3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(5):809-816. doi:10.7150/ijms.94485 This issue Cite
Review
Role of mitochondria in doxorubicin-mediated cardiotoxicity: from molecular mechanisms to therapeutic strategies
1. First Afliated Hospital, Heilongjiang University of Chinese Medicine, Harbin 150040, China.
2. Brandeis University, Waltham, MA 02453, USA.
3. Heilongjiang University of Chinese Medicine, Harbin 150040, China.
4. Department of Cardiology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, 100053, China.
Received 2024-1-19; Accepted 2024-2-27; Published 2024-3-11
Abstract
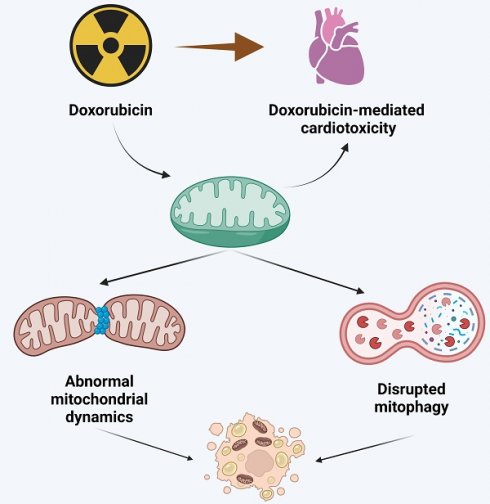
This comprehensive review delves into the pivotal role of mitochondria in doxorubicin-induced cardiotoxicity, a significant complication limiting the clinical use of this potent anthracycline chemotherapeutic agent. Doxorubicin, while effective against various malignancies, is associated with dose-dependent cardiotoxicity, potentially leading to irreversible cardiac damage. The review meticulously dissects the molecular mechanisms underpinning this cardiotoxicity, particularly focusing on mitochondrial dysfunction, a central player in this adverse effect. Central to the discussion is the concept of mitochondrial quality control (MQC), including mitochondrial dynamics (fusion/fission balance) and mitophagy. The review presents evidence linking aberrations in these processes to cardiotoxicity in doxorubicin-treated patients. It elucidates how doxorubicin disrupts mitochondrial dynamics, leading to an imbalance between mitochondrial fission and fusion, and impairs mitophagy, culminating in the accumulation of dysfunctional mitochondria and subsequent cardiac cell damage. Furthermore, the review explores emerging therapeutic strategies targeting mitochondrial dysfunction. It highlights the potential of modulating mitochondrial dynamics and enhancing mitophagy to mitigate doxorubicin-induced cardiac damage. These strategies include pharmacological interventions with mitochondrial fission inhibitors, fusion promoters, and agents that modulate mitophagy. The review underscores the promising results from preclinical studies while advocating for more extensive clinical trials to validate these approaches in human patients. In conclusion, this review offers valuable insights into the intricate relationship between mitochondrial dysfunction and doxorubicin-mediated cardiotoxicity. It underscores the need for continued research into targeted mitochondrial therapies as a means to improve the cardiac safety profile of doxorubicin, thereby enhancing the overall treatment outcomes for cancer patients.
Keywords: doxorubicin, cardiotoxicity, mitochondria, mitochondrial quality control
Introduction
Advances in drug development have enhanced significantly the survival of cancer patients. However, numerous chemotherapy drugs can induce adverse reactions, among which cardiovascular toxicity is the most prevalent and life-threatening. Anthracyclines are widely used chemotherapy drugs utilized in both adults and children for treating malignant tumors such as lymphoma, sarcoma, and breast cancer [1]. The most frequently prescribed anthracycline drug is doxorubicin. Nevertheless, despite its effectiveness, up to a quarter of patients may develop doxorubicin-induced cardiotoxicity, which limits the utilization of this medication in clinical management [2, 3]. Doxorubicin can induce cardiotoxicity through various mechanisms, including oxidative stress, induction of cell death via apoptosis or pyroptosis, abnormal intracellular calcium signaling, disrupted autophagy, metabolic disorder, endoplasmic reticulum stress, and mitochondrial structural/functional damage [4, 5]. Elucidating the intrinsic correlations among these mechanisms is crucial for identifying therapeutic targets and designing strategies to reduce the side effects of doxorubicin on the cardiovascular system. Extensive research has focused on reducing intracellular oxidative stress to mitigate the occurrence of doxorubicin-mediated cardiotoxicity [6, 7]. However, these efforts have largely failed [6, 8], indicating that additional mechanisms need to be addressed. It thus became clear that multiple and interdependent processes are involved in the cardiotoxic effects of doxorubicin.
The aim of this review is to summarize the pathological roles of mitochondrial dysfunction in the setting of doxorubicin-mediated cardiotoxicity. Since mitochondrial dynamics (determined by fission/fusion events), as well as mitophagy, are main targets of the mitochondrial quality control (MQC) program that sustains mitochondrial structure and function during organ and cellular stress [8], this review mainly discusses the molecular mechanisms underlying abnormal mitochondrial dynamics and disrupted mitophagy in cardiac cells exposed to doxorubicin. In addition, to provide insight and perspective for clinical management, we discuss potential therapeutics that aim to normalize mitochondrial dysfunction in patients with doxorubicin-mediated cardiotoxicity.
Doxorubicin and its pharmacology
Doxorubicin is derived from the bacterium Streptomyces peucetius [9] and belongs to the anthracycline class of antibiotics [10]. Chemically, it is composed of a naphthoquinone nucleus linked to a primary amine in its daunosamine sugar via a glycosidic bond, making it a cytotoxic drug. Pharmacologically, doxorubicin inhibits topoisomerase II [11, 12], thereby preventing DNA and RNA synthesis, which disrupts tumor proliferation and growth. Additionally, the anthracycline ring structure of doxorubicin intercalates into the DNA of human cells, covering the nucleotides [13]. Within the anthracycline skeleton there is also a lipophilic, saturated hydroxyl-rich moiety adjacent to the amino sugar [14]. Due to the hydrophilic nature of the amino sugar and the lipophilic properties of the napthoquinone core, doxorubicin can dissolve in both hydrophilic and lipophilic solvents. The lipophilic moiety of doxorubicin is acidic, while the hydrophilic amino sugar exhibits alkaline properties. In aqueous solution, maximum stability of the drug is observed at about pH 4.
The cytotoxic mechanism of doxorubicin on tumor cells is believed to be associated with its nucleotide base insertion and lipid binding activity [15]. The insertion of doxorubicin hinders nucleotide replication and the activity of DNA and RNA polymerases [16]. In addition, the interaction of doxorubicin with topoisomerase II forms a DNA-damaging complex, which further contributes to doxorubicin's tumor-killing actions [17]. In vitro experiments have shown that proliferating cells treated with doxorubicin exhibit specific morphological changes and activate programmed cell death [17]. As cancer is defined by uncontrolled cellular proliferation, doxorubicin has been found to effectively reduce tumor volume and size, thereby extending the survival of patients with cancer [17].
Adverse effects of doxorubicin on cardiac muscle and heart function
The main limitation of doxorubicin use in clinical practice is its cardiotoxicity, as it has been widely reported to cause dose-dependent, progressive, and potentially fatal myocardial damage [18]. Cardiotoxicity typically manifests within one year of treatment and is usually evident in the form of a decrease in left ventricular ejection fraction (LVEF) [19] [20]. It is worth noting that there are few studies reporting delayed cardiac toxicity in children years after doxorubicin treatment [21, 22]. Doxorubicin-induced cardiotoxicity is primarily characterized by left ventricular dysfunction, dilated cardiomyopathy (DCM), and heart failure [23-25]. Once it progresses to congestive heart failure, approximately 50% of patients die within two years [25]. Numerous studies have confirmed the cumulative dose of anthracycline chemotherapy drugs, including doxorubicin, as a determining factor for the development of congestive heart failure. Mitry et al. reported that the risk of heart failure is 4% when the cumulative dose is below 500 mg/m2, but this risk increases to 36% when it exceeds 600 mg/m2 [26]. In a retrospective study, the incidence of heart failure was 5% at a cumulative dose of 400 mg/m2, 16% at 500 mg/m2, 26% at 550 mg/m2, and rose to 48% when the dose reached 700 mg/m2 [19]. A study by Lefrak et al. showed that the incidence of congestive heart failure was only 0.27% in patients receiving DOX treatment with a dose of no more than 550 mg/m2, compared to 30% in patients receiving doses exceeding 550 mg/m2 [27]. At the molecular level, the cardiotoxic effects of doxorubicin include myocardial cell injury and apoptotic and necrotic cell death [28]. Dexrazoxane is currently the only drug approved by the U.S. Food and Drug Administration (FDA) for the prevention of cardiotoxicity in anthracycline-based chemotherapy patients [29]. However, its use is greatly limited by its association with reduced tumor response rates and potential risk of secondary malignancies.
Role of mitochondrial dynamics in doxorubicin-induced cardiotoxicity
Mitochondria maintain their dynamic network through continuous fission and fusion reactions, which determine what is known as mitochondrial dynamics [30]. Mitochondria undergo these processes to regulate their quantity, morphology, exchange of energy substrates, stability of genetic material, and conversion of extracellular signals to counteract external stress [31]. Physiological mitochondrial fission mainly separates dysfunctional mitochondria from the healthy mitochondrial network, maintaining the stability of the mitochondrial population through the process of mitochondrial autophagy (i.e. mitophagy) [32]. In contrast, pathological mitochondrial fission leads to a breakdown of the mitochondrial network and accumulation of fragmented mitochondria; this results in oxidative stress, via reactive oxygen species (ROS) overproduction, and impairs ATP synthesis, ultimately leading to cellular energy crisis [33]. The regulation of mitochondrial fission is mainly controlled by the GTPase dynamin-related protein 1 (Drp1) [34]. Drp1 translocates from the cytoplasm to the surface of mitochondria, where it firmly anchors to various Drp1 receptors [35]. The receptors for Drp1 include mitochondrial fission 1 protein (Fis1), mitochondrial fission factor (Mff), and mitochondrial dynamics proteins of 49 and 51 kDa (Mid49 and Mid51) [33]. Upon localization on the mitochondrial surface, Drp1 can assemble into multimeric ring-like structures around the mitochondrial surface, consuming GTP to constrict and divide the organelle to accomplish mitochondrial fission [35].
Mitochondrial fusion is the opposite process of mitochondrial fission, and proceeds via sequential merging of the outer and inner membranes of contiguous mitochondria [33]. Physiological mitochondrial fusion fundamentally helps newly formed mitochondria to mature quickly, enhances the exchange and sharing of energy substrates, and reduces damage to mitochondrial genetic material [33]. The fusion of mitochondrial membranes is regulated by two outer membrane fusion factors (mitofusin 1/2, Mfn1/2) and an inner membrane fusion factor (optic atrophy 1, Opa1). The outer membranes of adjacent mitochondria are tethered by dimeric structures involving Mfn1/2, followed by GTP consumption to promote outer membrane fusion. Although Opa1 has been shown to regulate inner membrane fusion in numerous studies, the specific mechanism is not yet fully understood [33].
Exposure to doxorubicin was shown to activate mitochondrial fission, evidenced by increased transcription of Drp1and mitochondrial fragmentation, in cardiomyocytes in vitro [36]. Suggesting that mitochondrial fission contributes to doxorubicin-induced cardiomyocyte death, siRNA-mediated Drp1 knockdown was associated with a decrease in caspase-3 expression [36]. Accordingly, Drp1-deficient mice were resistant to doxorubicin-mediated myocardial damage [36]. Another study in a mouse model of doxorubicin-induced DCM confirmed that doxorubicin treatment triggered Drp1-related mitochondrial fission in cardiac cells, which subsequently activated the NLRP3 inflammasome and elicited pyroptosis [37]. Nearly simultaneously, Liang et al. demonstrated that phosphorylation at Ser616 triggered Drp1-dependent unchecked mitochondrial fission, leading to cytochrome c leakage and cardiomyocyte death in mice treated with doxorubicin [38]. Several studies reported also the important role played by Drp1 receptors in mitochondrial fission during doxorubicin-mediated myocardial damage. Zhou et al. showed that doxorubicin administration suppresses the expression of Foxo3a, a transcriptional inhibitor of Mid49, in the mouse heart and in cultured cardiomyocytes, resulting in Mdi49 upregulation and increased mitochondrial fission. Accordingly, cardiac-specific Foxo3a transgenic mice and cardiomyocytes transfected with Mid49 siRNA showed resistance to doxorubicin-mediated toxicity [39].
Interestingly, the stimulatory effect of doxorubicin on mitochondrial fission was reported to be compounded by negative regulation of the expression of key markers of mitochondrial fusion, namely Mfn1/2 and Opa1, in heart tissue [40]. In addition to these changes in protein expression, mass spectrometry and mutagenesis analysis showed that doxorubicin induces Opa1 acetylation at Lys926/931 residues, possibly due to reduced Sirt3 expression [41]. By comparison, either Sirt3 overexpression or transfection of a deacetylation-mimetic version of Opa1 was able to normalize mitochondrial morphology and preserve mitochondrial networking, attenuating cardiomyocyte death in the presence of doxorubicin [41]. Similarly, administration of ethanolic extracts of Rhaponticum uniflorum (L.) DC inflorescence (loulu flowers) to H9c2 cardiomyocytes upregulated Mfn1 and Opa1 expression, prevented doxorubicin-mediated NF-κB activation, and reduced mitochondria-dependent apoptosis [42]. Notably, a study in mice suggested that physical exercise may be an effective way to balance mitochondrial fission and fusion, prevent mitochondrial dysfunction, and neutralize oxidative damage in cardiac tissue following doxorubicin exposure [43].
Mitophagy
Mitophagy is a protective process by which damaged mitochondria are degraded to prevent the accumulation of non-functional mitochondrial fragments [33]. Moreover, through mitophagy, mitochondrial membranes and internal macromolecules are broken down into amino acids, glucose, and nucleotides, used in cellular energy, metabolic, and biosynthetic reactions [44]. Mitophagy is broadly classified into two types, i.e. receptor-dependent and receptor-independent. The first is mainly mediated by specific proteins on the surface of mitochondria [45], and results in the formation of mitophagosomes. Receptor-independent mitophagy is mainly mediated by Parkin [46], which promotes the ubiquitination of mitochondrial proteins and fusion of mitochondria with lysosomes [33]. Mitochondrial surface proteins involved in mitophagy include BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3) [47], FUN14 domain-containing protein 1 (Fundc1) [48], and Nix [33].
Assays employing the novel dual-fluorescent mitophagy reporter mt-Rosella showed that doxorubicin exposure stimulates mitophagy in cardiomyocytes [36]. Interestingly, deletion of Drp1 significantly suppressed mitophagy activation, suggesting that Drp1-dependent mitochondrial fission stimulates mitophagy in the setting of doxorubicin-mediated myocardial dysfunction. Highlighting the central role of mitophagy in this clinical condition, deletion and overexpression of Parkin in doxorubicin-treated cardiomyocytes were linked to decreased and increased cell death, respectively [36]. In accordance with these findings, a study found that inhibition of mitophagy using liensinine, a plant-derived alkaloid, attenuated doxorubicin-caused cardiotoxicity by inhibiting cardiomyocyte apoptosis [38]. Similarly, a recent study in a rat model of doxorubicin-induced chronic heart failure demonstrated that application of moxibustion, a therapy used in traditional Chinese medicine (TCM), to certain acupressure points had cardioprotective effects related to mitophagy inhibition. The latter was evidenced by decreased LC3II/LC3I ratio, attenuated Fundc1 expression, and increased p62 levels in heart tissue [49]. However, as reviewed by Wallace et al. [50], some studies suggested that doxorubicin actually halts autophagic fluxes in cardiac cells, pointing to inhibition of lysosome acidification and subsequent accumulation of autophagosomes and autolysosomes as important determinants of DOX-induced cardiotoxicity.
In fact, a study by Hull et al. suggested that the activation and inactivation of Parkin/PINK1-mediated mitophagy may be related to the duration of doxorubicin treatment [51]. Specifically, these authors reported that Parkin/PINK1 protein expression levels show a biphasic pattern in the hearts of mice treated with doxorubicin: initially (up to day 8 post-treatment), a significant decrease in Parkin/PINK1 expression levels, indicative of mitophagy suppression, was observed; however, by day 14 post-treatment there was a significant increase in Parkin/PINK1 expression, suggesting enhanced mitophagy. Moreover, coincident with delayed mitophagy stimulation, upregulated cardiac expression of the mitochondrial fission-related protein Fis1 was recorded 14 days after doxorubicin treatment [51]. These results suggest that early suppression of mitophagy contributes to the cardiotoxic effects of doxorubicin, by determining late and ineffective removal of damaged mitochondria.
Meanwhile, there is evidence that doxorubicin administration is associated with over-activation of mitophagy, albeit with concomitant disruption of lysosomal function and interrupted mitophagy completion [33]. Thus, it is believed that upon doxorubicin exposure, acute inhibition of mitophagy in heart tissue contributes to the accumulation of dysfunctional mitochondria, which promotes oxidative damage and cardiomyocyte apoptosis. In turn, late and chronic activation of mitophagy would lead to depletion of the mitochondrial population, which drastically lowers ATP synthesis and triggers myocardial dysfunction [52].
Mitochondria-targeted therapies for doxorubicin-induced cardiotoxicity
In light of the essential roles of mitochondrial dynamics and mitophagy in regulating mitochondrial homeostasis, several investigations sought to evaluate the cardioprotective effect of pharmacological targeting of mitochondrial dynamics/mitophagy on doxorubicin-induced myocardial dysfunction (Table 1). Mdivi-1 is a mitochondrial fission inhibitor that interrupts the interaction between Drp1 and its receptors. Supplementation of Mdivi-1 was shown to prevent doxorubicin-induced mitochondrial fission and consequently reduce apoptosis in rat H9c2 [53] and human AC16 [54] cardiomyocytes.
Likewise, a study in rats revealed that Mdivi-1 treatment significantly attenuated doxorubicin-induced mitochondrial dysfunction, decreased cardiac inflammation, reduced cardiomyocyte apoptosis, and improved heart function [53]. Although the beneficial effects of Mdivi-1 against mitochondrial stressors are broadly confirmed by cellular and animal studies, toxicity concerns significantly limit its clinical utilization. Therefore, and in view of Mdivi-1 pre-clinical success, development of safer mitochondrial fission inhibitors is of great interest in cardiovascular medicine.
Oseltamivir is an inhibitor of influenza virus neuraminidase, widely used for the treatment of flu. Treatment with oseltamivir is reported to repress Drp1-mediated mitochondrial division and thus reduce heart dysfunction in doxorubicin-treated rats [55]. Similarly, Shenmai injection, a TCM formulation based on Panax ginseng and Ophiopogon japonicus extracts, was shown to not only inhibit Drp1-related mitochondrial fission but also promote Opa1-induced mitochondrial fusion, hence reducing doxorubicin-mediated cardiomyocyte death [56].
Melatonin is an endogenous hormone that acts a s a central regulator of the circadian rhythm. Several studies have reported that melatonin is also a powerful antioxidant agent. Incubation with melatonin effectively reduced doxorubicin-induced mitochondrial fragmentation and thus maintained viability in cardiomyocytes in vitro [57]. Importantly, in doxorubicin-injected rats, melatonin supplementation greatly enhanced mitochondrial fusion through upregulation of Mfn1/2 expression [57]. These results suggested that melatonin regulates mitochondrial dynamics through restricting mitochondrial fission and restoring mitochondrial fusion. Considering its endogenous and non-toxic nature, exogenous supplementation of melatonin may prove to be beneficial for cancer patients receiving doxorubicin therapy.
Sacubitril/valsartan is a novel inhibitor of the rennin-angiotensin system (RAS) which is now widely prescribed for patients with heart failure. Treatment with LCZ696, a component of sacubitril/valsartan, was found to reduce Drp1 activation and decrease cardiomyocyte death in mice with doxorubicin-induced DCM [58]. Due to its recognized cardioprotective actions, especially prevention of progression of heart failure, the use of sacubitril/valsartan during doxorubicin therapy may be a promising strategy to prevent or attenuate myocardial dysfunction.
Supplementation with vitamin D, an essential micronutrient with high antioxidant properties, was shown to not only reduce oxidative stress but also alleviate Drp1-dependent mitochondrial fission in a mouse model of doxorubicin-induced cardiomyopathy [7]. However, human studies are required to further validate the efficacy of Vitamin D in treating this condition.
Luteolin, a widely occurring flavonoid found in various plant sources such as broccoli, pepper, thyme, and celery, has been extensively studied for its neuroprotective effects. Luteolin exhibits antioxidant properties and possesses immunomodulatory activity in both in vitro and in vivo settings, making it a promising compound for therapeutic applications for doxorubicin-elicited cardiomyopathy. In doxorubicin-treated H9C2 cells, incubation with luteolin relieved mitochondrial fission, improved mitochondrial performance, and attenuated apoptosis [59].
Mitochondria-targeted therapies for doxorubicin-induced cardiotoxicity
| Drug | Model | Effects on mitochondrial dynamics/mitophagy | Cardiac phenotype | References |
|---|---|---|---|---|
| Mdivi-1 | Rats | Inhibition of mitochondrial fission | Decreased oxidative stress, inflammation, and myocardial injury | [53] |
| Mdivi-1 | Human AC16 cells | Inhibition of mitochondrial fission | Increased mitochondrial membrane potential, mtDNA copy number, and cardiomyocyte survival | [54] |
| Oseltamivir | Rats | Inhibition of mitochondrial fission and Parkin-related mitophagy | Decreased oxidative stress, reduced cardiomyocyte apoptosis | [55] |
| Shenmai injection | Mice | Inhibition of mitochondrial fission and activation of mitochondrial fusion | Improved mitochondrial respiratory function, attenuated oxidative stress, and decreased cardiomyocyte apoptosis | [56] |
| Melatonin | Rats | Inhibition of mitochondrial fission and activation of mitochondrial fusion | Improved mitochondrial metabolism, attenuated cardiomyocyte death | [57] |
| LCZ696 | Mice | Inhibition of mitochondrial fission | Increased mitochondrial respiration complex I activity and ATP production, attenuated cardiomyocyte apoptosis | [58] |
| Vitamin D | Mice | Inhibition of mitochondrial fission | Decreased ROS production and attenuated mitochondrial damage | [7] |
| Luteolin | Zebrafish and mice | Inhibition of mitochondrial fission | Decreased cardiomyocyte death, preserved ventricular function | [59]. |
| Paeonol | Rats | Activation of mitochondrial fusion | Decreased mitochondrial oxidative stress and cardiomyocyte apoptosis, improved heart function | [60] |
| Honokiol | Mice | Activation of mitochondrial fusion | Reduced oxidative stress and cardiomyocyte death, improved heart function | [61] |
| Total flavonoids of Selaginella tamariscina Spring | Mice | Activation of mitochondrial fusion | Decreased levels of myocardial injury markers, attenuated oxidative stress, preserved mitochondrial potential, increased cardiomyocyte survival | [63] |
| Ellagic acid | Rats | Inhibition of mitophagy and mitochondrial fission | Decreased mPTP opening, preserved mitochondrial membrane potential, reduced cardiomyocyte death | [65] |
Paeonol is a natural antioxidant derived from the root bark of Paeonia suffruticosa that is approved in China to alleviate pain and treat inflammatory conditions. Administration of paeonol was found to reverse Mfn2-related mitochondrial fusion in a manner depending on increased Stat3 expression, improving mitochondrial function and cardiac behavior in rats with doxorubicin-induced cardiomyopathy [60]. Given the important role played by Sirt3 in regulating the activity of mitochondrial fusion, the use of Sirt3 agonists has potential therapeutic value in this medical condition. Administration of honokiol (HKL), a natural extract of the bark of the magnolia tree that acts as a Sirt3 activator, enhanced Sirt3 activity and reduced mitochondrial damage and cell death in rat neonatal cardiomyocytes by inhibiting ROS production and stimulating Mfn1/Opa1-mediated mitochondrial fusion. Moreover, these mechanisms accounted for effective cardioprotection in rats exposed to doxorubicin [61]. Similarly, HKL treatment mitigated doxorubicin-induced cardiotoxicity in mice by suppressing cardiac inflammation and oxidative stress, resulting in improved cardiac function and reduced cardiomyocyte apoptosis [62].
Treatment with total flavonoids derived from Selaginella tamariscina (P.Beauv.) Spring (TFST) was found to attenuate doxorubicin-induced cardiotoxicity in mice [63]. This effect was correlated with enhanced Mfn2-mediated mitochondrial fusion, reduced mitochondrial dysfunction, as well as Mfn2/PERK pathway activation leading to inhibition of endoplasmic reticulum stress [63].
Considering the deleterious effects on heart function of exacerbated mitophagy during long-term doxorubicin treatment, pre-clinical research has also addressed the therapeutic potential of selective/specific inhibitors of mitophagy. Acetylcholine receptors (AChRs) were proposed to participate in the regulation of mitophagy, and experimental attempts have been made to evaluate whether activation of AChRs correlates with mitophagy inhibition. In a mouse model of doxorubicin-induced cardiomyopathy, administration of PNU-282987 or bethanechol, two AChR agonists acting respectively on 7nAChR and mAChR, was shown to confer cardioprotection related to prevention of abnormal mitophagy activation, improved ATP synthesis, and enhanced cardiomyocyte survival [64].
Ellagic acid, a natural polyphenolic compound abundant in fruits and vegetables, shows potential as a therapeutic agent to modulate mitophagy. In a mouse model of doxorubicin-induced cardiomyopathy, treatment with ellagic acid reduced the levels of mitochondria-associated Bnip3, resulting in attenuation of both mitophagy and cardiomyocyte death. Moreover, ellagic acid suppressed also mitochondrial injury and cell death in Bnip3-overexpressing or hypoxic cardiomyocytes in vitro [65].
In their review [66], Cocetta et al. elucidate resveratrol's potential as a chemosensitizer in cancer treatment. This polyphenolic compound, ubiquitous in various plants, has been shown to interact with conventional chemotherapeutics, spanning alkylating agents to mitotic inhibitors, enhancing their efficacy through modulation of cancer pathways. This work illuminates the promise of resveratrol in augmenting therapeutic outcomes and underscores the imperative for further clinical inquiry to address its bioavailability challenges. Notably, resveratrol emerges as a viable candidate for mitigating doxorubicin-induced cardiac events, heralding a significant stride in oncological adjunct therapy.
Quagliariello et al. delve into the creation of nano-encapsulated Coenzyme Q10 (CoQ10) formulations, aimed at bolstering cardioprotective and hepatoprotective responses against anthracycline-induced toxicities, notably from doxorubicin and trastuzumab [67]. This research [67] addresses the critical issue of anthracyclines triggering adverse cardiac and hepatic outcomes, proposing an innovative nano-emulsion technique to enhance CoQ10 delivery. Their findings reveal that such nano-encapsulated CoQ10 formulations significantly reduce oxidative stress and inflammation, suggesting a promising avenue to improve cancer therapy patient outcomes by minimizing cardiotoxic and hepatotoxic side effects [67].
Outlook and conclusion
Doxorubicin has broad-spectrum anti-tumor effects and is widely used in clinical practice to improve the prognosis of cancer patients. However, its use is restricted by cardiotoxicity associated with cumulative dose-dependent mitochondrial damage, evidenced by dysregulated mitochondrial dynamics, disruption of mitochondrial fusion, and mitophagy imbalance. Disruption of mitochondrial structure and function triggers cellular oxidative stress, intracellular calcium overload, endoplasmic reticulum stress, and damages enzymes, lipids, and nucleic acids in cardiac cells.
Although the roles of abnormal mitochondrial dynamics and disrupted mitophagy in doxorubicin-induced cardiotoxicity have been extensively studied, effective preventive strategies and clinically applicable cardioprotective drugs are limited. Some studies have explored the cardioprotective effects of small molecule compounds that target mitochondrial dynamics and mitophagy. However, these compounds have limited evidence of safety and efficacy for clinical translation. Likewise, although promising results have been reported using natural plant and animal extracts, as well as chemically synthesized drugs, their efficacies have been so far mostly assessed only in animal and cellular studies. The characteristics of cancer patients are admittedly far more complex than those of laboratory animals. In cancer patients, doxorubicin-mediated cardiotoxicity may be more pronounced and severe due to advanced age and pre-existing organ damage. Therefore, clinical data are needed to assess the effectiveness of drug interventions in preventing doxorubicin-induced cardiotoxicity. Likewise, further research on the molecular actions of anthracycline derivatives is needed to reduce off-target toxicity. Developing safer and more tumor-targeted doxorubicin analogues that may be used in combination with cardioprotective therapy will help curtail the incidence of doxorubicin-induced cardiotoxicity in cancer treatment.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kerr AJ, Dodwell D, McGale P, Holt F, Duane F, Mannu G. et al. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat Rev. 2022;105:102375
2. Henderson IC, Frei TE 3rd. Adriamycin and the heart. N Engl J Med. 1979;300:310-2
3. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63-75
4. Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104:971-7
5. Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263-302
6. Legha SS, Wang YM, Mackay B, Ewer M, Hortobagyi GN, Benjamin RS. et al. Clinical and pharmacologic investigation of the effects of alpha-tocopherol on adriamycin cardiotoxicity. Ann N Y Acad Sci. 1982;393:411-8
7. Lee KJ, Wright G, Bryant H, Wiggins LA, Dal Zotto VL, Schuler M. et al. Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer. Int J Mol Sci. 2021 22
8. Elitok A, Oz F, Cizgici AY, Kilic L, Ciftci R, Sen F. et al. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: A prospective randomized controlled study with six-month follow-up. Cardiol J. 2014;21:509-15
9. Booser DJ, Hortobagyi GN. Anthracycline antibiotics in cancer therapy. Focus on drug resistance. Drugs. 1994;47:223-58
10. Frederick CA, Williams LD, Ughetto G, van der Marel GA, van Boom JH, Rich A. et al. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry. 1990;29:2538-49
11. Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45:649-56
12. Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36:2891-5
13. Cutts SM, Nudelman A, Rephaeli A, Phillips DR. The power and potential of doxorubicin-DNA adducts. IUBMB Life. 2005;57:73-81
14. Zhang W, Jin X, Li H, Wei CX, Wu CW. Onion-structure bionic hydrogel capsules based on chitosan for regulating doxorubicin release. Carbohydr Polym. 2019;209:152-60
15. Nicoletto RE, Ofner CM 3rd. Cytotoxic mechanisms of doxorubicin at clinically relevant concentrations in breast cancer cells. Cancer Chemother Pharmacol. 2022;89:285-311
16. Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE. et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440-6
17. Speth PA, van Hoesel QG, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet. 1988;15:15-31
18. Mordente A, Meucci E, Silvestrini A, Martorana GE, Giardina B. Anthracyclines and mitochondria. Adv Exp Med Biol. 2012;942:385-419
19. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-79
20. Kim YA, Cho H, Lee N, Jung SY, Sim SH, Park IH. et al. Doxorubicin-induced heart failure in cancer patients: A cohort study based on the Korean National Health Insurance Database. Cancer Med. 2018;7:6084-92
21. Kumar S, Marfatia R, Tannenbaum S, Yang C, Avelar E. Doxorubicin-induced cardiomyopathy 17 years after chemotherapy. Tex Heart Inst J. 2012;39:424-7
22. Govender J, Loos B, Marais E, Engelbrecht AM. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: a review of the protective role of melatonin. J Pineal Res. 2014;57:367-80
23. Willis MS, Parry TL, Brown DI, Mota RI, Huang W, Beak JY. et al. Doxorubicin Exposure Causes Subacute Cardiac Atrophy Dependent on the Striated Muscle-Specific Ubiquitin Ligase MuRF1. Circ Heart Fail. 2019;12:e005234
24. Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155-62
25. Kamphuis JAM, Linschoten M, Cramer MJ, Doevendans PA, Asselbergs FW, Teske AJ. Early- and late anthracycline-induced cardiac dysfunction: echocardiographic characterization and response to heart failure therapy. Cardiooncology. 2020;6:23
26. Mitry MA, Edwards JG. Doxorubicin induced heart failure: Phenotype and molecular mechanisms. Int J Cardiol Heart Vasc. 2016;10:17-24
27. Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302-14
28. Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV. et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124:617-30
29. Kolaric K, Bradamante V, Cervek J, Cieslinska A, Cisarz-Filipcak E, Denisov LE. et al. A phase II trial of cardioprotection with Cardioxane (ICRF-187) in patients with advanced breast cancer receiving 5-fluorouracil, doxorubicin and cyclophosphamide. Oncology. 1995;52:251-5
30. Dorn GW 2nd, Vega RB, Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981-91
31. Vásquez-Trincado C, García-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA. et al. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594:509-25
32. Quiles JM, Gustafsson Å B. The role of mitochondrial fission in cardiovascular health and disease. Nat Rev Cardiol. 2022;19:723-36
33. Kraus F, Roy K, Pucadyil TJ, Ryan MT. Function and regulation of the divisome for mitochondrial fission. Nature. 2021;590:57-66
34. Jin JY, Wei XX, Zhi XL, Wang XH, Meng D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin. 2021;42:655-64
35. Hu C, Huang Y, Li L. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int J Mol Sci. 2017 18
36. Catanzaro MP, Weiner A, Kaminaris A, Li C, Cai F, Zhao F. et al. Doxorubicin-induced cardiomyocyte death is mediated by unchecked mitochondrial fission and mitophagy. Faseb j. 2019;33:11096-108
37. Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X. et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020;34:101523
38. Liang X, Wang S, Wang L, Ceylan AF, Ren J, Zhang Y. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of Drp1-mediated maladaptive mitochondrial fission. Pharmacol Res. 2020;157:104846
39. Zhou L, Li R, Liu C, Sun T, Htet Aung LH, Chen C. et al. Foxo3a inhibits mitochondrial fission and protects against doxorubicin-induced cardiotoxicity by suppressing MIEF2. Free Radic Biol Med. 2017;104:360-70
40. Marques-Aleixo I, Santos-Alves E, Torrella JR, Oliveira PJ, Magalhães J, Ascensão A. Exercise and Doxorubicin Treatment Modulate Cardiac Mitochondrial Quality Control Signaling. Cardiovasc Toxicol. 2018;18:43-55
41. Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D. et al. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol. 2014;34:807-19
42. Hu B, Zhen D, Bai M, Xuan T, Wang Y, Liu M. et al. Ethanol extracts of Rhaponticum uniflorum (L.) DC flowers attenuate doxorubicin-induced cardiotoxicity via alleviating apoptosis and regulating mitochondrial dynamics in H9c2 cells. J Ethnopharmacol. 2022;288:114936
43. Santos-Alves E, Rizo-Roca D, Marques-Aleixo I, Coxito P, Martins S, Guimarães JT. et al. Physical exercise positively modulates DOX-induced hepatic oxidative stress, mitochondrial dysfunction and quality control signaling. Mitochondrion. 2019;47:103-13
44. Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31-42
45. Terešak P, Lapao A, Subic N, Boya P, Elazar Z, Simonsen A. Regulation of PRKN-independent mitophagy. Autophagy. 2022;18:24-39
46. Nguyen TN, Padman BS, Lazarou M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016;26:733-44
47. Wu X, Zheng Y, Liu M, Li Y, Ma S, Tang W. et al. BNIP3L/NIX degradation leads to mitophagy deficiency in ischemic brains. Autophagy. 2021;17:1934-46
48. Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25:1080-93
49. Xia R, Wang W, Gao B, Ma Q, Wang J, Dai X. et al. Moxibustion alleviates chronic heart failure by regulating mitochondrial dynamics and inhibiting autophagy. Exp Ther Med. 2022;23:359
50. Wallace KB, Sardão VA, Oliveira PJ. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ Res. 2020;126:926-41
51. Hull TD, Boddu R, Guo L, Tisher CC, Traylor AM, Patel B. et al. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight. 2016;1:e85817
52. Koleini N, Kardami E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget. 2017;8:46663-80
53. Maneechote C, Khuanjing T, Ongnok B, Arinno A, Prathumsap N, Chunchai T. et al. Promoting mitochondrial fusion in doxorubicin-induced cardiotoxicity: a novel therapeutic target for cardioprotection. Clin Sci (Lond). 2022;136:841-60
54. Yin J, Guo J, Zhang Q, Cui L, Zhang L, Zhang T. et al. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicol In vitro. 2018;51:1-10
55. Qin Y, Lv C, Zhang X, Ruan W, Xu X, Chen C. et al. Neuraminidase1 Inhibitor Protects Against Doxorubicin-Induced Cardiotoxicity via Suppressing Drp1-Dependent Mitophagy. Front Cell Dev Biol. 2021;9:802502
56. Li L, Li J, Wang Q, Zhao X, Yang D, Niu L. et al. Shenmai Injection Protects Against Doxorubicin-Induced Cardiotoxicity via Maintaining Mitochondrial Homeostasis. Front Pharmacol. 2020;11:815
57. Govender J, Loos B, Marais E, Engelbrecht AM. Melatonin improves cardiac and mitochondrial function during doxorubicin-induced cardiotoxicity: A possible role for peroxisome proliferator-activated receptor gamma coactivator 1-alpha and sirtuin activity? Toxicol Appl Pharmacol. 2018;358:86-101
58. Xia Y, Chen Z, Chen A, Fu M, Dong Z, Hu K. et al. LCZ696 improves cardiac function via alleviating Drp1-mediated mitochondrial dysfunction in mice with doxorubicin-induced dilated cardiomyopathy. J Mol Cell Cardiol. 2017;108:138-48
59. Shi Y, Li F, Shen M, Sun C, Hao W, Wu C. et al. Luteolin Prevents Cardiac Dysfunction and Improves the Chemotherapeutic Efficacy of Doxorubicin in Breast Cancer. Front Cardiovasc Med. 2021;8:750186
60. Ding M, Shi R, Fu F, Li M, De D, Du Y. et al. Paeonol protects against doxorubicin-induced cardiotoxicity by promoting Mfn2-mediated mitochondrial fusion through activating the PKCε-Stat3 pathway. J Adv Res. 2023;47:151-62
61. Pillai VB, Kanwal A, Fang YH, Sharp WW, Samant S, Arbiser J. et al. Honokiol, an activator of Sirtuin-3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin-induced cardiomyopathy in mice. Oncotarget. 2017;8:34082-98
62. Huang L, Zhang K, Guo Y, Huang F, Yang K, Chen L. et al. Honokiol protects against doxorubicin cardiotoxicity via improving mitochondrial function in mouse hearts. Sci Rep. 2017;7:11989
63. Gao L, Yuan P, Wei Y, Fu Y, Hou Y, Li P. et al. Total flavonoids of Selaginella tamariscina (P.Beauv.) Spring ameliorates doxorubicin-induced cardiotoxicity by modulating mitochondrial dysfunction and endoplasmic reticulum stress via activating MFN2/PERK. Phytomedicine. 2022;100:154065
64. Prathumsap N, Ongnok B, Khuanjing T, Arinno A, Maneechote C, Apaijai N. et al. Acetylcholine receptor agonists provide cardioprotection in doxorubicin-induced cardiotoxicity via modulating muscarinic M(2) and α7 nicotinic receptor expression. Transl Res. 2022;243:33-51
65. Dhingra A, Jayas R, Afshar P, Guberman M, Maddaford G, Gerstein J. et al. Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes. Free Radic Biol Med. 2017;112:411-22
66. Cocetta V, Quagliariello V, Fiorica F, Berretta M, Montopoli M. Resveratrol as Chemosensitizer Agent: State of Art and Future Perspectives. Int J Mol Sci. 2021 22
67. Quagliariello V, Vecchione R, De Capua A, Lagreca E, Iaffaioli RV, Botti G. et al. Nano-Encapsulation of Coenzyme Q10 in Secondary and Tertiary Nano-Emulsions for Enhanced Cardioprotection and Hepatoprotection in Human Cardiomyocytes and Hepatocytes During Exposure to Anthracyclines and Trastuzumab. Int J Nanomedicine. 2020;15:4859-76
Author contact
![]() Corresponding authors: Ying Tong: email: tongyingedu.cn. Xing Chang: email: xingchang_tcmcom.
Corresponding authors: Ying Tong: email: tongyingedu.cn. Xing Chang: email: xingchang_tcmcom.

 Global reach, higher impact
Global reach, higher impact