3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(4):703-713. doi:10.7150/ijms.87870 This issue Cite
Research Paper
Roxadustat Versus Erythropoietin: The Comparison of Efficacy in Reversing Ventricular Remodeling in Dialysis Patients with Anaemia
1. Institute of Nephrology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, 210009, China.
2. John Moorhead Research Laboratory, Department of Renal Medicine, University College London (UCL) Medical School, Royal Free Campus, London, NW3 2PF, UK.
3. Department of Nephrology, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310003, China.
Received 2023-7-7; Accepted 2024-1-30; Published 2024-2-12
Abstract
Background: Renal anaemia and left ventricular hypertrophy are the main complications of chronic kidney disease and are shared among dialysis patients. This retrospective study aimed to compare the efficacies of the hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat and recombinant human erythropoietin in reversing ventricular remodeling in dialysis patients with renal anaemia.
Methods: A total of 204 participants underwent baseline examinations, including echocardiograms and laboratory tests, before being administered either treatment for at least 24 weeks from January 2018 to October 2021, after which follow-up examinations were conducted at 6 months. Propensity score matching based on key variables included age, gender, cardiovascular diseases, cardiovascular medications, dialysis course and the vascular access at baseline was performed to include populations with similar characteristics between groups.
Results: In total, 136 patients were included with roxadustat or recombinant human erythropoietin. The left ventricular mass index after treatment with roxadustat and recombinant human erythropoietin both significantly decreased after 6 months, but there was no significant difference in the change in left ventricular mass index between the two groups. In addition, the left ventricular end-diastolic diameters and left ventricular wall thickness, systolic blood pressure, and diastolic blood pressure significantly decreased in the roxadustat group. Roxadustat and recombinant human erythropoietin also increased haemoglobin significantly, but there was no significant difference in the change in haemoglobin between the two groups. The results of multiple linear regression showed that the change in haemoglobin was independent factor affecting the improvement of left ventricular mass index.
Conclusions: The increase of haemoglobin was associated with improving left ventricular hypertrophy in dialysis patients. However, the beneficial effects between roxadustat and recombinant human erythropoietin on left ventricular mass index did not show clear superiority or inferiority in six months
Keywords: anaemia, roxadustat, erythropoietin, ventricular remodeling, dialysis
Introduction
As one of the most common complications of chronic kidney disease (CKD), cardiovascular disease (CVD) is also one of the main causes of death in patients with CKD (1). CVD in these patients is related to many risk factors, such as hypertension, diabetes, dyslipidemia, anaemia, and abnormal calcium and phosphorus metabolism associated with uremia. Furthermore, cardiac remodeling, characterized by left ventricular hypertrophy (LVH) and myocardial fibrosis, is common in CKD patients. Studies have indicated that patients undergoing long-term renal replacement therapy have a high incidence of LVH(2, 3). Increased in left ventricular volume is independently associated with CVD incidence and mortality in end-stage renal disease (ESRD) patients(4, 5). It was previously reported that a decrease in the concentration of haemoglobin (Hb) by 1 g/dL resulted in an 18% increase in the risk of heart failure in patients on dialysis (6). Renal anaemia in patients with CKD can lead to a reduced myocardial oxygen supply, resulting in increased myocardial cell necrosis and apoptosis, which induces oxidative stress, activating various inflammatory pathways and the sympathetic nervous system and ultimately leading to pathological cardiac remodeling(7).
Erythrocyte-forming stimulants (ESA) have long been the primary treatment option for patients with renal anaemia and improve the quality of life in CKD patients receiving dialysis and non-dialysis treatment and reduce the incidence of anaemia-related CVD and the need for blood transfusion(8). According to Parfrey's meta-analysis(9), in patients with severe anaemia before treatment (at baseline) (defined as a concentration of Hb < 10g/dL, with the average baseline Hb concentration in each study as low as 5.9 g/dL), increasing the concentration of Hb to ≤ 12g/dL by ESA could significantly reduce the left ventricular mass index (LVMI). One study comparing the baseline and post-renal transplantation echocardiographs in 232 patients with ESRD showed that patients with left ventricular dysfunction had improved cardiac structure and function and higher levels of Hb after renal transplantation compared to pre-renal transplantation(10). Therefore, improving the level of Hb may be effective for reversing cardiac remodeling and cardiovascular prognosis.
Despite the clinical significance of ESA for the treatment of renal anaemia, some studies confirmed that supraphysiological doses of ESA were associated with an increased risk of cardiovascular events, CKD progression, vascular pathway thrombosis, and mortality. One study in nephrectomized mice recently showed that hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) reduced myocardial hypertrophy and fibrosis in these mice by restoring capillary density and improving mitochondrial morphology(11). HIF-PHIs are a novel class of orally available drugs that have been associated with a lower risk of cardiovascular events in dialysis patients while improving renal anaemia(12). Nevertheless, to date, the ability of HIF-PHIs to aid in the reversal of cardiac remodeling in dialysis patients with renal anaemia has not been explored. Therefore, this research aims at probing and comparing the clinical efficacy of two different drugs for renal anaemia (the HIF-PHI roxadustat and ESA) in ventricular remodeling in dialysis patients with renal anaemia through clinical retrospective analysis.
Methods
Study population and study design
The hospital's electronic medical record data of dialysis patients receiving either oral roxadustat or parenteral ESA three times per week for 6 months were retrospectively reviewed in a single tertiary healthcare centre (Department of Nephrology, Zhongda Hospital Affiliated to Southeast University, Nanjing, China) between January 2018 and October 2021. All patients had pre- and post-treatment echocardiograms at six months. Patients (age > 18 years) in stage 5 CKD who were previously diagnosed with renal anaemia (Hb < 100g/L) and began long-term haemodialysis (HD) (dialysis time ≥ 12 weeks) were eligible for the study. Exclusion criteria for the current analysis included: (1) any clinically significant or active underlying infection; (2) chronic liver disease; (3) acute coronary syndrome or thromboembolic event within 1 year prior to medication; (4) anaemia is caused by chronic inflammatory diseases (e.g. systemic lupus erythematosus and rheumatoid arthritis) or hematological diseases (e.g. myelodysplastic syndrome and multiple myeloma), or other non-renal anaemia; (5) blood transfusion within 12 weeks of study or expected need for blood transfusion; (6) poor long-term blood pressure control; and (7) congenital heart disease, valve disease, rheumatic heart disease, or other heart diseases that result in potential changes to heart structure and/or function. All eligible dialysis patients with renal anaemia in the electronic medical record system were reviewed. Eligible patients were divided into roxadustat and rhEPO groups based on treatment, with oral roxadustat three times weekly (TIW), or ESAs administered intravenously or subcutaneously. In roxadustat group, for ESA-untreated patients, the starting dose was 100mg for patients weighing 45-60kg or 120mg for patients weighing ≥60kg. For patients who previously received ESA, the starting dose was 70-120 mg TIW based on the previous ESA dose. In rhEPO group, patients treated with ESA were dosed according to the ESA dose before entering the study, and ESA-naive patients were dosed according to 50 IU/kg TIW, with the dose adjusted based on local prescribing information.
Information related to the demographics, dialysis-related medical history, medications currently being taken, comorbidities, blood pressure, echocardiogram, and laboratory data of the patients in the study were collected directly from the patients' electronic medical records. All patients were evaluated before treatment (baseline) and 6 months after treatment. The manuscript was prepared based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (13)statement. The Ethics Committee approved the study of Zhongda Hospital, Southeast University. The number of the ethics certificate is 2018ZDSYLL093-P01. The study was performed in accordance with the declaration of Helsinki. The data were anonymous, and the requirement for informed consent was waived.
Anaemia is diagnosed in adults with CKD when the Hb concentration is < 13.0 g/dL (< 130 g/L) in males and < 12.0 g/dL (< 120 g/L) in females based on the Kidney Disease - Improving Global Outcomes (KDIGO) guidelines(14). Hypertension is defined as systolic blood pressure (SBP) higher than 130 or a diastolic blood pressure (DBP) higher than 80 mm Hg based on the American College of Cardiology/American Heart Association (ACC/AHA) guidelines(15). Left ventricular (LV) parameters obtained by 2D echocardiography are employed to assess the estimated left ventricular mass. The following LVH evaluation criteria based on LV wall thickness and cavity dimensions of the LV were published by the American Society of Echocardiography (ASE) in conjunction with the European Society of Echocardiography(16): estimated LVMI > 103 g/m2 for males and > 89 g/m2 for females is abnormal. LVH was defined as LVMI> 115g/m2 in males and LVMI> 95g/m2 in females. Severe LVH was defined as LVMI>148 g/m2 in males and LVMI>121g/m2 in females.
Measurements and calculations
The primary outcome of this study was the changes in LVMI, left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameters (LVEDd), interventricular septal thickness (IVST), and left ventricular wall thickness (LVPWT) over 6 months after initiation of the treatment interventions. All patients were examined by Philips IE33 echocardiography diagnostic instrument and S5-1 heart probe with a frequency of 3.5-5mhz in a Core Echo Laboratory (Division of Cardiology, Zhongda Hospital Affiliated to Southeast University, Nanjing, China), to ensure consistent measurement methodology. Patients were placed in the supine position or left decubital position. Measurements were performed on three to five consecutive beats, from which the mean values were calculated. All readings were taken by qualified technicians and subsequently reviewed by senior echocardiographers. These changes were assessed by echocardiographic imaging by performing the biplane method of disk summation (modified Simpson's rule) according to the recommendations by the ASE. The LVMI was calculated using the following formula:
LVMI = LVM/body surface area (BSA) (1)
in which the Left Ventricular Mass (LVM) was a calculated based on the following formula:
LVM = 0.8 × (1.04 × [IVST + LVPWT + LVEDd]3 - [LVEDd3]) + 0.6 (2)
Propensity score matching
Propensity score matching was performed on the two cohorts to minimize the deviation caused by confounding factors between patients treated with roxadustat and rhEPO in the dialysis population. Matching based on clinical covariates considered to impact on the patient's outcomes, including age, gender, cardiovascular diseases, cardiovascular medications, dialysis course and the vascular access, was employed using 1:1 nearest neighbor algorithm with a caliper value of 0.1.
Statistical analysis
The Shapiro-Wilk test was adopted to assess the normality of continuous variables. The missing variables conforming to the normal distribution were filled with the mean, otherwise filled with the median. Variables of normal distribution reported herein were represented as the mean ± standard deviation (SD), and variables of skewness distribution were expressed as the median value (interquartile range, IQR). Pearson's χ2 test or Fisher's exact were employed to compare the categorical data expressed as numbers and percentages. Comparisons between groups were performed by adopting independent samples t-test or Mann-Whitney U test. As appropriate, within-group comparisons were performed using a paired-samples t-test or Wilcoxon signed-rank test. The primary analysis consisted of a comparing the mean change in the echocardiographic parameters between the baseline values and the six-month follow-up values between the 2 treatment groups. Bivariate correlation analysis was performed to analyze the correlation between clinical variables and the change of LVMI. Multiple linear regression analysis was employed to evaluate the effect of different clinical factors on the change in LVMI. Statistical analysis was performed using the SAS software package (version 9.4, SAS Institute Inc., Cary, NC), and the statistical significance was evaluated at a two-tailed probability level of < 0.05.
Results
Characteristics of study subjects
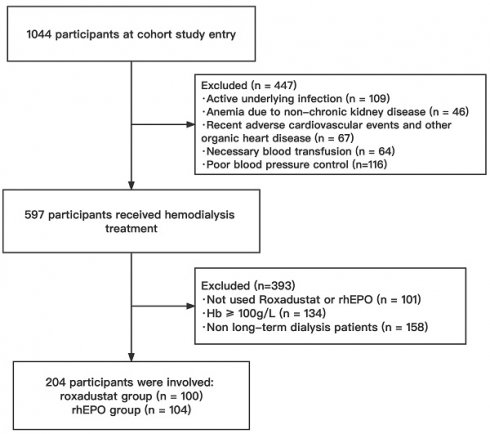
1044 patients were screened from an electronic medical record database between January 2018 to October 2021. A total of 840 patients were excluded based on study inclusion and exclusion criteria (Figure 1), and the remaining eligible 204 patients were divided into two groups based on different interventions: roxadustat group (n = 100) and rhEPO group (n = 104).
Propensity score matching identified 68 patients in each cohort. The baseline characteristics of the eligible HD patients are shown in Table 1. The male participants accounted for 51.5% of the total patient population in the HD cohort. The mean ± SD age of the final HD cohort was 60 ± 14 years, and the mean ± SD body mass index (BMI) was 23.8 ± 4.2 kg/m2. The median duration of dialysis in all HD patients was 3 years (IQR: 2 to 5). The most common comorbidity of these patients was hypertension (89.7%), followed by diabetes mellitus (39.7%) and coronary artery disease (30.2%). Of the 136 HD patients, 82 (60.3%) were taking calcium channel blockers (CCBs), 72 (52.9%) were taking β-blockers, and 29 (21.3%) patients were taking angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). After propensity score matching, there was no significant difference in the baseline clinical, laboratory, or echocardiographic characteristics between these two groups.
Baseline characteristics of the hemodialysis population
| rhEPO group(n=68) | Roxadustat group(n=68) | P- value | |
|---|---|---|---|
| Age, yr | 59±14 | 62±13 | 0.203 |
| Male, n (%) | 33(48.5) | 37(54.4) | 0.493 |
| BMI, kg/m2 | 22.9(21.2, 26.7) | 23.8(21.5, 25.7) | 0.588 |
| Dialysis duration, yr | 3(2, 5) | 3(2, 4) | 0.307 |
| Arteriovenous fistula, n (%) | 51(75.0) | 53(77.9) | 0.686 |
| Comorbidities, n (%) | 66(97.1) | 66(97.1) | 1.000 |
| Hypertension | 61(89.7) | 61(89.7) | 1.000 |
| Coronary artery disease | 21(30.9) | 20(29.4) | 0.852 |
| Atrial fibrillation | 15(22.1) | 13(19.1) | 0.671 |
| Diabetes mellitus | 26(38.2) | 28(41.2) | 0.726 |
| Cardiovascular medication, n (%) | 46(67.7) | 45(66.2) | 0.855 |
| ACEI/ARB | 14(20.6) | 15(22.1) | 0.834 |
| Beta-blocker | 35(51.5) | 37(54.4) | 0.731 |
| Aldosterone receptor blocker | 8(11.8) | 7(10.3) | 0.784 |
| ARNI | 12(17.7) | 11(16.2) | 0.819 |
| CCB | 39(57.4) | 43(63.2) | 0.483 |
| SBP, mmHg | 148(128, 160) | 154(138, 162) | 0.095 |
| DBP, mmHg | 78(68, 86) | 79 (75, 89) | 0.197 |
HD, hemodialysis; EPO, erythropoietin; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; Data are presented as mean ± SD or median (interquartile range).
Flowchart of the study population. HD, hemodialysis; rhEPO, recombinant human erythropoietin.
Changes in left ventricular parameters and blood pressure after 6 month or treatment
| rhEPO group (n=68) | roxadustat group (n=68) | |||||
|---|---|---|---|---|---|---|
| baseline | 6-month | P value | baseline | 6-month | P value | |
| IVST, cm | 1.18 ± 0.18 | 1.18 ± 0.19 | 0.744 | 1.20(1.07, 1.30) | 1.20(1.03, 1.25) | 0.319 |
| LVEDd, cm | 5.06±0.62 | 4.97±0.61 | 0.080 | 5.16±0.63 | 4.98±0.64 | <0.001 |
| LVPWT, cm | 1.10(0.98, 1.20) | 1.10(0.97, 1.20) | 0.318 | 1.12±0.14 | 1.08±0.13 | <0.001 |
| LVEF | 0.66(0.61, 0.74) | 0.69(0.59, 0.73) | 0.408 | 0.67(0.61, 0.73) | 0.69(0.60, 0.72) | 0.676 |
| LVMI, g/m2 | 135.39 ± 31.89 | 129.21 ± 28.08 | 0.005 | 133.99(122.47, 156.14) | 124.43(105.94, 148.72) | <0.001 |
| SBP, mmHg | 148(128, 160) | 141(128, 160) | 0.806 | 154(138, 162) | 143(127, 155) | <0.001 |
| DBP, mmHg | 78(68, 86) | 78(68, 85) | 0.768 | 81 ± 12 | 75 ± 10 | 0.001 |
IVST, interventricular septal thickness; LVEDd, left ventricular end-diastolic diameter; LVPWT, left ventricular posterior wall thickness; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Effects of Roxadustat and rhEPO on ventricular remodeling in dialysis patients
The changes in the echocardiographic outcomes of the HD population from baseline to 6-months-post treatment are summarized in Table 2. Compared to the baseline, there was a notable decrease in the LVMI of the roxadustat-treated group from 133.99 (122.47, 156.14) (baseline) to 124.43 (105.94, 148.72) g/m2 after the 6-month treatment duration (P < 0.001). The LVEDd and LVPWT also notably decreased from 5.16 ± 0.63 (baseline) to 4.98 ± 0.64 cm after the 6-month roxadustat treatment duration (P < 0.001) and from 1.12 ± 0.14 (baseline) to 1.08 ± 0.13 cm after the 6-month treatment duration (P < 0.001), respectively. The impact of rhEPO on LVMI was significant, decreasing from 135.39 ± 31.89 to 129.21 ± 28.08 g/m2 (P = 0.005), but rhEPO had no significant effect on the other echocardiographic indices. Our data showed that roxadustat was associated with obvious improvements in both SBP and DBP [from 154(138, 162) to 143(127,155) mm Hg (P < 0.001) and from 81 ± 12 to 75 ± 10 mm Hg (P = 0.001), respectively], whereas rhEPO group had no significant changes.
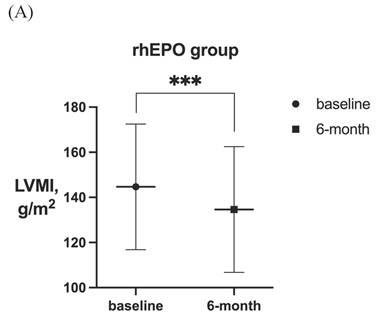
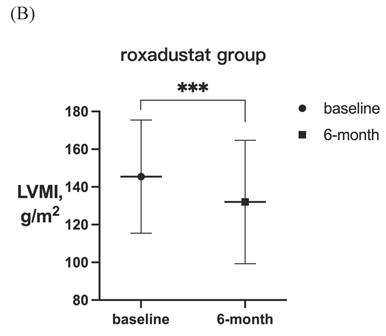
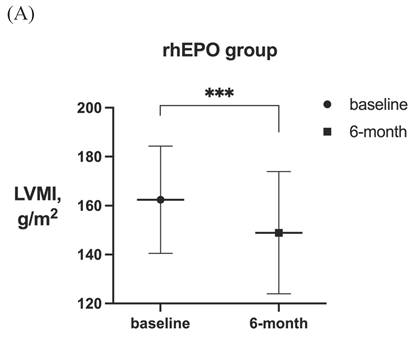
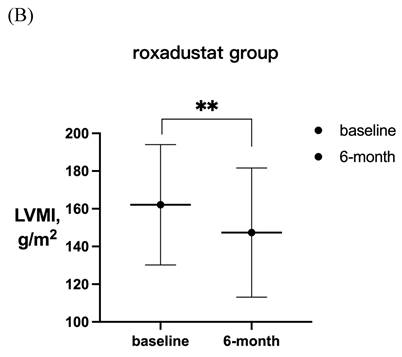
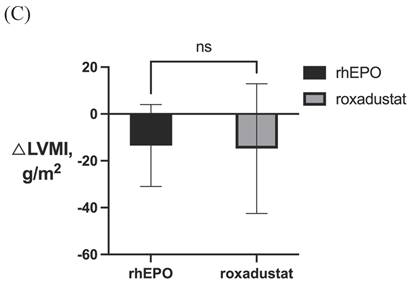
After 6-month treatment, LVMI of 60 patients with LVH in the roxadustat group decreased from 145.46 ± 30.01 g / m2 to 132.03 ± 32.7 g / m2 (P < 0.001). LVMI of 55 patients with LVH in rhEPO group decreased from 144.67 ± 27.85 g / m2 to 134.62 ± 27.85 g / m2 (P < 0.001). There was no significant difference in △LVMI (LVMI difference before and after treatment) between the two interventions in LVH patients (Figure 2). The LVMI of 31 patients with severe LVH was decreased from 162.16 ± 31.92 g/m2 to 147.36 ± 34.23 g/m2 after 6 months of roxadustat treatment (P = 0.006), and the LVMI of 31 patients with severe LVH was reduced from 162.40 ± 21.91 g/m2 to 148.93 ± 24.99g/m2 after using rhEPO (P<0.001). There was no significant difference in △LVMI between the two interventions in patients diagnosed with severe LVH (Figure 3).
Table 3 shows the magnitude of the changes in the echocardiography outcomes in dialysis population. The influences of roxadustat and rhEPO on the evaluation indices of cardiac structure and function, including the changes of LVMI, LVEDd, IVST, LVPWT, and LVEF, were not significantly different between these two groups. However, compared to rhEPO, roxadustat might result in a more significant reduction in the SBP from baseline to 6-month treatment [-12.7 ± 18.8 mm Hg (roxadustat) vs 0.9 ± 24.7 mmHg (rhEPO), P = 0.001]. There was no significant difference in DBP change between the two groups.
Changes in parameters measured by Echocardiography after 6 months treatment
| rhEPO group (n= 68) | roxadustat group (n= 68) | P value | |
|---|---|---|---|
| △IVST, cm | -0.01 ± 0.15 | -0.02 ± 0.17 | 0.530 |
| △LVEDd, cm | -0.09 ± 0.43 | -0.19 ± 0.45 | 0.224 |
| △LVPWT, cm | -0.02 ± 0.16 | -0.03 ± 0.15 | 0.533 |
| △LVEF | 0.01 ± 0.09 | -0.01 ± 0.08 | 0.257 |
| △LVMI, g/m2 | -6.98(-16.81, 6.99) | -8.86(-24.24, 3.58) | 0.297 |
| △SBP, mmHg | -1 ± 24 | -11 ± 19 | 0.011 |
| △DBP, mmHg | -1 ± 16 | -5 ± 12 | 0.078 |
With changes in LVMI as dependent variables, and treatment methods (roxadustat and rhEPO), gender, age, BMI, dialysis course, cardiovascular history, cardiovascular drug history, arterio-venous fistula, changes in SBP, DBP and Hb, as independent variables, bivariate correlation analysis was conducted to analyze the correlation between various clinical variables and the changes in LVMI. Table 4a summarizes the correlation between clinical variables and reverse remodeling. Changes in LVMI were significantly negatively correlated with changes in Hb. Multiple linear regression analysis (Table 4b) showed that the change in Hb was an independent factor influencing the change in LVMI, that is, the improvement of Hb was correlated with the improvement of ventricular remodeling.
Laboratory outcomes
The changes in laboratory indexes before and after the two treatments are shown in Table 5. Compared to baseline, statistically significant improvements in the concentrations of Hb, albumin (Alb), lipoprotein(a) [Lp(a)], unsaturated iron and total iron binding capacity (TIBC) after treatment for 6 months in both treatment groups were observed. Notably, the roxadustat group experienced a significant reduction in the levels of total cholesterol (TC) [ from 3.92(3.27, 4.50) to 3.51(2.89, 4.01) mmol/L, P = 0.01] and low-density lipoprotein (LDL) [ from 1.99(1.61, 2.68) to 1.89(1.45, 2.29) mmol/L, P = 0.014] compared to the baseline. Compared with patients treated with roxadustat, patients treated with rhEPO had a more significant increase in Alb [ 2.66 ± 4.54 mmol/L(rhEPO) vs 1.07 ± 4.6 mmol/L(roxadustat), P = 0.045]. However, roxadustat did not differ significantly from rhEPO in its effect on remaining laboratory indices after the 6-month treatments (Table 5).
Relationships between changes in LVMI and clinical parameters
| Correlation coefficient | P | |
|---|---|---|
| Treatment | -0.132 | 0.126 |
| Age, yr | -0.063 | 0.467 |
| BMI, g/m2 | -0.041 | 0.64 |
| Dialysis course, yr | 0.043 | 0.623 |
| Cardiovascular history | -0.032 | 0.715 |
| Cardiovascular medications | -0.027 | 0.753 |
| Arterio-venous fistula | -0.107 | 0.213 |
| △SBP, mmHg | 0.004 | 0.966 |
| △DBP, mmHg | 0.097 | 0.262 |
| △Hb, g/L | -.0268 | 0.002 |
Relationships between changes in LVMI and clinical parameters in the hemodialysis population according to multiple linear regression analysis
| Regression coefficients | 95.0% CI | P | ||
|---|---|---|---|---|
| (Constant) | 11.689 | -20.169 | 43.548 | 0.469 |
| Treatment | -4.721 | -11.74 | 2.298 | 0.186 |
| Age, yr | -0.052 | -0.325 | 0.221 | 0.709 |
| BMI, g/m2 | 0.065 | -0.789 | 0.92 | 0.88 |
| Dialysis course, yr | 0.272 | -1.338 | 1.881 | 0.739 |
| Cardiovascular history | -6.086 | -27.129 | 14.957 | 0.568 |
| Cardiovascular medications | -3.508 | -11.189 | 4.173 | 0.368 |
| Arterio-venous fistula | -5.918 | -14.296 | 2.46 | 0.165 |
| △SBP, mmHg | -0.137 | -0.34 | 0.066 | 0.185 |
| △DBP, mmHg | 0.261 | -0.06 | 0.582 | 0.11 |
| △Hb, g/L | -0.299 | -0.487 | -0.112 | 0.002 |
95% CI, 95% confidence interval.
LVMI in patients with LVH before and after 6-month treatment in (A) rhEPO group (n =55) and (B) roxadustat group (n=60). (C) Change in LVMI between rhEPO group and roxadustat group. LVMI, left ventricular mass index. * P<0.033, ** P<0.002, *** P<0.001.
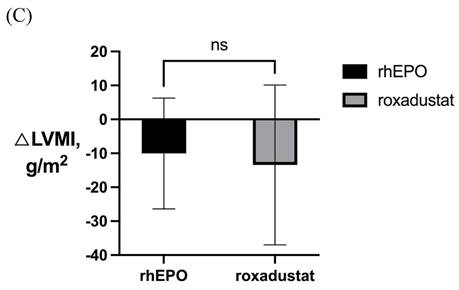
LVMI in patients with severe LVH before and after 6-month treatment in (A) rhEPO group (n=31) and (B) roxadustat group (n=31). (C) Change in LVMI between rhEPO group and roxadustat group. LVMI, left ventricular mass index. * P<0.033, ** P<0.002, *** P<0.001.
Laboratory parameters at baseline and 6 months
| rhEPO group (n=68) | roxadustat group (n=68) | |||||
|---|---|---|---|---|---|---|
| baseline | 6-month | P value | baseline | 6-month | P value | |
| Hb, g/L | 91(82, 95) | 103(95, 112) | <0.001 | 83(73.50, 93.75) | 99(88, 111) | <0.001 |
| Alb, mmol/L | 35.51 ± 5.65 | 38.17 ± 4.61 | <0.001 | 33.38 ± 4.99 | 34.45 ± 5.29 | 0.001 |
| TG, mmol/L | 1.43(0.95, 1.97) | 1.32(0.96, 2.04) | 0.837 | 1.55(1.11, 2.26) | 1.47(1.11, 1.83) | 0.364 |
| TC, mmol/L | 4.09 ± 1.06 | 4.06 ± 1.28 | 0.822 | 3.92(3.27, 4.50) | 3.51(2.89, 4.01) | 0.010 |
| HDL, mmol/L | 1.14(0.87, 1.33) | 1.15(0.95, 1.34) | 0.222 | 1.05(0.85, 1.30) | 0.95(0.80, 1.24) | 0.077 |
| LDL, mmol/L | 2.31 ± 0.77 | 2.26 ± 0.93 | 0.651 | 1.99(1.61, 2.68) | 1.89(1.45, 2.29) | 0.014 |
| Lp(a), mg/L | 236(95, 413) | 186(65, 299) | 0.011 | 228(107, 445) | 195(75, 391) | 0.005 |
| Uric acid, μmol/L | 345.6 ± 120.2 | 351.3 ± 111.3 | 0.691 | 410.0(325.3, 468.0) | 366.9(307.5, 449.8) | 0.460 |
| PTH, pg/mL | 218.6(94.0, 431.3) | 229.8(113.4, 499.5) | 0.319 | 205.9(102.1, 403.7) | 191.0(84.4, 349.6) | 0.935 |
| SF, ug/dL | 132(71.58, 258.92) | 82.25(45.46, 186.25) | 0.001 | 100.11(53.03, 245.02) | 77.1(44.27, 206.93) | 0.004 |
| EPO, mIU/mL | 10.30(8.21, 36.90) | 16.91(7.51, 48.77) | 0.263 | 8.89(5.88, 18.68) | 13.21(7.43, 35.03) | 0.158 |
| SI, ug/dL | 8.64(6.70, 12.01) | 8.8(5.38, 11.75) | 0.559 | 8.31(6.11, 10.10) | 8.8(5.60, 12.86) | 0.209 |
| TIBC, ug/dL | 37.72(27.51, 47.12) | 46.36(35.67, 57.76) | 0.001 | 43.7(29.15, 55.74) | 51.19(44.35, 60.10) | <0.001 |
| Unsaturated iron, ug/dL | 28.94(18.04, 33.50) | 38.81(24.36, 48.80) | <0.001 | 32.71(23.41, 48.08) | 41.35(30.26, 50.57) | 0.002 |
Hb, haemoglobin; Alb, albumin; TG, total triglycerides; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); SF, serum ferritin; SI, serum iron; TIBC, total iron binding capacity.
Discussion
Evidence shows that improving anaemia effectively improves cardiac structure and function(9, 17, 18). Parameters such as LV volume and mass, among others related to cardiac structure and function, can be accurately and conveniently evaluated by echocardiography. It was previously reported that LV hypertrophy (LVH), a deleterious change in cardiac structure and function indicative of CVD, occurs in approximately 75% of ESRD patients receiving long-term dialysis(19). Furthermore, increases in the LV volume were independently and positively related to the incidence of CVD and mortality in ESRD patients, and reductions in the LV mass were also correlated to reductions in cardiovascular events and an increased survival rate in ESRD patients (1). Other studies indicated that LVH might precede adverse cardiovascular events. Therefore, our study selected the left ventricular mass index change as the primary endpoint to assess the cardiovascular prognosis in renal anaemia patients receiving long-term dialysis.
At present, rhEPO is the most common therapeutic agent administered to treat renal anaemia because it stimulates the production of erythrocytes, and the receptors to which it binds are widely expressed in many non-erythrocyte blood cells. However, the excessive administration of rhEPO can lead to non-hematopoietic effects, such as hypertension, thrombosis, vascular remodeling, and even tumour growth. Previously, it was demonstrated that the risk of cardiovascular-related death in dialysis patients increased significantly as the dose of rhEPO increased(20, 21). HIF-PHIs, including roxadustat, represent a novel class of orally available drugs for renal anaemia and can regulate the expression of hypoxia-inducible factor (HIF), promoting the production of endogenous EPO and improving iron absorption and utilization (22). However, current studies related to the clinical efficacy of HIF-PHIs have primarily focused on their effects on the improvement of anaemia and the occurrence of cardiovascular adverse events. There were no studies on the efficacy of these new drugs for improving cardiac hypertrophy (i.e., LVH) in patients with ESRD receiving long-term dialysis. Therefore, we assessed whether targeting anaemia would lead to overall improvements in cardiac structure and function by monitoring the change of echocardiographic indices in patients with renal anaemia treated with roxadustat or rhEPO for six months. We hypothesized that the renal anaemia and metabolic disorders that the patients treated in this study with two different therapeutics might be associated with cardiac dysfunction.
There is a bidirectional interaction between heart and kidney disease in acute and chronic dysfunction. From a pathophysiological perspective, CVD and CKD share many common pathways (23) , such as anaemia, microinflammation, and dyslipidemia, to name a few. In one observational study involving 432 dialysis patients, anaemia was associated with an increase in LVMI (24). Patients with renal anaemia may experience pathological changes in cardiac structure and function after long-term renal replacement therapy due to irreversible reduction of renal function, the persistence of micro-inflammatory states, oxidative stress, the activation of inflammatory pathways, and the role of the sympathetic nervous system. However, any analysis of the association between LVH and anaemia may be confused by other factors affecting the results, such as low blood pressure control and poor cardiac structure (25).
Previous studies confirmed that early-stage LVH was reversible if haemoglobin levels could be increased quickly and effectively(9). It was consistent with previous clinical trials (12) that roxadustat and rhEPO both significantly engendered similar increases in haemoglobin levels in the studied dialysis patients. Although the results demonstrated that both drugs had a positive effect on reducing LVMI, the two drugs did not show clear superiority or inferiority in six months in this study. In addition, our paper found that the LVMI of patients diagnosed with LVH or severe LVH at baseline decreased significantly after 6-month treatments. Further analysis showed that the increase of Hb was an important factor in reversing cardiac remodeling. Therefore, our study shows that cardiac structure and functional parameters may be reversed with the improvement of anaemia. The results further strengthened the hypothesis that improving anaemia would have an overall beneficial effect on improving cardiac structure and function. However, the increase in haemoglobin level could not fully explain the changes in cardiac structure in the dialysis population, as there might be other factors unrelated to anaemia.
There are many complex factors affecting the change of LVMI in the dialysis population. Poor blood pressure control due to overload of capacity, water-sodium retention, and renin-angiotensin-aldosterone system (RAAS) sympathetic excitation are independent risk factors of LVH and cardiac remodeling from which dialysis patients suffer (26, 27). The pathogenesis of hypertension is more complex in dialysis patients than in non-dialysis patients, and the risk of cardiovascular events due to hypertension is higher in dialysis patients. Therefore, blood pressure management is integral in managing a good quality of life and a long-term cardiovascular prognosis in dialysis patients. Notably, our data showed that roxadustat was superior to rhEPO for improving SBP and DBP, suggesting that roxadustat in contrast to rhEPO, had a blood pressure lowering effect in dialysis patients. When blood pressure elevates, resulting in increased left ventricular afterload, adequate cardiac output can be maintained by enhancing myocardial contractility. However, prolonged increased cardiac afterload can lead to myocardial hypertrophy, thickening of muscle fibers, and decreased relative capillary density, ultimately promoting LVH. If hypertension is well controlled, as mentioned above, cardiac pathophysiological changes may be reversed. Therefore, roxadustat is recommended for patients with poor blood pressure control.
Concerning iron metabolism, two therapies both positively impacted serum ferritin and TIBC levels. It was previously demonstrated that HIF-PHI was different from rhEPO and intravenous iron in treating renal anaemia (28). The level of serum EPO in patients receiving HIF-PHIs was lower than that in the patients receiving rhEPO, while HIF-PHIs led to a decrease in the level of hepcidin, improving the utilization of existing iron storage and promoting the absorption of oral iron. Patients would not have to rely on the intravenous administration of high doses of iron, thereby mitigating inflammation and ultimately preventing mortality (29). However, the effect of the two drugs on iron metabolism did not appear to be significantly different in our study, and this result may need to be explored in more trials. It was worth noting that roxadustat was associated with decreased TC and LDL, while the rhEPO group had no significant effect on lipid metabolism, which confirmed the positive effect of HIF-PHIs on lipid metabolism. Current studies(30) have shown that HIF-1 α is involved in regulating the degradation of 3-hydroxy-3-menthyl glutarate mono-acyl-CoA reductase, reducing cholesterol synthesis and regulating lipid metabolism under hypoxia. Therefore, compared with traditional ESA, roxadustat has apparent advantages in terms of mechanism, improving lipid metabolism to a greater extent. In addition, malnutrition can aggravate the degree of anaemia in hemodialysis patients, and severe anaemia further deteriorates the nutritional status of patients. In this study, the serum Alb level of the patients after treatment with both drugs was higher than before, which may be related to the improvement of the patient's appetite after the anaemia was corrected.
Limitations
There are some limitations to this retrospective, single-centre cohort study. Firstly, as treatment is an exposure factor and a clinical decision, the administration of a particular treatment requires the consideration of a variety of confounding factors, such as the age and socioeconomic status of the patient and the severity of their disease. Although no clear difference in the effects of the two drugs on LVMI was observed in this study, it is only a 6-month assessment, and it has not been confirmed whether this difference would not occur at a 12-month assessment. Strict inclusion and exclusion criteria were developed, but there might still be selection bias in the cohorts. Secondly, the follow-up data of some patients are missing, especially data on the levels of hypersensitive C-reactive protein, interleukin-6, and tumor necrosis factor-α, which resulted in the inability to evaluate the influence of the two treatments on microinflammation indicators more comprehensively. Thirdly, although all ultrasound examinations were conducted by qualified technicians, there were also unavoidable measurement errors. Echocardiography may have some limitations in the dialysis population, given the cardiac structural abnormalities in patients receiving long-term dialysis and the changes in volume load during dialysis. Although data on the changes in left ventricular systolic volume were not acquired, LVEF can also evaluate systolic cardiac function well to some extent(31, 32). The study failed to obtain clinical symptoms and adverse side effects from the electronic medical record system, so this paper did not discuss the safety of the drugs.
Conclusion
This study is the first to compare the effects of roxadustat and rhEPO on cardiac structure and function. Although causality was not established in this observational study, extensive analyses were conducted to confirm the effects of anaemia, blood pressure management, and metabolic factors on cardiac structure and clinical outcomes. This paper demonstrated that increased haemoglobin was associated with improved left ventricular diastolic function in dialysis patients, and cardiac hypertrophy due to anaemia is reversible. Roxadustat can simultaneously improve patients' lipid and iron metabolism, with a less effect on blood pressure. Our assessment of subclinical CVD will provide a reference for the cardiovascular safety assessment of HIF-PHIs. Therefore, it is essential to conduct more extensive, multi-centre, randomized controlled trials to evaluate better the impact of roxadustat on cardiac structure and function in the future.
Abbreviations
CKD: chronic kidney disease; CVD: cardiovascular disease; LVH: left ventricular hypertrophy; ESRD: end-stage renal disease; Hb: haemoglobin; ESA: erythrocyte-forming stimulants; LVMI: left ventricular mass index; HIF-PHIs: hypoxia-inducible factor prolyl hydroxylase inhibitors; HD: haemodialysis; rhEPO: recombinant human erythropoietin; STROBE: the Strengthening the Reporting of Observational Studies in Epidemiology; KDIGO: Kidney Disease - Improving Global Outcomes; SBP: systolic blood pressure; DBP: diastolic blood pressure; ACC/AHA: American College of Cardiology/American Heart Association; left ventricular: LV; ASE: American Society of Echocardiography; LVEF: left ventricular ejection fraction; LVEDd: left ventricular end-diastolic diameters; IVST: interventricular septal thickness; LVPWT: left ventricular wall thickness; SD: standard deviation; IQR: interquartile range; BMI: body mass index; CCBs: calcium channel blockers; ACEI: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; Alb: albumin; TG: total triglycerides; TC: total cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Lp(a): lipoprotein(a); SF: serum ferritin; SI: serum iron; TIBC: total iron binding capacity; RAAS: renin-angiotensin-aldosterone system.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82170736, 82370716, 81970629), and the Natural Science Foundation of Zhejiang Province (LHDMY23H070002).
Author contributions
All authors listed made substantial contributions to the article presented here. Research idea and study design: KLM, MYW; data acquisition: MYW, XQL, TTJ, WTL, YH, YLH, FYJ, QZ, QYW; data analysis/interpretation: MYW, XQL, TTJ, WTL; statistical analysis: MYW; draft and revise the article: MYW, KLM; supervision or mentorship: KLM, BCL, XZR, GHW. All authors wrote and approved the final manuscript.
Reporting checklist
The authors have completed the Strengthening the Reporting of Observational Studies in Epidemiology reporting checklist.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhongda Hospital, Southeast University (NO.: 2018ZDSYLL093-P01) and individual consent for this retrospective analysis was waived.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Go A, Chertow G, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-305
2. Tian JP, Wang T, Wang H. et al. The prevalence of left ventricular hypertrophy in Chinese hemodialysis patients is higher than that in peritoneal dialysis patients. Ren Fail. 2008;30(4):391-400
3. McCullough PA, Chan CT, Weinhandl ED. et al. Intensive Hemodialysis, Left Ventricular Hypertrophy, and Cardiovascular Disease. Am J Kidney Dis. 2016;68(5s1):S5-s14
4. Zoccali C, Benedetto F, Mallamaci F. et al. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol. 2004;15(4):1029-37
5. Zoccali C, Benedetto F, Mallamaci F. et al. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65(4):1492-8
6. Walker A, Schneider G, Yeaw J. et al. Anemia as a predictor of cardiovascular events in patients with elevated serum creatinine. Journal of the American Society of Nephrology: JASN. 2006;17(8):2293-8
7. Kaesler N, Babler A, Floege J. et al. Cardiac Remodeling in Chronic Kidney Disease. Toxins (Basel). 2020 12(3)
8. Drüeke T, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071-84
9. Parfrey P, Lauve M, Latremouille-Viau D. et al. Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: a meta-analysis. Clin J Am Soc Nephrol. 2009;4(4):755-62
10. Finkelstein F, Story K, Firanek C. et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(1):33-8
11. Uchida L, Tanaka T, Saito H. et al. Effects of a prolyl hydroxylase inhibitor on kidney and cardiovascular complications in a rat model of chronic kidney disease. Am J Physiol Renal Physiol. 2020;318(2):F388-f401
12. Chen N, Hao C, Liu B. et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381(11):1011-22
13. von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-9
14. group Kaw. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl (2011). 2012;2(4):279-335
15. Whelton PK, Carey RM, Aronow WS. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115
16. Lang RM, Badano LP, Mor-Avi V. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14
17. Hawwa N, Shrestha K, Hammadah M. et al. Reverse Remodeling and Prognosis Following Kidney Transplantation in Contemporary Patients With Cardiac Dysfunction. J Am Coll Cardiol. 2015;66(16):1779-87
18. Ayus JC, Go AS, Valderrabano F. et al. Effects of erythropoietin on left ventricular hypertrophy in adults with severe chronic renal failure and hemoglobin <10 g/dL. Kidney Int. 2005;68(2):788-95
19. Foley RN, Parfrey PS, Harnett JD. et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186-92
20. Smith KJ, Bleyer AJ, Little WC. et al. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59(3):538-48
21. Boyle SM, Berns JS. Erythropoietin and resistant hypertension in CKD. Semin Nephrol. 2014;34(5):540-9
22. Sugahara M, Tanaka T, Nangaku M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int. 2017;92(2):306-12
23. Boudoulas KD, Triposkiadis F, Parissis J. et al. The Cardio-Renal Interrelationship. Prog Cardiovasc Dis. 2017;59(6):636-48
24. Foley R, Parfrey P, Harnett J. et al. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28(1):53-61
25. Eckardt K, Scherhag A, Macdougall I. et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol. 2009;20(12):2651-60
26. Georgianos P, Agarwal R. Aortic Stiffness, Ambulatory Blood Pressure, and Predictors of Response to Antihypertensive Therapy in Hemodialysis. Am J Kidney Dis. 2015;66(2):305-12
27. Kim ED, Sozio SM, Estrella MM. et al. Cross-sectional association of volume, blood pressures, and aortic stiffness with left ventricular mass in incident hemodialysis patients: the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease (PACE) study. BMC Nephrol. 2015;16:131
28. Akizawa T, Iwasaki M, Yamaguchi Y. et al. Phase 3, Randomized, Double-Blind, Active-Comparator (Darbepoetin Alfa) Study of Oral Roxadustat in CKD Patients with Anemia on Hemodialysis in Japan. J Am Soc Nephrol. 2020;31(7):1628-39
29. Haase VH. Hypoxia-inducible factor-prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl (2011). 2021;11(1):8-25
30. Hwang S, Nguyen AD, Jo Y. et al. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem. 2017;292(22):9382-93
31. Bosselmann H, Tonder N, Sölétormos G. et al. Influence of renal impairment on myocardial function in outpatients with systolic heart failure: an echocardiographic and cardiac biomarker study. Int J Cardiol. 2014;177(3):942-8
32. Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):260-74
Author contact
![]() Corresponding author: Kun Ling Ma; E-mail: klmaedu.cn.
Corresponding author: Kun Ling Ma; E-mail: klmaedu.cn.

 Global reach, higher impact
Global reach, higher impact