Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(3):474-482. doi:10.7150/ijms.90145 This issue Cite
Research Paper
Significance of Gastrokine-1 Polymorphism Rs4254535 as a Prognostic Marker and its Association with Clinical Characteristics in Chinese Lung Cancer Patients
1. School of Life Sciences, Fudan University, Shanghai, 200438, China.
2. Clinical College of Xiangnan University, Chenzhou, 423000, China.
3. Department of Surgery, Navy Military Medical University Affiliated to Changhai Hospital, Shanghai, China.
4. State Key Laboratory of Genetic Engineering, Collaborative Innovation Center for Genetics & Development, School of Life Sciences, Fudan University, Shanghai, 200438, China.
5. School of Basic Medicine, Navy Military Medical University, Shanghai, 200433, China.
6. Department of Respiratory and Critical Care Medicine, Shanghai East Hospital, Tongji University, Shanghai, 200120, China.
7. Department of Pulmonary Medicine, Zhongshan Hospital, Fudan University, Shanghai, 200032, China.
8. Nanjing Medical University, The Fourth Clinical Medical College, Nanjing, 211166, China.
9. Department of Thoracic Surgery, Navy Military Medical University Affiliated Changhai Hospital, Shanghai, 200433, China.
10. Department of Biochemistry and Molecular Biology, Hainan Medical University, Haikou, 571199, China.
11. Department of Respiratory Disease, Navy 905 Hospital, Shanghai, 200235, China.
12. Department of Respiratory and Critical Care Medicine, Shanghai Changhai Hospital, the First Afliated Hospital of Naval Medical University, Shanghai 200433, China.
13. Department of General Medicine, the First Afliated Hospital of Naval Medical University, Shanghai 200433, China.
14. Department of Pulmonary and Critical Care Medicine, Shanghai Geriatric Medical Center, Shanghai, 200032, China.
#These authors contributed equally to this work.
Received 2023-9-13; Accepted 2023-12-1; Published 2024-1-1
Abstract
Background: The single nucleotide polymorphism (SNP) of Gastrokine-1 (GKN1) is associated with lung cancer but its association with prognosis is not clear.
Methods: Genomic DNA was extracted from the blood samples of 888 patients with lung cancer. The association between GKN1 polymorphism rs4254535 and prognostic was analyzed by the Kaplan-Meier (KM) method, Log-rank test, and Cox proportional hazards model.
Results: In females and patients diagnosed with late-stage lung cancer, the CC genotype (CC vs TT, adjusted odds ratio [HR] = 0.57, 95% Confidence Interval [CI]: 0.33-0.99, P = 0.045; HR = 0.66, 95% CI: 0.48-0.92, P = 0.014) and recessive CC genotype (CC vs TT + TC, HR = 0.55, 95% CI: 0.32-0.94, P = 0.028; HR = 0.64, 95% CI: 0.47-0.89, P = 0.006) of rs4254535 conferred a better prognosis, compared with the TT and TT + TC genotype. Rs4254535 dominate TC + CC genotype, recessive CC genotype, and C allele who were adenocarcinoma patients had a significantly better prognosis. The recessive CC genotype of non-smoking patients has a better prognosis, compared to the TT + TC genotype. Additionally, in the dominant TT + TC genotype and C allele, no family history patients had a significantly better prognosis, compared to the TT genotype.
Conclusion: For lung cancer patients, GKN1 polymorphism rs4254535 may be a protective genetic marker and predicts the prognosis of lung cancer patients.
Keywords: GKN1, rs4254535, lung cancer, single nucleotide polymorphism, prognosis
Introduction
Lung cancer is the second most commonly diagnosed cancer worldwide (11.4%), and remains the leading cause of cancer death (18%), which poses a serious threat to the life span and living quality of people [1, 2]. Lung cancer is classified into Small Cell Lung Cancer (SCLC) and Non-Small Cell Lung Cancer (NSCLC) according to histopathology [3]. SCLC is the most invasive lung cancer histopathological subtype, accounts for 13-15% of new lung cancer, and has less than 7% 5-year survival rate [4, 5]. NSCLC accounts for about 85% of new lung cancer cases. Although there has been a tremendous advance in the treatment of NSCLC, the overall survival rate remains low [6]. The complication of metastatic tumors is the critical factor in most deaths associated with lung cancer [7, 8]. In addition, genetic polymorphisms and clinical characteristics significantly affect the prognosis of lung cancer patients.
GKN1 is also called Antrum Mucosal Protein (AMP)-18, which is secreted by mucus cells on the surface of the gastric antrum and base and has growth factor or cytokine-like activity on gastric epithelial cells [9]. Studies have shown that expression of GKN1 can regulate cell proliferation and differentiation [10-12], up-regulation of GKN1 can inhibit gastric epithelial cells developing into cancer cells by down-regulating NF-κB expression [13-20] and overexpression of GKN1 promotes lung cancer cell metastasis in vitro [21]. A high level of GKN1 protein, with which Keratin 14 (K14) - high cancer cells could resist anoikic, plays a vital role in promoting metastasis of lung cancer cells with low K14 expression. Meanwhile, GKN1 is a specific up-regulated gene in adenocarcinoma [22]. As a common genetic variation, Single nucleotide polymorphism (SNP) proves to be associated with the prognosis of lung cancer [23-30]. The rs4254535 is about 3 Kbp upstream of GKN1 and 20 Kbp upstream of GKN2, which are located on a pair of complementary chains. Previous studies have found that the GKN1 polymorphism rs4254535 is related to the chemotherapy response of lung cancer patients. A study on the Chinese Han population find that compared with "TT" genotype carriers, "CT" carriers have worse responses to cisplatin-based chemotherapy [31].
To further study whether GKN1 polymorphism rs4254535 is associated with the prognosis of lung cancer, we collected 888 blood samples from patients diagnosed with lung cancer. The blood samples were taken before treatment to detect GKN1 polymorphism rs4254535 and a follow-up investigation was conducted to explore the association between GKN1 polymorphism rs4254535 and disease progression and prognosis of patients with various types of lung cancer. The results are based on accurate data and rigorous statistical analysis.
Patients and methods
Study Group
From January to November 2009, a total of 888 patients with primary lung cancer were included, and 839 cases were retained after excluding 49 cases of incomplete data. The follow-up period ended in 2019. The patients in this cohort were from Changhai Hospital Affiliated to the Naval Military Medical university (Second Military Medical University) (n = 536) and Taizhou Institute of Health Sciences of Fudan University (n = 352). Eligibility criteria: Primary lung cancer diagnosed by histopathological diagnosis, no history of other malignancy, no age and sex restrictions. Patient clinical data were obtained based on patient history and telephone follow-up. The study was approved by the Ethics Committee of the School of Life Sciences of Changhai University, and all the subjects received informed consent.
SNP Genotyping
All selected patients collected 5 ml blood samples before treatment, genomic DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, 51106) and genotyping was performed using the 2×48-plex SNPscan TM Kit (Genesky Biotechnologies, G0104) [32, 33]. We used a detailed procedure to determine genotyping quality, guaranteed a call rate of more than 99%, reproduced genotype detection, internal positive control samples, and performed Hardy-Weinberg equilibrium (HWE) testing. Genotype analysts were blinded to the patients' clinical information.
Statistical analysis
Pearson's chi-square test was used to check for Hardy-Weinberg equilibrium (HWE). Overall survival (OS) was calculated from the date of sample collection until the date the patient died from any cause or the last time of follow-up visit. In this study, the median survival time (MST) was estimated using the Kaplan-Meier (KM) method, the Log-rank test compared the differences between groups, and the multivariate Cox proportional hazards model estimated the hazard ratio (HR) and 95% confidence interval (CI) of age and sex adjustment. Four SNP genetic models (allele, genotype, dominant, and recessive) were analyzed. The data were also analyzed by age, sex, smoking status, family history of lung cancer, histological type of lung cancer, and TNM stage. The statistical significance was considered at P < 0.05. All respective tests were bilateral and performed using R version 3.6.2 (Vienna, Austria).
Results
Demographic and general clinical Characteristics
The follow-up period was from the enrollment until November 15, 2019. Due to incomplete data, 49 patients were excluded, and the remaining 839 patient data were analyzed. The study sample was an ethnically homogeneous group of Han Chinese individuals. A total of 668 deaths (79.6%), 103 (12.3%) survived for more than 5 years, 68 cases (8.1%) were lost follow-up, 610 males (72.7%), 524 (62.5%) were aged ≥ 60 years, 582 (69.4%) had smoking history, 537 (64%) had no family history of malignant tumors, 154 (18.4%) and 625 (74.5%) were staged I-II and III-IV respectively. As for the cancer subtypes, 367 (43.7%) patients were diagnosed with adenocarcinoma, 282 (33.6%) with squamous cell carcinoma (SCC), 72 (8.6%) with small cell lung cancer (SCLC), and 118 (14.1%) with other cancer types, including adenosquamous carcinoma (ASC), large cell carcinoma (LCC), carcinosarcoma (CS) and mucoepidermoid carcinoma (MEC) (Table 1).
Association between patients' Characteristics and lung cancer outcomes
MST varied from a few months (M) to tens of months across different stratifications. As shown in Table 1, the MST was much lower for men than for women (34.27 vs. 40.17 M; P = 0.01), The MST of patients aged 60 and over is much lower than that of patients under 60. (33.2 vs. 40.87 M; P = 0.003), smokers were much lower than non-smokers (33.9 vs. 41.03 M; P < 0.001), patients with advanced tumor stage were much lower than those with early-stage tumors (29.4 vs. 113.93 M; P < 0.001).
Association between GKN1 polymorphism rs4254535 and lung cancer prognosis
A total of 839 genotypes were detected, with a call rate of 99.64%, of which 439 were TT, 329 TC, and 68 CC genotypes. Among all the patients who died, the proportion of genotype TT and dominant TT + TC were 52.03% (346/665) and 92.48% (615/665), respectively, which were significantly different from CC genotype (P = 0.02), suggesting that the latter had a lower prognosis. The MST of the CC genotype was 47.43 M, which was much higher than that of the former two at 33.63 M and 34.73 M (Table 2).
Characteristic distribution in Chinese patients with lung cancer and prognosis analysis.
| Variables | N (%) | MST | Log-rank P |
|---|---|---|---|
| All | 839 | 36.73 | |
| Sex | 0.01 | ||
| Female | 229 (27.3 %) | 40.17 | |
| Male | 610 (72.7 %) | 34.27 | |
| Age | 0.003 | ||
| Age < 60 | 315 (37.5 %) | 40.87 | |
| Age >= 60 | 524 (62.5 %) | 33.2 | |
| Smoking status | < 0.001 | ||
| Nonsmoker | 237 (28.2 %) | 41.03 | |
| Smoker | 582 (69.4 %) | 33.9 | |
| unknown | 20 (2.4 %) | 67 | |
| Family History | 0.462 | ||
| Yes | 302 (36 %) | 33.63 | |
| No | 537 (64 %) | 38.03 | |
| Stage | < 0.001 | ||
| Early stage | 154 (18.4 %) | 113.93 | |
| Late stage | 625 (74.5 %) | 29.4 | |
| Unknown | 60 (7.2 %) | 66.43 | |
| Subtype | 0.211 | ||
| ADC | 367 (43.7 %) | 38.8 | |
| SCC | 282 (33.6 %) | 33.63 | |
| SCLC | 72 (8.6 %) | 33.9 | |
| Other* | 118 (14.1 %) | 36.2 |
* Other carcinomas include ASC, LCC, CS and MEC.
Association analysis between GKN1 polymorphism rs4254535 and prognosis of lung cancer patients.
| SNP | Model | Death/survival | MST | HR (Cl) | P | HR* (95% CI) | P* |
|---|---|---|---|---|---|---|---|
| rs4254535 | Allele | ||||||
| T (ref) | 961/246 | 34.27 | 1 | 1 | |||
| C | 369/96 | 39.50 | 0.91 (0.80-1.02) | 0.105 | 0.90 (0.80-1.02) | 0.100 | |
| Genotype | |||||||
| T/T (ref) | 346/93 | 33.63 | 1 | 1 | |||
| T/C | 269/60 | 37.10 | 0.97 (0.83-1.14) | 0.754 | 0.96 (0.82-1.12) | 0.593 | |
| C/C | 50/18 | 47.43 | 0.74 (0.55-1.00) | 0.049 | 0.76 (0.56-1.02) | 0.065 | |
| Dominate | |||||||
| T/T (ref) | 346/93 | 33.63 | 1 | 1 | |||
| T/C+C/C | 319/78 | 38.5 | 0.93 (0.80-1.08) | 0.345 | 0.92 (0.79-1.07) | 0.272 | |
| Recessive | |||||||
| T/T+T/C (ref) | 615/153 | 34.73 | 1 | 1 | |||
| C/C | 50/18 | 47.43 | 0.75 (0.56-1.00) | 0.052 | 0.77 (0.58-1.03) | 0.077 | |
* CI, confidence interval; HR, hazard ratio; ref, reference. a Adjusted by age, sex.
Association of GKN1 polymorphism with lung cancer prognosis
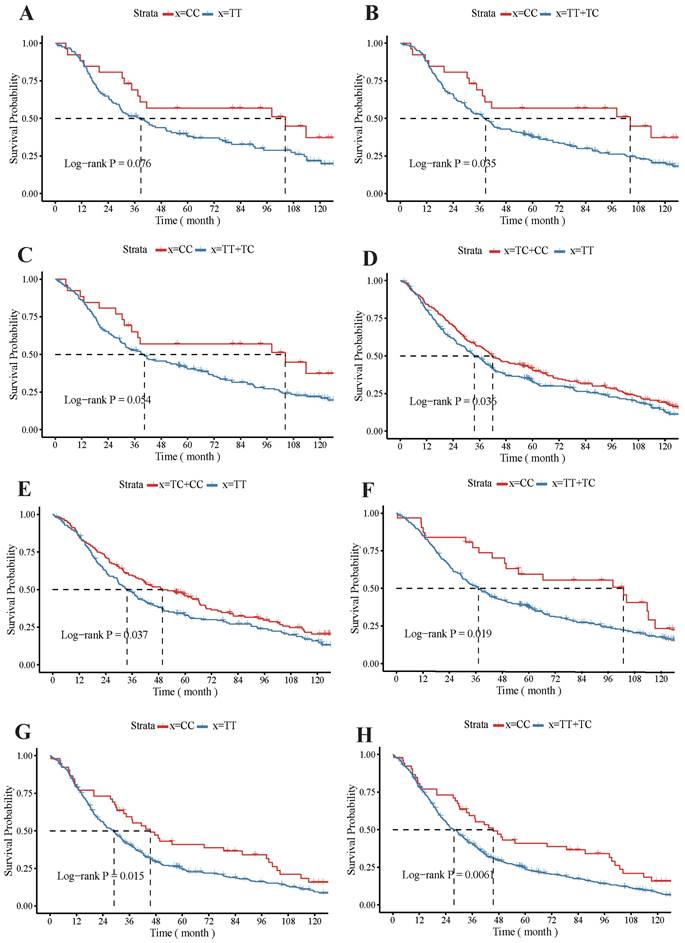
The KM survival curve showed that the rs4254535 polymorphism T > C could significantly increase the MST of several kinds of patients. For example, the Kaplan-Meier curves demonstrated that MST was longer in female patients with the CC (MST: 104.3 M) genotype compared to those with the TT (MST: 38.77 M) genotype (Log-rank P = 0.076; Figure 1 A), and MST was longer in the CC genotype (MST: 104.3 M) who female patients, compared to the recessive TT + TC (MST: 38.77 M) genotype (Log-rank P = 0.035; Figure 1 B). For non-smoker patients with CC genotype (MST: 104.3 M; Log-rank P = 0.054; Figure 1 C), their median survival time was higher than that with recessive TT + TC genotype (MST: 40.4 M). In no family history patients, the dominant TC + CC genotype (MST: 41.9 M) could also highly improve survival rate compared with the TT genotype (MST: 33.63 M; Log-rank P = 0.036146; Figure 1 D). Additionally, in the dominant TT + TC genotype (MST: 49.77 M) adenocarcinoma patients had a significantly better MST, compared to the TT genotype (MST: 33.63M; Log-rank P = 0.037; Figure 1 E), and MST was longer in the CC genotype (MST: 102.9 M) who adenocarcinoma, compared to the recessive TT + TC genotype (MST: 37.17 M; Log-rank P = 0.019; Figure 1 F). In patients with late-stage (stage III + IV) lung cancer, the MST was significantly longer in patients with the CC genotype (MST: 45.5 M) than TT genotype (MST: 37.17 M; Log-rank P = 0.015; Figure 1 G), and CC genotype (MST: 45.5 M) of MST compared to the recessive TT+TC genotype longer (MST: 26.3 M; Log-rank P = 0.006; Figure 1 H).
Association between GKN1 polymorphism rs4254535 and lung cancer prognosis stratified by patients' characteristics
The GKN1 rs4254535 allele C significantly increased the prognosis of no family history patients of lung cancer (MST: 42.3 M; P = 0.0281), and adenocarcinoma (MST: 55.37 M; P = 0.008) patients, and effectively prolonged MST (Table 3). Compared with the TT genotype, the CC genotype had a significantly better prognosis of females (P = 0.045), and patients diagnosed with late-stage lung cancer (P = 0.014) (Table 4). Compared with the TT genotype, the dominant TC + CC genotype had a better effect on the prognosis of patients without a family history (P = 0.046) and significantly increased the median survival time of patients with adenocarcinoma (P = 0.0396; MST: TT, 33.63 M; TC + CC, 49.77 M; Table 5). Compared with the recessive TT + TC genotype, the CC genotype in non-smoke (MST: 104.3 M; P = 0.050) and late-stage patients (MST: 45.5 M; P = 0.0069) improved prognosis and significantly prolonged MST, particularly in patients with adenocarcinoma (MST: 102.9 M; P = 0.0255; Table 6).
Association between GKN1 polymorphism rs4254535 in allele model and prognosis in Chinese patients with lung cancer.
| Variables | Death/survive | HR (95% CI) | P | HR* (95% CI) | P* | |
|---|---|---|---|---|---|---|
| T (ref) | C | |||||
| Sex | ||||||
| Female | 243/89 | 89/37 | 0.84 (0.66-1.07) | 0.159 | 0.82 (0.65-1.05) | 0.118 |
| Male | 718/157 | 280/59 | 0.93 (0.81-1.07) | 0.306 | 0.93 (0.81-1.07) | 0.322 |
| Age | ||||||
| Age < 60 | 339/122 | 121/46 | 0.91 (0.74-1.12) | 0.358 | 0.86 (0.69-1.06) | 0.146 |
| Age >= 60 | 622/124 | 248/50 | 0.91 (0.78-1.05) | 0.195 | 0.91 (0.78-1.06) | 0.214 |
| Smoking status | ||||||
| Nonsmoker | 247/96 | 91/40 | 0.82 (0.64-1.04) | 0.097 | 0.81 (0.64-1.04) | 0.096 |
| Smoker | 694/139 | 274/51 | 0.94 (0.82-1.08) | 0.408 | 0.95 (0.82-1.09) | 0.372 |
| Family History | ||||||
| Yes | 336/80 | 152/30 | 1.04 (0.86-1.26) | 0.690 | 1.03 (0.85-1.25) | 0.775 |
| No | 625/166 | 217/66 | 0.82 (0.71-0.96) | 0.014 | 0.84 (0.72-0.98) | 0.028 |
| Stage | ||||||
| Early stage | 117/107 | 35/49 | 0.79 (0.54-1.15) | 0.216 | 0.79 (0.54-1.16) | 0.234 |
| Late stage | 771/131 | 303/39 | 0.91 (0.80-1.04) | 0.160 | 0.90 (0.79-1.03) | 0.135 |
| Subtype | ||||||
| ADC | 407/123 | 143/59 | 0.77 (0.63-0.93) | 0.006 | 0.77 (0.64-0.94) | 0.008 |
| SCC | 331/65 | 145/21 | 1.06 (0.87-1.29) | 0.545 | 1.06 (0.87-1.28) | 0.587 |
| SCLC | 86/20 | 30/6 | 0.75 (0.49-1.15) | 0.188 | 0.73 (0.47-1.13) | 0.156 |
| NSCLC | 875/226 | 339/90 | 0.92 (0.81-1.04) | 0.190 | 0.92 (0.81-1.04) | 0.198 |
* Adjusted by age, gender. CI, confidence interval; HR, hazard ratio; ref, reference.
Association between GKN1 polymorphism rs4254535 in genotype models and prognosis of Chinese patients with lung cancer.
| Variables | Death/Survive | T/C vs T/T | C/C vs T/T | ||||
|---|---|---|---|---|---|---|---|
| T/T (ref) | T/C | C/C | HR* (95% Cl) P* | HR* (95% Cl) P* | |||
| Sex | |||||||
| Male | 254/56 | 210/45 | 35/7 | 0.92 (0.77-1.10) | 0.370 | 0.88 (0.62-1.25) | 0.477 |
| Female | 92/37 | 59/15 | 15/11 | 1.11 (0.80-1.54) | 0.547 | 0.57(0.33-0.99) | 0.045 |
| Age(year) | |||||||
| Age < 60 | 126/47 | 87/28 | 17/9 | 0.96(0.73-1.26) | 0.778 | 0.76 (0.46-1.27) | 0.301 |
| Age >= 60 | 220/46 | 182/32 | 33/9 | 0.98 (0.80-1.19) | 0.806 | 0.77 (0.53-1.11) | 0.160 |
| Smoking status | |||||||
| Yes | 245/51 | 204/37 | 35/7 | 0.97(0.80-1.16) | 0.711 | 0.87 (0.61-1.24) | 0.427 |
| No | 93/39 | 61/18 | 15/11 | - | - | 0.58 (0.33-1.01) | 0.056 |
| Family cancer history | |||||||
| Yes | 116/30 | 104/20 | 24/5 | 1.22(0.93-1.61) | 0.144 | 0.88 (0.57-1.38) | 0.588 |
| No | 230/63 | 165/40 | 26/13 | 0.85(0.69-1.04) | 0.134 | 0.68(0.45-1.03) | 0.065 |
| Histological type | |||||||
| ADC | 151/44 | 105/35 | 19/12 | 0.84 (0.66-1.09) | 0.189 | 0.55 (0.34-0.88) | 0.013 |
| SCC | 110/25 | 111/15 | 17/3 | 1.03 (0.79-1.35) | 0.810 | 1.20 (0.72-1.99) | 0.494 |
| SCLC | 32/9 | 22/2 | 4/2 | 1.06 (0.61-1.83) | 0.841 | 0.33(0.10-1.12) | 0.076 |
| NSCLC | 314/84 | 247/58 | 46/16 | 0.95 (0.80-1.13) | 0.561 | 0.81 (0.59-1.10) | 0.179 |
| staging | |||||||
| Early tage | 45/34 | 27/39 | 4/5 | 0.65 (0.40-1.05) | 0.078 | 0.98 (0.35-2.73) | 0.963 |
| Late stage | 275/57 | 221/17 | 41/11 | 1.08 (0.90-1.29) | 0.426 | 0.66 (0.48-0.92) | 0.014 |
* Adjusted by age, gender. CI, confidence interval; HR, hazard ratio; ref, reference.
Impacts of GKN1 polymorphism rs4254535 on the prognosis of patients with lung cancer patients. The Kaplan-Meier survival curve analysis of the GKN1 polymorphism rs4254535 and TT genotype (A) and the recessive TT + TC genotype in female (B), the CC genotype non-smoker patients (C), and no family history patients with dominant TC + CC genotype (D) survival probability. Impacts of GKN1 polymorphism rs4254535 on the prognosis of patients with dominate TT + TC genotype (E) and CC genotype (F) who adenocarcinoma patients, the CC genotype (G) and recessive TT+TC genotype (H) patients with late-stage (stage III + IV) lung cancer.
Association between GKN1 polymorphism rs4254535 in dominant genotype and prognosis of Chinese patients with lung cancer.
| Variables | Death/survive | HR (95% CI) | P | HR* (95% CI) | P* | |
|---|---|---|---|---|---|---|
| T/T (ref) | T/C + C/C | |||||
| Sex | ||||||
| Female | 92/37 | 74/26 | 0.95 (0.70-1.29) | 0.731 | 0.93 (0.68-1.27) | 0.647 |
| Male | 254/56 | 245/52 | 0.91 (0.76-1.09) | 0.297 | 0.91 (0.77-1.09) | 0.314 |
| Age | ||||||
| Age < 60 | 126/47 | 104/37 | 0.93 (0.72-1.21) | 0.587 | 0.92 (0.71-1.20) | 0.545 |
| Age >= 60 | 220/46 | 215/41 | 0.93 (0.77-1.12) | 0.450 | 0.94 (0.77-1.13) | 0.487 |
| Smoking stage | ||||||
| Nonsmoker | 93/39 | 76/29 | - | - | - | - |
| Smoker | 245/51 | 239/44 | 0.95 (0.79-1.13) | 0.536 | 0.95 (0.79-1.14) | 0.569 |
| Family History | ||||||
| Yes | 116/30 | 128/25 | 1.14 (0.89-1.47) | 0.295 | 1.14 (0.88-1.47) | 0.325 |
| No | 230/63 | 191/53 | 0.81 (0.67-0.99) | 0.036 | 0.82 (0.68-1.00) | 0.046 |
| Stage | ||||||
| Early stage | 45/34 | 31/44 | 0.68 (0.43-1.08) | 0.102 | 0.68 (0.43-1.08) | 0.100 |
| Late stage | 275/57 | 262/28 | 0.99 (0.84-1.18) | 0.931 | 0.98 (0.83-1.16) | 0.817 |
| Subtype | ||||||
| ADC | 151/44 | 124/47 | 0.78 (0.61-0.98) | 0.037 | 0.78 (0.61-0.99) | 0.039 |
| SCC | 110/25 | 128/18 | 1.07 (0.83-1.38) | 0.598 | 1.05 (0.81-1.36) | 0.694 |
| SCLC | 32/9 | 26/4 | 0.89 (0.53-1.51) | 0.671 | 0.86 (0.50-1.46) | 0.574 |
| NSCLC | 314/84 | 293/74 | 0.93 (0.80-1.09) | 0.393 | 0.93 (0.79-1.09) | 0.339 |
* Adjusted by age, gender. CI, confidence interval; HR, hazard ratio; ref, reference.
Association between GKN1 polymorphism rs4254535 in recessive genotype and prognosis of Chinese patients with lung cancer.
| Variables | Death/survive | HR (95% CI) | P | HR* (95% CI) | P* | |
|---|---|---|---|---|---|---|
| T/T + T/C (ref) | C/C | |||||
| Sex | ||||||
| Female | 151/52 | 15/11 | 0.57 (0.33-0.97) | 0.037 | 0.55 (0.32-0.94) | 0.028 |
| Male | 464/101 | 35/7 | 0.91 (0.65-1.29) | 0.597 | 0.91 (0.65-1.29) | 0.610 |
| Age | ||||||
| Age < 60 | 213/75 | 17/9 | 0.76 (0.46-1.25) | 0.283 | 0.78 (0.47-1.28) | 0.320 |
| Age >= 60 | 402/78 | 33/9 | 0.75 (0.53-1.08) | 0.118 | 0.78 (0.54-1.11) | 0.166 |
| Smoking stage | ||||||
| Nonsmoker | 154/57 | 15/11 | 0.60 (0.35-1.01) | 0.056 | 0.58 (0.34-1.00) | 0.050 |
| Smoker | 449/88 | 35/7 | 0.87 (0.61-1.22) | 0.414 | 0.88 (0.62-1.24) | 0.468 |
| Family History | ||||||
| Yes | 220/50 | 24/5 | 0.84 (0.55-1.28) | 0.409 | 0.81 (0.53-1.25) | 0.345 |
| No | 395/103 | 26/13 | 0.68 (0.46-1.02) | 0.059 | 0.73 (0.49-1.09) | 0.124 |
| Stage | ||||||
| Early stage | 72/73 | 4/5 | 1.09 (0.40-3.00) | 0.860 | 1.18 (0.43-3.23) | 0.751 |
| Late stage | 496/74 | 41/11 | 0.64 (0.47-0.88) | 0.006 | 0.64 (0.47-0.89) | 0.006 |
| Subtype | ||||||
| ADC | 256/79 | 19/12 | 0.58 (0.36-0.92) | 0.020 | 0.59 (0.37-0.94) | 0.025 |
| SCC | 221/40 | 17/3 | 1.14 (0.70-1.87) | 0.602 | 1.18 (0.72-1.93) | 0.519 |
| SCLC | 54/11 | 4/2 | 0.34 (0.11-1.10) | 0.072 | 0.32 (0.10-1.07) | 0.064 |
| NSCLC | 561/142 | 46/16 | 0.80 (0.59-1.08) | 0.149 | 0.83 (0.61-1.12) | 0.214 |
* Adjusted by age, gender. CI, confidence interval; HR, hazard ratio; ref, reference.
Discussion
The research investigated the association between the prognosis and the GKN1 polymorphism rs4254535 by using blood samples from 839 Chinese patients with lung cancer. The authors observed that rs4254535 polymorphism T > C, an SNP in GKN1, was significantly associated with better prognosis in female, non-smoking, No Family History, and adenocarcinoma patients diagnosed with late-stage (stage III + IV) lung cancer.
Expression of GKN can be used as a good prognostic marker and therapeutic target for lung and stomach cancer, and the OS rate of patients with low GKN2 mRNA expression is poor [34, 35]. The ectopic expression of GKN1 can inactivate the NF-κB signaling pathway and activate lκB to increase GKN2 expression by time-dependent means [36]. GKN1 also has a clear anti-proliferative effect and plays the role of antitumor [37]. In addition, in the development of gastric cancer in humans and mice, the highly coordinated deletion of GKN1/GKN2 genes and the abnormal expression (deletion) of GKN are closely related to the occurrence of precancerous inflammation and gastric cancer [38]. In a study on the effect of GKN1 polymorphism on the efficacy of cisplatin chemotherapy in lung cancer, it has been found that GKN1 polymorphism significantly reduce the chemotherapy response of lung cancer patients [39]. These studies have shown that GKN polymorphism or loss of expression is closely related to the occurrence and development of cancer. In our study, GKN1 polymorphism rs4254535 was found to be significantly associated with lung cancer prognosis, especially in adenocarcinoma and non-smoking patients.
Sex and cancer clinical stage are important clinical characteristics that affect the prognosis of lung cancer patients, and the difference in prognosis due to gender are mainly closely related to estrogen, the effect of estrogen is more obvious in women and is an important biological factor leading to lung cancer [40, 41]. In addition, female cancer patients generally have lower morbidity and mortality compared with males, mainly due to higher expression of tumor suppressor genes encoded by the X chromosome [42]. At the same time, gender synergistic genetic polymorphisms significantly affect the occurrence and development of lung cancer patients [43]. However, another study has found women have a higher prevalence of lung cancer than men, because of the high expression of Cytochrome P-450 1A1(CYPlA1) and P53 genes, which are genetically susceptible to tobacco and airborne pollutants [44]. A study of 36,658 patients with primary lung cancer in West China Hospital from 1995 to 2015 also finds that the incidence and number of lung cancer in women increased year by year, especially in adenocarcinoma [45]. The clinical stage is also an important clinical feature that differentiates cancer prognosis. In a study on the association between TNFRSF19 expression and lung cancer risk, it has been found that the differentiated expression of TNFRSF19 is statistically significantly associated with tumor TNM stage and patient survival [46]. In our study, GKN1 polymorphism rs4254535 was significantly associated with female and better prognosis in patients with advanced lung cancer.
In stratified analysis, we observed that GKN1 polymorphism rs4254535 was significantly associated with better prognosis in lung cancer patients with no smoking history and those diagnosed with lung adenocarcinoma cancer. Many compounds in tobacco smoke, such as nicotine, are classified as lung carcinogens and have proven to be closely related to the development and prognosis of lung cancer [47, 48]. Through the collation and analysis of 383 research literature on the association between smoking and lung cancer in the past 20 years, the study has found that smoking can affect the proliferation and differentiation of lung cancer cells by affecting the acetylcholine system and thus affect the progression of lung cancer [49]. Smoking can also affect gene expression through epigenomic modification and thus affect lung cancer prognosis [50]. Genetic polymorphisms cooperate with smoking to significantly affect the prognosis of lung cancer patients. In a study on the impact of genetic polymorphisms and smoking on the prognosis of lung cancer patients, it is found that genetic polymorphisms synergize with smoking to significantly reduce patient survival prognosis [51]. In addition, another important factor affecting prognosis is cancer tissue type. Multiple studies have found that patients diagnosed with adenocarcinoma significantly change their prognosis under the influence of genetic polymorphisms [52, 53]. Our study also found that the rs4254535 genetic polymorphism significantly increases the prognosis of patients with lung adenocarcinoma.
The association of genetic tools and clinical characteristics of specific cancers is giving medicine a new focus by means of “personalized cancer drugs” [54, 55]. A new and effective biomarker gives a new target for personalized cancer drug treatment, helps to select appropriate individualized patients to evaluate target regulation, and guides evidence-based treatment of early disease, and it is valuable in improving the prognosis of cancer patients [56]. Moreover, strengths of this study, we tested all lung cancer patients for polymorphisms in known genetic loci, reduced the possibility of missing important genomic possibilities as well as avoided data complexity. In addition, there were certain limitations in our study: only the association analysis between the rs4254535 single locus and lung cancer prognosis on the GKN1 gene, which lacked the exploration of genome-wide interactions [57]. Cancer prognosis is related to several factors [58], but we did not involve the influence of psychological and social factors on the progression and prognosis of patients' diseases in our study.
Conclusions
The study showed that GKN1 polymorphism rs4254535 T > C significantly reduced the prognosis of lung cancer patients and was relatively strongly associated with non-smoking, no family history, female, and adenocarcinoma patients. Although there were still some deficiencies in this study, it still might have potential clinical significance for the prognosis and treatment strategy of lung cancer patients. Patients with poor prognosis, such as non-smokers, females, and no family history of cancer, should undergo lung cancer testing for clinical characteristics identification and genetic polymorphisms. Based on different clinical characteristics and specific nucleotide site polymorphisms, they should receive personalized high-quality treatment as early as possible, and improve prognosis. In addition, our research model provides a new target for the treatment of lung cancer at the rs4254535 nucleotide site.
Abbreviations
SNP: single nucleotide polymorphism; GKN1: Gastrokine-1; KM: Kaplan-Meier; CI: Confidence Interval; ADC: adenocarcinoma; SCLC: Small Cell Lung Cancer; NSCLC: Non-Small Cell Lung Cancer; AMP: Antrum Mucosal Protein; K14: Keratin 14; HWE: Hardy-Weinberg equilibrium; OS: Overall survival; MST: Median Survival Time; HR: hazard ratio; TNM: Tumor Node Metastasis; SCC: squamous cell carcinoma; ASC: adenosquamous carcinoma; LCC: large cell carcinoma; CS: Carcinosarcoma; MEC: mucoepidermoid carcinoma; M: months; CYPlA1: cytochrome P-450 1A1.
Acknowledgements
The authors acknowledge all the volunteers involved in this study, the staff of Changhai Hospital, and the Taizhou Institute of Health Sciences for their support during the sample collection process. We thank Dr. Hao Hu, Dr. Wei Feng, and Dr. Zunyi Gao for their help in recruiting subjects. We thank Dr. Zhenhong Zhao and Dr. Yang Bao for their help in the lab.
Funding
This work was funded by the Shanghai Municipal Science and Technology Commission (Approval No. 20Z11901002). the National Natural Science Foundation of China (Grant No. 8217003, 81372236, 81360359, 82160847, 81972822, 82272863), and the Natural Science Foundation of Shanghai (21ZR1479200) Shanghai Changhai Hospital Scientific Research Fund (2019SLZ002, 2019YXK018).
Ethics approval and consent to participate
The School of Life Sciences Ethics Committee at Fudan University provided its approval to the research procedure (approval number: CHEC2015-100). All study subjects signed an informed consent form.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lebrett MB, Crosbie EJ, Smith MJ, Woodward ER, Evans DG, Crosbie PAJ. Targeting lung cancer screening to individuals at greatest risk: the role of genetic factors. Journal of Medical Genetics. 2021;58:217-26
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
3. Liang J, Guan X, Bao G, Yao Y, Zhong X. Molecular subtyping of small cell lung cancer. Seminars in Cancer Biology. 2022;86:450-62
4. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. The Lancet Oncology. 2015;16:e165-e72
5. Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes & Development. 2015;29:1447-62
6. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-54
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018: Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30
8. Lung Cancer Metastasis. New York, NY: Springer New York; 2010
9. Toback FG, Walsh-Reitz MM, Musch MW, Chang EB, Del Valle J, Ren H. et al. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;285:G344-G53
10. Martin TE, Powell CT, Wang Z, Bhattacharyya S, Walsh-Reitz MM, Agarwal K. et al. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;285:G332-G43
11. Lacy ER, Morris GP, Cohen MM. Rapid Repair of the Surface Epithelium in Human Gastric Mucosa After Acute Superficial Injury: Journal of Clinical Gastroenterology. 1993; 17: S125-S35.
12. Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S. et al. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. The Journal of Pathology. 2004;203:789-97
13. Yoon JH, La Cho M, Choi YJ, Back JY, Park MK, Lee SW. et al. Gastrokine 1 regulates NF-κB signaling pathway and cytokine expression in gastric cancers. Journal of Cellular Biochemistry. 2013;114:1800-9
14. Yoon JH, Song JH, Zhang C, Jin M, Kang YH, Nam SW. et al. Inactivation of the Gastrokine 1 gene in gastric adenomas and carcinomas: Inactivation of GKN1 in gastric tumours. The Journal of Pathology. 2011;223:618-25
15. Xing R, Li W, Cui J, Zhang J, Kang B, Wang Y. et al. Gastrokine 1 induces senescence through p16/Rb pathway activation in gastric cancer cells. Gut. 2012;61:43-52
16. Rippa E, La Monica G, Allocca R, Romano MF, De Palma M, Arcari P. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. Journal of Cellular Physiology. 2011;226:2571-8
17. Yoon JH, Seo HS, Choi WS, Kim O, Nam SW, Lee JY. et al. Gastrokine 1 induces senescence and apoptosis through regulating telomere length in gastric cancer. Oncotarget. 2014;5:11695-708
18. Guo X-Y. Decreased expression of gastrokine 1 in gastric mucosa of gastric cancer patients. World Journal of Gastroenterology. 2014;20:16702
19. Yoon JH, Choi WS, Kim O, Park WS. The Role of Gastrokine 1 in Gastric Cancer. Journal of Gastric Cancer. 2014;14:147
20. Yoon JH, Kang YH, Choi YJ, Park IS, Nam SW, Lee JY. et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. Journal of Cancer Research and Clinical Oncology. 2011;137:1697-704
21. Yao S, Huang H-Y, Han X, Ye Y, Qin Z, Zhao G. et al. Keratin 14-high subpopulation mediates lung cancer metastasis potentially through Gkn1 upregulation. Oncogene. 2019;38:6354-69
22. Bao L, Zhang Y, Wang J, Wang H, Dong N, Su X. et al. Variations of chromosome 2 gene expressions among patients with lung cancer or non-cancer. Cell Biology and Toxicology. 2016;32:419-35
23. Broderick P, Wang Y, Vijayakrishnan J, Matakidou A, Spitz MR, Eisen T. et al. Deciphering the Impact of Common Genetic Variation on Lung Cancer Risk: A Genome-Wide Association Study. Cancer Research. 2009;69:6633-41
24. Dong S, Guo A-L, Chen Z-H, Wang Z, Zhang X-C, Huang Y. et al. RRM1 single nucleotide polymorphism -37C→A correlates with progression-free survival in NSCLC patients after gemcitabine-based chemotherapy. Journal of Hematology & Oncology. 2010;3:10
25. Hong MJ, Lee SY, Choi JE, Kang H-G, Do SK, Lee JH. et al. Intronic variant of EGFR is associated with GBAS expression and survival outcome of early-stage non-small cell lung cancer: Polymorphism located in intron of EGFR. Thoracic Cancer. 2018;9:916-23
26. Lee SY, Choi JE, Jeon H-S, Choi Y-Y, Lee WK, Lee EB. et al. A Panel of Genetic Polymorphism for the Prediction of Prognosis in Patients with Early Stage Non-Small Cell Lung Cancer after Surgical Resection. PLOS ONE. 2015;10:e0140216
27. Liu Y, Qing H, Su X, Wang C, Li Z, Liu S. Association of CD44 Gene Polymorphism with Survival of NSCLC and Risk of Bone Metastasis. Medical Science Monitor. 2015;21:2694-700
28. Ren MM, Xu S, Wei YB, Yang JJ, Yang YN, Sun SS. et al. Roles of HOTAIR in lung cancer susceptibility and prognosis. Molecular genetics & genomic medicine. 2020 8
29. Wang G, Shen Y, Cheng G, Bo H, Lin J, Zheng M. et al. Lysyl Oxidase Gene G473A Polymorphism and Cigarette Smoking in Association with a High Risk of Lung and Colorectal Cancers in a North Chinese Population. International Journal of Environmental Research and Public Health. 2016;13:635
30. Yu T, Xue P, Cui S, Zhang L, Zhang G, Xiao M. et al. Rs3212986 polymorphism, a possible biomarker to predict smoking-related lung cancer, alters DNA repair capacity via regulating ERCC1 expression. Cancer Medicine. 2018;7:6317-30
31. Zhang S, Thakur A, Liang Y, Wang T, Gao L, Yang T. et al. Polymorphisms in C-Reactive Protein and Glypican-5 Are Associated with Lung Cancer Risk and Gartrokine-1 Influences Cisplatin-Based Chemotherapy Response in a Chinese Han Population. Disease markers. 2015;2015:1-8
32. Spinola M, Meyer P, Kammerer S, Falvella FS, Boettger MB, Hoyal CR. et al. Association of the PDCD5 Locus With Lung Cancer Risk and Prognosis in Smokers. Journal of Clinical Oncology. 2006;24:1672-8
33. Yin J, Wang L, Shi Y, Shao A, Tang W, Wang X. et al. Interleukin 17A rs4711998 A>G polymorphism was associated with a decreased risk of esophageal cancer in a Chinese population: IL17A polymorphism and esophageal cancer. Diseases of the Esophagus. 2014;27:87-92
34. Dai J, Zhang N, Wang J, Chen M, Chen J. Gastrokine-2 is downregulated in gastric cancer and its restoration suppresses gastric tumorigenesis and cancer metastasis. Tumor Biology. 2014;35:4199-207
35. Liu F, Wu H. Prognostic Value of Gastrokine-2 (GKN2) and Its Correlation with Tumor-Infiltrating Immune Cells in Lung Cancer and Gastric Cancers. Journal of Inflammation Research. 2020 Volume 13: 933-44
36. Kim O, Yoon JH, Choi WS, Ashktorab H, Smoot DT, Nam SW. et al. GKN2 Contributes to the Homeostasis of Gastric Mucosa by Inhibiting GKN1 Activity: GKN2 IS AN INHIBITOR OF GKN1. Journal of Cellular Physiology. 2014;229:762-71
37. Baus-Loncar M, Lubka M, Pusch CM, Otto WR, Poulsom R, Blin N. Cytokine Regulation of the Trefoil Factor Family Binding Protein GKN2 (GDDR/TFIZ1/blottin) in Human Gastrointestinal Epithelial Cells. Cellular Physiology and Biochemistry. 2007;20:193-204
38. Chung Nien Chin S, O'Connor L, Scurr M, Busada JT, Graham AN, Alipour Talesh G, et al. Coordinate expression loss of GKN1 and GKN2 in gastric cancer via impairment of a glucocorticoid-responsive enhancer
39. Zhang S, Thakur A, Liang Y, Wang TA-O, Gao L, Yang T, et al. Polymorphisms in C-reactive protein and Glypican-5 are associated with lung cancer risk and Gartrokine-1 influences Cisplatin-based chemotherapy response in a Chinese Han population
40. Siegfried JA-O. Sex and Gender Differences in Lung Cancer and Chronic Obstructive Lung Disease. LID - bqab254 [pii] LID - 10.1210/endocr/bqab254 [doi].
41. Qian Y, Xie LA-O, Li L, Feng T, Zhu T, Wang R, et al. Association between sex hormones regulation-related SNP rs12233719 and lung cancer risk among never-smoking Chinese women
42. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero J-J, DeMeo DL. et al. Sex and gender: modifiers of health, disease, and medicine. The Lancet. 2020;396:565-82
43. Pan SH, Chien WC, He JL, Shih LC, Hsu CL, Hsia TC, et al. The Contribution of Flap Endonuclease 1 Genotypes to Oral Cancer Risk
44. Kligerman S, White C. Epidemiology of Lung Cancer in Women: Risk Factors, Survival, and Screening. American Journal of Roentgenology. 2011;196:287-95
45. Zhang X, Wu L, Xu Y, Zhang B, Wu X, Wang Y. et al. Trends in the incidence rate of lung cancer by histological type and gender in Sichuan, China, 1995-2015: A single-center retrospective study: LC incidence rate trends in Sichuan. Thoracic Cancer. 2018;9:532-41
46. Shao L, Zuo X, Yang Y, Zhang Y, Yang N, Shen B, et al. The inherited variations of a p53-responsive enhancer in 13q12.12 confer lung cancer risk by attenuating TNFRSF19 expression
47. Scherübl H. [Smoking tobacco and cancer risk]
48. Xue J, Yang S, Seng S. Mechanisms of Cancer Induction by Tobacco-Specific NNK and NNN
49. Friedman JR, Richbart SD, Merritt JC, Brown KC, Nolan NA, Akers AT. et al. Acetylcholine signaling system in progression of lung cancers. Pharmacology & Therapeutics. 2019;194:222-54
50. Chen Y, Widschwendter M, Teschendorff AE. Systems-epigenomics inference of transcription factor activity implicates aryl-hydrocarbon-receptor inactivation as a key event in lung cancer development. Genome Biology. 2017;18:236
51. Jiang LP, Zhu ZT, He CY. Effects of CYP3A5 genetic polymorphism and smoking on the prognosis of non-small-cell lung cancer
52. Zhang H, Li Y, Guo S, Wang Y, Wang H, Lu D, et al. Effect of ERCC2 rs13181 and rs1799793 polymorphisms and environmental factors on the prognosis of patients with lung cancer
53. Yeo MK, Choi SY, Seong IO, Suh KS, Kim JM, Kim KH. Association of PD-L1 expression and PD-L1 gene polymorphism with poor prognosis in lung adenocarcinoma and squamous cell carcinoma
54. Chin L, Andersen Jn Fau - Futreal PA, Futreal PA. Cancer genomics: from discovery science to personalized medicine
55. Tran B, Dancey Je Fau - Kamel-Reid S, Kamel-Reid S Fau - McPherson JD, McPherson Jd Fau - Bedard PL, Bedard Pl Fau - Brown AMK, Brown Am Fau - Zhang T, et al. Cancer genomics: technology, discovery, and translation
56. Balistreri CR, Candore G, Lio D, Carruba G. Prostate cancer: from the pathophysiologic implications of some genetic risk factors to translation in personalized cancer treatments
57. Lee D, Lee GK, Yoon K-A, Lee JS. Pathway-Based Analysis Using Genome-wide Association Data from a Korean Non-Small Cell Lung Cancer Study. PLoS ONE. 2013;8:e65396
58. Pierzynski JA, Ye Y, Lippman SM, Rodriguez MA, Wu X, Hildebrandt MAT. Socio-demographic, Clinical, and Genetic Determinants of Quality of Life in Lung Cancer Patients. Scientific Reports. 2018;8:10640
Author contact
![]() Corresponding author: wjjxcccom (Junjie Wu); xfs2000com (Fan Xia); shangyan751200com (Yan Shang); *Address: Shanghai Geriatric Medical Center, Zhongshan Hospital, Fudan University. No. 512 Huashan Road, Jing'an District, Shanghai, China.
Corresponding author: wjjxcccom (Junjie Wu); xfs2000com (Fan Xia); shangyan751200com (Yan Shang); *Address: Shanghai Geriatric Medical Center, Zhongshan Hospital, Fudan University. No. 512 Huashan Road, Jing'an District, Shanghai, China.

 Global reach, higher impact
Global reach, higher impact