Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(4):674-680. doi:10.7150/ijms.91804 This issue Cite
Research Paper
Development and validation of a diagnostic nomogram to evaluate tubular atrophy/interstitial fibrosis of IgA nephropathy
Department of Nephrology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
† These authors contributed equally to this work.
Received 2023-11-2; Accepted 2024-1-10; Published 2024-2-4
Abstract
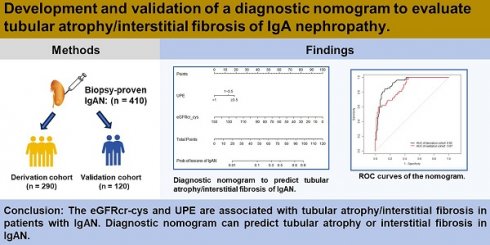
Background: IgA nephropathy (IgAN) is a cause of chronic kidney disease (CKD). Tubular atrophy/interstitial fibrosis is associated with IgAN prognosis. However, simple tools for predicting pathological lesions of IgAN remain limited. Our objective was to develop a tool for evaluating tubular atrophy/interstitial fibrosis in patients with IgAN.
Methods: In this cross-sectional study, 410 biopsy-verified IgAN patients were included. The factors associated with the incident interstitial fibrosis or tubular atrophy in IgAN were confirmed by using logistic regression analysis. A nomogram was developed using logistic regression coefficients to evaluate tubular atrophy or interstitial fibrosis. Receiver operating characteristic curves (ROC) and calibration curves were used to determine the discriminative ability and predictive accuracy of the nomogram.
Results: In this study, the IgAN patients with tubular atrophy or interstitial fibrosis were older and had a higher percentage of males, hypertension and urinary protein excretion (UPE), with high levels of serum cystatin C, serum creatinine, high-sensitivity C-reactive protein and serum C4. The eGFRcr-cys equation calculated using serum creatinine, cystatin C and UPE were considered independent influencing factors of tubular atrophy or interstitial fibrosis in patients with IgAN. Furthermore, the nomogram demonstrated good discrimination (AUC: 0.87, 95% CI 0.81 to 0.93) and calibration in the validation cohort.
Conclusion: The eGFRcr-cys and UPE are associated with tubular atrophy or interstitial fibrosis in patients with IgAN. Diagnostic nomogram can predict tubular atrophy or interstitial fibrosis in IgAN.
Keywords: estimated glomerular filtration rate, IgA nephropathy, nomogram, pathological change
Introduction
Immunoglobulin A nephropathy (IgAN) is a type of glomerulonephritis with a high incidence in the world [1]. IgA deposition in glomeruli is the main characteristic of IgAN [2]. IgA is commonly deposited in the mesangial region of the glomerulus, which induces an inflammatory response that promotes mesangial proliferation and interstitial damage that progresses to end-stage renal disease (ESRD) in nearly 40% of IgAN patients [3]. Patients with IgAN have many different clinical symptoms, ranging from asymptomatic hematuria and proteinuria to massive proteinuria to acute renal failure [4]. Currently, renal biopsy is crucial for the diagnosis and prognosis of IgAN. The Oxford classification is also used in clinical practice to assess the condition of patients with IgAN. Tubular atrophy or interstitial fibrosis lesions are vital indicators of the outcome of IgAN [5, 6]. Kidney puncture is an invasive examination that is difficult to repeat frequently. Simple tools for predicting tubular atrophy or interstitial fibrosis in IgAN are limited.
Estimated glomerular filtration rate (eGFR) is also an indicator of renal function [7]. There was evidence that the equation of eGFR incorporating cystatin C will improve the accuracy of predicting outcomes such as death and ESRD [8, 9]. Cystatin C can also elevate the accuracy of eGFR especially in patients with muscle wasting or chronic illness [10]. The eGFRcr-cys, which uses both markers, including creatinine and cystatin C, has higher accuracy than either eGFRcr or eGFRcys alone [11]. In this process, eGFR is a method frequently used to evaluate the renal function of IgAN, but its value in evaluating pathological lesions was unclear. Therefore, we aimed to explore the value of eGFRcr-cys in IgAN and establish a diagnostic nomogram to predict tubular atrophy or interstitial fibrosis in patients with IgAN.
Methods
Study population and design
This was a cross-sectional study. Patients diagnosed with IgAN by renal biopsy at Sun Yat-sen Memorial Hospital from 2015 to 2023 were included in the study. Patients with secondary IgAN, such as systemic lupus erythematosus, Henoch-Schonlein purpura nephritis, hepatitis B virus-related glomerulonephritis, diabetic nephropathy, and missing data were excluded. Ultimately, 410 patients with IgAN were included and randomly divided into derivation and validation cohorts at a ratio of 7:3.
Clinical and laboratory data
The clinical and laboratory information of the patients included in this study was collected. The clinical indicators included sex, age, height, weight, blood pressure, and disease course. Laboratory data included hemoglobin, serum creatinine, serum cystatin C, serum albumin, high-sensitivity C-reactive protein (hs-CRP), serum immunoglobulin, and complement levels. The BMI was calculated as weight (kg)/height (m2).
The equations of eGFR
Creatinine-based eGFR was calculated using the equation described in previous research [7]. eGFR was calculated using cystatin C and creatinine according to equations described by the chronic kidney disease epidemiology collaboration [11].
Renal pathology evaluation
Light microscopy and immunofluorescence were used to test all IgAN specimens. We used the Oxford Classification Scoring System to assess and classify histologic lesions, including mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), interstitial fibrosis/tubular atrophy (T), and cellular/fibrocellular crescents (C) [12]. At least two pathologists independently evaluated histopathological manifestations.
Statistical analysis
Continuous variables were expressed as mean ± SD or median and interquartile range. Categorical variables were expressed as frequencies and percentages. The Χ2 test was used to compare differences between categorical variables. Binary logistic regression analysis was used to evaluate factors associated with incident interstitial fibrosis or tubular atrophy in IgAN. Nomogram construction was performed according to logistic regression analysis. Receiver operating characteristic (ROC) and calibration curves were used to determine the discriminative ability and predictive accuracy of the nomogram. Statistical Product and Service Solutions (SPSS) version 25.0, and R 4.0.4, were utilized for statistical analyses. P<0.05.
Results
Clinicopathological characteristics of the included patients
The present study included 410 patients with IgAN who met the inclusion criteria. The characteristics of the study population are summarized in Table 1. Among the 410 patients, 133 (32.4%) were male and 277 (67.6%) were female, with a median age of 34 years (range 28-45 years). There were no significant differences between the derivation and validation cohorts (all P > 0.05).
Characteristics in patients with tubular atrophy or interstitial fibrosis of IgAN
In the derivation cohort, 34 (11.7%) and 48 (16.6%) patients were assigned to the T2 and T1 groups, respectively. The remaining patients were classified as T0 group. Table 2 presents the results. The T ≥ 1 group was older, had a higher proportion of male and had hypertension, which was also characterized by a significantly lower level of serum albumin, eGFRcr-cys, serum IgG, and serum IgM, with a higher level of urinary protein excretion (UPE), serum creatinine, serum cystatin C, Hs-CRP and serum C4.
Nomogram development
Logistic regression was used to determine the factors influencing tubular atrophy or interstitial fibrosis in IgAN, as shown in Table 3. eGFRcr-cys is composed of cystatin C, creatinine, age, and sex. Finally, hypertension, UPE, serum albumin, serum IgG, serum C4, and eGFRcr-cys were incorporated into the analysis showed in Figure 2. After adjusting for confounding factors, independent influencing factors were eGFRcr-cys and UPE. Based on the results of the multivariate analysis, we built a nomogram for predicting tubular atrophy or interstitial fibrosis of IgAN (Figure 2). First, we project the values of UPE and eGFRcr_cys onto the positions corresponding to the line of “Points” to get the score of each factor, and then sum them to obtain the total points. Based on the total points projection onto the last row, the risk of tubular atrophy/interstitial fibrosis is calculated. Higher total points indicate an elevated risk of tubular atrophy/interstitial fibrosis.
Nomogram validation
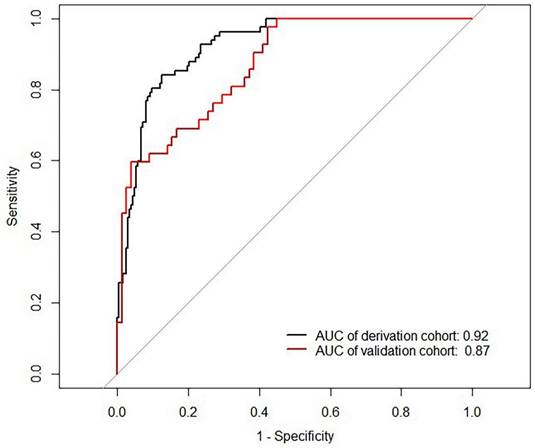
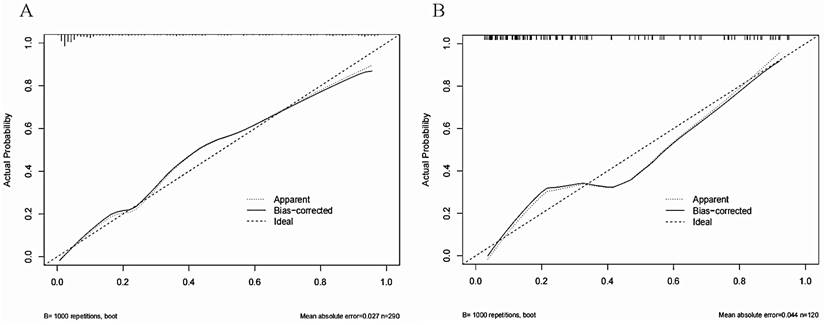
The Area Under Curve was 0.92[ 95% CI (0.90 - 0.95), P< 0.001] in the derivation cohort and 0.87[ 95% CI (0.81 - 0.93), P < 0.001] in the validation cohort (Figure 3). The calibration curves also revealed that our nomogram accurately predicted tubular atrophy or interstitial fibrosis of IgAN (Figure 4A, B).
Baseline characteristics of the patients in the derivation and validation cohorts
| Characteristic | Total (N=410) | Derivation cohort (N=290) | Validation cohort (N=120) | P |
|---|---|---|---|---|
| Age (years) | 34(28, 45) | 34(28, 44) | 34(27, 47) | 0.569 |
| Men, n (%) | 133(32.44) | 93(32.07) | 40(33.33) | 0.803 |
| BMI (kg/m2) | 22.50(20.50, 24.96) | 22.42(20.70, 25.13) | 22.50(20.30, 24.30) | 0.552 |
| Hypertension, n (%) | 135(32.93) | 98(33.79) | 37(30.83) | 0.562 |
| UPE (g/d) | 0.182 | |||
| <1 | 261(63.66) | 185(63.79) | 76(63.33) | |
| 1-3.5 | 118(28.78) | 79(27.24) | 39(32.50) | |
| ≥3.5 | 31(7.56) | 26(8.97) | 5(4.17) | |
| Hematuria (RBCs/ul) | 54.00(20.00, 149.25) | 54.00(18.00, 150.00) | 61.00(22.25, 146.25) | 0.341 |
| Serum creatinine (μmol/L) | 89.00(71.75, 125.00) | 89.00(71.00, 125.00) | 89.00(73.00, 121.00) | 0.632 |
| Serum cystatin C | 1.00(0.82, 1.37) | 0.97(0.82, 1.36) | 1.02(0.84, 1.39) | 0.504 |
| Serum albumin (g/L) | 36.40(32.70, 40.02) | 36.75(32.78, 40.30) | 35.60(32.60, 39.48) | 0.272 |
| Hs-CRP (mg/L) | 0.78(0.32, 1.99) | 0.80(0.31, 1.99) | 0.66(0.33, 2.01) | 0.936 |
| eGFRcr-cys (mL/min/1.73m2) | 80.44(50.48, 103.30) | 81.27(50.30, 104.00) | 78.60(54.69, 101.44) | 0.552 |
| Serum IgG (g/L) | 11.80(9.55, 13.80) | 11.90(9.77, 13.83) | 11.60(9.35, 13.48) | 0.307 |
| Serum IgA (g/L) | 3.16(2.51, 3.99) | 3.15(2.49, 3.98) | 3.18(2.60, 4.07) | 0.744 |
| Serum IgM (g/L) | 1.08(0.79, 1.47) | 1.08(0.80, 1.43) | 1.04(0.76, 1.55) | 0.938 |
| Serum C3 (mg/L) | 1030.00(916.75, 1170.00) | 1040.00(920.00, 1170.00) | 1005(895, 1178) | 0.785 |
| Serum C4 (mg/L) | 246.00(201.00, 305.25) | 247.50(203.00, 305.00) | 243(198, 308) | 0.809 |
| Oxford MEST-C, n (%) | ||||
| M1 | 385(93.90) | 270(93.10) | 115(95.8) | 0.293 |
| E1 | 81(19.80) | 58(20.00) | 23(19.2) | 0.874 |
| S1 | 196(47.80) | 137(47.20) | 59(49.2) | 0.723 |
| T1/2 | 124(30.20) | 82(28.30) | 42(35.0) | 0.177 |
| C1/2 | 221(53.90) | 149(51.40) | 72(60.0) | 0.111 |
Results are presented as mean SD, median (interquartile range), or number (percentage).
Groups were compared by using Kruskal-Wallis or F test
BMI body mass index; UPE urinary protein excretion; eGFR estimated glomerular filtration rate; hs-CRP high-sensitivity C-reactive protein; MEST-C, M mesangial hypercellularity; E endocapillary hypercellularity; S segmental glomerulosclerosis; T interstitial fibrosis/tubular atrophy; C crescent formation.
Baseline pathological characteristics stratified by Oxford T lesions of IgAN patients
| Variables | T0 (n ) ) | T1/2(n ) ) | P |
|---|---|---|---|
| Age (years) | 33(27, 42) | 37(31, 46) | 0.018 |
| Men, n (%) | 59(28.37) | 34(41.46) | 0.023 |
| BMI (kg/m2) | 22.15(20.53, 24.80) | 23.35(21.08, 25.80) | 0.072 |
| Hypertension, n (%) | 46(22.12) | 52(63.41) |  0.001 0.001 |
| UPE (g/d) |  0.001 0.001 | ||
| <1 | 164(78.84) | 21(25.61) | |
| 1-3.5 | 38(18.27) | 41(50.00) | |
| ≥3.5 | 6(2.88) | 20(24.39) | |
| Hematuria (RBCs/ul) | 58.00(19.00,176.50) | 45.00(14.75,104.00) | 0.205 |
| Serum creatinine (μmol/L) | 78.00(66.00, 96.00) | 17.50(117.75, 229.75) |  0.001 0.001 |
| Serum albumin (g/L) | 37.50(34.20, 40.55) | 34.60(30.28, 38.80) |  0.001 0.001 |
| Serum cystatin C | 0.88(0.75, 1.07) | 1.61(1.28, 2.43) |  0.001 0.001 |
| Hs-CRP (mg/L) | 0.69(0.30, 1.74) | 0.96(0.34, 3.06) | 0.094 |
| eGFRcr-cys (mL/min/1.73m2) | 95.39(77.55, 108.14) | 40.21(22.08, 61.42) |  0.001 0.001 |
| Serum IgG (g/L) | 12.15(10.23, 13.88) | 10.85(8.48, 13.83) | 0.008 |
| Serum IgA (g/L) | 3.25(2.51, 3.98) | 2.89(2.46, 4.03) | 0.296 |
| Serum IgM (g/L) | 1.12(0.82, 1.49) | 0.97(0.71, 1.37) | 0.049 |
| Serum C3 (mg/L) | 1040.00(929.25, 1170.00) | 1030.00(916.25, 1142.50) | 0.677 |
| Serum C4 (mg/L) | 237.00(198.00, 289.00) | 278.00(222.75, 336.75) |  0.001 0.001 |
Results are presented as mean SD, median (interquartile range), or number (percentage).
Groups were compared by using Kruskal-Wallis or F test
BMI body mass index; UPE urinary protein excretion; eGFR estimated glomerular filtration rate; hs-CRP high-sensitivity C-reactive protein; MEST-C, M mesangial hypercellularity; E endocapillary hypercellularity; S segmental glomerulosclerosis; T interstitial fibrosis/tubular atrophy; C crescent formation.
Analysis of influencing factors about Oxford T lesions in derivation cohort
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 1.02 | (1.00-1.04) | 0.05 | |||
| Gender (Men/Women) | 0.56 | (0.33, 0.96) | 0.03 | |||
| Hypertension | 6.10 | (3.53-10.76) | <0.001 | 1.16 | (0.51-2.58) | 0.72 |
| UPE(g/d) | <0.001 | |||||
| <1 | 1 | 1 | ||||
| 1-3.5 | 8.43 | (4.53, 16.14) | <0.001 | 3.49 | (1.53 -8.05) | 0.003 |
| ≥3.5 | 26.03 | (9.91, 78.17) | <0.001 | 6.98 | (1.63 - 34.66) | 0.01 |
| Serum creatinine | 1.03 | (1.02, 1.04) | <0.001 | |||
| Serum albumin | 0.94 | (0.90, 0.97) | 0.001 | 1.03 | (0.97-1.09) | 0.31 |
| Serum cystatin C | 13.52 | (6.81, 30.15) | <0.001 | |||
| Hs-CRP | 1.00 | (0.98, 1.02) | 0.820 | |||
| eGFRcr-cys | 0.94 | (0.92, 0.95) | <0.001 | 0.95 | (0.93-0.96) |  |
| Serum IgG | 0.93 | (0.86, 1.01) | 0.085 | |||
| Serum IgM | 0.70 | (0.40, 1.17) | 0.181 | |||
| Serum C4 | 1.00 | (1.00, 1.01) | 0.001 | 1.00 | (0.97- 1.01) | 0.66 |
UPE urinary protein excretion; hs-CRP high-sensitivity C-reactive protein; eGFR estimated glomerular filtration rate
ORs for tubular atrophy/interstitial fibrosis associated with eGFRcr-cys and UPE. ORs are from a multivariable-adjusted model including hypertension, serum albumin, serum IgG, serum C4, eGFRcr-cys and UPE. Boxes in the graph represent the OR and lines/arrows represent the 95% CI.
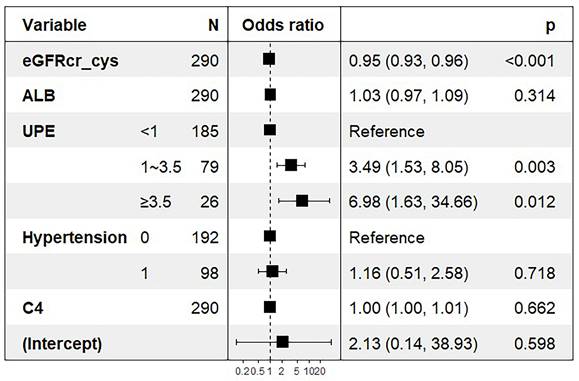
Diagnostic nomogram. To use the nomogram, draw a line perpendicular from the corresponding axis of each risk factor until it reaches the top line labeled “Points”. Sum up the number of points for all risk factors then draw a line descending from the axis labeled “Total points” until it determined the probabilities of tubular atrophy/interstitial fibrosis.
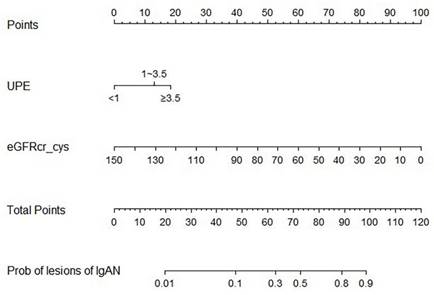
ROC curves of the nomogram. AUC: area under the curve.
The calibration curves of the nomogram for predicting the T1/2 lesions of IgAN patients at (A) in the derivation cohort and (B) in the validation cohort. The X-axis represents the nomogram-predicted probability of diagnosis, and the Y-axis represents the actual probability estimated with the logistic regression methods.
Discussion
The equation of eGFRcr-cys can estimate GFR more accurately than the equation using cystatin C or creatinine alone, and the differences from measured GFR are smaller [13]. In this study, we explored the value of eGFRcr-cys in patients with IgAN for Oxford T lesions. IgAN patients with decreased basal eGFRcr-cys had a high risk of renal fibrosis or tubular atrophy.
IgAN is a series of diseases mainly characterized by immune complex deposition and cell proliferation in the glomerular mesangial area [14]. Oxford T lesions, including T0/T1/T2, are important pathological features of IgAN, as evaluated by the Oxford classification. Studies have confirmed that Oxford T lesions are an independent risk factor for IgAN prognosis of IgAN[15, 16]. MEST score and clinical data can be used to predict the risk of IgAN patients [17]. Currently, Oxford T lesions can be determined through renal biopsy, which is invasive and difficult to repeat in IgAN patients [18]. IgAN is a chronic disease with variable clinical outcomes. The international glomerulonephritis guidelines recommend that the management of IgAN should focus on supportive care to slow disease progression of the disease [19]. In this process, eGFR is frequently used to evaluate the renal function of IgAN, but its value in evaluating pathological lesions is unclear.
eGFR is a common indicator used to assess kidney function in clinical practice worldwide. A decline in GFR to 60 ml/min/1.73 m2 or lower for 3 months or longer is a criterion to diagnose chronic kidney disease (CKD), which is also related to adverse outcomes including death [20, 21]. Thomas Knoop.et al found that inclusion of eGFR and age in prognostic models of ESRD and death can improve model accuracy in IgAN patients [22]. The eGFRcr equation is commonly used worldwide in clinical practice. However, a larger bias in the eGFRcr equations was observed in GFR estimates for different ethnic groups, which led to differences in the estimates of GFR stage [13]. Patient age, sex, and muscle mass may affect serum creatinine values [23]. Therefore, the function of the kidney based on serum creatinine level may be overestimated because of poor nutritional status and reduced muscle [24, 25]. Thio et al. found that a genetic risk score is strongly related to baseline CKD and eGFR independent of known risk factors, which may suggest the underlying genetics of renal function rather than creatinine metabolism or underlying etiology [26].
Compared to serum creatinine, cystatin C levels are not affected by factors such as sex and muscle mass [27]. Studies have found that cystatin C provides more accurate results in patients with an eGFRcr of 60 to 74 ml/minute/1.73 m2 without proteinuria, muscle wasting, or chronic disease [10]. Fleming et al. also found that serum cystatin C is a more useful biomarker for estimating eGFR in an observational cohort study, especially for patients with baseline eGFR< 60ml/min/1.73m2 [28]. Studies have found that eGFRcr-cys equations minimize inaccuracy in both ethnic groups, and the use of eGFRcr-cys may enhance the accuracy of CKD diagnosis and GFR staging [13]. The new eGFRcr-cys equation excluding race is more accurate in measuring GFR than equations using creatinine or cystatin C levels alone and resulted in lesser differences from measured GFR [11, 13]. However, it has not been extensively studied in patients with IgAN. This study found that the equation of eGFRcr-cys is an independent influencing factor for Oxford T lesions in patients with IgAN. Thus, eGFRcr-cys may be a useful marker for predicting Oxford T lesions in IgAN.
Predicting pathological lesions in IgAN patients is challenging because IgAN is highly heterogeneous. Thus, there is an urgent need to develop a simple tool to predict IgAN. In this study, we found that eGFRcr-cys and UPE were independent risk factors for IgAN with T lesions. Proteinuria is a widely studied risk factor for the progression to ESRD in patients with IgAN. Thompson et al. suggested the reduction of proteinuria as a surrogate endpoint in trials of IgAN[29]. We established a diagnostic nomogram based on the results of the logistic regression model. In the validation cohort, the Area Under Curve of the nomogram was 0.87 (95% CI 0.81 - 0.93, P < 0.001), indicating that our diagnostic nomogram had great internal validation and performed well in discrimination and calibration. This may provide vital clues for predicting T lesions of IgAN and may be useful in clinical practice.
Our study has some limitations. A causal relationship could not be drawn because of the cross-sectional design. Moreover, our data were all from renal biopsy patients, and some patients initially did not undergo renal biopsy for some reason. Therefore, missing data and selection bias might have been present in our study.
In conclusion, eGFRcr-cys and UPE were associated with tubular atrophy or interstitial fibrosis in patients with IgAN. Diagnostic nomograms can predict tubular atrophy or interstitial fibrosis in patients with IgAN.
Acknowledgements
Funding
This work was funded by the National Science Foundation of China (No. 82270743).
Author contributions
Yang designed the study; Yangang Gan, Yihuan Cai, Jiajia Li, Jianping Wu, Rui Zhang, Qianqian Han, and Wenchao Li collected data; Qiongqiong Yang, Yangang Gan, and Yihuan Cai analyzed and interpreted the data and drafted the manuscript; Yangang Gan and Yihuan Cai contributed equally to this work. All the authors have read and approved the manuscript for submission.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The source data of the article is available upon request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709-27
2. Rodrigues JC, Haas M, Reich HN. IgA Nephropathy. Clinical journal of the American Society of Nephrology: CJASN. 2017;12:677-86
3. Schena FP, Cerullo G, Rossini M, Lanzilotta SG, D'Altri C, Manno C. Increased risk of end-stage renal disease in familial IgA nephropathy. J Am Soc Nephrol. 2002;13:453-60
4. D'Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24:179-96
5. Chen T, Li X, Li Y, Xia E, Qin Y, Liang S. et al. Prediction and Risk Stratification of Kidney Outcomes in IgA Nephropathy. Am J Kidney Dis. 2019;74:300-9
6. Tanaka S, Ninomiya T, Katafuchi R, Masutani K, Tsuchimoto A, Noguchi H. et al. Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin J Am Soc Nephrol. 2013;8:2082-90
7. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-12
8. Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR. et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932-43
9. Stevens PE, Levin A, Kidney Disease. Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825-30
10. Stevens LA, Padala S, Levey AS. Advances in glomerular filtration rate-estimating equations. Curr Opin Nephrol Hypertens. 2010;19:298-307
11. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20-9
12. Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M. et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014-21
13. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y. et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737-49
14. Working Group of the International Ig ANN, the Renal Pathology S, Cattran DC, Coppo R, Cook HT, Feehally J. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534-45
15. Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J. et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828-36
16. Zhu X, Li H, Liu Y, You J, Qu Z, Yuan S. et al. Tubular atrophy/interstitial fibrosis scores of Oxford classification combinded with proteinuria level at biopsy provides earlier risk prediction in lgA nephropathy. Sci Rep. 2017;7:1100
17. Barbour SJ, Espino-Hernandez G, Reich HN, Coppo R, Roberts IS, Feehally J. et al. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int. 2016;89:167-75
18. Capretz T, Patel RM, Okhunov Z. Percutaneous renal biopsy: approach, diagnostic accuracy and risks. Curr Opin Urol. 2018;28:369-74
19. Kidney Disease. Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1-S276
20. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A. et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341-52
21. Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-81
22. Knoop T, Vagane AM, Vikse BE, Svarstad E, Magnusdottir BT, Leh S. et al. Addition of eGFR and Age Improves the Prognostic Absolute Renal Risk-Model in 1,134 Norwegian Patients with IgA Nephropathy. Am J Nephrol. 2015;41:210-9
23. Gounden V, Bhatt H, Jialal I. Renal Function Tests. StatPearls. Treasure Island (FL). 2022
24. Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933-53
25. Yoo JJ, Kim SG, Kim YS, Lee B, Lee MH, Jeong SW. et al. Estimation of renal function in patients with liver cirrhosis: Impact of muscle mass and sex. J Hepatol. 2019;70:847-54
26. Thio CHL, van der Most PJ, Nolte IM, van der Harst P, Bultmann U, Gansevoort RT. et al. Evaluation of a genetic risk score based on creatinine-estimated glomerular filtration rate and its association with kidney outcomes. Nephrol Dial Transplant. 2018;33:1757-64
27. Kar S, Paglialunga S, Islam R. Cystatin C Is a More Reliable Biomarker for Determining eGFR to Support Drug Development Studies. J Clin Pharmacol. 2018;58:1239-47
28. Fleming S, Perera-Salazar R, Taylor KS, Jones L, Hobbs FR, James T. et al. Frequency of Renal Monitoring - Creatinine and Cystatin C (FORM-2C): an observational cohort study of patients with reduced eGFR in primary care. Br J Gen Pract. 2021;71:e677-e84
29. Thompson A, Carroll K, L AI, Floege J, Perkovic V, Boyer-Suavet S. et al. Proteinuria Reduction as a Surrogate End Point in Trials of IgA Nephropathy. Clin J Am Soc Nephrol. 2019;14:469-81
Author contact
![]() Corresponding author: Qiongqiong Yang. Email: yangqqsysu.edu.cn; Address: Department of Nephrology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510000, China.
Corresponding author: Qiongqiong Yang. Email: yangqqsysu.edu.cn; Address: Department of Nephrology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510000, China.

 Global reach, higher impact
Global reach, higher impact