3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(5):784-794. doi:10.7150/ijms.92766 This issue Cite
Research Paper
The characterization and comorbidities of heterozygous Bardet-Biedl syndrome carriers
1. Division of Pediatric Genetics and Metabolism, Children's Medical Center, Taichung Veterans General Hospital, Taichung, Taiwan.
2. Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan.
3. Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan.
4. Division of Nephrology, Department of Pediatrics, Taichung Veterans General Hospital, Taichung, Taiwan.
5. School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
6. Division of Allergy, Immunology, and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan.
7. Institute of Biomedical Science and Rong Hsing Research Center for Translational Medicine, National Chung Hsing University, Taiwan.
Received 2023-12-1; Accepted 2024-2-10; Published 2024-2-25
Abstract
Introduction: Bardet-Biedl syndrome (BBS) is a rare autosomal recessive disorder with clinical features of retinal dystrophy, obesity, postaxial polydactyly, renal anomalies, learning disabilities, hypogonadism, and genitourinary abnormalities. Nevertheless, previous studies on the phenotypic traits of BBS heterozygous carriers have generated inconclusive results. The aim of our study was to investigate the impact of BBS heterozygosity on carriers when compared to non-carriers within the Taiwanese population.
Materials and Methods: This study follows a hospital-based case-control design. We employed the Taiwan Biobank version 2 (TWBv2) array to identify three specific loci associated with BBS (rs773862084, rs567573386, and rs199910690). In total, 716 patients were included in the case group, and they were compared to a control group of 2,864 patients who lacked BBS alleles. The control group was selected through gender and age matching at a ratio of 1:4. The association between BBS-related loci and comorbidity was assessed using logistic regression models.
Results: We found that BBS heterozygous carriers exhibited a significant association with elevated BMI levels, especially the variant rs199910690 in MKS1 (p=0.0037). The prevalence of comorbidities in the carriers' group was not higher than that in the non-carriers' group. Besides, the average values of the biochemistry data showed no significant differences, except for creatinine level. Furthermore, we conducted a BMI-based analysis to identify specific risk factors for chronic kidney disease (CKD). Our findings revealed that individuals carrying the CA/AA genotype of the BBS2 rs773862084 variant or the CT/TT genotype of the MKS1 rs199910690 variant showed a reduced risk of developing CKD, irrespective of their BMI levels. When stratified by BMI level, obese males with the MKS1 rs199910690 variant and obese females with the BBS2 rs773862084 variant exhibited a negative association with CKD development.
Conclusion: We found that aside from the association with overweight and obesity, heterozygous BBS mutations did not appear to increase the predisposition of individuals to comorbidities and metabolic diseases. To gain a more comprehensive understanding of the genetic susceptibility associated with Bardet-Biedl Syndrome (BBS), further research is warranted
Keywords: Bardet-Biedl syndrome, BBS2 rs773862084, MKS1 rs199910690, heterozygotes, obesity, BMI, CKD
Introduction
Bardet-Biedl syndrome (BBS) is a rare autosomal recessive disorder characterized by gene pleiotropy. Its features include retinal dystrophy, obesity, postaxial polydactyly, renal anomalies, learning disabilities, hypogonadism, and genitourinary abnormalities[1]. In the general population, the prevalence of BBS is 0.7 per 100,000[2], while the incidence varies among different regions, with a rate of 1 in 62,000 in Puerto Rico[3], 1 in 18,000 in Newfoundland due to high consanguinity[4], and 1 in 36,000 in Kuwait[5]. The occurrence of BBS in Europe is less frequent, affecting approximately 1 in 125,000 in the UK[6]. Among Asians, the incidence is notably lower, with rates as rare as 1 in 18 million[7]. As of July 31, 2023, there were a total of 45 confirmed BBS cases in Taiwan, as reported by the Health Promotion Administration, Ministry of Health and Welfare.
As BBS is a multisystem disorder, afflicted patients may commonly experience systemic effects, such as hypertension and metabolic abnormalities. To date, at least 26 genes associated with BBS have been identified[1, 8, 9]. These genes encode various components, including the BBSome complex, BBS-chaperonin complex, and other BBS proteins that function independently[10]. A number of studies have investigated the correlation between phenotype and genotype in BBS patients[9]. Forsythe et al. demonstrated the correlation between severe renal disease and BBS2, BBS10, and BBS12 patients[11]. In a study by Mujahid et al., patients with BBS10 were at increased risk of metabolic syndrome[12]. However, previous studies regarding the characteristics of BBS heterozygous carriers have yielded inconclusive results. Clinically, the relatives of BBS patients are not predisposed to developing the diagnostic features. Croft et al. established an association between obesity and male BBS heterozygous carriers[13]. Benzinou et al. also presented evidence of increased obesity risk associated with BBS2, BBS4, and BBS6 genes[14]. Nevertheless, in a recent study, no correlation between obesity and metabolic disorders was found in first-degree relatives of BBS patients[15]. Based on prior studies, the attributes of BBS heterozygous carriers remain unclear.
This study aimed to evaluate the impact of BBS heterozygotes on carriers as compared to non-carriers within the Taiwanese population, focusing on the metabolic characteristics between genotype and phenotype. A second objective was to examine the variant's effect on different genders and its association with obesity. In this investigation, we aimed to uncover trends that could inform better genetic counseling and disease prevention for both affected BBS families and inadvertent carriers.
Material and Methods
Study design and data source
This retrospective case-control study made use of data from the Taiwan Precision Medicine Initiative (TPMI), a nationwide genetic program managed by Academia Sinica and partner hospitals. The TPMI cohort comprised Taiwanese participants from 16 hospitals across the country, with a significant portion of the cohort consisting of patients from Taichung Veterans General Hospital (TCVGH), a tertiary medical center. The study enrolled a total of 58,091 patients aged 18 years and older, who had visited 28 surgical and medical outpatient clinics at TCVGH between June 2019 and May 2021.
For our specific study cohort, we included a total of 3,580 patients whose genetic profiles were linked with medical claims data from TCVGH. This comprehensive dataset contained a wide range of information, including demographic characteristics, laboratory examinations, and diagnoses. The analysis was performed based on age and gender distribution. Diagnoses were coded using the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM, ICD-10-CM) format. All of the participants provided informed consent and the study received approval from the Institutional Review Board (IRB No. SF19153A) of Taichung Veterans General Hospital's ethics committee. Written informed consent was obtained from all participants in accordance with the principles defined in the Declaration of Helsinki.
Genotyping
In our study, blood samples were obtained from a total of 58,091 TPMI participants. Genomic DNA was isolated from these samples using DNA isolation kits (TIANGEN Biotech, Beijing, China). The extracted DNA was then quantified using a NanoDrop 2000 Spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA). We utilized the Taiwan Biobank version 2 (TWBv2) array to conduct next-generation sequencing (NGS) for the participants. This sequencing approach specifically targeted 114,000 risk variants in 2,831 unusual disease genes, which were carefully selected from reputable sources such as ClinVar, ACMG, GWAS Catalog, HGMD, locus-specific databases, and published literature[16].
For rare variants genotyping and quality control, advanced normalization was performed to correct misclustering caused by the batch effect. This process was carried out using the advnorm package provided by Thermo Fisher Scientific (Santa Clara, CA, United States). Additionally, we applied a rare heterozygote adjustment to exclude probesets with inconsistent signals in replicated probes. The rare heterozygote adjustment was conducted using the axiomBestPractices-1.2.4 program, employing the "do-rare-het-adjustment” command[17].
Participants
After conducting a review of the literature, we selected three available loci associated with BBS matched with the Affymetrix Taiwan Biobank version 2 (TWBv2) array. There were two susceptibility alleles in BBS2 and one allele in MKS1, including rs773862084 (BBS2), rs567573386 (BBS2), and rs199910690 (MKS1). The MKS1 gene is not limited to Bardet-Biedl syndrome; it is also implicated in allelic disorders, such as Joubert syndrome and Meckel syndrome, which result in ciliary dysfunction[9]. A total of 716 patients who had variants associated with BBS were enrolled as the case group. A total of 2,864 patients without BBS alleles were matched to the case group by gender and age at a ratio of 1:4 to serve as the control group.
Covariates
We obtained comorbidity information from the electronic health records of TCVGH based on ICD-9 and ICD-10 diagnostic codes. Comorbidities were identified, including hyperlipidemia (ICD-9-CM code 272, ICD-10-CM code E78.1-E78.5), hypertension (ICD-9-CM code 401-405, ICD-10-CM code I10-I15), obesity (ICD-9-CM code 278, ICD-10-CM code E66), diabetes mellitus (DM) (ICD-9-CM code 250, ICD-10-CM code E10.9 and E11.9), DM comorbidity with retinopathy, neuropathy, or chronic kidney disease (ICD-9 code 250, ICD-10-CM code E08 and E11), chronic kidney disease (CKD) (ICD-9-CM code 585 and ICD-10 code N18.1-N18.9), renal cancer (ICD-9-CM code 189, ICD-10-CM code C64 and C65), acute myocardial infarction(AMI) (ICD-9 code 410, ICD-10-CM code I21.3 and I24.9), coronary artery disease (CAD) (ICD-9 code 411-413, 414.00, 414.01 and ICD-10 code I20.8, I24.1, I25.1, I25.2), and cerebrovascular accident (CVA) (ICD-9 code 433-438 and ICD-10 code I63.5, I63.9, I67, I69). These comorbidities were identified if the diagnostic code was used once during admission or at least twice in the outpatient service.
Biochemical data included lipid profiles (low-density lipoprotein [LDL], high density lipoprotein [HDL], triglyceride [TG], and total cholesterol), blood sugar status (fasting glucose level and hemoglobin A1c [HbA1c]), serum insulin level, uric acid level, serum creatinine, and liver enzymes (alanine aminotransferase [ALT], aspartate aminotransferase [AST]). We selected the initial biochemical data collected from each individual in our hospital. Logistic regression was employed to examine the associations between BBS variants and the collected biochemical data.
Statistics
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) version 24.0 (Armonk, NY: IBM Corp.). Statistical significance was defined as p-values less than 0.05. The demographic information is presented as mean ± standard deviation (SD) for continuous variables and as number (percent) for categorical variables. To compare variables between BBS carriers and non-BBS carriers, Student's t-test was conducted for continuous variables, while Chi-square test was performed for categorical variables. Fisher's exact test was utilized to compare variables between alleles in BBS2 and MKS1. Multivariate logistic regression analysis was employed to calculate odds ratios (OR) and 95% confidence intervals (95% CI) for the three variants, adjusting for potential confounders. Furthermore, the impact of these variants on comorbidity was explored.
Results
Baseline characteristics and genetic variations associated with Bardet-Biedl Syndrome
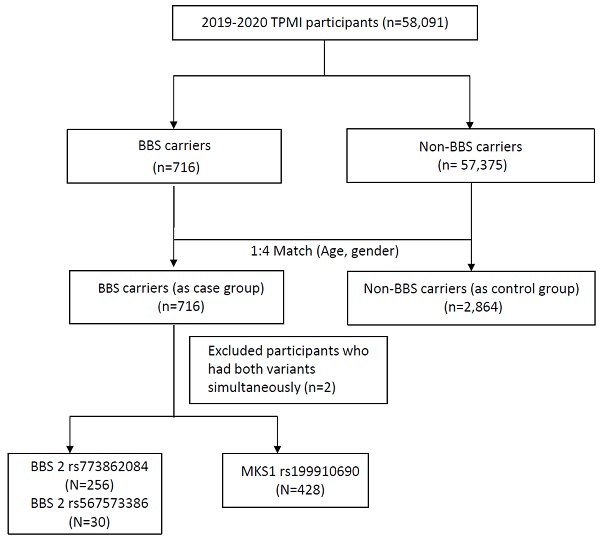
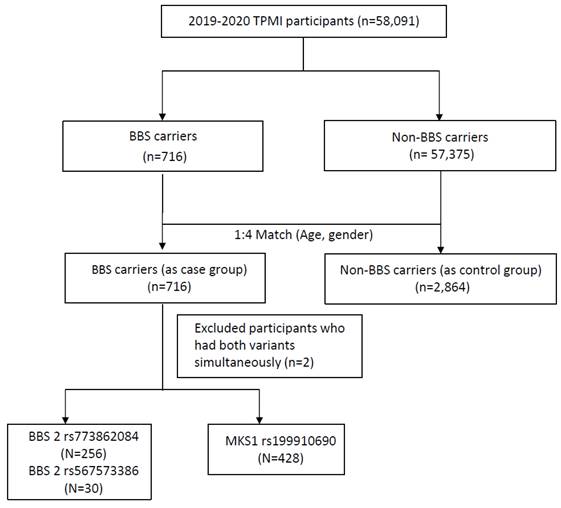
A total of 58,091 participants were included in the study, and the participant enrollment process is depicted in Figure 1. All recruited participants underwent genotyping. Among them, 716 individuals (1.23%) were identified as having susceptibility alleles associated with Bardet-Biedl syndrome (BBS carriers), while 57,375 individuals (98.77%) served as non-BBS carriers. After matching for age and gender at a 1:4 ratio, the BBS case group consisted of 716 subjects, and the control group consisted of 2,864 subjects. The baseline demographics of the study participants are shown in Table 1. Individuals with BBS variants exhibited higher percentages of overweight (BMI ≥ 24 kg/m2) compared to those without BBS variants (55.47% vs. 51.61%). The mean value of BMI in the BBS case group was 25.02±4.64 kg/m2 with significant differences observed. Conversely, hyperlipidemia (32.4% vs. 39.49%, p = 0.0005), hypertension (26.96% vs. 37.26%, p<0.0001), diabetes mellitus (DM) (25.28% vs. 34.67%, p<0.0001), diabetes mellitus comorbidity (4.47% vs. 7.93%, p = 0.0014), and chronic kidney disease (CKD) (18.72% vs. 36.94%, p<0.0001) were inversely associated with individuals without BBS variants, as they were more prevalent in the control group.
Furthermore, in Table 2, individuals with BBS variants displayed higher levels of the liver enzyme alanine aminotransferase (ALT) (32.42 ± 38.16 vs. 27.49 ±27.94 uIU/mL, p = 0.0026) and demonstrated better renal function, as indicated by the lower levels of serum creatinine (1.17 ± 1.64 vs. 1.66 ± 2.32 uIU/mL, p<0.0001) compared to their counterparts. Additionally, participants carrying BBS susceptibility alleles exhibited higher insulin levels (46.97 ± 119.36 vs. 17.69 ± 23.14 uIU/mL) and elevated levels of aspartate aminotransferase (AST) (30.94 ± 44.72 vs. 26.66 ± 34.87 uIU/mL), although the latter difference was not statistically significant.
Basic characteristics of the study population.
| Variables | With BBS (N=716) | Without BBS (N=2,864) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||||
| Age, years (mean/SD)a | 59.02 | 15.75 | 59.02 | 15.74 | 1 | ||||
| Gender (n, %)b | 1 | ||||||||
| Female | 380 | 53.07% | 1520 | 53.07% | |||||
| Male | 336 | 46.93% | 1344 | 46.93% | |||||
| BMI (n, %)b | |||||||||
| BMI (Mean/SD) | 25.02 | 4.64 | 24.59 | 5.65 | 0.0419 | ||||
| BMI<24 kg/m2 | 293 | 44.53 | 1305 | 48.39 | 0.0054 | ||||
| 24≦BMI≦27 kg/m2 | 171 | 25.99 | 760 | 28.18 | |||||
| BMI>27 kg/m2 | 194 | 29.48 | 632 | 23.43 | |||||
| Comorbidities (n, %)b | |||||||||
| Hyperlipidemia | 0.0005 | ||||||||
| No | 484 | 67.6 | 1733 | 60.51 | |||||
| Yes | 232 | 32.4 | 1131 | 39.49 | |||||
| Hypertension | <0.0001 | ||||||||
| No | 523 | 73.04 | 1797 | 62.74 | |||||
| Yes | 193 | 26.96 | 1067 | 37.26 | |||||
| Obesity | 0.2715 | ||||||||
| No | 705 | 98.46 | 2834 | 98.95 | |||||
| Yes | 11 | 1.54 | 30 | 1.05 | |||||
| DM | <0.0001 | ||||||||
| No | 535 | 74.72 | 1871 | 65.33 | |||||
| Yes | 181 | 25.28 | 993 | 34.67 | |||||
| DM comorbidity* | 0.0014 | ||||||||
| No | 684 | 95.53 | 2637 | 92.07 | |||||
| Yes | 32 | 4.47 | 227 | 7.93 | |||||
| CKD | <0.0001 | ||||||||
| No | 582 | 81.28 | 1806 | 63.06 | |||||
| Yes | 134 | 18.72 | 1058 | 36.94 | |||||
| Renal cancer | 0.1751 | ||||||||
| No | 708 | 98.88 | 2811 | 98.15 | |||||
| Yes | 8 | 1.12 | 53 | 1.85 | |||||
| AMI | 0.5151 | ||||||||
| No | 706 | 98.6 | 2814 | 98.25 | |||||
| Yes | 10 | 1.4 | 50 | 1.75 | |||||
| CAD | 0.3278 | ||||||||
| No | 603 | 84.22 | 2368 | 82.68 | |||||
| Yes | 113 | 15.78 | 496 | 17.32 | |||||
| CVA | 0.2599 | ||||||||
| No | 638 | 89.11 | 2508 | 87.57 | |||||
| Yes | 78 | 10.89 | 356 | 12.43 | |||||
a Continuous variables were expressed as mean ± standard deviation (SD) and were analyzed using Student's t-test for normal data distributions.
b Categorical variables were expressed as numbers (percent) and were analyzed using the Chi-square test.
*DM comorbidity with retinopathy, neuropathy, or chronic kidney disease
Abbreviations: BBS: Bardet-Biedl syndrome; BMI: Body mass index; DM: diabetes mellitus; CKD: chronic kidney disease; AMI: acute myocardial infarction; CAD: coronary artery disease; CVA: cerebrovascular accident
Study participants enrollment flow chart. BBS, Bardet-Biedl syndrome.
Basic biochemistry characteristics of the study population.
| Variables | With BBS (N=716) | Without BBS (N=2,864) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||||
| Biochemistry (mean/SD)a | |||||||||
| LDL (mg/dL) | 118.25 | 36.73 | 117.38 | 42.85 | 0.6682 | ||||
| HDL (mg/dL) | 52.72 | 15.79 | 54 | 16.8 | 0.2557 | ||||
| Triglyceride (mg/dL) | 137.8 | 101.15 | 145.24 | 149.8 | 0.1848 | ||||
| Total cholesterol (mg/dL) | 191.44 | 43.7 | 189.5 | 48.35 | 0.4367 | ||||
| Uric acid (mg/dL) | 6.38 | 2.02 | 6.5 | 2.18 | 0.3376 | ||||
| Fasting glucose (mg/dL) | 117.1 | 42.79 | 117.93 | 47.13 | 0.7218 | ||||
| HbA1c (%) | 6.58 | 1.73 | 6.67 | 2.04 | 0.3666 | ||||
| Insulin (uIU/mL) | 46.97 | 119.36 | 17.69 | 23.14 | 0.3597 | ||||
| Creatinine (mg/dL) | 1.17 | 1.64 | 1.66 | 2.32 | <0.0001 | ||||
| ALT (U/L) | 32.42 | 38.16 | 27.49 | 27.94 | 0.0026 | ||||
| AST (U/L) | 30.94 | 44.72 | 26.66 | 34.87 | 0.053 | ||||
a Continuous variables were expressed as mean ± standard deviation (SD) and were analyzed using Student's t-test for normal data distributions.
Abbreviations: BBS: Bardet-Biedl syndrome; SD: standard deviation; LDL: low density lipoprotein; HDL: high density lipoprotein; HbA1c: glycosylated hemoglobin; eGFR: estimated glomerular filtration rate; ALT: alanine aminotransferase; AST: aspartate transferase.
Comparisons of comorbidities and serology among each of the BBS risk alleles
Within the BBS carriers group, among the 716 participants with BBS variants, two cases were identified as double heterozygous carriers, possessing both BBS2 rs773862084 and MKS1 rs199910690 heterozygous mutations. The remaining 714 subjects were heterozygous carriers, each having a single copy of the mutated gene. Specifically, among the BBS2 variants, 286 patients (40.06%) had rs773862084 (256 subjects) or rs567573386 (30 cases), while the remaining 428 patients (59.94%) had MKS1 variants with rs199910690. The characteristics of comorbidities and serology in each of the BBS risk alleles are shown in Table 3 and Table 4.
For the majority of risk alleles in the BBS group, no statistically significant associations were found with any of the comorbidities. However, individuals carrying the MKS1 rs199910690 allele exhibited a noteworthy increase in the incidence of cerebrovascular accidents (14.49%, p = 0.0009). Furthermore, although the associations did not reach statistical significance, patients with the MKS1 rs199910690 allele showed the highest proportions of obesity (BMI > 27 kg/m2), hyperlipidemia, hypertension, diabetes mellitus, diabetes mellitus comorbidity, CKD, renal cancer, and CAD. Therefore, in Table 5, we subsequently conducted a comparison of the BMI levels between the single variant and the control group, respectively. The variant rs199910690 in MKS1 revealed an increased risk of overweight and obesity. Regarding the mean values of the biochemistry data, no significant differences were observed.
Comparisons of comorbidities among each of the BBS risk alleles.
| Variables | rs773862084 (N=256) | rs567573386 (N=30) | rs199910690 (N=428) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||||
| Age, years (mean/SD)a | 59.1 | 15.71 | 57.63 | 15.11% | 59.07 | 15.85 | 0.8852 | |||
| Gender (n, %)b | 0.582 | |||||||||
| Female | 131 | 51.17 | 18 | 60 | 231 | 53.97 | ||||
| Male | 125 | 48.83 | 12 | 40 | 197 | 46.03 | ||||
| BMI (n, %)b | 0.2507 | |||||||||
| BMI<24 kg/m2 | 111 | 47.23 | 18 | 60 | 163 | 41.69 | ||||
| 24≦BMI≦27 kg/m2 | 57 | 24.26 | 7 | 23.33 | 107 | 27.37 | ||||
| BMI>27 kg/m2 | 67 | 28.51 | 5 | 16.67 | 121 | 30.95 | ||||
| Comorbidities (n, %)b | ||||||||||
| Hyperlipidemia | 0.1283 | |||||||||
| No | 180 | 70.31 | 24 | 80 | 279 | 65.19 | ||||
| Yes | 76 | 29.69 | 6 | 20 | 149 | 34.81 | ||||
| Hypertension | 0.0978 | |||||||||
| No | 193 | 75.39 | 26 | 86.67 | 303 | 70.79 | ||||
| Yes | 63 | 24.61 | 4 | 13.33 | 125 | 29.21 | ||||
| Obesity | 0.3842 | |||||||||
| No | 254 | 99.22 | 29 | 96.67 | 420 | 98.13 | ||||
| Yes | 2 | 0.78 | 1 | 3.33 | 8 | 1.87 | ||||
| DM | 0.4185 | |||||||||
| No | 195 | 76.17 | 25 | 83.33 | 315 | 73.6 | ||||
| Yes | 61 | 23.83 | 5 | 16.67 | 113 | 26.4 | ||||
| DM comorbidity* | 0.8593 | |||||||||
| No | 246 | 96.09 | 29 | 96.67 | 408 | 95.33 | ||||
| Yes | 10 | 3.91 | 1 | 3.33 | 20 | 4.67 | ||||
| CKD | 0.9609 | |||||||||
| No | 208 | 81.25 | 25 | 83.33 | 348 | 81.31 | ||||
| Yes | 48 | 18.75 | 5 | 16.67 | 80 | 18.69 | ||||
| Renal cancer | c0.7994 | |||||||||
| No | 254 | 99.22 | 30 | 100 | 422 | 98.6 | ||||
| Yes | 2 | 0.78 | 0 | 0 | 6 | 1.4 | ||||
| AMI | c0.3959 | |||||||||
| No | 252 | 98.44 | 29 | 96.67 | 423 | 98.83 | ||||
| Yes | 4 | 1.56 | 1 | 3.33 | 5 | 1.17 | ||||
| CAD | 0.1517 | |||||||||
| No | 224 | 87.5 | 26 | 86.67 | 351 | 82.01 | ||||
| Yes | 32 | 12.5 | 4 | 13.33 | 77 | 17.99 | ||||
| CVA | 0.0009 | |||||||||
| No | 241 | 94.14 | 29 | 96.67 | 366 | 85.51 | ||||
| Yes | 15 | 5.86 | 1 | 3.33 | 62 | 14.49 | ||||
a Continuous variables were expressed as mean ± standard deviation (SD) and used ANOVA test for continuous variables.
b Categorical variables were expressed as numbers (percent) and were analyzed using Chi-square test for categorical variables.
c Using Fisher's exact test.
Excluded 2 participants with two variants
*DM comorbidity with retinopathy, neuropathy, or chronic kidney disease
Abbreviations: BBS: Bardet-Biedl syndrome; BMI: Body mass index; DM: diabetes mellitus; CKD: chronic kidney disease; AMI: acute myocardial infarction; CAD: coronary artery disease; CVA: cerebrovascular accident.
BMI associated with genetic variants
In order to examine the specific risk factors for each comorbidity based on BMI, we performed univariate analyses for hyperlipidemia, DM, hypertension (refer to Supplementary Tables 1-3), and CKD (Table 6). As shown in Supplementary Tables 1-3, it can be seen that males have a higher risk of developing diseases when stratified by BMI, as compared to females. The study revealed a significant reduction in the risk of hyperlipidemia, DM and hypertension among individuals carrying the rs773862084 alleles when BMI was <24 kg/m2. When stratified by BMI, all three genetic variants demonstrated protective associations with each comorbidity, except for coronary artery disease (CAD). In Table 6, the BBS2 rs773862084 CA/AA genotype demonstrated a lower risk of CKD compared to the CC genotype, with an OR of 0.45 (95% CI: 0.325-0.615, p < 0.0001). Similarly, the MKS1 rs199910690 CT/TT genotype was also associated with a reduced risk of CKD when compared to the CC genotype, with an OR of 0.43 (95% CI: 0.331-0.549, p < 0.0001).
BMI and genetic variants in different genders
We further divided participants according to different gender and BMI levels, as presented in Table 7a and Table 7b. We conducted a multivariate logistic regression analysis, to explore the relationship between genetic variants, BMI levels, and CKD. Additionally, potential confounding factors, such as diabetes mellitus (DM) and hyperlipidemia, were adjusted for in the analysis. Our findings found a statistically significant association indicating protection against the development of CKD in both males and females who carry either the BBS2 rs773862084 or MKS1 rs199910690 variant, when compared to their respective counterparts.
Comparisons of serology among each of the BBS risk alleles.
| Variables | rs773862084 (N=256) | rs567573386 (N=30) | rs199910690 (N=428) | P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||||
| Biochemistry | ||||||||||
| LDL (mg/dL) | 118.07 | 36.1 | 124.93 | 54.78 | 117.89 | 36.09 | 0.7707 | |||
| HDL (mg/dL) | 52.11 | 15.05 | 54.89 | 14.09 | 52.89 | 16.42 | 0.853 | |||
| Triglyceride (mg/dL) | 139.64 | 112.9 | 128.47 | 121.41 | 137.32 | 92.9 | 0.8954 | |||
| Total cholesterol (mg/dL) | 190.24 | 42.51 | 192.88 | 76.56 | 192.06 | 41.77 | 0.9198 | |||
| Uric acid (mg/dL) | 6.4 | 1.99 | 6.25 | 2.04 | 6.37 | 2.04 | 0.9625 | |||
| Fasting glucose (mg/dL) | 117.34 | 40.03 | 101.21 | 27.94 | 117.29 | 43.48 | 0.2628 | |||
| HbA1c (%) | 6.54 | 1.82 | 6.22 | 1.55 | 6.61 | 1.67 | 0.6458 | |||
| Insulin (uIU/mL) | 29.69 | 38.84 | - | - | 51.29 | 133.25 | 0.7907 | |||
| Creatinine (mg/dL) | 1.33 | 2.15 | 0.92 | 0.31 | 1.11 | 1.37 | 0.443 | |||
| ALT (U/L) | 35.92 | 54.15 | 31.41 | 21.85 | 30.47 | 26.43 | 0.2485 | |||
| AST (U/L) | 33.22 | 55.44 | 36.58 | 29.64 | 29.17 | 37.81 | 0.5579 | |||
a Using ANOVA test for continuous variables.
Excluded 2 participants with two variants
Abbreviations: BBS: Bardet-Biedl syndrome; LDL: low density lipoprotein; HDL: high density lipoprotein; HbA1c: glycosylated hemoglobin; eGFR: estimated glomerular filtration rate; ALT: alanine aminotransferase; AST: aspartate transferase.
Comparisons of the BMI level between carrier and non-carrier group, with variant BBS2 rs773862084 and MKS1 rs199910690 respectively.
| Variables | BBS2 rs773862084 (N=256) | Non-carrier (n=2864) | P value | MKS1 rs199910690 (N=428) | Non-carrier (n=2864) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||||
| BMI<24 kg/m2 | 111 | 47.23 | 1305 | 48.39 | 0.166 | 163 | 41.69 | 1305 | 48.39 | 0.0037 | |
| 24≦BMI≦27 kg/m2 | 57 | 24.26 | 760 | 28.18 | 107 | 27.37 | 760 | 28.18 | |||
| BMI>27 kg/m2 | 67 | 28.51 | 632 | 23.43 | 121 | 30.95 | 632 | 23.43 | |||
Abbreviations: BMI: Body mass index
Stratification by BMI and CKD.
| Variables | CKD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI<24 kg/m2 | 24≦BMI≦27 kg/m2 | BMI>27 kg/m2 | Overall | |||||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P valuea | |
| Age, years | ||||||||||||
| Gender | ||||||||||||
| Female | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Male | 1.92 | 1.549-2.386 | <0.0001 | 1.56 | 1.183-2.063 | 0.0017 | 1.49 | 1.106-1.995 | 0.0086 | 1.69 | 1.469-1.944 | <0.0001 |
| rs773862084 | ||||||||||||
| CC | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| CA / AA | 0.52 | 0.352-0.823 | 0.0053 | 0.43 | 0.221-0.846 | 0.0144 | 0.35 | 0.184-0.662 | 0.0013 | 0.45 | 0.325-0.615 | <0.0001 |
| rs567573386 | ||||||||||||
| GG | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| GA / AA | 0.24 | 0.056-1.066 | 0.0608 | 0.76 | 0.146-3.913 | 0.7378 | 0.43 | 0.048-3.895 | 0.4554 | 0.40 | 0.152-1.042 | 0.0607 |
| rs199910690 | ||||||||||||
| CC | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| CT / TT | 0.49 | 0.327-0.718 | 0.0003 | 0.35 | 0.204-0.584 | <0.0001 | 0.34 | 0.207-0.547 | <0.0001 | 0.43 | 0.331-0.549 | <0.0001 |
a Comparisons of categorical variables were analyzed using logistic regression adjusted by age and gender.
Abbreviations: CI: confidence interval; OR: odds ratio; BMI: Body mass index; CKD: chronic kidney disease
Specifically, among participants with BMI > 27 kg/m2, obese males carrying the MKS1 rs199910690 variant exhibited a negative association with the development of CKD (OR: 0.25, 95% CI: 0.123-0.523, p = 0.0002). Similarly, obese females carrying the BBS2 rs773862084 variant had a lower risk of developing CKD (OR: 0.09, 95% CI: 0.012-0.073, p = 0.0239).
Furthermore, in Supplementary Tables 4 and 5, these obese females carrying the BBS2 rs773862084 variant also showed a significant inverse association with development of hyperlipidemia (OR: 0.34, 95% CI: 0.113 - 0.994, p = 0.0487). For men with various BMI levels, the difference in developing hyperlipidemia was not statistically significant. As for developing diabetes mellitus (DM), presented in Supplementary Tables 7 and 8, we observed a statistically significant protective association in males who carried the MKS1 rs199910690 variant, indicating a protective effect. However, when considering different BMI levels and genders, we did not find any statistically significant associations with the development of diabetes mellitus (DM).
Stratification by BMI and CKD in females.
| Variables | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI<24 kg/m2 | 24≦BMI≦27 kg/m2 | BMI>27 kg/m2 | Overall | |||||||||
| ORb | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P valuea | |
| rs773862084 | ||||||||||||
| CC | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| CA / AA | 0.46 | 0.218-0.949 | 0.0358 | 0.37 | 0.119-1.163 | 0.089 | 0.09 | 0.012-0.730 | 0.0239 | 0.38 | 0.218-0.654 | 0.0005 |
| Age, years | 1.02 | 1.008-1.028 | 0.0003 | 1.02 | 0.999-1.035 | 0.0697 | 1.03 | 1.015-1.053 | 0.0004 | 1.02 | 1.012-1.028 | <0.0001 |
| DM | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 1.19 | 0.829-1.709 | 0.3451 | 1.11 | 0.658-1.857 | 0.7041 | 1.16 | 0.664-2.041 | 0.5963 | 1.15 | 0.891-1.472 | 0.2907 |
| hyperlipidemia | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 2.43 | 1.769-2.333 | <0.0001 | 3.91 | 2.377-6.419 | <0.0001 | 2.15 | 1.221-3.789 | 0.0081 | 2.70 | 2.131-3.411 | <0.0001 |
| rs199910690 | ||||||||||||
| CC | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| CT / TT | 0.45 | 0.266-0.769 | 0.0034 | 0.22 | 0.073-0.639 | 0.0056 | 0.53 | 0.258-1.099 | 0.0884 | 0.45 | 0.308-0.657 | <0.0001 |
| Age, years | 1.02 | 1.008-1.028 | 0.0004 | 1.02 | 0.998-1.035 | 0.0772 | 1.04 | 1.016-1.054 | 0.0002 | 1.02 | 1.013-1.028 | <0.0001 |
| DM | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 1.18 | 0.824-1.700 | 0.362 | 1.11 | 0.657-1.882 | 0.6938 | 1.10 | 0.629-1.924 | 0.7367 | 1.14 | 0.887-1.469 | 0.3023 |
| hyperlipidemia | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 2.52 | 1.832-3.453 | <0.0001 | 3.85 | 2.326-6.377 | <0.0001 | 2.30 | 1.309-4.030 | 0.0037 | 2.79 | 2.204-3.532 | <0.0001 |
a Comparisons of categorical variables were analyzed using logistic regression.
b OR was adjusted for all variables in the table.
Abbreviations: CI: confidence interval; OR: odds ratio; BMI: Body mass index; CKD: chronic kidney disease; DM: diabetes mellitus.
Stratification by BMI and CKD in males.
| Variables | Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI<24 kg/m2 | 24≦BMI≦27 kg/m2 | BMI>27 kg/m2 | Overall | |||||||||
| ORb | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P valuea | |
| rs773862084 | ||||||||||||
| CC | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| CA / AA | 0.54 | 0.271-1.075 | 0.0794 | 0.43 | 0.176-1.04 | 0.0609 | 0.51 | 0.239-1.086 | 0.0809 | 0.50 | 0.324-0.772 | 0.0018 |
| Age, years | 1.02 | 1.008-1.032 | 0.0014 | 1.03 | 1.015-1.043 | <0.0001 | 1.04 | 1.020-1.050 | <0.0001 | 1.03 | 1.02-1.035 | <0.0001 |
| DM | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 1.23 | 0.812-1.851 | 0.3326 | 1.45 | 0.987-2.141 | 0.058 | 1.86 | 1.231-2.806 | 0.0032 | 1.53 | 1.219-1.913 | 0.0002 |
| hyperlipidemia | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 3.21 | 2.167-4.745 | <0.0001 | 1.83 | 1.248-2.681 | 0.002 | 1.84 | 1.219-2.779 | 0.0037 | 2.24 | 1.794-2.796 | <0.0001 |
| rs199910690 | ||||||||||||
| CC | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| CT / TT | 0.53 | 0.268-1.062 | 0.0737 | 0.40 | 0.211-0.762 | 0.0053 | 0.25 | 0.123-0.523 | 0.0002 | 0.38 | 0.262-0.550 | <0.0001 |
| Age, years | 1.02 | 1.007-1.032 | 0.0016 | 1.03 | 1.015-1.043 | <0.0001 | 1.04 | 1.020-1.051 | <0.0001 | 1.03 | 1.020-1.035 | <0.0001 |
| DM | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 1.24 | 0.818-1.872 | 0.312 | 1.46 | 0.990-2.154 | 0.0563 | 1.72 | 1.128-2.609 | 0.0117 | 1.50 | 1.196-1.884 | 0.0005 |
| hyperlipidemia | ||||||||||||
| No | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ | 1.00 | ─ | ─ |
| Yes | 3.16 | 2.135-4.683 | <0.0001 | 1.84 | 1.252-2.702 | 0.0019 | 1.82 | 1.195-2.757 | 0.0052 | 2.25 | 1.800-2.815 | <0.0001 |
a Comparisons of categorical variables were analyzed using logistic regression.
b OR was adjusted for all variables in the table.
Abbreviations: CI: confidence interval; OR: odds ratio; BMI: Body mass index; CKD: chronic kidney disease; DM: diabetes mellitus.
Discussion
Our results demonstrate that BBS heterozygous carriers had increased BMI levels, particularly in individuals with the variant rs199910690 in MKS1. In this study, we investigated the distinct genetic risk variants for CKD, stratified by BMI levels. Our findings revealed that individuals carrying the CA/AA genotype of the BBS2 rs773862084 variant or the CT/TT genotype of the MKS1 rs199910690 variant did not exhibit an increased risk of developing CKD, irrespective of their BMI level. Moreover, among participants classified as obese (BMI > 27 kg/m²), males with the MKS1 rs199910690 variant and females with the BBS2 rs773862084 variant displayed a negative association with the development of CKD. Our dataset focused on the phenotype-genotype relationship of BBS heterozygous carriers in a hospital-based case-control study conducted in Taiwan. Notably, this study is the first to compare these associations within an Asian population. We sincerely hope that these results can be employed in the future to provide enhanced health guidance for individuals with the known variants, thereby offering valuable insights from a disease prevention perspective.
As is widely known, obesity is a prevalent manifestation in BBS patients, with approximately 89% of diagnosed individuals noted to have obesity[9]. For example, in individuals with BBS1, the prevalence of obesity is significantly higher compared to both carriers and non-carriers of the BBS1 gene[18]. Despite mostly having normal birth body weight, BBS patients experience rapid body weight gain during childhood. Among children and adolescents aged 2 to 19 years who were enrolled in the Clinical Registry Investigating Bardet-Biedl Syndrome (CRIBBS), the prevalence of overweight (BMI > 25 kg/m2) or obesity (BMI > 30 kg/m2) in BBS patients ranges from 86% to 95%[19]. With respect to adults, in a study by Mujahid et al., the average BMI of 152 BBS patients aged 16 to 58 years in United Kingdom was 35.7 ± 7.8 kg/m2[12]. The Asian-specific BMI cutoffs for overweight and obesity was relatively low in comparison to White adults. Compared to individuals of the same age, sex, and BMI among white populations, Asians typically exhibit a higher percentage of body fat. Asians are known to have elevated risks of developing type 2 diabetes and cardiovascular disease, even when their BMI is below the WHO-defined cutoff point of 25 kg/m² for overweight [20, 21]. As suggested by W. H. Pan et al.[22], for Taiwanese individuals, a BMI of 23.4 could be the threshold for optimizing both positive and negative predictive values for comorbidities. The BBS proteins, namely, ciliary‑related proteins, play a crucial role in ciliary function by directing vesicle trafficking to the ciliary membrane. When cilia dysfunction occurs, it can lead to hyperleptinemia and leptin resistance. The disruption in leptin action further hampers the proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus. This is followed by the inactivation of the melanocortin-4 receptor (MC4R), which results in hyperphagia (increased appetite) and ultimately contributes to obesity[23]. Patients with BBS were known to have hyperleptinemia with leptin resistance compared with individuals without BBS[24]. Benzinou et al. reported that variants in BBS2, BBS4, and BBS6 showed evidence of association with common obesity in French Caucasians[14]. In a study by Day et al., rs59252892 in BBS9 exhibited a statistically significant association with obesity. It has been hypothesized that these BBS9 variants may lead to a subtle increase in the shuttling of LEPR (leptin receptor), resulting in leptin resistance[25]. Therefore, obesity is recognized as a hallmark of BBS. Individuals with BBS also encounter the challenges associated with metabolic syndrome, that is, obesity related complications including hyperlipidemia, insulin resistance and fasting hyperglycemia[9]. In a study by Mujahid et al., the prevalence of metabolic syndrome among BBS patients was 54.3%[12]. For individuals with BBS, an annual assessment of fasting blood glucose, HbA1c, and lipid levels (triglycerides, HDL-C, LDL-C, and total cholesterol) is recommended. Additionally, for better quality of life, reduced-calorie diet and lifestyle changes such as regular exercise program are suggested[8]. For pharmaceutical management, it is noteworthy that setmelanotide, a melanocortin-4 receptor (MC4R) agonist, was the first drug approved by the United States Food and Drug Administration (FDA). It functions by binding to the melanocortin-4 receptor (MC4R), thereby reducing appetite and increasing energy expenditure. It is specifically indicated for the treatment of obesity resulting from genetic disorders such as proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), and leptin receptor (LEPR) deficiency[26, 27]. In the clinical trial by Forsythe et al.[28], individuals with BBS and obesity experienced a significant reduction in BMI levels and an improvement in health-related quality of life after 52 weeks of setmelanotide therapy. However, the efficacy of setmelanotide in patients with heterozygous variants remains inconclusive, as ongoing clinical trials focusing on heterozygous participants are underway[29-31]. Adverse effects associated with setmelanotide treatment included injection site reactions, skin hyperpigmentation, sexual dysfunction, and psychological issues such as depression and suicidal ideation[27]. In a study by Robert et al.[32], all BBS participants who received setmelanotide treatment experienced injection site reactions, with eighty percent developing hyperpigmentation.
In our study, we verified that adult individuals who were BBS heterozygous carriers exhibited higher percentages of elevated BMI level compared to those without BBS variants, which is consistent with findings from previous studies[13, 14]. With regard to the risk allele in the BBS group, more specifically in our study, the variant rs199910690 in MKS1 showed evidence of associations with overweight and obesity. Conversely, our investigation did not reveal any significant links between individuals carrying rs773862084 variants in BBS2 and overweight or obesity. This outcome contrasts with the findings reported by Benzinou et al., which suggested an association between BBS2 and obesity. We postulate that this difference might be attributed to the relatively limited number of participants in our study or the presence of diverse SNPs encoding proteins with varying functions. Furthermore, concerning BBS heterozygous carriers, prior reports have indicated that the incidence rates of hypertension and diabetes were similar when comparing carriers and non-carriers[15]. Our study aligns with these findings, as it revealed no significant disparities in the risk of metabolic diseases and comorbidities, which include hyperlipidemia, hypertension, diabetes mellitus, diabetes mellitus comorbidity, chronic kidney disease (CKD), acute myocardial infarction (AMI), coronary artery disease (CAD), and cerebrovascular accident (CVA). Furthermore, it is noteworthy that our results were not solely concerned with the risk of developing the disease, but our biochemical analysis also yielded consistent outcomes. This implies that the lipid levels and blood sugar homeostasis in BBS heterozygous carriers were comparable to those of the control groups.
Kidney disease is also a major features of BBS, encompassing structural anomalies, urologic complications, and chronic kidney disease (CKD). The prevalence of kidney disease in BBS is 52%, with chronic kidney disease (CKD) being a prominent contributor to both morbidity and mortality in individuals with BBS[9]. Several studies have explored the genotype‐phenotype analysis in Bardet‐Biedl syndrome. In a study by Niederlova et al., a strong correlation between genotype and renal anomalies was observed among individuals with BBS. Specifically, patients carrying mutations in the core BBSome subunits, such as BBS2, BBS7, or BBS9, exhibited a relatively high frequency of renal anomalies[33]. Forsythe et al. demonstrated that mutations in BBS2, BBS10, and BBS12 were more likely to be associated with severe renal disease. Among these, patients with BBS10 mutations were more frequently observed as the stage of CKD increased. The study further provided valuable findings insights that CKD4-5 mainly develops during childhood; otherwise, individuals in adulthood have a comparatively lower risk of experiencing severe renal disease[11]. Being ciliopathies, the renal impairment in BBS primarily arises from tubular ciliary dysfunction. This dysfunction disrupts the mammalian target of rapamycin(mTOR) signaling pathway and eventually contributes to the development of cystic kidney disease[10, 11, 34, 35]. Of note, a significant association was found between renal ciliary dysfunction and CKD. Using electron microscopy, Imhoff et al. also observed thickening of the basement membrane in BBS patients, which subsequently led to hypertension or diabetes[36]. There was no consensus regarding the risk of renal disorders in heterozygous carriers when compared to the general population. Concerning renal cancer differing opinions exist. A study by Beales et al. reported 3 cases of renal cancer among 180 parents of BBS patients, implying a 17-fold increased risk compared to the general population[37]. However, Hjortshøj et al. surveyed 428 relatives of BBS patients and found no elevated risk of renal cancer[38]. Our results also indicated no increased risk of developing renal cancer. Individuals carrying a single mutation variant of Bardet-Biedl syndrome, known as obligate carriers, were not prone to having renal function impairment. As previously noted by MP Webb et al., no significant difference in the incidence of stage-3 CKD was observed when comparing carriers and non-carriers, which was consistent with our results[15]. We demonstrated that the renal function of individuals with BBS variants was not inferior to that of the control group. Additionally, we established that the risk of CKD in heterozygous carriers with the BBS2 rs773862084 CA/AA genotype or the MKS1 rs199910690 CT/TT genotype was not higher compared to non-carriers, regardless of the BMI level. Obesity is a well-documented risk factor for chronic kidney diseases[39]. Overweight persons (25≤BMI<30) had an elevated risk for chronic kidney diseases, and persons with higher BMI (>30) had an even higher risk[40]. The findings were similar to those found in a Taiwanese population[41]. Renal hyperfiltration can occur in overweight or obese patients, resulting in an overestimation of renal function test with serum creatinine or cystatin C[42]. According to findings reported by Basolo et al., obese individuals were observed to have glomerular hyperfiltration, subsequently leading to an elevated creatinine clearance[43]. In our study, where we identified a risk of developing overweight and obesity in the BBS heterozygous carriers group, we also noted that the creatinine levels in the carriers' group were lower than those in the non-carriers' group, with significant differences observed. Nevertheless, renal hyperfiltration can only partially explain our finding that BBS heterozygous carriers have lower serum creatinine level and a lower rate of CKD. Whether partial mutation in BBSome subunits can lead to renal protection requires further research using experimental cell or animal models.
There were some limitations in our study. First, certain detailed information was not recorded in the study. There were several genes identified for obesity which could be classified as monogenic obesity and polygenic obesity[44]. Lifestyle and dietary habits are known to contribute to obesity as well. We were unable to comprehensively evaluate the potential confounders. Regarding the renal function evaluation, we did not conduct a complete assessment of renal structural anomalies and the severity of proteinuria in our study. Second, it is possible that we underestimated the impact of genetic variants on the BBS heterozygous carriers because of the selection bias inherent in hospital-based research. Third, the enrolled participants were exclusively East Asians, and therefore our conclusions may not be applicable to genetic studies involving other ethnic groups. Lastly, due to the rarity of BBS, the allele frequency remained relatively low, ranging from 1/250 to 1/2200[45], resulting in a low number of participants in our study. Longer-term prospective research is required to further confirm the consistent effect of the genetic variant on the general population. We recommend initiating a thorough management and monitoring approach for disease prevention as it could benefit BBS heterozygous carriers.
Conclusion
Our study revealed that individuals carrying heterozygous mutations in the MKS1 gene, specifically the rs199910690 variant associated with Bardet-Biedl syndrome, had an elevated risk of overweight and obesity. However, regardless of their body mass index (BMI), individuals possessing the CA/AA genotype for BBS2 rs773862084 or the CT/TT genotype for MKS1 rs199910690 did not exhibit an increased risk of developing chronic kidney disease (CKD). Furthermore, within the subset of participants with a BMI of > 27 kg/m², males carrying the MKS1 rs199910690 variant and females carrying the BBS2 rs773862084 variant demonstrated a negative correlation with the development of CKD. These findings underscore the need for further research to comprehensively explore the genetic susceptibility factors associated with Bardet-Biedl syndrome.
Supplementary Material
Supplementary tables.
Acknowledgements
We thank all the participants and investigators from the Taiwan Precision Medicine Initiative.
Funding
This study was funded by Academia Sinica 40-05-GMM and AS-GC-110-MD02, National Science and Technology Council, Taiwan [NSTC-111-2634-F-A49-014, NSTC-111-2218-E-039-001, and NSTC-111-2314-B-075A-003-MY3], and Taichung Veterans General Hospital, Taiwan [TCVGH-1127301C, TCVGH-1127302D, TCVGH-YM1120110, and TCVGH-1127304B].
Data sharing statement
All data used in this study are available in this article.
Author contributions
Research idea and study design: MHL and ICC; data acquisition: MHL, ICC, and HWY; data analysis/interpretation: ICC, HWY, HCY, YCH, CCH, YMC and YYK; statistical analysis: ICC and HWY; supervision or mentorship: HCY, YCH, CCH, YMC, and YYK. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual's own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Melluso A, Secondulfo F, Capolongo G, Capasso G, Zacchia M. Bardet-Biedl Syndrome: Current Perspectives and Clinical Outlook. Ther Clin Risk Manag. 2023;19:115-32
2. Florea L, Caba L, Gorduza EV. Bardet-Biedl Syndrome-Multiple Kaleidoscope Images: Insight into Mechanisms of Genotype-Phenotype Correlations. Genes (Basel). 2021 12
3. Guardiola GA, Ramos F, Izquierdo NJ, Oliver AL. A Genotype-Phenotype Analysis of the Bardet-Biedl Syndrome in Puerto Rico. Clin Ophthalmol. 2021;15:3757-64
4. Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA. et al. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A. 2005;132A:352-60
5. Farag TI, Teebi AS. High incidence of Bardet Biedl syndrome among the Bedouin. Clinical Genetics. 1989;36:463-5
6. P L Beales, A M Warner, G A Hitman, R Thakker, Flinter FA. Bardet-Biedl syndrome: a molecular and phenotypic study of 18 families. Journal of Medical Genetics. 1997;34:92-8
7. Rao AR, Nazir A, Imtiaz S, Paracha SA, Waryah YM, Ujjan ID. et al. Delineating the Spectrum of Genetic Variants Associated with Bardet-Biedl Syndrome in Consanguineous Pakistani Pedigrees. Genes (Basel). 2023 14
8. Caba L, Florea L, Braha EE, Lupu VV, Gorduza EV. Monitoring and Management of Bardet-Biedl Syndrome: What the Multi-Disciplinary Team Can Do. J Multidiscip Healthc. 2022;15:2153-67
9. Forsyth R, Gunay-Aygun M. Bardet-Biedl Syndrome Overview. GeneReviews. 2023
10. Priya S, Nampoothiri S, Sen P, Sripriya S. Bardet-Biedl syndrome: Genetics, molecular pathophysiology, and disease management. Indian J Ophthalmol. 2016;64:620-7
11. Forsythe E, Sparks K, Best S, Borrows S, Hoskins B, Sabir A. et al. Risk Factors for Severe Renal Disease in Bardet-Biedl Syndrome. J Am Soc Nephrol. 2017;28:963-70
12. Mujahid S, Hunt KF, Cheah YS, Forsythe E, Hazlehurst JM, Sparks K. et al. The Endocrine and Metabolic Characteristics of a Large Bardet-Biedl Syndrome Clinic Population. J Clin Endocrinol Metab. 2018;103:1834-41
13. Croft JB, Morrell D, Chase CL, Swift M. Obesity in Heterozygous Carriers of the Gene for the Bardet-Biedl Syndrome. American Journal of Medical Genetics. 1995;55:12-5
14. Benzinou M, Walley A, Lobbens S, Charles MA, Jouret B, Fumeron F. et al. Bardet-Biedl syndrome gene variants are associated with both childhood and adult common obesity in French Caucasians. Diabetes. 2006;55:2876-82
15. Webb MP, Dicks EL, Green JS, Moore SJ, Warden GM, Gamberg JS. et al. Autosomal recessive Bardet-Biedl syndrome: first-degree relatives have no predisposition to metabolic and renal disorders. Kidney Int. 2009;76:215-23
16. Wei CY, Yang JH, Yeh EC, Tsai MF, Kao HJ, Lo CZ. et al. Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom Med. 2021;6:10
17. Sun TH, Shao YJ, Mao CL, Hung MN, Lo YY, Ko TM, Hsiao TH. A Novel Quality-Control Procedure to Improve the Accuracy of Rare Variant Calling in SNP Arrays. Front Genet. 2021;12:736390
18. Fan Y, Rahman P, Peddle L, Hefferton D, Gladney N, Moore SJ. et al. Bardet-Biedl syndrome 1 genotype and obesity in the Newfoundland population. Int J Obes Relat Metab Disord. 2004;28:680-4
19. Pomeroy J, Krentz AD, Richardson JG, Berg RL, VanWormer JJ, Haws RM. Bardet-Biedl syndrome: Weight patterns and genetics in a rare obesity syndrome. Pediatr Obes. 2021;16:e12703
20. Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-63
21. Hsieh TH, Lee JJ, Yu EW, Hu HY, Lin SY, Ho CY. Association between obesity and education level among the elderly in Taipei, Taiwan between 2013 and 2015: a cross-sectional study. Sci Rep. 2020;10:20285
22. Pan WH, Lee MS, Chuang SY, Lin YC, Fu ML. Obesity pandemic, correlated factors and guidelines to define, screen and manage obesity in Taiwan. Obes Rev. 2008;9(Suppl 1):22-31
23. Guo DF, Rahmouni K. Molecular basis of the obesity associated with Bardet-Biedl syndrome. Trends Endocrinol Metab. 2011;22:286-93
24. Feuillan PP, Ng D, Han JC, Sapp JC, Wetsch K, Spaulding E. et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J Clin Endocrinol Metab. 2011;96:E528-35
25. Day SE, Muller YL, Koroglu C, Kobes S, Wiedrich K, Mahkee D. et al. Exome Sequencing of 21 Bardet-Biedl Syndrome (BBS) Genes to Identify Obesity Variants in 6,851 American Indians. Obesity (Silver Spring). 2021;29:748-54
26. Markham A. Setmelanotide: First Approval. Drugs. 2021;81:397-403
27. Hussain A, Farzam K. Setmelanotide - StatPearls - NCBI Bookshelf. StatPearls. 2023
28. Forsythe E, Haws RM, Argente J, Beales P, Martos-Moreno GA, Dollfus H. et al. Quality of life improvements following one year of setmelanotide in children and adult patients with Bardet-Biedl syndrome: phase 3 trial results. Orphanet J Rare Dis. 2023;18:12
29. Le Collen L DB, Poitou C, Vaxillaire M, Toussaint B, Dechaume A, Badreddine A, Boissel M, Derhourhi M, Clément K, Petit JM, Mau-Them FT, Bruel AL, Thauvin-Robinet C, Saveanu A, Cherifi BG, Le Beyec-Le Bihan J, Froguel P, Bonnefond A. Heterozygous pathogenic variants in POMC are not responsible for monogenic obesity Implication for MC4R agonist use. Genet Med. 2023
30. Voigtmann F, Wolf P, Landgraf K, Stein R, Kratzsch J, Schmitz S. et al. Identification of a novel leptin receptor (LEPR) variant and proof of functional relevance directing treatment decisions in patients with morbid obesity. Metabolism. 2021;116:154438
31. Clement K, Mosbah H, Poitou C. Rare genetic forms of obesity: From gene to therapy. Physiol Behav. 2020;227:113134
32. Haws R, Brady S, Davis E, Fletty K, Yuan G, Gordon G. et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020;22:2133-40
33. Niederlova V, Modrak M, Tsyklauri O, Huranova M, Stepanek O. Meta-analysis of genotype-phenotype associations in Bardet-Biedl syndrome uncovers differences among causative genes. Hum Mutat. 2019;40:2068-87
34. Tobin JL, Beales PL. Restoration of renal function in zebrafish models of ciliopathies. Pediatr Nephrol. 2008;23:2095-9
35. Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013;21:8-13
36. Imhoff O, Marion V, Stoetzel C, Durand M, Holder M, Sigaudy S. et al. Bardet-Biedl syndrome: a study of the renal and cardiovascular phenotypes in a French cohort. Clin J Am Soc Nephrol. 2011;6:22-9
37. P L Beales, H A Reid, M H Griffiths, E R Maher, F A Flinter, Woolf AS. Renal cancer and malformations in relatives of patients with Bardet-Biedl syndrome. Nephrology Dialysis Transplantation. 2000;15:1977-85
38. Hjortshoj TD, Gronskov K, Rosenberg T, Brondum-Nielsen K, Olsen JH. Risk for cancer in patients with Bardet-Biedl syndrome and their relatives. Am J Med Genet A. 2007;143A:1699-702
39. Kovesdy CP, Furth SL, Zoccali C, World Kidney Day Steering C. Obesity and Kidney Disease: Hidden Consequences of the Epidemic. Can J Kidney Health Dis. 2017;4:2054358117698669
40. Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19-33
41. Lai YJ, Hu HY, Lee YL, Ku PW, Yen YF, Chu D. Association between obesity and risk of chronic kidney disease: A nationwide Cohort study in Taiwan. Nutr Metab Cardiovasc Dis. 2017;27:1008-14
42. Stefansson VT, Schei J, Jenssen TG, Melsom T, Eriksen BO. Central obesity associates with renal hyperfiltration in the non-diabetic general population: a cross-sectional study. BMC Nephrol. 2016;17:172
43. Basolo A, Salvetti G, Giannese D, Genzano SB, Ceccarini G, Giannini R. et al. Obesity, hyperfiltration and early kidney damage: a new formula for the estimation of creatinine clearance. J Clin Endocrinol Metab. 2023
44. Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120-33
45. Sapp JC, Nishimura D, Johnston JJ, Stone EM, Héon E, Sheffield VC, Biesecker LG. Recurrence risks for Bardet-Biedl syndrome: Implications of locus heterogeneity. Genetics in Medicine. 2010;12:623-7
Author contact
![]() Corresponding authors: Yi-Ming Chen, M.D., Ph.D., Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung, Taiwan Veterans General Hospital, 1650, Section 4, Taiwan Boulevard, Xitun Dist., Taichung City 40705, Taiwan; Tel.: +886-4-2359-2525 ext. 3354; Fax: +886-4-2350-3285; E-mail: ymchen1gov.tw. Yu-Yuan Ke, M.D., Division of Pediatric Genetics and Metabolism, Children's Medical Center, Taichung Veterans General Hospital, Taichung, Taiwan; Veterans General Hospital, 1650, Section 4, Taiwan Boulevard, Xitun Dist., Taichung City 40705, Taiwan; Tel.: +886-4-2359-2525 ext. 5921; Fax: +886-4-2359-5046; E-mail: yuyuankegov.tw.
Corresponding authors: Yi-Ming Chen, M.D., Ph.D., Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung, Taiwan Veterans General Hospital, 1650, Section 4, Taiwan Boulevard, Xitun Dist., Taichung City 40705, Taiwan; Tel.: +886-4-2359-2525 ext. 3354; Fax: +886-4-2350-3285; E-mail: ymchen1gov.tw. Yu-Yuan Ke, M.D., Division of Pediatric Genetics and Metabolism, Children's Medical Center, Taichung Veterans General Hospital, Taichung, Taiwan; Veterans General Hospital, 1650, Section 4, Taiwan Boulevard, Xitun Dist., Taichung City 40705, Taiwan; Tel.: +886-4-2359-2525 ext. 5921; Fax: +886-4-2359-5046; E-mail: yuyuankegov.tw.

 Global reach, higher impact
Global reach, higher impact