8].
The risk of developing chronic kidney disease in elderly population is particularly high because of the increasing prevalence of other comorbid, predisposing risk factors, such as hypertension, diabetes, and cardiovascular disorders [9]. Because of the significant anti-inflammatory effects, MSC-based therapies are considered to be very effective in treating kidney disorders wherein inflammation is one of the major contributing factors [10, 11]. Several studies have been conducted to evaluate the effect of MSC-based treatments in various types of kidney disorders, such as acute kidney injury, focal segmental glomerulosclerosis, systemic lupus erythematosus, diabetic nephropathy, and kidney transplantation [12]. Mechanistically, MSCs work as regenerative medicine by secreting a large number of bioactive molecules and extracellular vesicles that are collectively responsible for the therapeutic effects [13]. However, the biological activity of MSCs can be drastically reduced in response to the challenging cellular microenvironment they encounter after transplantation [14, 15]. In this context, MSC preconditioning using various external stimuli, such as hypoxia, pro-inflammatory mediators, growth factors, and 3D culture, is the best way to preserve and improve the therapeutic efficacy of MSCs [14].
Bioactive plant molecules or phytochemicals have become an attractive choice for treating many health complications particularly because of beneficial therapeutic properties, including anti-oxidative, anti-inflammatory, and tissue regenerative effects [14, 16]. Moreover, a growing pool of evidence has suggested that phytochemicals can improve the basic characteristics of MSCs, including proliferation, differentiation, and migration [17].
Alpinate Oxyphyllae Fructus (AOF) is a Chinese herb used widely as traditional Chinese medicine for treating many health complications, including kidney, gastrointestinal, and cardiovascular disorders [18, 19]. Bioactive components present in AOF extract, such as flavonoids, terpenes, essential oils, steroids, and diarylheptanoids, are known to possess several health benefits, including antioxidative, anti-inflammatory, anticancer, anti-diuretic, anti-diabetic, anti-diarrheal, anti-aging, and neuroprotective properties [20-23]. Moreover, previous studies also indicated AOF can provide anti-aging effects in aging hearts by decreasing apoptosis and enhancing longevity gene expression [24]. Interestingly, a recent study has shown that preconditioning of adipose-derived mesenchymal stem cells (ADMSCs) with AOF extract causes enrichment in ADMSC stemness, an increase in longevity, and improvement in stem cell homing [23]. Moreover, AOF-preconditioned ADMSCs have been shown to protect cardiac cells from doxorubicin-induced cellular senescence. These hints suggested that AOF may provide beneficial effects in ADSC treated with aging renal.
Given the beneficial effect of AOF on the urinary system, as well as its ability to improve ADMSC properties, the present study was designed to evaluate the effect of AOF and ADMSC co-treatment on the kidneys of D-galactose-induced aging rats.
Materials and Methods
Alpinia extract
Alpinia was purchased from Shin-Long Pharmaceutical Company (Taiwan, ROC). For water extraction, 150 g of AOF was boiled in 600 ml of water for 2 h, followed by passing of the extract through Whatman filter paper at a reduced pressure. The filtrate was stored until become powder. The yield was approximately 7.4%. For preparing the stock solution, the powder was dissolved in water, and the solution was filter sterilized before administering to animals.
Animals and experimental design
The entire experimental procedures and animal handling were supervised and approved by the China Medical University - Animal Care and Use Committee (IACUC) (No. 2016-172). The experiments were conducted on 18-week-old Wistar male rats that were purchased from the National Laboratory Animal Centre, Taiwan. The rats were maintained at room temperature of 23 ± 2°C under a dark/light cycle and were supplied with drinking water and rat chow ad libitum. After acclimatization for a couple of weeks, WKY rats were randomly divided into 6 groups (n = 4 per group): Control group, D-galactose (150 mg/kg/day) (D-gal) group, D-gal + AoF group, D-gal + ADMSC group, D-gal + AoF Oral + ADMSC group, and D-gal + AoF treated ADMSC group. For the treatments, AoF extract (100 mg/kg/day) was administered using oral gavage, and ADMSCs (10,000,000 cells) were administered intravenously. D-gal will treat with 6 weeks, after 6 weeks will provide treatment for 4 weeks, the rats were anesthetized and sacrificed by decapitation. The kidneys were harvested from the rats and immediately kept at -80°C.
Hematoxylin-Eosin (H&E) Staining
The slides containing paraffin-embedded kidney sections were de-waxed by immersing in xylene (x 3) and dehydrated by dipping in ethanol gradients (100%, 95% and 75%). Then, the sections were incubated with hematoxylin staining solution for 5 min, followed by washing with double-distilled water (x 3) and submerging in 85% alcohol for 2 min. Next, the sections were incubated with eosin solution for 5 min, followed by submerging in 100% alcohol for 5 min and xylene for 1 min (x 2). The tissue sections were observed under microscope (Olympus Microscope), and the images were obtained.
PAS staining
The slides were de-paraffinized and dehydrated with ethanol. Then they were immersed in PAS solution (10 min). After incubation, slides were washed using water (X 4). Then the samples were treated with Schiff's solution an incubated for 15min. After washing, the samples were incubated with Hematoxylin (3 min). Again the samples were washed with water, then for few seconds samples were kept with Bluing reagent. Finally, after washing, Light Green Solution was added for 2 min. Samples were dehydrated again with alcohol. The tissue sections were observed under microscope (Olympus Microscope), and the images were documented.
TUNEL assay
The tissue sections were incubated with xylene, then the slides were washed in a series of alcohol. Slides were kept in proteinase K (2 μg/ml), followed by phosphate-buffered saline washing. Then they were treated with permeabilization solution and incubated in blocking buffer, and then washed with PBS. The terminal deoxynucleotidyl transferase and fluorescein isothiocyanate-dUTP apoptosis detection kit (Roche Applied Science, Indianapolis, IN, USA) was utilized for 60 min at 37 °C. Bright-green light at 450-500 nm was used to detect the number of apoptotic nuclei. The tissue sections were also stained with 0.1 mg/ml 4′,6-diamidino-2-phenylindole (DAPI) and detected by UV light at 340-380 nm. Photomicrographs were obtained using an Olympus® CKX53 microscope. All counts were performed by at least three independent individuals in a blinded manner.
Tissue extraction
The kidney tissue (100 mg) was excised, washed with 1X PBS (x 3), and homogenized in 1 ml of RIPA buffer using tissue homogenizer. The lysate was kept on ice for 20 min, followed by centrifugation at 1,200 rpm for 5 min (4°C). The clear upper layer of the lysate was collected. After a few seconds of vortex, the supernatant was centrifuged at 13,000 rpm for 30 min (4°C), and the clear supernatant was stored for experimental usage.
Western blotting
The protein samples were extracted from the harvested kidney tissues and quantified using Lowry's method. An appropriate amount of protein sample (40µg/lane) was mixed with 5X loading dye and boiled for 10 min at 95°C. The samples were vortexed, centrifuged, and loaded on the gel for SDS-PAGE analysis (current: 90 V; run time: 2 h). After the separation, the protein samples were transferred to a PVDF membrane, followed by blocking with 2.5% BSA for 1 h. Next, the membrane was rinsed in washing buffer and incubated with indicated primary antibodies overnight at 4°C. After washing with wash buffer (X3), the membrane was incubated with respective secondary antibodies for 1 h and washed with TBST. The immunoblot was developed with chemiluminescence ECL, and the bands were observed in LAS-4000 mini (GE Healthcare). The changes in protein expressions were compared using 'Image J' software.
Results
Synergistic effect of AOF and ADMSC cotreatment on D-galactose-induced aging kidneys
To investigate the effect of AOF and ADMSC cotreatment on aging kidney morphology, 18-week-old WKY male rats were treated with D-galactose (150 mg/kg/day) to induce aging phenotypes. After induction of aging, the rats were further treated with AOF extract alone (100 mg/kg/day); ADMSC cells alone (10,000,000 cells); AOF + ADMSC; and AOF-preconditioned ADMSC. The untreated rats were used as negative control.
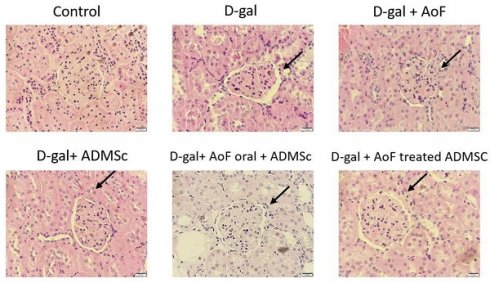
The kidney tissues excised from the aging rats were subjected to hematoxylin-eosin staining to investigate effects of AOF and ADMSC in ameliorating the histological changes induced by D-galactose. As observed in Figure 1, D-galactose treatment caused significantly increased Bowman's space and altered shape and size of glomeruli. These findings clearly indicate D-galactose-induced onset of glomerular aging. Interestingly, it was observed that cotreatment with AOF and ADMSC was capable of moderately ameliorating the aging-induced renal phenotypes.
Next, PAS staining was performed to further investigate the renal protective effects of AOF and ADMSC. As observed in Figure 2, D-gal treatment caused thickening of the capillary wall, which was recovered, at least in part, by AOF and ADMSC cotreatment. Taken together, these findings clearly depict renal protective effects of AOF and ADMSC cotreatment on D-gal-induced aging kidneys.
Protective effects of AOF and ADMSC cotreatment on oxidative and inflammatory stress markers in D-galactose-induced aging kidneys
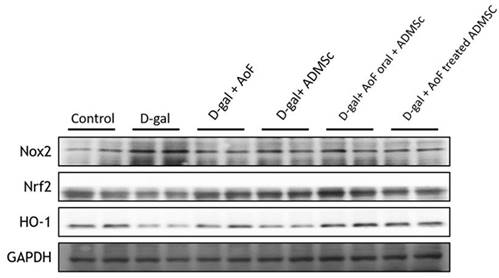
Since oxidative stress and inflammation play crucial roles in age-related renal pathologies [25], we next investigated the effects of AOF and ADMSC cotreatment on biomarkers of oxidative stress and inflammation in D-gal-induced aging kidneys. To evaluate cellular oxidative stress, the protein expressions of NADPH oxidase (NOX2), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) were analyzed using immunoblotting method. As observed in Figure 3, D-gal treatment caused significant induction of oxidative stress, which was characterized by increased NOX2 expression and decreased Nrf2 and HO-1 expressions. Interestingly, AOF and ADMSC cotreatment was found to restore the redox homeostasis by enhancing Nrf2 and HO-1 expressions.
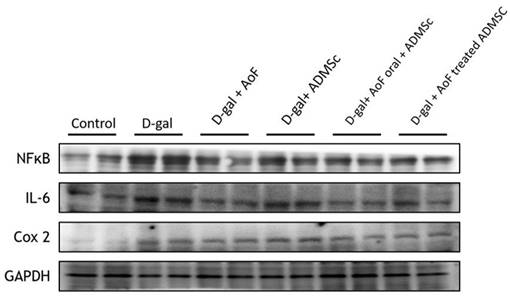
To investigate cellular inflammation, the protein expressions of pro-inflammatory mediators including nuclear factor NFkB, interleukin-6 (IL-6), and cyclooxygenase 2 (Cox2) were analyzed using kidney tissue extracts. As observed in Figure 4, D-gal-induced induction of inflammatory microenvironment in aging kidney tissues was well evidenced by the increased expressions of NFkB, IL-6, and Cox2. However, the cotreatment with AOF and ADMSC was found to considerably ameliorate renal inflammation by reducing the expressions of pro-inflammatory mediators.
Synergistic effect of AOF and ADMSC cotreatment on renal cell death
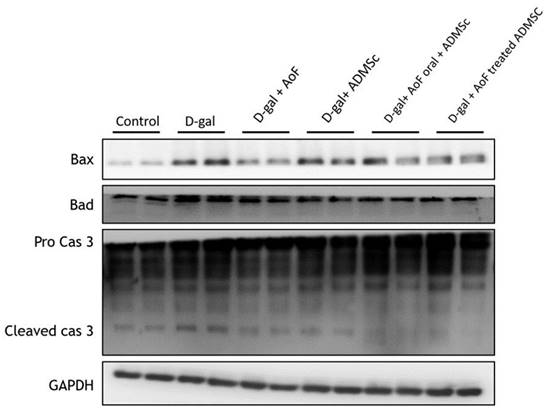
To investigate the effects of AOF and ADMSC on renal cell apoptosis, the protein expressions of intrinsic (mitochondria-mediated) apoptotic pathway components including Bax, Bad, and caspase 3 were analyzed using kidney tissue extracts. Mechanistically, induction of BH3 domain-containing pro-apoptotic protein (Bax and Bad) expression causes release of cytochrome c from mitochondria to the cytosol, leading to activation of caspase 3 and induction of apoptosis [26]. As observed in Figure 5, D-gal-induced increased expression of Bax, Bad, and caspase 3 was counterbalanced by the administration of AOF and ADMSC, indicating anti-apoptotic effects of AOF and ADMSC on aging renal cells.
AOF and ADMSC decrease aging-induced kidney injury. Kidney injury evaluated by tissue section and H&E staining. D-gal induced renal aging significant showed the organization is not neatly arranged (arrow site). After treated with AOF and ADMSc significant decrease the arranged sites compared with D-gal group (arrow site).
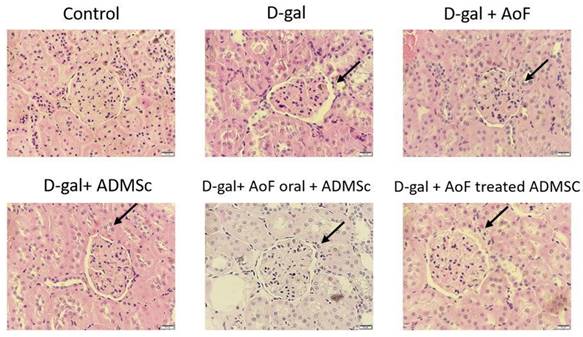
AOF and ADMSC prevent D-gal induce kidney aging. Kidney injury evaluated by tissue section and PAS staining (red color). D-gal induced kidney aging (arrow site), treated with AOF or ADMSc or co-treated both can decreased aging in kidney arrow site.
D-gal induce ROS injury by targeting Nrf2 molecular pathway. D-gal significantly increased Nox2 expression and decreased ROS-related molecular proteins Nrf2 an d HO-1 expression. Treatment with AOD or ADMSC significantly decreased injury from D-gal, analysis by western blotting.
AOF and ADMSC decrease D-gal induced inflammation in kidney. D-gal increased inflammation-related protein, and after treatment with AOF and ADMSC significantly decreased inflammation-related protein factor expression, analysis by western blotting.
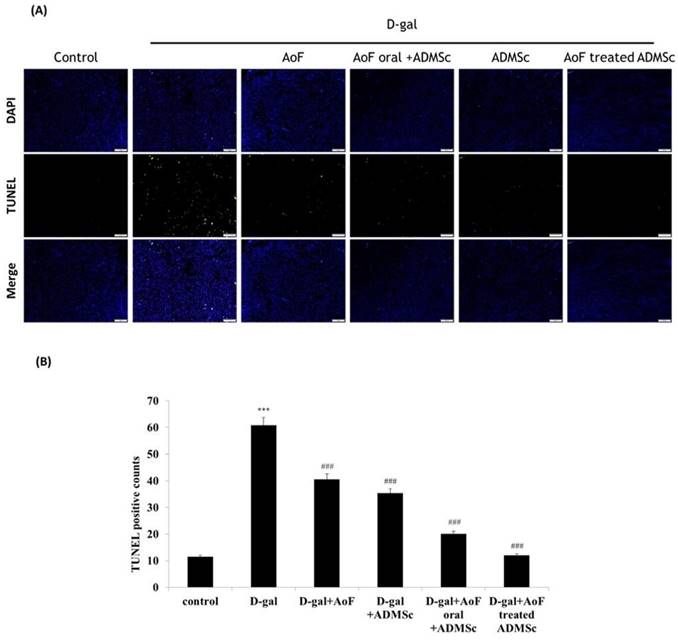
Similarly, the TUNEL assay data showed that the number of apoptotic cells reduced significantly upon administration of AOF and ADMSC, which were otherwise increased by D-gal treatment (Figure 6). Taken together, these observations clearly indicate the health benefits of AOF and ADMSC co-treatment on D-gal-induced renal aging phenotypes.
AOF and ADMSC decreased D-gal-induced apoptosis in kidney. Treated with AOF, ADMSC in Rat significantly decreased D-gal-induced apoptosis-related protein expression.
AOF and ADMSC provide protective effects in aging kidney. (A) D-gal induced kidney aging and cell death (green spots). After treatment with AOF or ADMSC in aging rats, significant decrease in cell death, analysis on tissue section by TUNEL assay. (B) Quantification of the TUNEL-positive cells by Image J software.
Discussion
Structural and functional alterations in the kidney are common consequences of aging. These changes grossly include reduced functionality of kidney blood vessels, increased number and size of nephrons, thickened glomerular basement membrane, reduced glomerular filtration rate, interstitial fibrosis, and tubular atrophy [27, 28]. The age-related structural alterations are commonly defined as nephrosclerosis, which is developed due to nonmalignant hypertension. Importantly, nephrosclerosis along with kidney dysfunction can make elderly people more susceptible to acute kidney injury and chronic kidney disease [29]. Thus, it is of prime importance to identify novel therapeutic interventions with minimal side-effects to manage age-related kidney deterioration.
Given the significant impact of MSC-based therapies in treating kidney disorders [10], the present study was designed to evaluate the effect of a traditional Chinese herb, AOF, in the potentiation of MSC functions, as well as to investigate the effects of AOF and ADMSC co-treatment in ameliorating age-related renal changes.
We used D-gal for the induction of aging phenotypes, and our data showed that D-gal caused age-related structural changes in the kidneys, such as increased Bowman's space and altered shape and size of glomeruli (Figure 1). Although treatment with AOF or ADMSC alone showed ameliorating effects on the aging kidney tissue, the maximum benefit was observed with AOF and ADMSC co-treatment (Figure 1). These findings suggested the possible effect of AOF extract in improving ADMSC characteristics, which is in line with a previous study indicating AOF pretreatment induced improvement in ADMSC longevity and homing ability [23]. Further analysis of the kidney morphology using PAS staining revealed that AOF and ADMSC co-treatment was capable of restoring D-gal-induced thickening of the capillary wall (Figure 2).
Similar to our findings, the renally protective role of AOF has been observed previously [30, 31]. By reducing the oxidative stress level and modulating PTEN expression, AOF extract has been found to improve kidney functions in diabetic mice [32]. Regarding renal morphological changes, AOF extract has been shown to prevent tubular necrosis in rat models of ischemia/reperfusion-induced acute renal failure [22].
Since inflammation and oxidative stress are two major predisposing factors for kidney disorders, we investigated several oxidative stress and inflammatory biomarkers in the present study. Our western blot data showed that D-gal treatment caused induction of NOX2 expression, which was accompanied with reduced Nrf2 and HO-1 expressions (Figure 3). This indicates that D-gal-induced renal aging is associated with induction of oxidative stress. However, co-treatment with AOF and ADMSC was found to reverse the D-gal effects by reducing NOX2 expression and increasing Nrf2 and HO-1 expressions in the kidney tissues (Figure 3). Regarding inflammation, we observed that co-treatment with AOF and ADMSC diminished renal inflammation by reducing the expressions of pro-inflammatory biomarkers including NFkB, IL-6, and Cox2, which were otherwise increased after the D-gal treatment (Figure 4).
Previous studies concerning health benefits of AOF extracts have also highlighted the antioxidant and anti-inflammatory properties of AOF [33, 34]. In a rat model of hypoxia-induced enuresis, treatment with AOF extract has been found to reduce urine output and increase bladder leak point pressure by increasing superoxide dismutase (SOD) activity, decreasing malondialdehyde (MDA) level, and regulating purinergic, muscarinic, and beta-adrenergic receptors [35]. Regarding inflammation, AOF extract has been shown to reduce the symptom severity of encephalomyelitis in mice by decreasing the infiltration of pro-inflammatory mediators in the spinal cord and regulating Th1/Th17 response [21]. Moreover, research also indicated AOF increased the longevity related genes such as SIRT1 molecular pathway to protect against aging injury in heart [24]. These results and our data suggested that AOF can provide a strong anti-aging effect in many organs.
The antioxidative and anti-inflammatory benefits of MSC are also we-evidenced in the literature [36]. Co-culture of MSCs with airway smooth muscles has been found to reduce mitochondrial ROS production, maintain mitochondrial membrane potential, and prevent cell death [37]. Moreover, MSC has been found to attenuate adriamycin-induced nephropathy by reducing oxidative stress, downregulating NFkB expression, and reducing inflammatory cytokine production [38]. In line with these observations, our findings reveal that the co-treatment with AOF and ADMSC synergistically ameliorates D-gal-induced renal aging by reducing inflammation and maintaining redox homeostasis.
Furthermore, we observed that the treatment with D-gal increased the expressions of Bax and Bad and activated caspase 3 (Figure 5), which was accompanied with increased numbers of apoptotic cells (Figure 6). Interestingly, AOF and ADMSC co-treatment was found to completely nullify the D-gal effects (Figure 5 and 6). These findings indicate that AOF and ADMSC collectively prevent D-gal-induced renal aging by reducing oxidative stress- and inflammation-induced renal cell death.
The present study observations are supported by previous studies showing the anti-apoptotic effects of MSCs against hypoxia-induced alveolar epithelial cell death [39]. Moreover, exosomes derived from MSC have been shown to reduce ER stress-induced apoptosis of nucleus pulposus cells [40]. Interestingly, ADMSC pretreated with AOF extract has been shown to protect hepatic cells from doxorubicin-induced senescence and aging.
Conclusion
The present study was designed to evaluate the synergistic effect of a traditional Chinese herb, AOF, and ADMSC in ameliorating D-gal-induced renal aging phenotypes. The study findings reveal that the co-treatment with AOF and ADMSC is capable of restoring D-gal-induced alterations in renal morphology. Mechanistically, AOF and ADMSC collectively ameliorate D-gal-induced renal aging by reducing the expression of inflammatory mediators (NFkB, IL-6, and Cox 2); increasing the expression of redox regulators (Nrf2 and HO-1); and preventing renal cell death. The present study findings can open up a new path toward developing novel therapeutic interventions using both AOF and ADMSC to effectively manage age-related renal deterioration.
Acknowledgements
This study was supported in part by a grant from the National Science and Technology Council, Taipei, Taiwan (107-2320-B-039-069).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jaul E, Barron J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front Public Health. 2017;5:335
2. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196-208
3. Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C. et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell. 2015;14:497-510
4. Abdrakhmanova S, Ospanova M, Turgambayeva A, Akhayeva M, Savchuk T, Tuyakova N. et al. Mesenchymal cord blood stem cells in regenerative medicine. Minerva Med. 2022;113:200-1
5. Raggi C, Berardi AC. Mesenchymal stem cells, aging and regenerative medicine. Muscles Ligaments Tendons J. 2012;2:239-42
6. Kassem M. Stem cells: potential therapy for age-related diseases. Ann N Y Acad Sci. 2006;1067:436-42
7. Yu Y. Application of Stem Cell Technology in Antiaging and Aging-Related Diseases. Adv Exp Med Biol. 2018;1086:255-65
8. Schulman IH, Balkan W, Hare JM. Mesenchymal Stem Cell Therapy for Aging Frailty. Front Nutr. 2018;5:108
9. Mallappallil M, Friedman EA, Delano BG, McFarlane SI, Salifu MO. Chronic kidney disease in the elderly: evaluation and management. Clin Pract (Lond). 2014;11:525-35
10. Bochon B, Kozubska M, Surygala G, Witkowska A, Kuzniewicz R, Grzeszczak W. et al. Mesenchymal Stem Cells-Potential Applications in Kidney Diseases. Int J Mol Sci. 2019 20
11. Fan M, Zhang J, Xin H, He X, Zhang X. Current Perspectives on Role of MSC in Renal Pathophysiology. Front Physiol. 2018;9:1323
12. Peired AJ, Sisti A, Romagnani P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016;2016:4798639
13. Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Goncalves RM. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front Immunol. 2018;9:2837
14. Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428-42
15. Forni C, Facchiano F, Bartoli M, Pieretti S, Facchiano A, D'Arcangelo D. et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed Res Int. 2019;2019:8748253
16. Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260
17. Zhang Q, Zheng Y, Hu X, Hu X, Lv W, Lv D. et al. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: A review. J Ethnopharmacol. 2018;224:149-68
18. Almubayedh H, Ahmad R. Ethnopharmacological uses, phytochemistry, biological activities of Debregeasia salicifolia: A review. J Ethnopharmacol. 2019;231:179-86
19. Ho TJ, Ahmed T, Shibu MA, Lin YJ, Shih CY, Lin PY. et al. A prospective review of the health-promoting potential of Jing Si Herbal Tea. Tzu Chi Med J. 2024;36:1-22
20. Lee YM, Son E, Kim SH, Kim DS. Effect of Alpinia oxyphylla extract in vitro and in a monosodium iodoacetate-induced osteoarthritis rat model. Phytomedicine. 2019;65:153095
21. Huang KK, Lin MN, Hsu YL, Lu IH, Pan IH, Yang JL. Alpinia oxyphylla Fruit Extract Ameliorates Experimental Autoimmune Encephalomyelitis through the Regulation of Th1/Th17 Cells. Evid Based Complement Alternat Med. 2019;2019:6797030
22. Kim EJ, Lee YJ, Ahn YM, Lee H, Kang DG, Lee HS. Renoprotective effect of Alpiniae oxyphyllae Fructus on ischemia/reperfusion-induced acute renal failure. Arch Pharm Res. 2013;36:1004-12
23. Chang YM, Asokan Shibu M, Tsai CT, Tsai CC, Lin SL, Chang CC. et al. Alpinate Oxyphyllae extracts enhance the longevity and homing of mesenchymal stem cells and augment their protection against senescence in H9c2 cells. J Cell Physiol. 2019;234:12042-50
24. Chang YM, Chang HH, Kuo WW, Lin HJ, Yeh YL, Padma Viswanadha V. et al. Anti-Apoptotic and Pro-Survival Effect of Alpinate Oxyphyllae Fructus (AOF) in a d-Galactose-Induced Aging Heart. Int J Mol Sci. 2016;17:466
25. Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int J Mol Sci. 2019 21
26. Pawlowski J, Kraft AS. Bax-induced apoptotic cell death. Proc Natl Acad Sci U S A. 2000;97:529-31
27. O'Sullivan ED, Hughes J, Ferenbach DA. Renal Aging: Causes and Consequences. J Am Soc Nephrol. 2017;28:407-20
28. Denic A, Glassock RJ, Rule AD. Structural and Functional Changes With the Aging Kidney. Adv Chronic Kidney Dis. 2016;23:19-28
29. James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW. et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376:2096-103
30. Lee YS, Sung YY, Yuk HJ, Son E, Lee S, Kim JS. et al. Anti-hyperuricemic effect of Alpinia oxyphylla seed extract by enhancing uric acid excretion in the kidney. Phytomedicine. 2019;62:152975
31. Li YH, Tan YF, Cai HD, Zhang JQ. Metabonomic study of the fruits of Alpinia oxyphylla as an effective treatment for chronic renal injury in rats. J Pharm Biomed Anal. 2016;124:236-45
32. Xie Y, Xiao M, Li D, Liu H, Yun F, Wei Y. et al. Anti-diabetic effect of Alpinia oxyphylla extract on 57BL/KsJ db-/db- mice. Exp Ther Med. 2017;13:1321-8
33. Shui G, Bao YM, Bo J, An LJ. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur J Pharmacol. 2006;538:73-9
34. Qi Y, Cheng X, Jing H, Yan T, Xiao F, Wu B. et al. Effect of Alpinia oxyphylla-Schisandra chinensis herb pair on inflammation and apoptosis in Alzheimer's disease mice model. J Ethnopharmacol. 2019;237:28-38
35. Su MS, Xu L, Gu SG, Huang N, Ren XK, Cai XH. et al. Therapeutic effects and modulatory mechanism of Alpiniae oxyphyllae Fructus in chronic intermittent hypoxia induced enuresis in rats. Sleep Breath. 2020;24:329-37
36. de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB. et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem. 2018;293:1957-75
37. Li X, Michaeloudes C, Zhang Y, Wiegman CH, Adcock IM, Lian Q. et al. Mesenchymal stem cells alleviate oxidative stress-induced mitochondrial dysfunction in the airways. J Allergy Clin Immunol. 2018;141:1634-45 e5
38. Song IH, Jung KJ, Lee TJ, Kim JY, Sung EG, Bae YC. et al. Mesenchymal stem cells attenuate adriamycin-induced nephropathy by diminishing oxidative stress and inflammation via downregulation of the NF-kB. Nephrology (Carlton). 2018;23:483-92
39. Bernard O, Jeny F, Uzunhan Y, Dondi E, Terfous R, Label R. et al. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am J Physiol Lung Cell Mol Physiol. 2018;314:L360-L71
40. Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K. et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084-100
Author contact
![]() Corresponding author: Chih-Yang Huang Ph.D. Chair Professor, Cardiovascular and Mitochondrial Related Disease Research Center, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Tzu Chi University of Science and Technology, Hualien, Taiwan. Tel.: +886-3-8561825 ext 15930. Fax: +886-4-22032295. E-mail address: cyhuangcmu.edu.tw.
Corresponding author: Chih-Yang Huang Ph.D. Chair Professor, Cardiovascular and Mitochondrial Related Disease Research Center, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Tzu Chi University of Science and Technology, Hualien, Taiwan. Tel.: +886-3-8561825 ext 15930. Fax: +886-4-22032295. E-mail address: cyhuangcmu.edu.tw.

 Global reach, higher impact
Global reach, higher impact