ISSN: 1449-1907International Journal of Medical Sciences
Int J Med Sci 2024; 21(4):755-764. doi:10.7150/ijms.93171 This issue Cite
Research Paper
Phosphoglycerate mutase 5 aggravates alcoholic liver disease through disrupting VDAC-1-dependent mitochondrial integrity
1. Chinese PLA General Hospital, Medical School of Chinese PLA, Beijing 100853, China.
2. Department of Clinical Laboratory Medicine, The First Medical Centre, Medical School of Chinese PLA, Beijing, China.
3. Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, 100053, China.
*The first two authors contributed equally to this article.
Abstract
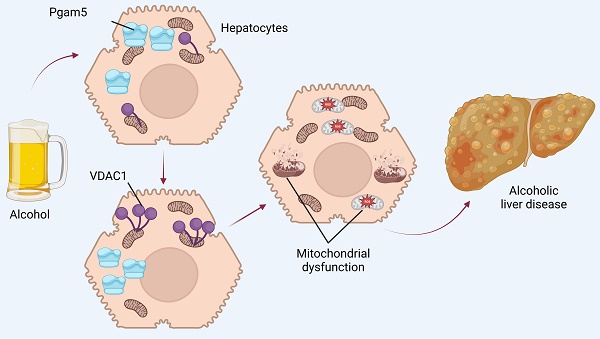
Alcoholic liver disease (ALD) poses a substantial global health challenge, with its pathogenesis deeply rooted in mitochondrial dysfunction. Our study explores the pivotal roles of Phosphoglycerate mutase family member 5 (Pgam5) and Voltage-Dependent Anion Channel 1 (VDAC1) in the progression of ALD, providing novel insights into their interplay and impact on mitochondrial integrity. We demonstrate that Pgam5 silencing preserves hepatocyte viability and attenuates ethanol-induced apoptosis, underscoring its detrimental role in exacerbating hepatocyte dysfunction. Pgam5's influence extends to the regulation of VDAC1 oligomerization, a key process in mitochondrial permeability transition pore (mPTP) opening, mitochondrial swelling, and apoptosis initiation. Notably, the inhibition of VDAC1 oligomerization through Pgam5 silencing or pharmacological intervention (VBIT-12) significantly preserves mitochondrial function, evident in the maintenance of mitochondrial membrane potential and reduced reactive oxygen species (ROS) production. In vivo experiments using hepatocyte-specific Pgam5 knockout (Pgam5hKO) and control mice reveal that Pgam5 deficiency mitigates ethanol-induced liver histopathology, inflammation, lipid peroxidation, and metabolic disorder, further supporting its role in ALD progression. Our findings highlight the critical involvement of Pgam5 and VDAC1 in mitochondrial dysfunction in ALD, suggesting potential therapeutic targets. While promising, these findings necessitate further research, including human studies, to validate their clinical applicability and explore broader implications in liver diseases. Overall, our study provides a significant advancement in understanding ALD pathophysiology, paving the way for novel therapeutic strategies targeting mitochondrial pathways in ALD.
Keywords: ALD, mitochondria, Pgam5, VDAC1

 Global reach, higher impact
Global reach, higher impact