Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(6):994-1002. doi:10.7150/ijms.93457 This issue Cite
Research Paper
Increased Risk of New-Onset Rheumatoid Arthritis Among Osteoarthritis Patients Received Total Knee Arthroplasty: a global federated health network analysis
1. Department of Medical Education and Research, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
2. Evidence-based Medicine Center, Chung Shan Medical University Hospital, Taichung, Taiwan.
3. Library, Chung Shan Medical University Hospital, Taichung, Taiwan.
4. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
5. Department of Neurosurgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
6. School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan.
7. Department of Medical Education, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
8. Orthopedics Department, Chi-Mei Medical Center, Tainan, Taiwan.
*Dr. Zong-Han Lin and Hui-Chin Chang contributed equally and share the first authorship equally.
#Dr. Yu-Lun Wu and Shuo-Yan Gau contributed equally and share the corresponding authorship equally.
Received 2023-12-21; Accepted 2024-3-28; Published 2024-4-8
Abstract
Background: Complications of total knee arthroplasty (TKA) had been widely discussed. However, whether TKA influence risk of rheumatoid arthritis (RA) in osteoarthritis patients remained uncertain. We intend to evaluate the risk of RA in osteoarthritis patients underwent TKA.
Methods: In this retrospective cohort study, data was retrieved from the US collaborative networks in TriNetX research network. Within the study period between 2005 and 2017, osteoarthritis patients underwent TKA were enrolled as case cohort whereas osteoarthritis patients never underwent TKA were enrolled as control cohort. Covariates were matched via propensity score matching. Risk of RA in TKA patients were valuated under various follow-up time and sensitivity models.
Results: Under 1-year, 3-year and 5-year of follow-up, TKA patients were associated with significantly elevated risk of RA, especially under 1-year follow-up (HR=1.74; 95% CI, 1.39-2.18). Subgroup analysis demonstrated a significant increase in the risk of RA following TKA in the female subgroup (HR=1.42; 95% CI, 1.24-1.63), the subgroup aged 18-64 years (HR=1.48; 95% CI, 1.11-1.97), and the subgroup aged greater than 65 years old (HR=1.38; 95% CI, 1.21-1.58) based on 5-year follow-up.
Conclusion: Clinicians should be concerned about uncharted association between TKA and RA reported our current study. Additional prospective studies and in-depth mechanistic inquiries were warranted to determine the causation.
Keywords: osteoarthritis, cohort, epidemiology, electronic medical records, rheumatoid arthritis, real world study
Introduction
Rheumatoid arthritis (RA) is an autoimmune ailment defined by long-term symmetrical inflammatory peripheral polyarthritis1. It invariably leads to joint destruction through cartilage and bone erosion2,3. If left untreated, RA can result in functional impairment, hindrance in daily tasks, and challenges in maintaining employment4-8. The uncontrolled inflammation associated with RA may contribute to adverse long-term outcomes and elevate the risk of various health issues, including cardiovascular disease9,10, interstitial lung disease11, peripheral vascular disease12-14, osteoporosis15 and osteoarthritis16. Epidemiologic and related studies have identified a range of genetic17,18, demographic19,20, lifestyle21 as risk factors for RA. The substantial impact of RA on healthcare systems and society is evident through regular medical visits, prolonged disease-modifying antirheumatic drug (DMARD) treatments, heightened disability and work loss, diminished quality of life, and premature mortality. These factors contribute to an estimated annual social cost exceeding $39 billion22. Given the intricate nature of RA, there is a compelling need to delve into the multifaceted origins and consequences of this chronic autoimmune disease.
Concurrently, Total Knee Arthroplasty (TKA) has emerged as a pivotal intervention for joint-related pathologies23, providing relief and enhanced functionality for numerous individuals. However, recent investigations have illuminated the interactive nexus between implants and autoimmune diseases. Research indicates an association between breast implants and an increased risk of RA24,25, with analogous effects potentially extending to metal implants26-28. Metal implants may instigate autoimmune disorders, and individuals with autoimmune disorders may experience more frequent allergic reactions to metal implants29, which, in turn, could contribute to the progression of connective tissue disorders29. Notably, there have been instances of systemic lupus erythematosus in patients sensitized to molybdenum30. Moreover, the use of bone cement has been linked to depigmentation31.
We have noted the intricate relationship between TKA as a crucial treatment for osteoarthritis and the potential of implants to trigger autoimmune diseases32. However, a comprehensive understanding of this phenomenon is lacking due to the absence of large-scale studies. To address this knowledge gap, we conducted a retrospective study utilizing the TriNetX - U.S. Collaborative Network. The primary objective of our study was to leverage a substantial database to investigate whether knee replacement surgery contributes to an increased risk of RA. By exploring the association between TKA and the onset of RA, our aim is to provide valuable insights into the complex interplay between orthopedic interventions and autoimmune disorders. We anticipate that our findings will inform future clinical practices, facilitating the optimization of patient care post-joint replacement surgeries.
Methods and Materials
This study was performed based on a retrospective cohort design. The TriNetX research network, a global-federated electronic health record database, was applied as the data source of this study. De-identified, prospectively updated electronic health records from patients in the collaborative healthcare organizations (HCOs) were available in the TriNetX research network for statistical analyses. In the current design, a subset of TriNetX, the US collaborative network, has been utilized. This subset retrieved the health data from 60 HCOs in the United States, and contained greater than 80 million patients' data. This subset of TriNetX was widely applied in epidemiological studies in various fields of clinical investigation33-35.
Patients with visit record and being diagnosed of osteoarthritis at the period between January 2005 and December 2017 were enrolled for further analyses. Since the database was prospectively updated, we ensured that each participant could be applied with a follow-up time greater than five years. For the enrolled osteoarthritis patients, those who underwent TKA were identified as the TKA cohort. Osteoarthritis patients with no TKA record before the index date were identified as non-TKA control cohort. For both cohorts, any individuals who being diagnosed of autoimmune diseases (including ankylosing spondylitis, dermatopolymyositis, Sjögren syndrome, systemic lupus erythematosus, psoriasis. ulcerative colitis, Crohn's disease, rheumatoid arthritis) or any cancers would not be included for further analyses. Moreover, people less than 18 years old or those who died before index date would be excluded from the study population. Applied administrative codes were reported in detail in Table S1.
In each analysis evaluating hazard ratio, propensity score matching was performed to address the potential baseline difference between the TKA cohort and control cohort. In every instance of the propensity score matching process, a greedy-nearest neighbor algorithm was employed, integrating the standard deviations with a caliper width set at 0.1. In the main analysis, matched covariates included age, sex, race, obesity status, inflammatory status, comorbidities, comedication use, lifestyle, socioeconomic issues, medical utilization status. Sensitivity analyses were performed based on multiple propensity score matching algorithms to address potential bias caused by overmatching. Moreover, exclusion of incident outcome event has been performed based on different wash-out period to address bias caused by reversed causality. To further investigate the influence of incident rheumatoid arthritis in TKA patients, we performed stratification analysis based on populations in different age and sex. Cox regression method were performed in each sensitivity and stratification analysis to provide comparison of hazard ratio in developing further RA in the TKA and non-TKA cohorts.
The formal analysis has been performed via the Analytics function of TriNetX research network. Hazard ratio (HR) has been calculated in each analysis evaluating the future risk of rheumatoid arthritis in TKA group while comparing with controls, with the 95% confidence intervals (95% C.I.) presented with the HR to evaluate the significance of the result. While presenting the baseline characteristics before and after matching, standardized difference (SD) were calculated to represent the difference of each covariates between TKA group and control group. When the value of SD more than 0.1, the difference between two groups were regarded as significant.
Results
Baseline characteristics of patients with and without TKA
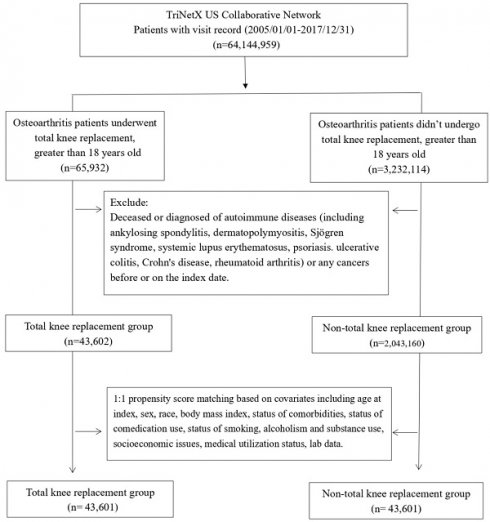
Patients diagnosed with OA were categorized into an experimental group (n=65,932) undergoing TKA and a control group (n=3,232,114) not receiving TKA. After excluding individuals with autoimmune diseases and cancer, a total of 43,602 patients were enrolled in the experimental group, and 2,043,160 patients were enrolled in the control group. Subsequently, employing 1:1 propensity score matching, 43,601 patients were included in the experimental group, and 43,601 patients were retained in the control group (Figure 1).
Flowchart of participant selection.
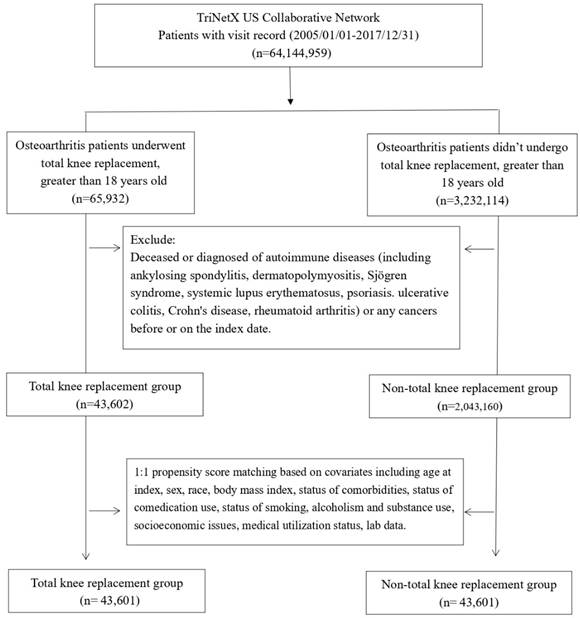
Following the matching process, there were no significant differences between patients who underwent TKA and unmatched patients regarding age, sex, race, and medication use (Glucocorticoids, Beta blockers), body mass index, and medical utilization status (Table 1). Nevertheless, the analysis of baseline characteristics revealed that the TKA group exhibited a higher percentage of patients with alcohol dependence, smoking, substance use, comorbidities, health hazards, and the use of antidepressants compared to the control group. Moreover, the proportion of C-reactive protein ≥ 3 (mg/L) in the TKA group was notably higher than that in the control group. In the baseline, the difference between erythrocyte sedimentation rate (ESR), antinuclear antibody (ANA), anti-cyclic citrullinated peptide antibody (anti-CCP) and rheumatoid factor (RF) did not present significant difference between TKA and non-TKA groups.
Risk of RA in osteoarthritis patients underwent TKA
The hazard ratio estimation, conducted using the analysis function of the TriNetX research network, examined the association between various follow-up periods and the risk of RA. The results consistently demonstrated a positive trend overall (Table 2). Under 1-year, 3-year and 5-year of follow-up, TKA patients were associated with significantly elevated risk of RA, especially under 1-year follow-up (HR=1.74; 95% CI, 1.39-2.18). The incidence rate of RA in TKA group was 0.000008 person-day, and in non-TKA group, the incidence rate of RA was 0.000008 person-day (Table S2). Subgroup analysis demonstrated a significant increase in the risk of RA following TKA in female subgroup (HR=1.42; 95% CI, 1.24-1.63), the subgroup aged 18-64 years (HR=1.48; 95% CI, 1.11-1.97), and the subgroup aged >64 years (HR=1.38; 95% CI, 1.21-1.58) (Table 3).
Baseline characteristics of study subjects (before and after propensity score matching)
| Before matching | After matchinga | ||||||
|---|---|---|---|---|---|---|---|
| TKA cohort (n=43,602) | Control cohort (n=2,043,160) | Standardized difference | TKA cohort (n=43,601) | Control cohort (n=43,601) | Standardized difference | ||
| Age at index | |||||||
| Mean±SD | 64.6±10.3 | 61.0±14.7 | 0.28 | 64.6±10.3 | 64.6±11.0 | 0.00 | |
| Sex | |||||||
| Male | 17096(39.2) | 800052(39.2) | 0.00 | 17096(39.2) | 17212(39.5) | 0.01 | |
| Female | 26258(60.2) | 1163594(57.0) | 0.07 | 26257(60.2) | 26162(60.0) | 0.00 | |
| Race, n (%) | |||||||
| White | 32064(73.5) | 1375618(67.3) | 0.14 | 32063(73.5) | 32224(73.9) | 0.01 | |
| Black or African American | 4558(10.5) | 255173(12.5) | 0.06 | 4558(10.5) | 4511(10.3) | 0.00 | |
| Asian | 1180(2.7) | 45086(2.2) | 0.03 | 1180(2.7) | 1183(2.7) | 0.00 | |
| Native Hawaiian or other Pacific Islander | 373(0.9) | 7450(0.4) | 0.06 | 373(0.9) | 419(1.0) | 0.01 | |
| Lifestyle | |||||||
| Alcohol dependence, smoking and substance use | 2274(5.2) | 124172(6.1) | 0.04 | 2274(5.2) | 2050(4.7) | 0.02 | |
| Comorbidities | |||||||
| Hypertension | 12093(27.7) | 452515(22.1) | 0.13 | 12092(27.7) | 11763(27.0) | 0.02 | |
| Diabetes mellitus | 4453(10.2) | 195589(9.6) | 0.02 | 4453(10.2) | 4288(9.8) | 0.01 | |
| Hyperlipidemia | 7191(16.5) | 270172(13.2) | 0.09 | 7190(16.5) | 6953(15.9) | 0.01 | |
| Ischemic heart diseases | 3076(7.1) | 122801(6.0) | 0.04 | 3076(7.1) | 2850(6.5) | 0.02 | |
| Cerebrovascular diseases | 1440(3.3) | 66269(3.2) | 0.00 | 1440(3.3) | 1282(2.9) | 0.02 | |
| Vitamin D deficiency | 1470(3.4) | 75566(3.7) | 0.02 | 1470(3.4) | 1307(3.0) | 0.02 | |
| Chronic kidney disease | 1084(2.5) | 53806(2.6) | 0.01 | 1084(2.5) | 840(1.9) | 0.04 | |
| Socioeconomic Status | |||||||
| Health hazards related to socioeconomic and psychosocial circumstances | 204(0.5) | 13797(0.7) | 0.03 | 204(0.5) | 163(0.4) | 0.01 | |
| Medications | |||||||
| Antidepressants | 6202(14.2) | 216859(10.6) | 0.11 | 6202(14.2) | 5963(13.7) | 0.02 | |
| Glucocorticoids | 12180(27.9) | 308489(15.1) | 0.32 | 12179(27.9) | 12100(27.8) | 0.00 | |
| Beta blockers | 7584(17.4) | 217008(10.6) | 0.20 | 7583(17.4) | 7334(16.8) | 0.02 | |
| Medical Utilization Status | |||||||
| Ambulatory visit | 28787(66.0) | 1148428(56.2) | 0.20 | 28786(66.0) | 28526(65.4) | 0.01 | |
| Inpatient visit | 11043(25.3) | 289312(14.2) | 0.28 | 11042(25.3) | 10876(24.9) | 0.01 | |
| Laboratory data | |||||||
| BMI, n (%) | |||||||
| ≥ 35 (kg/m2) | 3823(8.8) | 90924(4.5) | 0.17 | 3822(8.8) | 3602(8.3) | 0.02 | |
| C reactive protein, n (%) | |||||||
| ≥ 3 (mg/L) | 2982(6.8) | 58926(2.9) | 0.18 | 2981(6.8) | 2608(6.0) | 0.03 | |
| Rheumatoid factor, n (%) | |||||||
| ≥ 14 (IU/mL) | 208(0.5) | 4628(0.2) | 0.04 | 208(0.5) | 150(0.3) | 0.02 | |
| Antinuclear antibody, n (%) | |||||||
| ≥ 1:80 (titer) | 18(0.0) | 450(0.0) | 0.01 | 18(0.0) | 11(0.0) | 0.01 | |
| Anti Cyclic citrullinated peptide, n (%) | |||||||
| ≥ 20 (arbitrary unit/mL) | 10(0.0) | 106(0.0) | 0.01 | 10(0.0) | 10(0.0) | 0.00 | |
| Erythrocyte sedimentation rate | |||||||
| ≥ 20 (mm/hour) | 3300(7.6) | 64546(3.2) | 0.20 | 3300(7.6) | 2351(5.4) | 0.09 | |
Bold font represents a standardized difference was more than 0.1; In TriNetX research platform, if the amount of population in specific item is less than or equal to 10 people, the data will be presented as 10 to ensure full de-identification.
a Propensity score matching was performed on age at index, sex, race, body mass index, CRP level, status of comorbidities, comedication use, smoking, alcoholism and substance use, socioeconomic issues, medical utilization status.
Risk of rheumatoid arthritis under different follow-up timea
| Outcomes | Hazard ratio (95% Confidence interval)b | ||
|---|---|---|---|
| 1 year | 3 years | 5 years | |
| Rheumatoid arthritis | 1.74 (1.39,2.18) | 1.31 (1.15,1.50) | 1.33 (1.19,1.48) |
aData present here were the value of follow up from 90 days after index date to the respective following up years.
b Propensity score matching was performed on age at index, sex, race, body mass index, CRP level, status of comorbidities, comedication use, smoking, alcoholism and substance use, socioeconomic issues, medical utilization status.
Sensitivity analysis: risk of rheumatoid arthritis in total knee arthroplasty patients based on different covariate matching models, with 5-year follow up
| Outcomes | Hazard ratio (95% Confidence interval) | |||
|---|---|---|---|---|
| Crude | Model 1a | Model 2b | Model 3c | |
| Rheumatoid arthritis | 1.32 (1.22,1.43) | 1.25 (1.11,1.41) | 1.23 (1.10,1.38) | 1.25 (1.11,1.40) |
a Propensity score matching was performed on age at index and sex
b Propensity score matching was performed on age, sex and comorbidities
c Propensity score matching was performed on age, sex, substance use and comedications
Stratification analysis of rheumatoid arthritis in total knee arthroplasty patients
| Cases occurring new-onset rheumatoid arthritis | |||
|---|---|---|---|
| Subgroups | Total knee arthroplasty cohort (No. of event/ Total knee arthroplasty patient amount in each subgroup) | Control cohort (No. of event/ non-total knee arthroplasty patient amount in each subgroup) | HR (95% CI)a |
| Gender | |||
| Male | 170/14770 | 134/14770 | 1.24 (0.99,1.55) |
| Female | 493/22739 | 339/22739 | 1.42 (1.24,1.63) |
| Age at index date | |||
| 18-64 years old | 118/5719 | 79/5719 | 1.48 (1.11,1.97) |
| ≥ 65 years old | 547/31844 | 387/31844 | 1.38 (1.21,1.58) |
a Propensity score matching was performed on age at index, sex, race, body mass index, CRP level, status of comorbidities, comedication use, smoking, alcoholism and substance use, socioeconomic issues, medical utilization status.
Sensitivity analyses
Various sensitivity analyses were conducted to explore the association between TKA and RA risk (Table 4 and Table S3). In the first set of analyses applying various propensity matching algorithms (Table 4), Model 1 involved matching age at index and sex through propensity score matching, resulting in an HR of 1.25 (95% CI, 1.11- 1.41). Model 2 matched age, sex, and comorbidities, yielding an HR of 1.23 (95% CI, 1.10- 1.38). Model 3 involved matching age, sex, substance use, and comedications, resulting in an HR of 1.25 (95% CI, 1.11-1.40). In the second set of analyses (Table S3), patients diagnosed of RA within one year, two years, and three years after TKA were excluded. The calculated HRs were 1.30 (95% CI, 1.15-1.47), 1.39 (95% CI, 1.21-1.59), and 1.46 (95% CI, 1.26-1.70), respectively. In order to evaluate the generalizability of the TKA-RA association, aside from osteoarthritis population, we performed an additional sensitivity analysis to evaluate the risk of RA in TKA patients while comparing with general population. We found that under 5-year-follow-up, the risk of TKA group with OA has 1.87-fold of risk in developing future RA (95% CI, 1.66-2.11). Under 1-year-follow-up period, the risk elevates to 2.76-fold higher than general population (95% CI, 2.13-3.58) (Table S4).
Discussion
Findings of this study indicate a notable rise in the risk of subsequent RA in osteoarthritis patients who have undergone TKA compared to those who have not. Even with the consideration of multiple covariates, the observed association between post-TKA surgery and the reported heightened risk of RA persisted significantly in statistical models. Furthermore, the results retained their significance even after the exclusion of individuals diagnosed with RA within 1 to 3 years of surgery.
Studies have underscored a significant interplay between implants and autoimmune diseases. For instance, a case report documented a 24-year-old woman who manifested symptoms of systemic lupus erythematosus (SLE) following the implantation of a metal plate30. It was revealed that she exhibited delayed-type hypersensitivity to molybdenum, and her symptoms improved following the subsequent removal of the implant. Another case involved a 72-year-old woman who developed aseptic periprosthetic arthritis after TKA, suspected to be linked to metal release and cobalt sensitization from the artificial joint36. Additionally, a 23-year-old woman undergoing nickel-titanium chin implantation experienced a spectrum of symptoms, leading to a diagnosis of autoinflammatory syndrome, which could potentially be induced by adjuvants37.
A comprehensive retrospective study exploring the correlation between dental amalgam and arthritis, encompassing 86,305,425 weighted individuals in the exposed group, indicated a six-fold higher risk of arthritis compared to the control group26. Metals, with their potential to induce immunosuppression, immunotoxicity, or act as immune adjuvants, may incite allergy and autoimmunity, a phenomenon that has shown an escalating incidence38. Beyond metal implants, the utilization of intraoperative bone cements may also heighten the susceptibility to autoimmune diseases. Benzoyl Peroxide, a crucial constituent of bone cements, has been associated with conditions such as leukoplakia and allergic complications31. Moreover, individuals with autoimmune disorders exhibit an increased likelihood of experiencing allergic reactions to metal implants. Noteworthy is the observation of delayed-type hypersensitivity reactions to nickel, mercury, gold, palladium, titanium, or chromium in patients with connective tissue disorders like SLE and RA39. Chronic inflammatory reactions induced by metals can further exacerbate connective tissue disorders29.
Furthermore, C-reactive protein (CRP) is considered to be associated with systemic inflammation, and patients with osteoarthritis may have higher serum CRP levels, which are correlated with symptom severity 40. Similarly, elevated CRP levels can be observed in patients with rheumatoid arthritis (RA) and may vary with the severity of inflammation and synovitis 41. Untreated RA patients may also present with acute phase reactants; however, such findings are uncommon, and alternative diagnoses should be considered as a priority. Therefore, the influence of CRP levels on subsequent RA diagnosis is relatively minor42. Nonetheless, to minimize experimental bias resulting from differences in baseline inflammation, we matched patients with CRP levels ≥ 3 (mg/L) in our study. The results indicated that patients with high inflammatory indices constituted a lower proportion of the overall study population, with no significant differences observed between the two groups.
Joint replacement surgery, particularly hip and knee implants, has been linked to autoimmune diseases, particularly connective tissue diseases. A substantial body of evidence supports this association. A retrospective study reported that in post-implantation individuals, a heightened risk of developing autoimmune or connective tissue disease was observed27. Another large retrospective study in Denmark stated that compared with osteoarthritis patients who did not undergo joint replacement, those who underwent hip replacement had significantly higher rates of hospitalization for dryness and systemic lupus erythematosus. Patients undergoing knee replacement surgery had a significantly increased risk of hospitalization for RA, CTD, and dryness43. A small-scale case-control study found a increase in the incidence of undifferentiated connective tissue disease (UCTD) after exposure to silicone-free artificial joints, with the risk greater than 5-fold observed. Significant increase in the incidence of UCTD after exposure to orthopedic metallic fixation devices was also noted32. Exposure to silicone-containing devices (shunts and catheters) was observed to be significantly associated with CTD. In light of this compelling evidence, our study aims to provide a well-designed investigation into the risk ratio between artificial joint replacement and subsequent autoimmune diseases, particularly connective tissue diseases.
Silicone breast implants have been implicated in triggering chronic inflammatory responses and are linked to various symptoms, including fatigue, cognitive impairment, joint pain, muscle pain, fever, dry eyes, dry mouth, and Autoimmune/Inflammatory Syndrome by Adjuvants (ASIA). An analysis comparing 24,651 silicone breast implant recipients with 98,604 matched individuals without breast implants revealed an increased risk of autoimmune diseases post-implantation, with an odds ratio of 1.45 (95% CI, 1.21-1.73)24. Recent integrated evidences indicated that silicone breast implants could be associated with an increased risk of developing rheumatoid arthritis, Raynaud's syndrome, and dryness. The effect size for the development of rheumatoid arthritis was particularly notable at 1.38 (95% CI, 1.06-1.80)25.
While our research does not permit us to definitively determine the mechanisms underlying this phenomenon, insights gleaned from previous literature offer speculative explanations. One theory posits that breast implant-related ailments may be linked to a chronic inflammatory response triggered by the release of silicone particles from the implants. However, subsequent studies have not found a statistically significant difference in antibody levels between women with ruptured implants and those with intact implants, suggesting that silicone leakage might not be the primary cause of systemic symptoms post-implantation44. Another hypothesis suggests that the formation of bacterial biofilms on implants leads to prolonged interactions between the host and pathogens, potentially initiating chronic inflammation and subsequent systemic autoimmune symptoms. Bacterial biofilms have been implicated in various complications associated with implanted biomaterials, including pericondylar contracture, spondylolisthesis47,48, and pain49.
Furthermore, allergic reactions to metals are not uncommon, with prevalence rates ranging from 10% to 15%49-51. Metal reactions are linked to implant failure as metal ions accumulate and persistently release into the tissue surrounding the implant. This accumulation causes an inflammatory reaction, ultimately resulting in implant failure. The incidence of metal sensitization is up to six times greater in people with implant failure while comparing with the general population51. A macrophage-mediated immune response from joint replacement was previously identified. Some patients exhibit T lymphocytes specifically targeting metal particles. The production of excessive metal fragments is associated with type IV immune response, and T cells could be highly involved in this reaction52. Patients with osteolytic lesions show an elevated rate of sensitization to cobalt, and tissue sections around the joint prosthesis reveal CD3-positive T cells and CD68-positive macrophages. These findings strongly suggest that early osteolysis in metal hip replacement patients is associated with delayed-type hypersensitivity to metal53. Activation of the immune system by forming complexes with natural proteins triggers subsequent delayed-type hypersensitivity (DTH)54. These abnormal inflammatory responses may be significant risk factors for the development of autoimmune diseases. Although the detailed mechanisms are still uncertain, there is a substantial body of literature on the role of metal sensitization in contributing to the onset, progression, and severity of symptoms in these diseases (e.g., RA, chronic fatigue syndrome, and dryness)28. We anticipate further literature to help clarify the association between metal implants and disease.
This study possesses several notable strengths. Firstly, we utilized a robust global-federated, multi-center database for data acquisition and analysis. This platform offers precise healthcare system-specific diagnostic insights based on electronic medical records. Secondly, via conducting subgroup and sensitivity analyses, the evidences of the observed association could be further evaluated and solidified. Thirdly, we implemented robust control and adjustment measures for baseline and potential confounding factors, enabling more dependable conclusions from propensity-matched analyses conducted on substantial sample sizes. Lastly, to the best of our knowledge, this represents the pioneering study to employ a large-scale database for the analysis of the association between TKA and the risk of RA.
Inherent limitations are present in this retrospective study, stemming from its design and data source. First, our reliance on ICD-10-CM codes for patient identification and outcome assessment introduces the potential for misdiagnosis and misclassification. However, it is noteworthy that our study demonstrated a high degree of accuracy in identifying RA patients using ICD-10-CM codes, as supported by previous research55. Second, the severity of osteoarthritis may differ between the TKA and non TKA cohort as those who underwent TKA are likely to have a more severe disease. Nonetheless, in the current study, severity of osteoarthritis was also not able to be precisely stratified due to lacking related information. Third, despite adjustment through matching exposed and control populations, the possibility of residual confounding factors persists. Forth, the majority of participants were Americans, encompassing various ethnicities, yet the generalizability of our conclusions to Asian or European populations remains uncertain. Fifth, lack of information on the material composition of knee prostheses precludes an analysis of RA risk based on different materials. Lastly, the intricate mechanisms underlying the increased risk of RA after TKA remain undisclosed by our study alone, highlighting the need for further research to unravel this observed association.
In conclusion, our investigation has unveiled a previously uncharted connection between TKA and RA. While our discoveries offer valuable insights, additional prospective studies and in-depth mechanistic inquiries are imperative to substantiate and enhance our comprehension. This deeper understanding promises to propel our knowledge of autoimmune responses post-surgical interventions, ultimately fostering the development of more precise preventive and therapeutic strategies within the dynamic domain of joint arthroplasty.
Supplementary Material
Supplementary tables.
Acknowledgements
Author contributions
All the authors involved in drafting or revising the article and approved of the submitted version.
Study conception and design: Lin ZH, Chang HC, Wu YL, Gau SY.
Data acquisition: Lin ZH, Chang HC, Wu YL, Gau SY.
Data analysis and demonstration: Lin ZH, Chang HC, Wu YL, Gau SY.
Original draft preparation: Lin ZH, Chang HC, Wu YL, Gau SY.
Statement of ethics
The TriNetX database was previously approved by the Western Institutional Review Board (Western IRB). The subsequent determination regarding the de-identification process attested on December 2020 replaced the need of Western IRB approval in TriNetX studies. Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
“This retrospective study is exempt from informed consent. The data reviewed is a secondary analysis of existing data, does not involve intervention or interaction with human subjects, and is de-identified per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. This formal determination by a qualified expert refreshed on December 2020.”
Data sharing statement
Data in this study were retrieved from TriNetX Research Network. All data available in the database were administrated by the TriNetX platform. Detailed information can be retrieved at the official website of the research network (https://trinetx.com).
ORCID
Zong-Han Lin: 0000-0002-0954-0789.
Shuo-Yan Gau: 0000-0001-8897-5635.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001Sep15;358(9285):903-11 doi:10.1016/s0140-6736(01)06075-5
2. Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev. 2005Dec;208:228-51 doi:10.1111/j.0105-2896.2005.00338.x
3. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003May15;423(6937):356-61 doi:10.1038/nature01661
4. Olofsson T, Petersson IF, Eriksson JK. et al. Predictors of work disability after start of anti-TNF therapy in a national cohort of Swedish patients with rheumatoid arthritis: does early anti-TNF therapy bring patients back to work? Ann Rheum Dis. 2017Jul;76(7):1245-1252 doi:10.1136/annrheumdis-2016-210239
5. Chorus AM, Miedema HS, Wevers CJ, van Der Linden S. Labour force participation among patients with rheumatoid arthritis. Ann Rheum Dis. 2000Jul;59(7):549-54 doi:10.1136/ard.59.7.549
6. Aletaha D, Smolen J, Ward MM. Measuring function in rheumatoid arthritis: Identifying reversible and irreversible components. Arthritis Rheum. 2006Sep;54(9):2784-92 doi:10.1002/art.22052
7. Markusse IM, Akdemir G, Dirven L. et al. Long-Term Outcomes of Patients With Recent-Onset Rheumatoid Arthritis After 10 Years of Tight Controlled Treatment: A Randomized Trial. Ann Intern Med. 2016Apr19;164(8):523-31 doi:10.7326/m15-0919
8. Katz PP, Morris A, Yelin EH. Prevalence and predictors of disability in valued life activities among individuals with rheumatoid arthritis. Ann Rheum Dis. 2006Jun;65(6):763-9 doi:10.1136/ard.2005.044677
9. Voskuyl AE. The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology (Oxford). 2006Oct;45(Suppl 4):iv4-7 doi:10.1093/rheumatology/kel313
10. Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthritis Rheum. 2008Sep;58(9):2612-21 doi:10.1002/art.23811
11. Tanoue LT. Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med. 1998Dec;19(4):667-85 viii. doi:10.1016/s0272-5231(05)70109-x
12. del Rincón I, Haas RW, Pogosian S, Escalante A. Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Ann Rheum Dis. 2005Mar;64(3):425-32 doi:10.1136/ard.2003.018671
13. Kim SC, Schneeweiss S, Liu J, Solomon DH. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013Oct;65(10):1600-7 doi:10.1002/acr.22039
14. Holmqvist ME, Neovius M, Eriksson J. et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. Jama. 2012Oct3;308(13):1350-6 doi:10.1001/2012.jama.11741
15. Xue AL, Wu SY, Jiang L, Feng AM, Guo HF, Zhao P. Bone fracture risk in patients with rheumatoid arthritis: A meta-analysis. Medicine (Baltimore). 2017Sep;96(36):e6983 doi:10.1097/md.0000000000006983
16. Lee YH, Tsou HK, Kao SL. et al. Patients With Rheumatoid Arthritis Increased Risk of Developing Osteoarthritis: A Nationwide Population-Based Cohort Study in Taiwan. Front Med (Lausanne). 2020;7:392 doi:10.3389/fmed.2020.00392
17. Frisell T, Holmqvist M, Källberg H, Klareskog L, Alfredsson L, Askling J. Familial risks and heritability of rheumatoid arthritis: role of rheumatoid factor/anti-citrullinated protein antibody status, number and type of affected relatives, sex, and age. Arthritis Rheum. 2013Nov;65(11):2773-82 doi:10.1002/art.38097
18. Okada Y, Wu D, Trynka G. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014Feb20;506(7488):376-81 doi:10.1038/nature12873
19. Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010Jun;62(6):1576-82 doi:10.1002/art.27425
20. Cross M, Smith E, Hoy D. et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014Jul;73(7):1316-22 doi:10.1136/annrheumdis-2013-204627
21. Sugiyama D, Nishimura K, Tamaki K. et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010Jan;69(1):70-81 doi:10.1136/ard.2008.096487
22. Furneri G, Mantovani LG, Belisari A. et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol. Jul-Aug. 2012;30(4 Suppl 73):S72-84
23. Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum. 1998Jun;41(6):1072-82 doi:10.1002/1529-0131(199806)41:6<1072::Aid-art14>3.0.Co;2-g
24. Watad A, Rosenberg V, Tiosano S. et al. Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis. Int J Epidemiol. 2018Dec1;47(6):1846-1854 doi:10.1093/ije/dyy217
25. Balk EM, Earley A, Avendano EA, Raman G. Long-Term Health Outcomes in Women With Silicone Gel Breast Implants: A Systematic Review. Ann Intern Med. 2016Feb2;164(3):164-75 doi:10.7326/m15-1169
26. Geier DA, Geier MR. Dental Amalgams and the Incidence Rate of Arthritis among American Adults. Clin Med Insights Arthritis Musculoskelet Disord. 2021;14:11795441211016261 doi:10.1177/11795441211016261
27. Signorello LB, Ye W, Fryzek JP. et al. A nationwide followup study of autoimmune and connective tissue disease among hip and knee implant patients. J Long Term Eff Med Implants. 2002;12(4):255-62
28. Roach K, Roberts J. A comprehensive summary of disease variants implicated in metal allergy. J Toxicol Environ Health B Crit Rev. 2022Aug18;25(6):279-341 doi:10.1080/10937404.2022.2104981
29. Stejskal V, Reynolds T, Bjørklund G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J Trace Elem Med Biol. 2015;31:230-6 doi:10.1016/j.jtemb.2015.01.001
30. Federmann M, Morell B, Graetz G. et al. Hypersensitivity to molybdenum as a possible trigger of ANA-negative systemic lupus erythematosus. Ann Rheum Dis. 1994Jun;53(6):403-5 doi:10.1136/ard.53.6.403
31. Dudda M, Godau P, Al-Benna S, Schildhauer TA, Gothner M. Vitiligo and allergic complications from orthopaedic joint implants: the role of benzoyl peroxide. Recent Pat Inflamm Allergy Drug Discov. 2013May;7(2):176-82 doi:10.2174/1872213x11307020009
32. Laing TJ, Schottenfeld D, Lacey JV Jr. et al. Potential risk factors for undifferentiated connective tissue disease among women: implanted medical devices. Am J Epidemiol. 2001Oct1;154(7):610-7 doi:10.1093/aje/154.7.610
33. Chang H-C, Lin C-Y, Guo Y-C. et al. Association between hidradenitis suppurativa and atopic diseases: a multi-center, propensity-score-matched cohort study. International Journal of Medical Sciences. 2024;21(2):299-305 doi:10.7150/ijms.90086
34. Yong SB, Gau SY, Li CJ, Tseng CW, Wang SI, Wei JC. Associations between COVID-19 outcomes and asthmatic patients with inhaled corticosteroid. Front Pharmacol. 2023;14:1204297 doi:10.3389/fphar.2023.1204297
35. Chang H-C, Wu C-L, Chiu T-M, Liao W-C, Gau S-Y. Risk of osteoarthritis in patients with hidradenitis suppurativa: a global federated health network analysis. Original Research. Frontiers in Immunology. 2023-December-19 2023;14doi:10.3389/fimmu. 2023 1285560
36. Schoon J, Ort MJ, Huesker K, Geissler S, Rakow A. Diagnosis of Metal Hypersensitivity in Total Knee Arthroplasty: A Case Report. Front Immunol. 2019;10:2758 doi:10.3389/fimmu.2019.02758
37. Loyo E, Jara LJ, López PD, Puig AC. Autoimmunity in connection with a metal implant: a case of autoimmune/autoinflammatory syndrome induced by adjuvants. Auto Immun Highlights. 2013Apr;4(1):33-8 doi:10.1007/s13317-012-0044-1
38. Drenovska K, Shahid M, Vassileva S. Nickel and Skin: From Allergy to Autoimmunity. Endocr Metab Immune Disord Drug Targets. 2020;20(7):1032-1040 doi:10.2174/1871530320666191231115437
39. Bjørklund G, Dadar M, Aaseth J. Delayed-type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ Res. 2018Feb;161:573-579 doi:10.1016/j.envres.2017.12.004
40. Jin X, Beguerie JR, Zhang W. et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015Apr;74(4):703-10 doi:10.1136/annrheumdis-2013-204494
41. Pope JE, Choy EH. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. 2021Feb;51(1):219-229 doi:10.1016/j.semarthrit.2020.11.005
42. Funovits J, Aletaha D, Bykerk V. et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: methodological report phase I. Ann Rheum Dis. 2010Sep;69(9):1589-95 doi:10.1136/ard.2010.130310
43. Mellemkjaer L, Friis S, McLaughlin JK. et al. Connective tissue disease after hip and knee implant surgery. Scand J Rheumatol. 2001;30(2):82-6 doi:10.1080/03009740151095321
44. Hölmich LR, Kjøller K, Fryzek JP. et al. Self-reported diseases and symptoms by rupture status among unselected Danish women with cosmetic silicone breast implants. Plast Reconstr Surg. 2003Feb;111(2):723-32 discussion 733-4. doi:10.1097/01.Prs.0000041442.53735.F8
45. Suh LJ, Khan I, Kelley-Patteson C, Mohan G, Hassanein AH, Sinha M. Breast Implant-Associated Immunological Disorders. J Immunol Res. 2022;2022:8536149 doi:10.1155/2022/8536149
46. Allan JM, Jacombs ASW, Hu H, Merten SL, Deva AK. Detection of bacterial biofilm in double capsule surrounding mammary implants: findings in human and porcine breast augmentation. Plast Reconstr Surg. 2012Mar;129(3):578e-580e doi:10.1097/PRS.0b013e3182419c82
47. Prinz V, Bayerl S, Renz N. et al. High frequency of low-virulent microorganisms detected by sonication of pedicle screws: a potential cause for implant failure. J Neurosurg Spine. 2019May28;31(3):424-429 doi:10.3171/2019.1.Spine181025
48. Leitner L, Malaj I, Sadoghi P. et al. Pedicle screw loosening is correlated to chronic subclinical deep implant infection: a retrospective database analysis. Eur Spine J. 2018Oct;27(10):2529-2535 doi:10.1007/s00586-018-5592-2
49. Wawrzynski J, Gil JA, Goodman AD, Waryasz GR. Hypersensitivity to Orthopedic Implants: A Review of the Literature. Rheumatol Ther. 2017Jun;4(1):45-56 doi:10.1007/s40744-017-0062-6
50. Mitchelson AJ, Wilson CJ, Mihalko WM. et al. Biomaterial hypersensitivity: is it real?. Supportive evidence and approach considerations for metal allergic patients following total knee arthroplasty. Biomed Res Int. 2015;2015:137287 doi:10.1155/2015/137287
51. Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001Mar;83(3):428-36 doi:10.2106/00004623-200103000-00017
52. Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007Dec;28(34):5044-8 doi:10.1016/j.biomaterials.2007.06.035
53. Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am. 2005Jul;87(7):1515-21 doi:10.2106/jbjs.D.02641
54. Louis-Dit-Sully C, Schamel WW. Activation of the TCR complex by small chemical compounds. Exp Suppl. 2014;104:25-39 doi:10.1007/978-3-0348-0726-5_3
55. Almutairi K, Inderjeeth C, Preen DB, Keen H, Rogers K, Nossent J. The accuracy of administrative health data for identifying patients with rheumatoid arthritis: a retrospective validation study using medical records in Western Australia. Rheumatol Int. 2021Apr;41(4):741-750 doi:10.1007/s00296-021-04811-9
Author contact
![]() Corresponding authors: Yu-Lun Wu, MD, Department of Neurosurgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, No. 386, Dazhong 1st Rd., Zuoying Dist., Kaohsiung City 813414, Taiwan (R.O.C.); Email: ncage0417com. Shuo-Yan Gau, School of Medicine, Chung Shan Medical University, Taichung, Taiwan, No. 110, Sec. 1, Jianguo N. Rd., Taichung City 40201, Taiwan; Email: sixsamurai.shien15com.
Corresponding authors: Yu-Lun Wu, MD, Department of Neurosurgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, No. 386, Dazhong 1st Rd., Zuoying Dist., Kaohsiung City 813414, Taiwan (R.O.C.); Email: ncage0417com. Shuo-Yan Gau, School of Medicine, Chung Shan Medical University, Taichung, Taiwan, No. 110, Sec. 1, Jianguo N. Rd., Taichung City 40201, Taiwan; Email: sixsamurai.shien15com.

 Global reach, higher impact
Global reach, higher impact