3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(3):540-546. doi:10.7150/ijms.90273 This issue Cite
Research Paper
Prescription of glucagon-like peptide 1 agonists and risk of subsequent open-angle glaucoma in individuals with type 2 diabetes mellitus
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Department of Ophthalmology, Changhua Christian Hospital, Changhua, Taiwan.
3. Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan.
4. Department of Ophthalmology, Cathay General Hospital, Taipei, Taiwan.
5. Departments of Ophthalmology, Sijhih Cathay General Hospital, New Taipei City, Taiwan.
6. School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan.
7. Department of Ophthalmology, Nobel Eye Institute, Taipei, Taiwan.
8. Department of Optometry, Da-Yeh University, Chunghua 515, Taiwan.
9. Department of Ophthalmology, Jen-Ai Hospital Dali Branch, Taichung, Taiwan.
10. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
11. Program for Cancer Biology and Drug Discovery, China Medical University, Taichung, Taiwan.
12. Department of Family and Community Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan.
13. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Received 2023-9-18; Accepted 2024-1-3; Published 2024-1-12
Abstract
Background: The glucagon-like peptide 1 receptor agonist (GLP-1RA) is an antidiabetic medication with vascular protection and anti-inflammatory properties. Theoretically, the use of GLP-1RA should inhibit the development of open-angle glaucoma (OAG) as both vascular damage and inflammation are associated with OAG. Therefore, our objective was to investigate the association between the application of GLP-1RA and the subsequent OAG in individuals with type 2 diabetes mellitus (T2DM).
Methods: We conducted a retrospective cohort study by using data from the National Health Insurance Research Database (NHIRD) of Taiwan. Participants with T2DM were divided into those who used GLP-1RA and those who did not, forming the GLP-1RA and control groups. The primary outcome was the occurrence of OAG based on diagnostic codes. Cox proportional hazard regression was employed to calculate the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for OAG.
Results: 91 patients in the control group developed OAG, and 40 patients in the GLP-1RA group developed OAG. After adjustment for all covariates, the GLP-1RA group exhibited a significantly lower incidence of OAG compared with the control group (aHR: 0.712, 95% CI: 0.533-0.936. P = 0.0025). In the subgroup analyses, the association between GLP-1RA use and OAG incidence was more pronounced in patients with T2DM using GLP-1RA and aged younger than 60 years (P = 0.0438).
Conclusion: The prescription of GLP-1RA is associated with a lower incidence of subsequent OAG in individuals with T2DM, and this association was more significant in patients with T2DM under the age of 60 years.
Keywords: glucagon-like peptide 1 receptor agonist, open angle glaucoma, epidemiology, age, vascular
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance, resulting in hyperglycemia [1]. In advanced stages, T2DM can result in several comorbidities such as acute myocardial infarction, cerebrovascular disease, and diabetic retinopathy [2, 3]. Current management strategies for T2DM primarily involve the use of antidiabetic medications, with insulin injection reserved for more severe cases [4]. Among the antidiabetic medicines, glucagon-like peptide 1 receptor agonists (GLP-1RA) have been demonstrated to effectively reduce glycated hemoglobin levels by 2% [5].
In addition to its antihyperglycemic effects, GLP-1RA exhibits protective effects on various other organs [5]. The use of GLP-1RA has been shown to preserve the kidney function in T2DM patients [6, 7], and the use of GLP-1RA can also reduce the incidences of all-cause mortality, major adverse cardiovascular event and obesity [8, 9]. Studies have indicated that GLP-1RA can influence the development and progression of various ocular diseases, particularly those featuring neurosensory impairment [10, 11]. An experimental study demonstrated that the application of GLP-1RA can mitigate neurodegeneration in the retina [11]. GLP-1RA is associated with a reduction in the incidence of diabetic retinopathy as well as a decrease in retinal vascular leakage and damage to the blood-retinal barrier [12, 13]. However, another study reported a nonsignificant association between the use of GLP-1RA and retinal angiogenesis [10]. Additionally, patients with T2DM using GLP-1RA or sodium-glucose transport protein 2 inhibitors exhibit a significantly lower rate of dry eye disease than those using other antidiabetic medications such as metformin monotherapy [14, 15]. However, evidence regarding the relationship between GLP-1RA use and the incidence of open-angle glaucoma (OAG) is limited. Given the vascular-protective effects of GLP-1RA and the relationship between OAG and impaired ocular vasculature [16, 17], a relationship between GLP-1RA use and OAG incidence may exist.
This study investigated the relationship between GLP-1RA use and the incidence of OAG. The other risk factors for OAG development were also adjusted for in the analysis model.
Materials and Methods
Data Source
The current study adhered to the principles of the Declaration of Helsinki as revised in 1964 and its subsequent amendments. This study was approved by both the National Health Insurance Administration of Taiwan and Institutional Review Board of Chung Shan Medical University (Project code: CS1-20108). The requirement for written informed consent was waived by both institutions. We used data from the Taiwan National Health Insurance Research Database (NHIRD), which contains the data of approximately 23 million individuals in Taiwan for the period from January 1, 2015, to December 31, 2020. The accessible data in this Taiwan NHIRD include International Classification of Diseases-Ninth Revision (ICD-9) diagnostic codes, International Classification of Diseases-Tenth Revision (ICD-10) diagnostic codes, age, sex, income level, education level, urbanization level, occupation type, laboratory examination codes, medical department codes, surgery codes, image examination codes, procedure codes, and the international Anatomical Therapeutic Chemical (ATC) codes for medicines.
Participant Selection
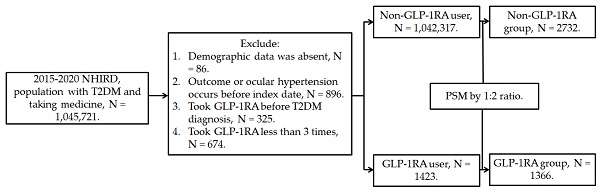
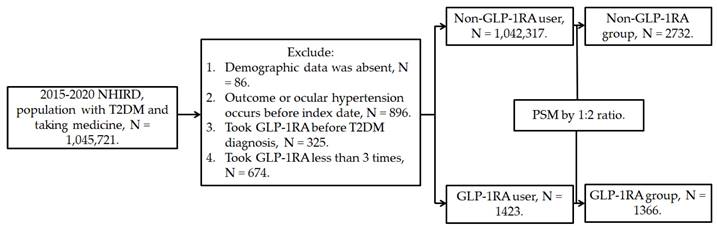
This retrospective population-based cohort study identified individuals with T2DM who used GLP-1RA based on the following criteria: (1) a diagnosis of T2DM according to ICD-9 and ICD-10 codes from 2015 to 2019, (2) follow-up appointments in either family medicine or internal medicine departments for more than 3 months based on department codes, and (3) the prescription of GLP-1RA, including exenatide and liraglutide, according to the relevant ATC codes. The index date was set 6 months after the initiation of GLP-1RA treatment. Furthermore, the following exclusion criteria were applied to enhance the homogeneity of the study population: (1) absence of demographic data, (2) presence of glaucoma or ocular hypertension before the index date, (3) use of GLP-1RA before the T2DM diagnosis, and (4) fewer than three prescriptions of GLP-1RA. For comparison, each individual with T2DM using GLP-1RA was matched to two individuals with T2DM who did not use GLP-1RA, and the latter group of individuals served as the control group. The propensity score-matching (PSM) method was adopted to match the two groups through the adjustment of demographic data, systemic disease factors, and medicine covariates. Following the selection process, a total number of 1366 and 2732 participants with T2DM were included in the GLP-1RA group and the control group, respectively. The patient selection flowchart is presented in Figure 1.
Primary Outcome
The primary outcome in this study was the incidence of newly developed OAG, and it was determined on the basis of the following criteria: (1) diagnosis of OAG according to related ICD-9 and ICD-10 diagnostic codes; (2) use of slit-lamp biomicroscopy and fundoscopic examinations before or at the same time as OAG diagnoses, as indicated by procedure codes; (3) use of optical coherence tomography or visual field examinations before or at the same time as OAG diagnoses, identified through examination codes; (4) use of topical or systemic antiglaucomatous medications after receiving the diagnosis of OAG, as documented using ATC codes; and (5) OAG diagnoses confirmed by an ophthalmologist. The individuals with T2DM in the current study were followed up until one of the following conditions occurred: (1) incidents of OAG, (2) participant withdrawal from the National Health Insurance program, or (3) the end of the NHIRD data collection period on December 31, 2020.
Flowchart of participant selection. NHIRD: National Health Insurance Research Database, N: number, T2DM: type 2 diabetes mellitus, GLP-1RA: glucagon-like peptide 1 receptor agonist, PSM: propensity score-matching.
Confounders
In our statistical analysis, we accounted for various demographic factors, systemic comorbidities, and medications to control for confounders that might influence the incidence of OAG: age, sex, urbanization level, hypertension, hyperlipidemia, ischemic heart diseases, ischemic stroke, hemorrhagic stroke, peripheral vascular disease, kidney disease, as well as medications such as sulfonylureas, biguanides which indicates the metformin, thiazolidinediones, alpha glucosidase inhibitors, dipeptidyl peptidase-4 inhibitor, insulin, statin and corticosteroids. These factors were identified based on ICD-9 and ICD-10 diagnostic codes as well as ATC codes in the NHIRD. The number of systemic diseases was incorporated into the adapted diabetes complications severity index (aDCSI) by using difference scores. To ensure that these confounders sufficiently influenced the risk of OAG, only factors with a disease or prescription interval exceeding 2 years prior to the index date were included in statistical analyses. Because nearly all the GLP-1RA users in our study were concurrently taking metformin as their antidiabetic treatment (~93%), the resulting high collinearity between GLP-1RA and metformin led to prominent statistical bias or error in the initial statistical analysis. Consequently, we did not include metformin as a covariate in our final statistical analysis.
Statistical Analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). Descriptive analyses were used to present basic demographic information, aDCSI, and related medications for both the groups. The absolute standardized difference (ASD) was calculated to compare the differences between the two groups, with an ASD >0.1 considered to be statistically significant. Following descriptive analyses, Cox proportional hazard regression was used to calculate the adjusted hazard ratios (aHR) with corresponding 95% confidence intervals (CIs) for determining the OAG incidence between the GLP-1RA group and control group. The demographic features, systemic morbidities, and medications were all adjusted for in the Cox proportional hazard regression. In subgroup analyses, patients with T2DM were categorized by age and sex, and the Cox proportional hazard regression was applied again to compare the aHR and 95% CI for OAG among different subgroups. Furthermore, interaction tests were conducted to illustrate the influence of GLP-1RA on OAG development in different subgroups. Statistical significance was set at P < 0.05, and a P value of <0.0001 was presented as P < 0.0001.
Results
Table 1 presents the basic characteristics of the GLP-1RA users and non-GLP-1RA users. The distribution of age and sex was similar between the two groups, which was due to PSM. Additionally, the urbanization level and the distribution of aDCSI were similar (ASD < 0.1). Regarding medications, a higher proportion of GLP-1RA users had insulin application than non-GLP-1RA users (36.44% versus 5.21%; ASD: 0.2148). However, the ratios of other medications were similar between the two groups (all ASD < 0.1; Table 1).
Clinical characteristics of the GLP-1RA users and the non-GLP-1RA population
| Characteristic | Non-GLP-1RA (N = 2732) | GLP-1RA (N = 1366) | ASD |
|---|---|---|---|
| Age (years) | 0.0235 | ||
| 20-39 | 703(25.73%) | 428 (31.33%) | |
| 40-49 | 852 (31.20%) | 374 (27.38%) | |
| 50-59 | 774 (28.33%) | 366 (26.79%) | |
| 60-69 | 345 (12.62%) | 153 (11.20%) | |
| 70-79 | 38 (1.39%) | 36 (2.64%) | |
| ≥80 | 20 (0.73%) | 9 (0.66%) | |
| Sex | 0.0000 | ||
| Male | 1540 (56.40%) | 770 (56.40%) | |
| Female | 1192 (43.60%) | 596 (43.60%) | |
| Urbanization | 0.0019 | ||
| 1 | 674 (24.67%) | 412 (30.14%) | |
| 2 | 906 (33.16%) | 453 (33.18%) | |
| 3 | 593 (21.71%) | 236 (17.29%) | |
| ≥4 | 559 (20.46%) | 265 (19.39%) | |
| aDCSI score# | 0.0556 | ||
| 0 | 1996 (73.04%) | 807 (59.09%) | |
| 1 | 426 (15.60%) | 285 (20.88%) | |
| 2 | 229 (8.37%) | 171 (12.51%) | |
| 3 | 53 (1.94%) | 69 (5.08%) | |
| 4 | 18 (0.67%) | 19 (1.40%) | |
| ≥5 | 10 (0.37%) | 15 (1.05%) | |
| Comedication | |||
| Biguanides | 2244 (82.15%) | 1262 (92.41%) | 0.0137 |
| Sulfonylureas | 844 (30.89%) | 581 (42.55%) | 0.0624 |
| Alpha glucosidase inhibitors | 96 (3.53%) | 110 (8.03%) | 0.0596 |
| Thiazolidinediones | 136 (4.98%) | 144 (10.52%) | 0.0723 |
| Dipeptidyl peptidase-4 | 655 (23.97%) | 695 (50.86%) | 0.0867 |
| Insulin | 142 (5.21%) | 498 (36.44%) | 0.2148* |
| Statin | 1072 (39.24%) | 769 (56.27%) | 0.0629 |
| Corticosteroids | 466 (17.06%) | 255 (18.67%) | 0.0533 |
GLP-1RA: glucagon-like peptide 1 receptor agonist, N: number, ASD: absolute standard difference, aDCSI: adapted diabetes complications severity index
* Significant difference between the two groups
Over the entire follow-up period, 91 and 40 patients had OAG in the non-GLP-1RA and GLP-1RA groups, respectively. The GLP-1RA group exhibited a significantly lower incidence of OAG than the control group after adjustment for all the confounders (aHR: 0.712, 95% CI: 0.533-0.936. P = 0.0025; Table 2). In subgroup analyses, patients with T2DM taking GLP-1RA, regardless of age (both younger and older than 60 years), exhibited a significantly lower incidence of OAG than the non-GLP-1RA users (both upper limits of 95% CI lower than 1). Moreover, the association between GLP-1RA use and OAG incidence was more pronounced in the patients with T2DM taking GLP-1RA and those aged younger than 60 years (P = 0.0438). However, the association between GLP-1RA use and OAG incidence did not significantly differ between sex subgroups (P = 0.5621; Table 3).
Incidence of glaucoma between the GLP-1RA and non-GLP-1RA groups
| Event | Non-GLP-1RA group | GLP-1RA group | P |
|---|---|---|---|
| Person-months | 116,292 | 59,078 | |
| Event | 91 | 40 | |
| cHR (95% CI) | Reference | 0.768 (0.598-1.059) | |
| aHR (95% CI) | Reference | 0.712 (0.533-0.936)* | 0.0025* |
GLP-1RA: glucagon-like peptide 1 receptor agonist, aHR: adjusted hazard ratio, CI: confidence interval, DME: diabetic macular edema, PDR: proliferative diabetic retinopathy
Results of the subgroup analyses of glaucoma development, stratified by age and sex
| Subgroup | aHR | 95% CI | P for interaction |
|---|---|---|---|
| Age (years) | 0.0438* | ||
| <60 | 0.624 | 0.527-0.921 | |
| ≥60 | 0.759 | 0.585-0.956 | |
| Sex | 0.5621 | ||
| Male | 0.735 | 0.520-0.942 | |
| Female | 0.686 | 0.543-0.901 |
aHR: adjusted hazard ratio, CI: confidence interval, DME: diabetic macular edema, PDR: proliferative diabetic retinopathy
* Significant difference between the two groups
Discussion
The current study revealed an association between the use of GLP-1RA and a lower incidence of OAG in individuals with T2DM compared with those not taking GLP-1RA. Notably, this association was more pronounced in patients with T2DM aged ≤60 years. Furthermore, the association between GLP-1RA and OAG development was consistent across different sexes in individuals with T2DM.
GLP-1RA has various beneficial various functions in addition to its antihyperglycemic ability [18, 19]. A previous study demonstrated the cardiovascular protective effects of GLP-1RA according to a lower incidence of cardiovascular death in individuals with T2DM patients under GLP-1RA treatment [20]. Additionally, the incidence rates of nonfatal myocardial infarction and cerebrovascular diseases were significantly lower in individuals using GLP-1RA [21]. The GLP-1RA has been demonstrated to exert renal protective effects through the amelioration of albumin excretion and the prevention of creatinine elevation [22-24]. Moreover, GLP-1RA has the potential for use in managing Alzheimer's disease and Parkinson's disease [25]. At the molecular level, the cardiovascular protection offered by GLP-1RA may be due to its anti-inflammatory properties and antioxidative effects [16, 22, 26]. Glaucoma, including OAG, is a neurodegenerative disease characterized by the death of retinal ganglion cells [27, 28]. It shares protein features with other neurodegenerative diseases such as Alzheimer's disease [29]. Although intraocular pressure is a crucial risk factor for OAG [30], the impairment of ocular vasculature has recently been recognized as another major factor contributing to OAG development [31]. In individuals with OAG, ocular blood flow decreases and can be reversed by dorzolamide [32]. Additionally, the vascular densities of the optic disc and macula are significantly lower in individuals with OAG [33, 34]. Furthermore, diseases that damage vasculature, such as hypertension, are significant risk factors for OAG [28]. GLP-1RA can generally preserve vasculature and suppress inflammation, which are both predisposing factors for OAG [28, 31]. Experimental glaucoma models have shown that GLP-1RA suppresses interleukin-1α production, tumor necrosis factor α production, astrocyte transformation, and the subsequent death of retinal ganglion cells [35]. Additionally, topical application of GLP-1RA has been found to reduce extracellular glutamate expression, preventing retinal neurodegeneration, including glial activation and neural apoptosis [11]. Consequently, we infer that the use of GLP-1RA could alter the incidence of subsequent OAG. This inference aligns with the results of the current study.
The current study revealed an association between the use of GLP-1RA and a lower incidence of OAG in individuals with T2DM. Although the potential protective effect of GLP-1RA on glaucoma development has been suggested [35, 36], research exploring this concept is limited. A previous study demonstrated the protective effect of GLP-1RA on the development of primary OAG, glaucoma suspect, and low-tension glaucoma; however, the specific effect of GLP-1RA on OAG was not evaluated [37]. To our knowledge, this study provides preliminary evidence of the possible correlation between GLP-1RA application and the incidence of subsequent OAG in individuals with T2DM. Additionally, individuals with previous OAG and ocular hypertension were excluded to ensure that the OAG episodes in the current study occurred after the application of GLP-1RA treatment. Moreover, the effects of several known risk factors of OAG, including age, hypertension, and corticosteroid usage, were adjusted in the multivariable analysis in the current study [28, 30], and the diabetes duration were all between one to five years in both the GLP-1RA and control groups. Consequently, the application of GLP-1RA may serve as an independent protective factor for the development of subsequent OAG, possibly due to its vascular protection effects. Notably, T2DM itself is considered a risk factor for OAG [38], and severe T2DM is correlated with a higher incidence of OAG. In the current study, the percentage of antidiabetic medications was numerically higher in the GLP-1RA group, whereas the ratio of insulin prescription was significantly higher in the GLP-1RA group. This observation might suggest that the severity of T2DM was higher in the GLP-1RA group, which would theoretically be associated with a higher risk of OAG. However, the study found a reduced risk of OAG in this population after GLP-1RA prescription, implying a potential protective effect of GLP-1RA on OAG development. About the generalizability of our results, most of the Taiwanese are collateral to the Chinese ethnicity. Consequently, our result could be applied to the other nation that mainly consists of Chinese like the China and Singapore.
In the subgroup analyses, all age and sex subgroups with T2DM using GLP-1RA demonstrated a significantly lower risk of OAG development compared with the T2DM population not using GLP-1RA. This aligns with a study that demonstrated a reduced incidence of OAG, glaucoma suspect, and low-tension glaucoma in the T2DM population using GLP-1RA [37]. The current study further supports these findings, indicating a consistent and universal effect of GLP-1RA on the development of subsequent OAG in T2DM populations across various age and sex subgroups. In particular, patients with T2DM aged younger than 60 years with GLP-1RA application exhibited a significantly lower risk of subsequent OAG development compared with their older counterparts. Because age is a well-established risk factor for OAG development [30], it is reasonable to expect that older individuals with T2DM exhibited a higher incidence of OAG. However, the aHRs for both age subgroups were not markedly different, suggesting that the observed difference may be clinically nonsignificant. Furthermore, the incidence of OAG after GLP-1RA prescription did not show a significant difference between the two sex subgroups. Although previous studies have proposed conflicting findings regarding the vulnerability of males or females to the development of OAG [27, 30], the results of the current study indicated that the incidence of OAG was not affected by the different sexes in the T2DM population.
The epidemiology of T2DM underscores its status as one of the most prevalent systemic diseases globally, with an overall prevalence exceeding 8% in a previous study [39]. The presence of T2DM is associated with major morbidities, including ischemic heart disease, cerebrovascular accidents, diabetic kidney disease, and diabetic retinopathy [40]. Among these complications, the mortality rate of patients with T2DM-associated complications can reach 60.2 per 100,000 individuals [41], and diabetic retinopathy accounts for 2.5% of legal blindness [42]. OAG is also a prevalent ocular disease, affecting approximately 2.5% of the European population according to previous research [30]. Moreover, advanced OAG is a major cause of legal blindness, contributing to 11% of cases, further contributing to a huge economic burden [42]. Because both the T2DM and OAG are common diseases and can impair the vision to a large extent [27, 39, 42], management strategies that could reduce the possibility of OAG development in the T2DM population should be identified.
The current study has several limitations. First, the use of claims data as the primary data resource rather than real medical documents prevents the analysis of crucial information, including the initial blood sugar and glycated hemoglobin levels in patients with T2DM, subsequent blood sugar and glycated hemoglobin levels after the prescription of GLP-1RA and other antidiabetic medications, fundoscopic images of all individuals with T2DM, initial intraocular pressure among patients with OAG, results of optical coherence tomography and visual field examinations in patients with OAG, treatment outcomes of patients with OAG, and changes in intraocular pressure after anti-glaucomatous treatment in patients with OAG. Moreover, the retrospective design of the current study, despite the application of PSM, may reduce homogeneity compared with a study with a prospective design. We did not consider the metformin application as a covariate in the statistical analysis since the high collinearity between GLP-1RA and metformin (about 93% patients with GLP-1RA also took metformin) would cause prominent statistical bias/error even in interaction test or stratified analysis. However, the metformin is an important medication for T2DM control and the exclusion of metformin usage in the statistical analysis due to any reason will decrease the integrity of both data and results of the current study. Furthermore, misdiagnosis of OAG may occur as some patients with normal tension glaucoma could be included with the ICD-9 or ICD-10 diagnostic codes of OAG in clinical practice. Finally, the exclusion and matching process resulted in the loss of approximately 40% of individuals with GLP-1RA application. Although this was done to enhance diagnostic accuracy and homogeneity, the impact of patient loss might not be significant as the GLP-1RA group still included over 1000 patients, a number not inferior to previous studies [32, 34].
In conclusion, the use of GLP-1RA is associated with a lower incidence of subsequent OAG development in individuals with T2DM, even after adjustment for several risk factors for OAG. Furthermore, this association was more pronounced in patients with T2DM who were younger than 60 years. Consequently, GLP-1RA may be considered as a potential recommendation for patients with T2DM with known risk factors for OAG development. However, further large-scale prospective studies are essential to comprehensively evaluate the influence of GLP-1RA usage on glaucoma progression.
Acknowledgements
This research was funded by China Medical University, Taiwan (CMU109-MF-47; CMU110-MF-18).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH. et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50
2. Han SB, Yang HK, Hyon JY. Influence of diabetes mellitus on anterior segment of the eye. Clin Interv Aging. 2019;14:53-63
3. Htay T, Soe K, Lopez-Perez A, Doan AH, Romagosa MA, Aung K. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. Curr Cardiol Rep. 2019;21:45
4. Melmer A, Laimer M. Treatment Goals in Diabetes. Endocr Dev. 2016;31:1-27
5. Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262-76
6. Caruso I, Cignarelli A, Sorice GP, Natalicchio A, Perrini S, Laviola L. et al. Cardiovascular and Renal Effectiveness of GLP-1 Receptor Agonists vs. Other Glucose-Lowering Drugs in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Real-World Studies. Metabolites. 2022 12
7. Thomas MC. The potential and pitfalls of GLP-1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017;43(Suppl 1):2s20-2s7
8. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-85
9. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV. et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. Jama. 2015;314:687-99
10. Gaborit B, Julla JB, Besbes S, Proust M, Vincentelli C, Alos B. et al. Glucagon-like Peptide 1 Receptor Agonists, Diabetic Retinopathy and Angiogenesis: The AngioSafe Type 2 Diabetes Study. J Clin Endocrinol Metab. 2020 105
11. Hernández C, Bogdanov P, Corraliza L, García-Ramírez M, Solà-Adell C, Arranz JA. et al. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes. 2016;65:172-87
12. Marchand L, Luyton C, Bernard A. Glucagon-like peptide-1 (GLP-1) receptor agonists in type 2 diabetes and long-term complications: FOCUS on retinopathy. Diabet Med. 2021;38:e14390
13. Wei L, Mo W, Lan S, Yang H, Huang Z, Liang X. et al. GLP-1 RA Improves Diabetic Retinopathy by Protecting the Blood-Retinal Barrier through GLP-1R-ROCK-p-MLC Signaling Pathway. J Diabetes Res. 2022;2022:1861940
14. Su YC, Hung JH, Chang KC, Sun CC, Huang YH, Lee CN. et al. Comparison of Sodium-Glucose Cotransporter 2 Inhibitors vs Glucagonlike Peptide-1 Receptor Agonists and Incidence of Dry Eye Disease in Patients With Type 2 Diabetes in Taiwan. JAMA Netw Open. 2022;5:e2232584
15. Pan LY, Kuo YK, Chen TH, Sun CC. Dry eye disease in patients with type II diabetes mellitus: A retrospective, population-based cohort study in Taiwan. Front Med (Lausanne). 2022;9:980714
16. Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR. et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72-130
17. Demirtaş AA, Karahan M, Ava S, Çilem Han Ç, Keklikçi U. Evaluation of Diurnal Fluctuation in Parafoveal and Peripapillary Vascular Density Using Optical Coherence Tomography Angiography in Patients with Exfoliative Glaucoma and Primary Open-Angle Glaucoma. Curr Eye Res. 2021;46:96-106
18. Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-56
19. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 2017;136:849-70
20. Aroda VR. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(Suppl 1):22-33
21. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ. et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105-13
22. Winiarska A, Knysak M, Nabrdalik K, Gumprecht J, Stompór T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int J Mol Sci. 2021 22
23. Prattichizzo F, de Candia P, Ceriello A. Diabetes and kidney disease: emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metabolism. 2021;120:154799
24. Mosterd CM, Bjornstad P, van Raalte DH. Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol. 2020;33:965-75
25. Kopp KO, Glotfelty EJ, Li Y, Greig NH. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol Res. 2022;186:106550
26. Cornell S. A review of GLP-1 receptor agonists in type 2 diabetes: A focus on the mechanism of action of once-weekly agents. J Clin Pharm Ther. 2020;45(Suppl 1):17-27
27. Gupta D, Chen PP. Glaucoma. Am Fam Physician. 2016;93:668-74
28. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711-20
29. Sen S, Saxena R, Tripathi M, Vibha D, Dhiman R. Neurodegeneration in Alzheimer's disease and glaucoma: overlaps and missing links. Eye (Lond). 2020;34:1546-53
30. Schuster AK, Wagner FM, Pfeiffer N, Hoffmann EM. Risk factors for open-angle glaucoma and recommendations for glaucoma screening. Ophthalmologe. 2021;118:145-52
31. WuDunn D, Takusagawa HL, Sit AJ, Rosdahl JA, Radhakrishnan S, Hoguet A. et al. OCT Angiography for the Diagnosis of Glaucoma: A Report by the American Academy of Ophthalmology. Ophthalmology. 2021;128:1222-35
32. Nivean PD, Ariga M, Chithra MR, Gohil P, Das S, Jaideep G. Efficacy of dorzolamide in improving ocular blood flow in patients with open-angle glaucoma: The Indian carbonic anhydrase inhibitor trial. Indian J Ophthalmol. 2022;70:4164-7
33. Rao HL, Pradhan ZS, Suh MH, Moghimi S, Mansouri K, Weinreb RN. Optical Coherence Tomography Angiography in Glaucoma. Journal of glaucoma. 2020;29:312-21
34. Lee CY, Liu CH, Chen HC, Sun CC, Yao YP, Chao SC. Correlation between Basal Macular Circulation and Following Glaucomatous Damage in Progressed High-Tension and Normal-Tension Glaucoma. Ophthalmic Res. 2019;62:46-54
35. Sterling JK, Adetunji MO, Guttha S, Bargoud AR, Uyhazi KE, Ross AG. et al. GLP-1 Receptor Agonist NLY01 Reduces Retinal Inflammation and Neuron Death Secondary to Ocular Hypertension. Cell Rep. 2020;33:108271
36. Mouhammad ZA, Vohra R, Horwitz A, Thein AS, Rovelt J, Cvenkel B. et al. Glucagon-Like Peptide 1 Receptor Agonists - Potential Game Changers in the Treatment of Glaucoma? Front Neurosci. 2022;16:824054
37. Sterling J, Hua P, Dunaief JL, Cui QN, VanderBeek BL. Glucagon-like peptide 1 receptor agonist use is associated with reduced risk for glaucoma. Br J Ophthalmol. 2023;107:215-20
38. Zhou M, Wang W, Huang W, Zhang X. Diabetes mellitus as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. PLoS One. 2014;9:e102972
39. Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The Growing Epidemic of Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:104-9
40. Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De Cosmo S. GLP-1 Receptor Agonists and Kidney Protection. Medicina (Kaunas). 2019 55
41. Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia. 2022;65:3-13
42. Causes of blindness, vision impairment in 2020, trends over 30 years, prevalence of avoidable blindness in relation to VISION 2020. the Right to Sight: an analysis for the Global Burden of Disease Study. The Lancet Global health. 2021;9:e144-e60
Author contact
![]() Corresponding authors: Shun-Fa Yang, Ph.D. or Po-Jen Yang, MD, Ph.D., Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: cshy1030org.tw (Po-Jen Yang).
Corresponding authors: Shun-Fa Yang, Ph.D. or Po-Jen Yang, MD, Ph.D., Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: cshy1030org.tw (Po-Jen Yang).

 Global reach, higher impact
Global reach, higher impact