3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(4):725-731. doi:10.7150/ijms.92222 This issue Cite
Review
The Protein Acetylation after Traumatic Spinal Cord Injury: Mechanisms and Therapeutic Opportunities
Department of Spine Surgery, Lanzhou University Second Hospital; Orthopaedics Key Laboratory of Gansu Province, Lanzhou 730030, China.
Received 2023-11-14; Accepted 2024-2-1; Published 2024-2-12
Abstract
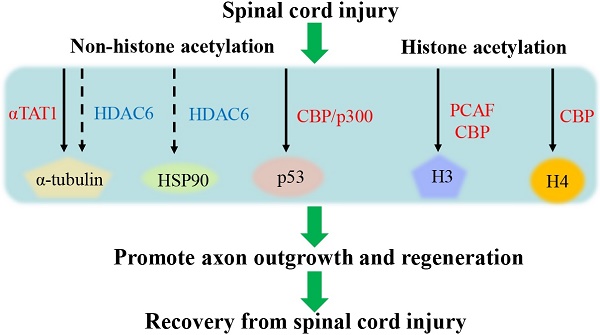
Spinal cord injury (SCI) leads to deficits of various normal functions and is difficult to return to a normal state. Histone and non-histone protein acetylation after SCI is well documented and regulates spinal cord plasticity, axonal growth, and sensory axon regeneration. However, our understanding of protein acetylation after SCI is still limited. In this review, we summarize current research on the role of acetylation of histone and non-histone proteins in regulating neuron growth and axonal regeneration in SCI. Furthermore, we discuss inhibitors and activators targeting acetylation-related enzymes, such as α-tubulin acetyltransferase 1 (αTAT1), histone deacetylase 6 (HDAC6), and sirtuin 2 (SIRT2), to provide promising opportunities for recovery from SCI. In conclusion, a comprehensive understanding of protein acetylation and deacetylation in SCI may contribute to the development of SCI treatment.
Keywords: spinal cord injury, protein acetylation, HAT, HDAC, inhibitors
1. Introduction
Spinal cord injury (SCI) is a devastating and irreversible disease of the central nervous system (CNS), which can lead to permanent deficits in motor, sensory, and autonomic functioning [1]. The most common causes of traumatic SCI are car accidents, sports accidents, broken bones, and gunshot wounds [2-4]. The severity of an injury is determined by physical forces, such as compression, shearing, laceration, and acute stretch/distraction [2]. Generally, spinal fractures caused by the above factors are often accompanied by SCI, ultimately leading to severe paraplegia, varying degrees of respiratory dysfunction of cervical fractures, and varying degrees of motor and sensory dysfunction below the injury plane due to thoracolumbar fractures.
SCI can be classified into two phases: primary injury and secondary injury [5, 6]. Primary SCI occurs in the initial stage immediately after the injury, which includes the disruption of axons, blood vessels, and cell membranes [7]. After the primary injury phase, secondary injury has a crucial effect on the pathophysiological process of SCI and is a destructive and processive cascade occurring later with neuronal necrosis and apoptosis, axonal rupture, demyelination, immune inflammatory reaction, and activation of aberrant molecular signaling [8]. During the secondary injury cascade, the release of chemicals, such as reactive oxygen, glutamate, and aspartate, leads to neuronal excitotoxicity and additional loss of neurons and glia by both necrotic and apoptotic cell death [9, 10]. Furthermore, these chemicals can negatively affect nucleic acid, proteins, and phospholipids around the lesion, resulting in neurological dysfunction [11].
Due to disruption of the blood-spinal cord barrier, various inflammatory cells infiltrate into the injury sites and contribute to the release of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1α, IL-1β, IL-6, and IL-17 after 6-10 hours following injury [12, 13]. Researchers have shown that these cytokines promote necrosis, apoptosis, and neurodegeneration, impeding SCI repair [13]. Meanwhile, ion imbalance after SCI aggravates the secondary injury zone, activates calcium-dependent proteases, and triggers mitochondrial dysfunction, which is closely related to the activation of necrotic and apoptotic cell deaths [14]. Therefore, more intervention on the pathogenesis of secondary injury can represent a potentially effective therapeutic target to overcome repair failure by SCI.
Recent studies have suggested that epigenetic regulations are involved in the mechanism of gene modification, thus influencing cell growth, differentiation, apoptosis, autophagy, and transformation [15, 16]. A variety of diseases arising from inflammation, internal or external damages, alter epigenetic regulations, including DNA methylation, histone methylation, and histone acetylation. Evidence has demonstrated that epigenetic regulations play an essential role in mediating spinal cord plasticity and neuronal differentiation during SCI [17-19]. The epigenetic modification processes are associated with mechanisms that underlie the repair and therapy of SCI.
This review focuses on the evidence for modification of protein acetylation in SCI. We summarize the regulating effects of histone and non-histone acetylation and outline possible pharmacological interventions that could be used to modulate and target this acetylation modification in SCI.
2. Protein acetylation
Protein acetylation is one of the most common protein modifications. It can be divided into the two major types: co-translational Nα-terminal acetylation and post-translational Nε-lysine acetylation [20, 21]. Nα-terminal acetylation occurs on the α-amino group at the N-terminus of proteins and the process is considered to be irreversible [22]. Nε-lysine acetylation refers to the acetylation of the ε-amino group of a lysine residue (more typically referred to as lysine acetylation) [21]. Nε-lysine acetylation has been thoroughly investigated and is a widespread and versatile regulation form. In this review, acetylation refers only to post-translational Nε-lysine acetylation. It is acetylation that enables proteins to possess abundant activities and functions by modulating protein stability, enzyme activity, subcellular localization, and interactions with other PTMs [23, 24]. Because each modified protein is distinct, acetylation can be classified as histone acetylation or non-histone acetylation. Histone acetylation and non-histone acetylation are involved in regulating transcription, autophagy, metabolism, signal transduction, protein folding, differentiation, and neural function [25].
2.1 Acetylation-related enzymes
Acetylation is regulated by two types of enzymes: the lysine acetyltransferases (KATs), which catalyze the transfer of an acetyl group from acetyl coenzyme A to the lysine residue on a polypeptide chain, and the lysine deacetylases (KDACs), which catalyze the removal of acetyl group [26, 27]. Taking into consideration histone acetylation, KATs and KDATs were customarily called histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. Manifold studies firmly demonstrated that apart from histones, acetylation has been implicated in tens of thousands of non-histone proteins in biological processes [28]. KATs whose enzymatic activities as well as biological structures and functions were well characterized in mammalians are classified into three main families: the GCN5/PCAF family; the p300/CBP family; and the MYST family [29, 30]. These subfamilies of KATs share a conserved central core region that contributes to the acetyl-CoA-binding (KAT) domain [31]. The other remaining KATs will not be referred to in this review. Another class of enzymes, KDACs (known as HDACs), are divided into two major subgroups: Zn2+-dependent HDACs (HDAC1-11) and NAD+-dependent sirtuins (SIRT1-7, among which only SIRT1, SIRT2, and SIRT3 function as strong deacetylases) [32]. Besides, KDACs are also divided into four subtypes according to structure and function. Class I HDACs, including HDAC1, HDAC2, HDAC3, and HDAC8, are mainly located in the nucleus. Class II HDACs, which are grouped into class IIa (HDAC4, HDAC5, HDAC7, and HDAC9) and class IIb (HDAC6 and HDAC10), shuttle between the nucleus and cytoplasm. Class III HDACs are NAD+-dependent sirtuins.
2.2 Histone acetylation
It is well known that acetylation occurs in more than 40 different lysine residues within the four classic core histones, H2A, H2B, H3, and H4, which play a fundamental role in the initiation and elongation of transcriptional regulation [33, 34]. This process is dynamically and reversibly regulated by a balance between HATs and HDACs. Research has reported that histone acetylation can regulate gene transcription. For instance, hyper-acetylation of histone H3 on the promoter region of MIP-2 and CXCR2 may lead to augmentation of the MIP-2/CXCR2 axis and induce chronic neuroinflammation [35]. Histone acetylation also contributes to the recruitment of chromatin remodeling factors, which are ATP-dependent enzymes capable of sliding, expelling, or modifying nucleosome spacing to expose specific DNA segments [36]. Histone acetylation has a profound effect on the response to epileptic insults, cerebral trauma, and neuropathic pain [17, 37, 38].
2.3 Non-histone acetylation
With the further study of histone acetylation for more than half a century, researchers have gradually paid attention to non-histone acetylation. Research has shown that non-histone acetylation participates in physiological or pathological processes relevant to various diseases, such as cell growth, autophagy, apoptosis, and DNA damage repair. Acetylation of non-histone is mediated by KATs and removed by KDACs. Non-histone acetylation could affect protein stability, enzymatic activity and subcellular localization of the targeted proteins. For example, studies have demonstrated that the acetylation of transcription factor p53 increases its protein stability, interaction with other proteins, and binding ability to promoters [39, 40]. Cell cycle regulators, including cyclin-dependent kinase 1 (CDK1), CDK2, the protein kinases BUBR1, and Aurora kinase A and Aurora kinase B are also modulated by acetylation to influence cell cycle [41, 42]. Acetylation of tau by CBP/p300 has been suggested to impair its function and contribute to its pathological aggregation in Alzheimer's disease [43]. Another discovery suggested that the kinases AKT and PDK1 in their pleckstrin homology domains are acetylated by p300/CBP-associated factor (PCAF) and p300, obstructing their membrane localization and activation [44]. Thus, non-histone acetylation serves as an attractive regulation form and a therapeutic target in the pathophysiological process.
3. Histone acetylation after SCI
Histone acetylation and deacetylation act as a mediator of axonal and neuronal regeneration in SCI by modeling developmental gene expression and chromatin remodeling. A study has clarified that acetylation of histone H4 plays an important role in neural plasticity, synaptogenesis, synaptic plasticity, learning, and memory. After SCI, the level of global histone H4 acetylation was time-related and influenced glial fibrillary acidic protein level, thus H4 acetylation can be further used for tissue remodeling after SCI [45]. It was confirmed in previous studies that histone H4 acetylation induced by HDAC inhibitors could module a variety of regeneration-associated genes (RAGs) to improve spinal cord plasticity, axonal growth, and sensory addxon regeneration after SCI [46]. Research found that there was increased acetylation of histone H3K9 via PCAF at the promoters of growth-associated protein 43 (GAP-43), galanin, and BDNF following a peripheral axonal injury, thus contributing to axonal regeneration [47]. Moreover, histone acetylation mediated by Creb-binding protein also increased the expression of genes related to regenerative process and program, further promoting axon regeneration after SCI [48]. On the contrary, a paclitaxel-induced neuropathic pain model revealed that enhanced histone H4 acetylation might upregulate the expression of CX3CL1, which is implicated in neuropathic pain following SCI [49]. Evidence suggested that the acetylation levels of histone H3 at the promoter of the inflammatory molecules such as MIP-2 and CXCR2 were increasing. The status contributed to the expressions of MIP-2 and CXCR2, thus inducing chronic neuroinflammation and neuropathic pain [35]. CBP/p300 exerts a crucial role as a HAT of H3K9 and H4K5 in upregulating the expression of the pro-nociceptive molecules, BDNF and cyclooxygenase-2 in a rat model with chronic constriction injury [50, 51]. Taken together, histone acetylation plays a dual role in neuronal development and recovery after SCI. The overview of the role of histone acetylation after SCI is shown in Table 1.
Overview of the major roles of protein acetylation after SCI.
| Type of modification | Modified enzymes | Specific substrates | State of acetylation after SCI | Major roles | References |
|---|---|---|---|---|---|
| Histone acetylation | histone H4 | Increase | Improve spinal cord plasticity, axonal growth, and sensory addxon regeneration after SCI | [45, 46] | |
| PCAF | histone H3 | Increase | Contribute to axonal regeneration | [47] | |
| histone H4 | Increase | Implicated in neuropathic pain following SCI | [49] | ||
| histone H3 | Increase | Induce chronic neuroinflammation and neuropathic pain | [35] | ||
| CBP | histone H3 and H4 | Increase | Promote axon regeneration after SCI | [50, 51] | |
| Non-histone acetylation | αTAT1 | α-tubulin Lys40 | Increase | Affect the stability of microtubules; Promote axonal growth in vitro | [60, 62, 68] |
| HDAC6 | α-tubulin | Decrease | Inhibition of HDAC6 to recover the normal α-tubulin acetylation levels and axonal transport | [66] | |
| CBP/p300 | P53 | Increase | Promote axon outgrowth and regeneration | [74] | |
| HDAC6 | HSP90 | Decrease | Resist hypoxia-ischemia-induced oxidative stress after SCI | [72, 73] |
4. Non-histone acetylation after SCI
Acetylation of non-histone proteins, including α-tubulin, heat shock protein 90 (HSP90) and p53, plays a fundamental role in axon regeneration and neuron differentiation in SCI. The PTM of these proteins through acetylation refers to various KATs and KDACs, which regulates their interaction with proteins, subcellular distribution, stability, and activity [52]. Non-histone acetylation is further discussed to provide new insights into the mechanism of the secondary damage and therapeutic target after SCI. The overview of the role of non-histone acetylation after SCI is shown in Table 1.
4.1 Acetylation of α-tubulin after SCI
Microtubules, as the most important component of cytoskeleton, consist of α-tubulin and β-tubulin. They not only affect changes in cell morphology, transports of intracellular substances, and expression of intracellular proteins [53], but also provide environmental information within the organization and energy transfer [54]. Microtubules are subject to tubulin acetylation, a crucial epigenetic modification in regulating microtubule structure and stability. The level of tubulin acetylation is mainly mediated by α-tubulin acetyltransferase1 (αTAT1)[55]. On the contrary, the reverse reaction of the process is catalyzed by HDAC6 [56, 57]. Abnormal level of α-tubulin acetylation is associated with neurological disorders, including SCI. Research has demonstrated that the expression of α-tubulin was decreased after SCI and that remodeling microtubules could repair SCI [58, 59]. Targeting α-tubulin acetylation as well as the corresponding enzymes offers a promising insight into SCI.
αTAT1 is a microtubule-specific acetyltransferase that catalyzes α-tubulin Lys40 acetylation and directly affects the stability of microtubules in cells. αTAT1 plays a positive role in promoting axonal growth in vitro by acetylating α-tubulin [60]. In addition, elongator protein 3 regulates α-tubulin acetylation to contribute to the migration and differentiation of cortical neurons [61].
HDAC6, a member of the HDAC family, is mainly localized and is actively maintained in the cytoplasm [62]. HDAC6 has a dynamic effect on reversible acetylation that the overexpression of HDAC6 contributes to α-tubulin deacetylation, whereas inhibition of HDAC6 increases α-tubulin acetylation. α-Tubulin acetylation was significantly decreased, and this phenomenon was associated with loss of axonal transport in these neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis [63-65]. Targeting inhibition of HDAC6 can recover the normal α-tubulin acetylation levels and axonal transport in these neurodegenerative diseases [66]. A study inhibiting HDAC6 via the administration of tubastatin A, a special selectivity compound for HDAC6, certified that the levels of acetylated tubulin were increased and axonal regeneration was promoted [67]. In addition, SIRT2 is NAD+-dependent deacetylase and is mainly found in the cytoplasm. SIRT2 deacetylates α-tubulin dependent on the CDK5 phosphorylation in neurons [68]. A study found that alternatively polarized macrophages 2 (M2) increase SIRT2 expression, which deacetylates α-tubulin in microtubules to further facilitate the differentiation of ependymal stem cells toward neurons following SCI [69].
Therefore, the regulation of α-tubulin acetylation and deacetylation by related enzymes plays a role in neural functional recovery and provides new insights into neurological disease interventions, including SCI.
4.2 Acetylation of other non-histone proteins after SCI
HSP90 and its co-chaperones orchestrate essential physiological processes including cell survival, cell cycle, and apoptosis [70]. HSP90 acetylation can modulate its interaction with both client and co-chaperone proteins [71]. Existing report has demonstrated that HDAC6 is a crucial deacetylase of HSP90 [72]. HDAC6 regulates chaperone-mediated autophagy to resist hypoxia-ischemia-induced oxidative stress by affecting HSP90 acetylation after SCI [73]. HSP90 acetylation may play a role in neuroprotection and cell antioxidant activity after SCI. Another study found that the acetylation of p53 via CBP/p300 promotes axon outgrowth and regeneration by augmenting the GAP-43 expression [74].
5. Target therapy
In this review, we further discuss the target treatment involved in HDAC inhibitors and HAT activator in various neurological diseases, including SCI. The overview of the therapies targeting protein acetylation after SCI is shown in Table 2.
5.1 HDAC inhibitors
A growing body of literature has suggested that HDAC inhibitors exert indispensable effects on neuroprotection and anti-inflammation in the CNS [75]. For example, CI-994, known as a benzamide-based class I HDAC inhibitor, has effects on neuroprotection and contributes to functional recovery by targeting HDAC1 and HDAC3 in an experimental model of SCI [76, 77]. Consistent with this, tubastatin A, which has a pharmacological inhibitory effect on HDAC6, promotes acetylation of tubulin and partially repairs autophagic flux impaired by SCI, thus facilitating axon regeneration and functional recovery after SCI [67]. According to one study, HDAC inhibitors, such as MS-275 and MGCD0103, reduce behavioral hypersensitivity and alleviate neuropathic pain in a rat model of traumatic nerve injury [78]. Another research has demonstrated that trichostatin A (TSA) inhibits the protein expression of HDAC2 through promoting ubiquitination-mediated degradation of HDAC2, and thus plays an analgesic effect in nerve injury [79]. Valproic acid (VPA) exerts various therapeutic advantages in diseases, including epilepsy, SCI, and cancers [80-82]. Recent evidence has suggested that VPA, as a HDAC inhibitor, mediates high acetylation of the STAT1/NF-κB pathway by suppressing HDAC3 expression and activity. As a result, acetylation of the complex with NF-κB p65 and STAT1 inhibited the transcriptional activity of NF-κB p65 and attenuated the central inflammatory response mediated by microglia after SCI [83]. Moreover, sodium valproate, a global HDAC inhibitor, contributes to anti-hypersensitivities in rats by mediating glutamate transporters following peripheral nerve injury [84].
The overview of the therapies targeting protein acetylation after SCI.
| HDAC inhibitors or HAT activator | Substrate | Proposed functions | References |
|---|---|---|---|
| CI-994 | HDAC1 HDAC3 | Neuroprotection and contribute to functional recovery | [76, 77] |
| tubastatin A | HDAC6 | Promote acetylation of tubulin; Facilitate axon regeneration and functional recovery from SCI | [67] |
| MS-275 MGCD0103 | HDAC1 | Reduce behavioral hypersensitivity and alleviate neuropathic pain | [78] |
| trichostatin A (TSA) | HDAC2 | Play an analgesic effect in nerve injury | [79] |
| Valproic acid (VPA) | HDAC3 | Attenuate the central inflammatory response mediated by microglia after SCI | [80-82] |
| CSP-TTK21 | CBP/p300 | Promote axon growth, sprouting, and synaptic plasticity | [85] |
5.2 HAT activator
In a chronic SCI model, pharmacological activation of CBP/p300 by CSP-TTK21 enhances histone acetylation and the expression of RAGs. Furthermore, CSP-TTK21 triggers motor and sensory axon growth, sprouting, and synaptic plasticity, suggesting that it may be a molecular regenerative intervention to recover from SCI [85].
6. Conclusions
The last two decades have witnessed tremendous advances in clarifying the molecular mechanisms and targeting therapies for SCI. Acetylation of histone and non-histone proteins has been extensively and deeply studied in many diseases, and it plays an important role in various biological processes and development of diseases. Many enzymes, including αTAT1, HDAC6, and SIRT2, are not only involved in protein acetylation and deacetylation, but also maintain a balance between the two. Studies have provided substantial evidence suggesting that epigenetic modification through protein acetylation regulates gene transcription, signal transduction, and interaction with other developmental proteins after SCI. Protein acetylation excerts a crucial role in SCI and becomes the entry point for SCI treatment. Targeting protein acetylation-related enzymes, such as HAT activators and HDAC inhibitors, can provide new ideas for SCI treatment as well as new therapeutic targets for axon growth and regeneration.
However, the current research on the epigenetic modulation of SCI has several limitations. First, targeting protein acetylation primarily focuses on the level of animal models and rarely involves humans. Second, the role of protein acetylation differs according to the time points after SCI, making it difficult to determine the optimal time to intervene. Finally, SCI recovery requires the involvement of multiple regulatory mechanisms, of which protein acetylation is only one. Thus, the development of single-cell sequencing, high-resolution quantitative mass spectrometry, and selective chemical probes aids in defining specific regulatory genes and precise mechanisms.
Although complete recovery is difficult for patients with SCI, therapeutic strategies targeting epigenetic regulation can reduce the pain caused by SCI and enhance neuronal repair as much as possible. With the advancement of cell transplantation and gene therapy, it is promising to target protein acetylation modification to recover from SCI.
Acknowledgements
The work was supported by National Natural Science Foundation of China (No.31960175).
Author contributions
All authors contributed to the manuscript and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cowan H, Lakra C, Desai M. Autonomic dysreflexia in spinal cord injury. BMJ (Clinical research ed). 2020;371:m3596
2. Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. Journal of neurotrauma. 2004;21:1355-70
3. Papadopoulos SM, Selden NR, Quint DJ, Patel N, Gillespie B, Grube S. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. The Journal of trauma. 2002;52:323-32
4. Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal cord. 2012;50:365-72
5. McDonald JW, Sadowsky C. Spinal-cord injury. Lancet (London, England). 2002;359:417-25
6. Eckert MJ, Martin MJ. Trauma: Spinal Cord Injury. The Surgical clinics of North America. 2017;97:1031-45
7. Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A. et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. International journal of molecular sciences. 2020 21
8. Rogers WK, Todd M. Acute spinal cord injury. Best practice & research Clinical anaesthesiology. 2016;30:27-39
9. Liu M, Wu W, Li H, Li S, Huang LT, Yang YQ. et al. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. The journal of spinal cord medicine. 2015;38:745-53
10. Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:1190-8
11. Dimitrijevic MR, Danner SM, Mayr W. Neurocontrol of Movement in Humans With Spinal Cord Injury. Artificial organs. 2015;39:823-33
12. Hill F, Kim CF, Gorrie CA, Moalem-Taylor G. Interleukin-17 deficiency improves locomotor recovery and tissue sparing after spinal cord contusion injury in mice. Neuroscience letters. 2011;487:363-7
13. Ren H, Chen X, Tian M, Zhou J, Ouyang H, Zhang Z. Regulation of Inflammatory Cytokines for Spinal Cord Injury Repair Through Local Delivery of Therapeutic Agents. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2018;5:1800529
14. Schmidt J, Quintá HR. Mitochondrial dysfunction as a target in spinal cord injury: intimate correlation between pathological processes and therapeutic approaches. Neural regeneration research. 2023;18:2161-6
15. Gupta S, Dutta S, Hui SP. Regenerative Potential of Injured Spinal Cord in the Light of Epigenetic Regulation and Modulation. Cells. 2023 12
16. Pal S, Tyler JK. Epigenetics and aging. Science advances. 2016;2:e1600584
17. Ghosh K, Pan HL. Epigenetic Mechanisms of Neural Plasticity in Chronic Neuropathic Pain. ACS chemical neuroscience. 2022;13:432-41
18. Wang Y, Li WY, Li ZG, Guan LX, Deng LX. Transcriptional and Epigenetic Regulation in Injury-Mediated Neuronal Dendritic Plasticity. Neuroscience bulletin. 2017;33:85-94
19. Kameda T, Imamura T, Nakashima K. Epigenetic regulation of neural stem cell differentiation towards spinal cord regeneration. Cell and tissue research. 2018;371:189-99
20. Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N. et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8157-62
21. Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science (New York, NY). 2009;325:834-40
22. Constantinou M, Klavaris A, Koufaris C, Kirmizis A. Cellular effects of NAT-mediated histone N-terminal acetylation. Journal of cell science. 2023 136
23. Marín-Hernández Á, Rodríguez-Zavala JS, Jasso-Chávez R, Saavedra E, Moreno-Sánchez R. Protein acetylation effects on enzyme activity and metabolic pathway fluxes. Journal of cellular biochemistry. 2022;123:701-18
24. Narita T, Weinert BT, Choudhary C. Functions and mechanisms of non-histone protein acetylation. Nature reviews Molecular cell biology. 2019;20:156-74
25. Shvedunova M, Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nature reviews Molecular cell biology. 2022;23:329-49
26. Verdin E, Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nature reviews Molecular cell biology. 2015;16:258-64
27. Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T. et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633-6
28. Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nature reviews Molecular cell biology. 2014;15:536-50
29. Li P, Ge J, Li H. Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease. Nature reviews Cardiology. 2020;17:96-115
30. Drazic A, Myklebust LM, Ree R, Arnesen T. The world of protein acetylation. Biochimica et biophysica acta. 2016;1864:1372-401
31. Srivastava S, Kumar S, Bhatt R, Ramachandran R, Trivedi AK, Kundu TK. Lysine Acetyltransferases (KATs) in Disguise: Diseases Implications. Journal of biochemistry. 2023;173:417-33
32. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature reviews Genetics. 2009;10:32-42
33. Zhao Y, Garcia BA. Comprehensive Catalog of Currently Documented Histone Modifications. Cold Spring Harbor perspectives in biology. 2015;7:a025064
34. Ali I, Conrad RJ, Verdin E, Ott M. Lysine Acetylation Goes Global: From Epigenetics to Metabolism and Therapeutics. Chemical reviews. 2018;118:1216-52
35. Kiguchi N, Kobayashi Y, Maeda T, Fukazawa Y, Tohya K, Kimura M. et al. Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. The Journal of pharmacology and experimental therapeutics. 2012;340:577-87
36. Clapier CR, Iwasa J, Cairns BR, Peterson CL. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nature reviews Molecular cell biology. 2017;18:407-22
37. Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:8422-8
38. Liao Y, Cheng J, Kong X, Li S, Li X, Zhang M. et al. HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics. 2020;10:9644-62
39. Reed SM, Quelle DE. p53 Acetylation: Regulation and Consequences. Cancers. 2014;7:30-69
40. Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612-26
41. Nishiyama T, Sykora MM, Huis in 't Veld PJ, Mechtler K, Peters JM. Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13404-9
42. Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. The EMBO journal. 2009;28:2077-89
43. Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ. et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nature communications. 2011;2:252
44. Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V. et al. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Science signaling. 2011;4:ra46
45. de Menezes MF, Nicola F, Vital da Silva IR, Vizuete A, Elsner VR, Xavier LL. et al. Glial fibrillary acidic protein levels are associated with global histone H4 acetylation after spinal cord injury in rats. Neural regeneration research. 2018;13:1945-52
46. Finelli MJ, Wong JK, Zou H. Epigenetic regulation of sensory axon regeneration after spinal cord injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:19664-76
47. Puttagunta R, Tedeschi A, Sória MG, Hervera A, Lindner R, Rathore KI. et al. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nature communications. 2014;5:3527
48. Hutson TH, Kathe C, Palmisano I, Bartholdi K, Hervera A, De Virgiliis F. et al. Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Science translational medicine. 2019 11
49. Li D, Huang ZZ, Ling YZ, Wei JY, Cui Y, Zhang XZ. et al. Up-regulation of CX3CL1 via Nuclear Factor-κB-dependent Histone Acetylation Is Involved in Paclitaxel-induced Peripheral Neuropathy. Anesthesiology. 2015;122:1142-51
50. Zhu X, Li Q, Chang R, Yang D, Song Z, Guo Q. et al. Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PloS one. 2014;9:e91303
51. Zhu XY, Huang CS, Li Q, Guo QL, Wang Y, He X. et al. Temporal distribution of p300/CBP immunoreactivity in the adult rat spinal dorsal horn following chronic constriction injury (CCI). Cellular and molecular neurobiology. 2013;33:197-204
52. Park JM, Jo SH, Kim MY, Kim TH, Ahn YH. Role of transcription factor acetylation in the regulation of metabolic homeostasis. Protein & cell. 2015;6:804-13
53. Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiological reviews. 1998;78:763-81
54. Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. Journal of cell science. 2003;116:1157-73
55. Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21517-22
56. Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D. et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. The EMBO journal. 2002;21:6820-31
57. Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S. et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. The EMBO journal. 2003;22:1168-79
58. Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A. et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science (New York, NY). 2015;348:347-52
59. Crunkhorn S. CNS injury: Microtubule stabilizer repairs spinal cord injury. Nature reviews Drug discovery. 2015;14:310
60. Lin S, Sterling NA, Junker IP, Helm CT, Smith GM. Effects of αTAT1 and HDAC5 on axonal regeneration in adult neurons. PloS one. 2017;12:e0177496
61. Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O. et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551-64
62. Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A. et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455-8
63. Silva DF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondrial Metabolism Power SIRT2-Dependent Deficient Traffic Causing Alzheimer's-Disease Related Pathology. Molecular neurobiology. 2017;54:4021-40
64. Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM. et al. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nature communications. 2014;5:5245
65. Taes I, Timmers M, Hersmus N, Bento-Abreu A, Van Den Bosch L, Van Damme P. et al. Hdac6 deletion delays disease progression in the SOD1G93A mouse model of ALS. Human molecular genetics. 2013;22:1783-90
66. d'Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP. et al. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nature medicine. 2011;17:968-74
67. Zheng Z, Zhou Y, Ye L, Lu Q, Zhang K, Zhang J. et al. Histone deacetylase 6 inhibition restores autophagic flux to promote functional recovery after spinal cord injury. Experimental neurology. 2020;324:113138
68. Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo. PloS one. 2012;7:e34805
69. Ma Y, Deng M, Zhao XQ, Liu M. Alternatively Polarized Macrophages Regulate the Growth and Differentiation of Ependymal Stem Cells through the SIRT2 Pathway. Experimental neurobiology. 2020;29:150-63
70. Hoter A, El-Sabban ME, Naim HY. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. International journal of molecular sciences. 2018 19
71. Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K. et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Molecular cell. 2007;25:151-9
72. Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV. et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular cell. 2005;18:601-7
73. Su M, Guan H, Zhang F, Gao Y, Teng X, Yang W. HDAC6 Regulates the Chaperone-Mediated Autophagy to Prevent Oxidative Damage in Injured Neurons after Experimental Spinal Cord Injury. Oxidative medicine and cellular longevity. 2016;2016:7263736
74. Tedeschi A, Nguyen T, Puttagunta R, Gaub P, Di Giovanni S. A p53-CBP/p300 transcription module is required for GAP-43 expression, axon outgrowth, and regeneration. Cell death and differentiation. 2009;16:543-54
75. Didonna A, Opal P. The promise and perils of HDAC inhibitors in neurodegeneration. Annals of clinical and translational neurology. 2015;2:79-101
76. Zhang S, Fujita Y, Matsuzaki R, Yamashita T. Class I histone deacetylase (HDAC) inhibitor CI-994 promotes functional recovery following spinal cord injury. Cell death & disease. 2018;9:460
77. Harrison IF, Dexter DT. Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease? Pharmacology & therapeutics. 2013;140:34-52
78. Denk F, Huang W, Sidders B, Bithell A, Crow M, Grist J. et al. HDAC inhibitors attenuate the development of hypersensitivity in models of neuropathic pain. Pain. 2013;154:1668-79
79. Ouyang B, Chen D, Hou X, Wang T, Wang J, Zou W. et al. Normalizing HDAC2 Levels in the Spinal Cord Alleviates Thermal and Mechanical Hyperalgesia After Peripheral Nerve Injury and Promotes GAD65 and KCC2 Expression. Frontiers in neuroscience. 2019;13:346
80. Romoli M, Mazzocchetti P, D'Alonzo R, Siliquini S, Rinaldi VE, Verrotti A. et al. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Current neuropharmacology. 2019;17:926-46
81. Heers H, Stanislaw J, Harrelson J, Lee MW. Valproic acid as an adjunctive therapeutic agent for the treatment of breast cancer. European journal of pharmacology. 2018;835:61-74
82. Hao HH, Wang L, Guo ZJ, Bai L, Zhang RP, Shuang WB. et al. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neuroscience bulletin. 2013;29:484-92
83. Chen S, Ye J, Chen X, Shi J, Wu W, Lin W. et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. Journal of neuroinflammation. 2018;15:150
84. Hobo S, Eisenach JC, Hayashida K. Up-regulation of spinal glutamate transporters contributes to anti-hypersensitive effects of valproate in rats after peripheral nerve injury. Neuroscience letters. 2011;502:52-5
85. Müller F, De Virgiliis F, Kong G, Zhou L, Serger E, Chadwick J. et al. CBP/p300 activation promotes axon growth, sprouting, and synaptic plasticity in chronic experimental spinal cord injury with severe disability. PLoS biology. 2022;20:e3001310
Author contact
![]() Corresponding author: Hai-hong Zhang, Email: ery_zhanghheredu.cn; Tel.: 86-0931-8943701.
Corresponding author: Hai-hong Zhang, Email: ery_zhanghheredu.cn; Tel.: 86-0931-8943701.

 Global reach, higher impact
Global reach, higher impact