3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(7):1337-1343. doi:10.7150/ijms.90642 This issue Cite
Research Paper
Interleukin-25 as a Potential Biomarker in Lung Metastasis of Hepatocellular Carcinoma with HBV History in Chinese Patients: A Single Center, Case-control Study
1. Department of Laboratory Medicine, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China.
2. School of Medicine, Shanghai Jiao Tong University, Shanghai 200025, China.
3. Department of Oncology, State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
* These authors contributed equally to this work.
Received 2023-9-29; Accepted 2024-4-22; Published 2024-5-19
Abstract
Background: Interleukin-25 (IL-25) has been proved to play a role in the pathogenesis and metastasis of Hepatocellular carcinoma (HCC), but the relationship between the level of IL-25 and the metastasis and prognosis of HCC is still not clear. This study aimed to investigate the expression of IL-25 and other potential biochemical indicators among healthy people, HBV-associated HCC patients without lung metastasis and HBV-associated HCC patients with lung metastasis.
Methods: From September 2019 to November 2021, 33 HCC patients without lung metastasis, 37 HCC patients with lung metastasis and 29 healthy controls were included in the study. IL-25 and other commonly used biochemical markers were measured to establish predictors of overall survival (OS) and progression-free survival (PFS) after treatment.
Results: The serum level of IL-25 was increased in HCC patients than healthy controls (p < 0.001) and HCC patients with lung metastasis had higher IL-25 level than HCC patients without metastasis (p = 0.035). Lung metastasis also indicated higher death rate (p < 0.001) by chi-square test, higher GGT level (p = 0.024) and higher AFP level (p = 0.049) by non-parametric test. Kaplan-Meier analysis demonstrated that IL-25 was negatively associated with PFS (p = 0.024). Multivariate Cox-regression analysis indicated IL-25 (p = 0.030) and GGT (p = 0.020) to be independent predictors of poorer PFS, while IL-25 showed no significant association with OS.
Conclusion: The level of IL-25 was significantly associated with disease progression and lung metastasis of HBV-associated HCC. The high expression of IL-25 predicted high recurrence rate and death probability of HCC patients after treatment. Therefore, IL-25 may be an effective predictor of prognosis in HCC.
Keywords: Interleukin-25, hepatocellular carcinoma, lung metastasis, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers and the third leading cause of cancer-related death in China [1]. HCC has a high mortality due to the occurrence of intrahepatic or systemic metastasis. AFP is the only biomarker that is most commonly used to predict the occurrence and prognosis of HCC patients, but its sensitivity and specificity are not satisfactory [2, 3]. Thus, new and reliable biomarkers are needed to improve the diagnostic level of HCC. A few recent studies have suggested GGT as an independent prognostic indicator in cases with HCC. GGT was suggested to promote tumor progression and poor prognosis through inducing DNA damage and genome instability, releasing reactive oxygen species to activate invasion-related signaling pathways, blocking chemotherapy, regulating microRNAs, and managing CpG island methylation [4]. A series of studies showed that the onset and progression of HCC was closely associated with the interaction between tumor cells and the inflammatory microenvironment [5]. The inflammatory liver microenvironment also promotes the metastasis of HCC [6]. Many studies reported that some inflammatory cytokines were included in the regulation of cancer cells, including IL-6 in breast cell and IL-20RB in bone metastasis of lung cancer [7, 8]. IL-17 promoted the secretion of CAL27 cell and increased the infiltration of CD11c+ cells in the peritumoral area of basal cell carcinomas (BCCs) and squamous-cell carcinomas (SCCs) in mice [9].
HCC is prone to both intrahepatic and extrahepatic metastasis. Extrahepatic metastasis has been reported in 13.5 to 42% of HCC patients [10]. Lung is the most common metastatic organ of primary liver cancer, accounting for 39.5%-53.8% of extrahepatic metastases. Lung metastasis seriously affects the prognosis of patients and is difficult to treat [11, 12]. Inflammatory microenvironment has been proven to be crucial in HCC metastasis. Studied have shown that exosomal miR-1247-3p, which converts fibroblasts to cancer-associated fibroblasts (CAFs), could increase the secretion of IL-6 and IL-8 and promoted the lung metastasis of HCC [13]. HIF-1α/IL-1β loop mediates the crosstalk between HCC cells and TAMs in a hypoxia-inflammatory microenvironment, which induces lung metastasis [14].
Interleukin-25 (IL-25, also called IL-17E) maps to chromosome 14q11 and it is an inflammatory IL-17 family cytokine. IL-25 was originally described as a Th2-produced cytokine involved in the induction of Th2-like responses [15]. IL-25 has been proved to influence the promotion of nonmelanoma skin cancer, colorectal cancer and breast cancer [9, 16-18]. Studies have showed that sustained innate IL-25 signaling helped to maintain the cancer permissive microenvironment of colorectal adenocarcinoma by preventing anti-tumor T cells and IFNγ-mediated immunity [17]. Previous studies have demonstrated that IL-25 activate macrophages alternatively, secreted CXCL10 and activated the EMT pathway of HCC [19]. However, there are few studies on the level of IL-25 in patients with lung metastasis of HCC and the prognosis on HCC.
This study was designed to determine the difference in IL-25 levels between HCC and HCC with lung metastasis and its association with the survival of patients with HBV-associated HCC.
Materials and Methods
Patients
From September 2019 to November 2021, a total of 99 patients were enrolled in the third affiliated hospital of Sun Yat-sen University, including 33 HCC patients without lung metastasis, 37 HCC patients with lung metastasis and 29 healthy controls (HC). The diagnosis of HCC was based on the diagnostic criteria for HCC used by the European Association for the Study of the Liver [20]. Lung metastasis was diagnosed by chest computed tomography (CT) or magnetic resonance imaging (MRI). Patients with concurrent autoimmune disease or without history of HBV infection were excluded. All the patients underwent transcatheter arterial chemoembolization (TACE) as initial treatment.
Clinical and laboratory data collection
The baseline data was taken within one week before treatment. Tumor location and TNM (tumor, lymph node and metastasis) staging were judged from the results of CT or MRI in accordance with the 7th edition of the AJCC Cancer Staging Manual [21]. AST, ALT, GGT and TBA were included in serological detection. Serum levels of IL-25 were measured in HCC patient before treatment by ELISA (Cloud-Clone Corp. Wuhan, China) in accordance with the product instructions. Child-Pugh score was assessed in accordance with the 2021 NCCN guidelines [22]. All consecutive parameters were categorized for further analysis as follows: age (≤65 or >65years), AFP (≤200 U/L or >200 U/L), nodule size (≤70cm or >70cm), AST (≤56U/L or >56U/L), ALT (≤40U/L or >40U/L), GGT (≤100U/L or >100U/L), PLT (≤130U/L or >130U/L). Cut-off values were set base on the previous studies (age [23, 24], tumor size [25] and AFP [26]) or median of the data (AST, ALT, GGT, PLT).
Follow‑up and outcome measures
US and computed tomography (CT) were conducted at one month after treatment and every 3-6 months thereafter. Extrahepatic organ examination was also carried out if patients had extrahepatic metastases. Liver magnetic resonance imaging was also used to define suspicious lesions demonstrated on CT. We defined progression as the appearance of new lesions with radiological features typical of HCC, as confirmed by at least two imaging methods for patients underwent curative treatment. For patients who underwent palliative treatment, progression was defined as at least a 20% increase in the sum of the longest diameter of the target lesions, or the appearance of new lesions or metastasis. The secondary outcome was overall survival (OS), which defined as the time from treatment to death for any reason.
Statistical analysis
Mann-Whitney U-test was used to compare quantitative variables, and differences between categorical variables were analyzed by chi-squared or Fisher exact test. The associations between IL-25 and PFS as well as OS were evaluated. Univariate and multivariate Cox-regression analyses were done to discriminate clinicopathological predictors of PFS and OS, including group, age, gender, history of cirrhosis, nodule size and number, cancer embolus, number of metastases, TNM stage, AST, ALT, GGT, AFP, PLT, Child-Pugh score and IL-25. The differences of survival analysis were compared using the Kaplan-Meier method with log-rank tests for survival plot depiction. Hazard ratio (HR) and 95% confidence interval (95% CI) was evaluated. Only variables with statistical significance in univariate analysis were included in multivariate analysis.
Receiver operating characteristic (ROC) curves were depicted to set the cut-off points of IL-25. The classification variable was PFS. The reason for the choice of classification variables was that there were clinical trials demonstrating the association between IL-25 and PFS2. IL-25 was divided into high-level group and low-level group to investigate its relationship with other predictors.
The statistical significance level of all tests was p<0.05. Analyses and calculation were done by IBM SPSS Statistics 24.0.
Results
Patient characteristic
The demographics and characteristics of HCC patients were shown in Table 1. Among the 70 HCC patients in the study, 63 were male and 7 were female. The median age at baseline was 54.5 years (range, 25-81). During follow-up, tumor progression occurred in 67.1% of patients and 24.3% of patients died. All patients had a history of HBV infection and 91.4% had a history of cirrhosis. There were 31.4% of patients with a single primary nodule in liver, 37.1% with cancer embolus and 42.9% with more than 2 nodules of metastasis.
Serum levels of IL-25 in HCC patients
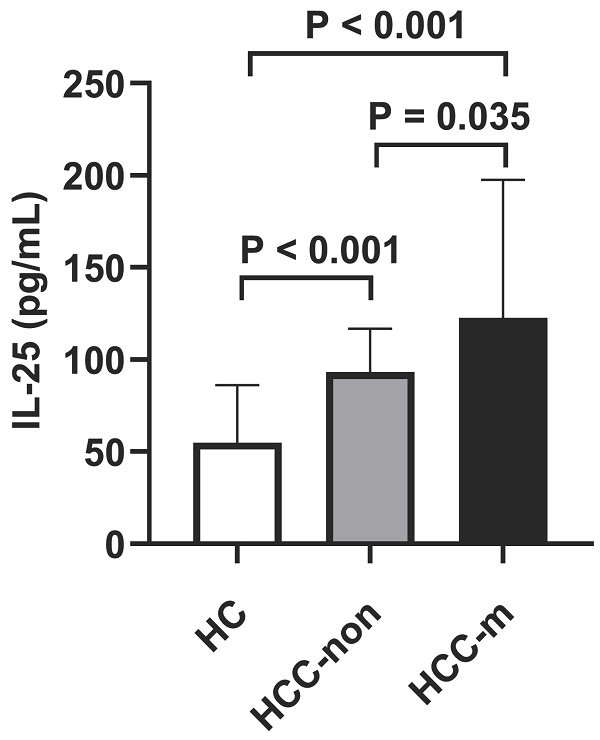
It was uncovered that HCC group suggested higher IL-25 levels than healthy control group (p < 0.001) and lung metastasis group suggested higher IL-25 levels than HCC group (p = 0.035, Fig. 1), which indicated that lung metastasis of HCC was significantly associated with IL-25. Lung metastasis also indicated higher death rate (p < 0.001), higher GGT (p = 0.024) and higher AFP (p = 0.049) (Table 1). Child-Pugh score also showed a marginal significance (p = 0.058).
Effect of IL-25 on prognosis
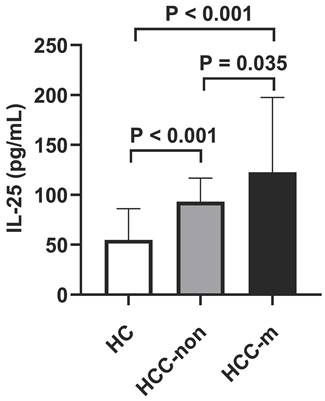
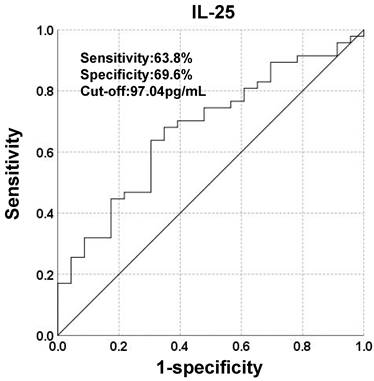
To investigate the potential of IL-25 in predicting prognosis, a ROC curve was constructed to find the cut-off point of IL-25 based on tumor progression. The area under curve (AUC) was 0.675. A fixed cut-off value of 97.04 pg/mL was taken for the analysis, yielding a sensitivity of 63.8% and a specificity of 69.6% (Fig. 2). Kaplan-Meier analysis revealed that IL-25 level was significantly associated with PFS, with median PFS of 7 months and 4 months for patients with IL-25 ≤ 97.04 pg/mL and > 97.04 pg/mL respectively (p = 0.024) (Fig. 3A). However, IL-25 showed no association with OS. The median OS of patients with IL-25 ≤ 97.04 pg/mL was 12 months and that of patients with IL-25 > 97.04 pg/mL was 11 months (p = 0.591) (Fig. 3B). These results might indicate that HCC patients with higher levels of IL-25 tend to have worse prognosis.
Levels of IL-25 in healthy control (HC) group vs non-metastatic HCC (HCC-non) group vs lung metastatic HCC (HCC-m) group. Bars represent the medians and interquartile range.
Patient characteristics
| Variables | HCC without metastasis (n=33) | HCC with metastasis (n=37) | p value |
|---|---|---|---|
| Sex | 0.565 | ||
| Male (%) | 30 (90.9) | 33 (89.2) | |
| Female (%) | 3 (9.1) | 4 (10.8) | |
| Age, median (IQR), years | 55 (27) | 53 (17) | 0.489 |
| Progression | 0.199 | ||
| Present (%) | 20 (60.6) | 27 (73.0) | |
| Absent (%) | 13 (39.4) | 10 (27.0) | |
| Death | < 0.001 | ||
| Present (%) | 2 (6.1) | 15 (40.5) | |
| Absent (%) | 31 (93.9) | 22 (59.5) | |
| Liver cirrhosis | 0.607 | ||
| Present (%) | 30 (90.9) | 34 (91.9) | |
| Absent (%) | 3 (9.1) | 3 (8.1) | |
| Tumor multiplicity | 0.494 | ||
| Single (%) | 11 (33.3) | 11 (29.7) | |
| Multiple (%) | 22 (66.7) | 19 (51.4) | |
| Cancer embolus | 0.086 | ||
| Present (%) | 9 (27.3) | 17 (45.9) | |
| Absent (%) | 24 (72.7) | 20 (54.1) | |
| TNM Stage | < 0.001 | ||
| Ⅰ/Ⅱ/Ⅲ (%) | 32 (97.0) | 0 (0.0) | |
| Ⅳ (%) | 1 (3.0) | 37 (100.0) | |
| Child-Pugh score | 0.058 | ||
| ≤6 (%) | 13 (39.4) | 7 (18.9) | |
| >6 (%) | 15 (45.5) | 23 (62.2) | |
| AFP (U/L) | 0.049 | ||
| ≤200 (%) | 17 (51.5) | 12 (32.4) | |
| >200 (%) | 13 (39.4) | 24 (64.9) | |
| AST, median (IQR), U/L | 54.0 (65.0) | 61.0 (56.0) | 0.441 |
| ALT, median (IQR), U/L | 39.0 (49.0) | 39.5 (45.0) | 0.746 |
| GGT, median (IQR), U/L | 61.50 (95.00) | 94.5 (158.0) | 0.024 |
| PLT, median (IQR), ×10^9/L | 130.5 (117.0) | 122.5 (120.0) | 0.430 |
| IL-25, median (IQR), pg/mL | 94.21 (52.27) | 124.89 (121.19) | 0.035 |
HCC, hepatocellular carcinoma; HC, healthy control; AFP, alpha fetoprotein; AST, aspartate transaminase; IQR, interquartile range; ALT, alanine aminotransferase; GGT, γ-glutamyl transpeptidase; PLT, platelet; IL-25, interleukin-25.
Receiver operating characteristic (ROC) curve analysis of IL-25 for the discrimination between progressed and non-progressed patients.
To evaluate whether IL-25 was independent predictors of prognosis, univariate and multivariate cox-regression analyses for both PFS and OS were performed (Table 2). In the assessment of potential prognostic variables, GGT (p = 0.012) and IL-25 (p = 0.039) were found to be significantly associated with PFS by univariate cox-regression analysis. In addition, nodule size (p = 0.066) and AST (p = 0.056) showed marginally significant association with PFS in univariate analysis. At multivariate analysis, both GGT (p = 0.020) and IL-25 (p = 0.030) showed statistically significant association with PFS. In the assessment of OS, only cancer embolus showed marginally significant association in univariate cox-regression analysis (p = 0.084). IL-25 didn't show any association with OS in univariate analysis (p = 0.974).
Associations between serum IL-25 levels and patient characteristics
With 97.04 pg/mL as the cut-off point, IL-25 was divided into high and low groups and the associations between IL-25 levels and clinicopathologic parameters were assessed by the chi-squared test (Table 3). Tumor size, PLT and serological indicators including AST, ALT and GGT were separated by the median of the data. High IL-25 level was found to correlate with high progression rate (p = 0.009) and more lung metastases nodes (p = 0.048). The death rate exhibits marginal significance with IL-25 (p = 0.079). However, we found no significant association between IL-25 and other clinical parameters including AST, ALT, GGT, AFP and PLT. IL-25 also had no association with cancer embolus, tumor size, TNM stage and Child-Pugh score.
Discussion
IL-25 is an inflammatory IL-17 family cytokine that is confirmed to sustain type 2 immunity [15]. Accumulated evidence indicates a key role of IL-25 in diseases including acute hepatitis, liver fibrosis and liver cirrhosis [2, 27]. IL-25 was also confirmed to be associated with the onset and progression of various cancers, but it is rarely reported in the lung metastasis and the prognosis of HCC [28]. In our study, we found that the serum level of IL-25 was significantly associated with the disease progression and lung metastasis of HBV-associated HCC.
Our results demonstrated that the serum level of IL-25 was increased in HCC patients than healthy controls and HCC patients with lung metastasis had higher IL-25 level than HCC patients without metastasis.
A tumor-infiltrating MMTV-PyMT tumor mouse model showed that tumor macrophages were the primary source of IL-25 and demonstrated the critical role of IL-25 in regulating the type 2 immune response by targeting Th2 cells in a breast cancer model, thereby promoting tumor metastasis [29]. Another study confirmed that c-RAF phosphorylation, ERK1/2 and p70 S6 kinase induced by IL-17A and IL-17E were involved in tumor cell proliferation and survival [30]. IL-25 can also induce JAK/STAT3 signaling pathway to promote self-renewal of cancer cells [31].
Some previous studies have initially uncovered the relationship between IL-25 and HCC. It was reported that the serum levels of IL-25 were significantly higher in patients with HCC than in patients with chronic hepatitis C and IL-25 was negatively correlated with serum zinc level, which promotes fibrosis of the liver [32]. IL-25 could indirectly promote the progression of HCC cells and induce the alternative activation of macrophages to secrete CXCL 10 and activate the EMT pathway. It was uncovered that the alternative activation of macrophages induced by IL-25 promoted the migration, invasion and tumorigenesis of hepatoma cells, increased the expression of vimentin, Snail and phospho-ERK, and decreased the expression of E-cadherin in hepatoma cells [19]. Moreover, another study also reported that IL-25 could predict the recurrence of patients with HBV-HCC after resection and help to diagnose HCC as a supplement to AFP [2]. It indicated that IL-25 might be a potential tumor marker for the diagnosis of liver cancer. As a type 2 cytokine, IL-25 was also upregulated in many inflammatory disorders such as atopic dermatitis, psoriasis, and asthma [15]. Thus, the specificity of IL-25 as a HCC biomarker was relatively low. In our study, we confirmed the relationship between HCC and IL-25 and further found that IL-25 provided independent prognostic information of prognosis in terms of PFS, whereas no significance in OS. It indicates that IL-25 may be a prognostic indicator of HCC progression and a potential target for gene therapy.
Univariate and multivariate cox-regression analysis of factors associated with the progression and survival of HCC patients
| Variables | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| PFS | OS | PFS | |
| HR (95% CI), p | HR (95% CI), p | HR (95% CI), p | |
| Sex (female vs. male) | 0.825 (0.326-2.091), 0.685 | 2.982 (0.628-14.168), 0.169 | |
| Age (>65 vs. ≤65 years) | 0.476 (0.202-1.124), 0.090 | 0.648 (0.084-5.014), 0.678 | |
| Liver cirrhosis (present vs. absent) | 1.870 (0.580-6.029), 0.295 | 1.113 (0.249-4.984), 0.889 | |
| Tumor size (>7cm vs.≤7) | 1.823 (0.962-3.456), 0.066 | 1.299 (0.266-6.348), 0.746 | |
| Tumor multiplicity (multiple vs. single) | 1.257 (0.648-2.440), 0.499 | 1.422 (0.417-4.846), 0.574 | |
| Cancer embolus (present vs. absent) | 1.335 (0.743-2.397), 0.334 | 2.495 (0.883-7.045), 0.084 | |
| Number of metastases (>2 vs. ≤2) | 1.177 (0.647-2.141), 0.593 | 0.403 (0.106-1.534), 0.183 | |
| TNM stage (Ⅳ vs. Ⅰ-Ⅲ) | 1.163 (0.636-2.129), 0.624 | 0.946 (0.102-8.782), 0.961 | |
| AST (>56 vs. ≤56 U/L) | 1.770 (0.986-3.175), 0.056 | 1.392 (0.423-4.575), 0.586 | |
| ALT (>40 vs. ≤40 U/L) | 1.248 (0.703-2.215), 0.449 | 1.031 (0.384-2.768), 0.952 | |
| GGT (>80 vs. ≤80 U/L) | 2.215 (1.189-4.127), 0.012 | 1.725 (0.455-6.546), 0.423 | 2.104 (1.127-3.928), 0.020 |
| AFP (>200 vs. ≤200 U/L) | 1.460 (0.804-2.651), 0.214 | 1.241 (0.420-3.671), 0.696 | |
| PLT (>130 vs.≤130 ×10^9/L) | 1.007 (0.566-1.791), 0.981 | 1.596 (0.606-4.202), 0.344 | |
| Child-Pugh score (>6 vs. ≤6) | 1.090 (0.565-2.104), 0.798 | 0.821 (0.274-2.459), 0.724 | |
| IL-25 (>97.04 vs. ≤97.04 pg/mL) | 1.877 (1.033-3.411), 0.039 | 1.019 (0.327-3.175), 0.974 | 1.986 (1.069-3.688), 0.030 |
Abbreviation: AFP, alpha fetoprotein; AST, aspartate transaminase; ALT, alanine aminotransferase; GGT, γ-glutamyl transpeptidase; PLT, platelet; IL-25, interleukin-25; PFS, progression-free survival; OS, overall survival.
Kaplan-Meier curves stratified by the level of IL-25. (A) Progression-free survival (PFS) and (B) Overall survival (OS) of patients classified by IL-25 ≤ 97.04 pg/mL and > 97.04 pg/mL
Associations between IL-25 levels and patients' characteristics
| Characteristics | Low IL-25 group (n=33) | High IL-25 group (n=37) | p value |
|---|---|---|---|
| Gender | |||
| Male | 30 (90.9) | 33 (89.2) | 0.565 |
| Female | 3 (9.1) | 4 (10.8) | |
| Age (yrs) | |||
| ≤65 | 27 (81.8) | 28 (75.7) | 0.371 |
| >65 | 6 (18.2) | 9 (24.3) | |
| Progression | |||
| Present | 17 (51.5) | 30 (81.1) | 0.009 |
| Absent | 16 (48.5) | 7 (18.9) | |
| Death | |||
| Present | 5 (15.2) | 12 (32.4) | 0.079 |
| Absent | 28 (84.8) | 25 (67.6) | |
| Liver cirrhosis | |||
| Present | 30 (90.9) | 34 (91.9) | 0.607 |
| Absent | 3 (9.1) | 3 (8.1) | |
| Tumor size (cm) | |||
| ≤7 | 14 (42.4) | 16 (43.2) | 0.351 |
| >7 | 17 (51.5) | 14 (37.8) | |
| Tumor multiplicity | |||
| Single | 11 (33.3) | 11 (29.7) | 0.568 |
| Multiple | 21 (63.6) | 20 (54.1) | |
| Cancer embolus | |||
| Present | 15 (45.5) | 11 (29.7) | 0.133 |
| Absent | 18 (54.5) | 26 (70.3) | |
| Number of lung metastases nodes | |||
| ≤2 | 22 (66.7) | 17 (45.9) | 0.048 |
| >2 | 10 (30.3) | 20 (54.1) | |
| TNM stage | |||
| Ⅰ-Ⅲ | 18 (54.5) | 14 (37.8) | 0.123 |
| Ⅳ | 15 (45.5) | 23 (62.2) | |
| AST (U/L) | |||
| ≤56 | 18 (54.5) | 18 (48.6) | 0.400 |
| >56 | 15 (45.5) | 19 (51.4) | |
| ALT (U/L) | |||
| ≤40 | 18 (54.5) | 20 (54.1) | 0.579 |
| >40 | 15 (45.5) | 17 (45.9) | |
| GGT (U/L) | |||
| ≤80 | 16 (48.5) | 17 (45.9) | 0.596 |
| >80 | 15 (45.5) | 16 (43.2) | |
| AFP (U/L) | |||
| ≤200 | 12 (36.4) | 17 (45.9) | 0.368 |
| >200 | 18 (54.5) | 19 (51.4) | |
| PLT (×10^9/L) | |||
| ≤130 | 17 (51.5) | 18 (48.6) | 0.500 |
| >130 | 16 (48.5) | 19 (51.4) | |
| Child-Pugh score | |||
| ≤6 | 10 (30.3) | 10 (27.0) | 0.457 |
| >6 | 17 (51.5) | 21 (56.8) |
Abbreviation: AFP, alpha fetoprotein; AST, aspartate transaminase; ALT, alanine aminotransferase; GGT, γ-glutamyl transpeptidase; PLT, platelet; IL-25, interleukin-25.
We also focused on the effect of IL-25 on HCC patients with lung metastasis. Lung metastasis is the most common distant invasive progression of HCC, and it is also one of the main causes of cancer-related death [33]. The tumor microenvironment is a complex mixture, including various cytokines and stromal cells and extracellular matrix, which has important impacts on tumor metastasis [34]. Recently, cancer associated fibroblast-derived CCL5 was reported to promote hepatocellular carcinoma metastasis through activating HIF1α/ZEB1 axis [35]. Both in vitro and in vivo investigations identified that osteopontin had an important role in metastasis of HCC and was an attractive potential therapeutic target for combating HCC metastasis [36]. Our study revealed that the increased expression of IL-25 was significantly associated with the lung metastasis of HBV-associated HCC, which indicates that IL-25 may be a predictor of lung metastasis in HCC.
We recognized several limitations in our research. The first was that it was a single-center retrospective analysis, which means a small sample size. Thus, the significance of multivariate cox-regression analysis was not high. The second was that no blood samples were taken during follow-up, thus the relationship between the change of IL-25 and the prognosis was not analyzed. The third was that more clinically proven HCC makers should be included to study their relationship with IL-25. Therefore, large-scale multi-center studies are warranted to validate and extend our results to confirm the clinical relevance of serum IL-25 as a prognostic biomarker in HBV-associated HCC patients in the future.
In conclusion, the serum level of IL-25 was significantly associated with the occurrence and lung metastasis of HBV-associated HCC and IL-25 may be an effective biomarker for HCC. The high expression of IL-25 had a negative effect on the progression probability of HCC patients after treatment, and a higher IL-25 level shortened the PFS time of HBV-associated HCC. Our findings suggest that IL-25 might be a promising independent outcome predictor and potential therapeutic target for HBV-associated HCC.
Acknowledgements
Funding
This work was supported by grants from the National Natural Science Foundation of China (82271883, 82073195); Science and Technology Program of Guangzhou (2023A04J1810); Hospital-Pharma Integration Project on Innovative Achievement Translation (SHDC2022CRD043); Special Funds for Technological Innovation of Shanghai Baoshan Science and Technology Commission (No.20-E-32); Clinical Research Innovation Cultivation Fund of Baoshan Branch of Renji Hospital, Shanghai Jiaotong University School of Medicine (2019-rbcxjj-001).
Ethics approval
This study was approved by the Ethic Committee of the Third Affiliated Hospital, Sun Yat-sen University as stipulated by the Declaration of Helsinki ([2019]02-309-01).
Author contributions
Conceptualization: [Yuan Liao]; Methodology: [Yuan Liao], [Ziying Mo]; Formal analysis and investigation: [Yuan Liao], [Yixin Xu]; Writing - original draft preparation: [Yixin Xu]; Writing - review and editing: [Yuan Liao]; Funding acquisition: [Qing Xia], [Yuan Liao]; Resources: [Wenying Zhou], [Huimin Dong]; Supervision: [Qing Xia]. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
2. Chen SH, Wang X. A high preoperative serum IL-25 level is a negative prognosis predictor after liver resection for HBV-HCC. Front Oncol. 2022;12:858151
3. Nault JC, Villanueva A. Biomarkers for Hepatobiliary Cancers. Hepatology. 2021;73(Suppl 1):115-27
4. Xia J, Song P, Sun Z, Sawakami T, Jia M, Wang Z. Advances of diagnostic and mechanistic studies of gamma-glutamyl transpeptidase in hepatocellular carcinoma. Drug Discov Ther. 2016;10:181-7
5. Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401-35
6. Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis. 2019;39:26-42
7. Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT. et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26:1446-51
8. He Y, Luo W, Liu Y, Wang Y, Ma C, Wu Q. et al. IL-20RB mediates tumoral response to osteoclastic niches and promotes bone metastasis of lung cancer. J Clin Invest. 2022 132
9. Nardinocchi L, Sonego G, Passarelli F, Avitabile S, Scarponi C, Failla CM. et al. Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. Eur J Immunol. 2015;45:922-31
10. Yang B, Li M, Tang W, Liu W, Zhang S, Chen L. et al. Dynamic network biomarker indicates pulmonary metastasis at the tipping point of hepatocellular carcinoma. Nat Commun. 2018;9:678
11. Chen S, Li X, Liang Y, Lu X, Huang Y, Zhu J. et al. Short-term prognosis for hepatocellular carcinoma patients with lung metastasis: A retrospective cohort study based on the SEER database. Medicine (Baltimore). 2022;101:e31399
12. Ou L, Lu G, Cao M, Hu M. Lung metastases after liver cancer resection cured by immunotherapy: case report and literature review. Anticancer Drugs. 2023;34:e1-e8
13. Fang T, Lv H, Lv G, Li T, Wang C, Han Q. et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191
14. Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, Wei T. et al. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67:1872-89
15. Borowczyk J, Shutova M, Brembilla NC, Boehncke WH. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148:40-52
16. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345-8
17. Jou E, Rodriguez-Rodriguez N, Ferreira AF, Jolin HE, Clark PA, Sawmynaden K. et al. An innate IL-25-ILC2-MDSC axis creates a cancer-permissive microenvironment for Apc mutation-driven intestinal tumorigenesis. Sci Immunol. 2022;7:eabn0175
18. Liao Y, Zhao J, Bulek K, Tang F, Chen X, Cai G. et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat Commun. 2020;11:900
19. Li Q, Ma L, Shen S, Guo Y, Cao Q, Cai X. et al. Intestinal dysbacteriosis-induced IL-25 promotes development of HCC via alternative activation of macrophages in tumor microenvironment. J Exp Clin Cancer Res. 2019;38:303
20. EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236
21. Chen Y, Liao Y, Lam LM, He L, Tsang YS, Di YS. et al. Pretreatment biomarkers as prognostic predictors of survival in patients with Pancreatic Cancer treated with Gemcitabine-based Therapy and 5-Fluorouracil: Neutrophil-to-lymphocyte ratio vs Platelet-to-lymphocyte ratio. Int J Med Sci. 2020;17:1449-57
22. Hou Z, Liu J, Jin Z, Qiu G, Xie Q, Mi S. et al. Use of chemotherapy to treat hepatocellular carcinoma. Biosci Trends. 2022;16:31-45
23. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-96
24. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W. et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808-20
25. Öcal O, Zech CJ, Fabritius MP, Loewe C, van Delden O, Vandecaveye V. et al. Non-hypervascular hepatobiliary phase hypointense lesions detected in patients with hepatocellular carcinoma: a post hoc analysis of SORAMIC trial to identify risk factors for progression. Eur Radiol. 2023;33:493-500
26. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL. et al. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: A subanalysis of the REFLECT study. J Hepatol. 2023;78:133-41
27. Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D. et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765-76.e3
28. Gorczynski RM. IL-17 Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1240:47-58
29. Jiang Z, Chen J, Du X, Cheng H, Wang X, Dong C. IL-25 blockade inhibits metastasis in breast cancer. Protein & cell. 2017;8:191-201
30. Mombelli S, Cochaud S, Merrouche Y, Garbar C, Antonicelli F, Laprevotte E. et al. IL-17A and its homologs IL-25/IL-17E recruit the c-RAF/S6 kinase pathway and the generation of pro-oncogenic LMW-E in breast cancer cells. Sci Rep. 2015;5:11874
31. Luo Y, Yang Z, Su L, Shan J, Xu H, Xu Y. et al. Non-CSCs nourish CSCs through interleukin-17E-mediated activation of NF-κB and JAK/STAT3 signaling in human hepatocellular carcinoma. Cancer Lett. 2016;375:390-9
32. Askoura M, Abbas HA, Al Sadoun H, Abdulaal WH, Abu Lila AS, Almansour K. et al. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens (Basel, Switzerland). 2022 11
33. Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K. et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-20
34. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19:9-31
35. Xu H, Zhao J, Li J, Zhu Z, Cui Z, Liu R. et al. Cancer associated fibroblast-derived CCL5 promotes hepatocellular carcinoma metastasis through activating HIF1alpha/ZEB1 axis. Cell Death Dis. 2022;13:478
36. Qin L. Osteopontin is a promoter for hepatocellular carcinoma metastasis: a summary of 10 years of studies. Front Med. 2014;8:24-32
Author contact
![]() Corresponding authors: Qing Xia, Department of Oncology, State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, P.R. China. Tel.: +86-21-68385559; E-mail: xiaqingedu.cn; Wenying Zhou, Department of Laboratory Medicine, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, PR China. Tel.: +86-20-85253225; Email: zhouwy3sysu.edu.cn; Huimin Dong, Department of Laboratory Medicine, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, PR China. Tel.: +86-20-85253225; Email: donghminsysu.edu.cn.
Corresponding authors: Qing Xia, Department of Oncology, State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, P.R. China. Tel.: +86-21-68385559; E-mail: xiaqingedu.cn; Wenying Zhou, Department of Laboratory Medicine, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, PR China. Tel.: +86-20-85253225; Email: zhouwy3sysu.edu.cn; Huimin Dong, Department of Laboratory Medicine, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, PR China. Tel.: +86-20-85253225; Email: donghminsysu.edu.cn.

 Global reach, higher impact
Global reach, higher impact