3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(2):404-412. doi:10.7150/ijms.90012 This issue Cite
Review
Research progress of absorbable stents
1. Department of Neurovascular oncology Surgery, First Hospital of Jilin University, 1 Xinmin Avenue Changchun 130021, Jilin Province, China.
2. Department of Neurovascular Surgery, First Hospital of Jilin University, 1 Xinmin Avenue Changchun 130021, Jilin Province, China.
Received 2023-9-10; Accepted 2023-12-2; Published 2024-1-1
Abstract
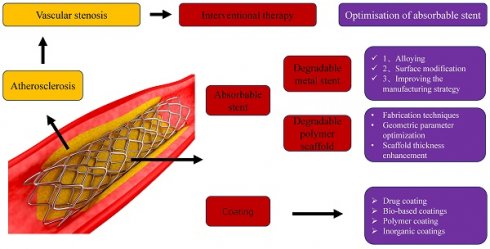
Atherosclerosis, a chronic inflammation of blood vessel walls, is a progressive pathophysiological process characterized by lipid deposition and innate adaptive immune responses. Arteriosclerosis often leads to narrowing of blood vessels. At present, interventional stent therapy is the main treatment method for vascular stenosis, which has the advantages of less trauma, less risk and faster recovery. However, atherosclerosis occurs in a complex pathophysiological environment. Stenting inevitably causes local tissue damage, leading to complications such as inflammation, intimal hyperplasia, late thrombosis, stent restenosis and other complications. It is urgent to optimize interventional therapy program. This article summarizes the advantages and disadvantages of absorbable metal scaffolds and the research progress of absorbable polymer scaffolds. The optimization strategy of stent is proposed. The status quo of drug coating was summarized. The prospect of new stent. To improve the therapeutic effect of arteriosclerosis.
Keywords: Atherosclerosis, Bioresorbable stents, coating, Stent optimization
1. Introduction
Atherosclerosis, the chronic inflammation of blood vessel walls, is a progressive pathophysiological process characterised by lipid deposition and innate adaptive immune responses. In response to blood flow disturbances, endothelial cells shift from a resting phenotype to a pro-atherosclerotic phenotype, which is often described as the starting point of atherosclerosis. It also causes excessive activation of the oxidative system, thrombosis, foam cell formation, inflammatory release, and sensitisation of SMC [1], gradually forming arteriosclerotic plaques and leading to stroke, coronary heart disease, and other diseases. It is a major cause of death in developed countries [2, 3].
Currently, interventional stenting is the most commonly used treatment for atherosclerotic stenosis. This method has the advantages of low trauma, low risk, and fast recovery and is clinically effective [4-6]. However, atherosclerosis is characterised by a complex pathophysiological environment that includes low pH, high oxidative stress, and chronic inflammation. Stenting therapy inevitably causes local tissue damage, leading to complications such as inflammation, intimal hyperplasia, late thrombosis, and intrastent restenosis [7-9]. Therefore, researchers continue to explore whether the implanted stent not only inhibits thrombus formation and promotes endothelialisation in the early stage but also inhibits cell proliferation in the later stage of treatment. Therefore, new stent technologies are being continuously developed. In this paper, we summarize the current research progress of absorbable stents and propose a scheme for material optimization. At the same time, the development of coating technology has also increased the effect of stent therapy. There are great expectations for bioabsorbable scaffolds.
2. Absorbable stent
2.1. Degradable metal stent
In recent decades, the use of bare-metal stents in clinical practice has improved the efficacy of arterial interventions [10, 11]. However, the long-term presence of traditional permanent metal stents in patients can cause chronic inflammation, leading to intrastent restenosis and stent thrombosis [12]. Moreover, long-term dual antiplatelet therapy after stent implantation may increase the risk of bleeding [13]. Developing and improving a new generation of absorbable metal scaffolds (AMS) can replace permanent scaffolds and mitigate the associated risks. Ideal AMS can induce an appropriate host reaction and gradual corrosion, and its material and degradation products have sufficient safety and suitable biocompatibility [14-17] and can provide adequate mechanical integrity with appropriate elastic modulus, radial support strength, and ductility [18-20]. Recently, magnesium, iron, and zinc, essential elements of the human body, have been considered candidates for making AMS. Stents made of these three materials are safe for implantation in the body [15, 20, 21]. In addition, each of these three metals has unique characteristics. Magnesium stents release negligible amounts of magnesium ion (Mg2+) during degradation, which may inhibit abnormal nerve excitation and reduce the risk of atherosclerosis [22]. Iron does not cause an excessive release of H+ or a sharp increase in pH; therefore, it has little impact on the local microenvironment [23]. Zinc plays an important role in cell proliferation [20] and has demonstrated potential antibacterial and anti-atherosclerotic effects [24, 25].
However, in studies on biodegradable metal stents, differences in mechanical properties in vivo have limited their development. The radial strength and degradation rate of magnesium-based scaffolds are extremely high, and a large amount of H2 is generated during the degradation process, which creates an acidic environment in the local part of the scaffold and increases the corrosion rate [26]. In addition, the mechanical properties of the support are reduced during corrosion. However, the degradation rate of iron-based scaffolds is extremely slow, resulting in the accumulation of corrosion products [15] (mainly iron oxide [Fe-O]) that remain in the encased neointima and inhibit vascular tissue regeneration [27]. In addition, the magnetic properties of iron cannot be detected using magnetic resonance imaging [28]. Moreover, the direct interaction between blood vessel cells and iron can generate harmful free radicals during corrosion [29], which can promote the oxidation and modification of nucleic acids and proteins, leading to oxidative stress and a range of harmful systemic events, such as ischaemia, inflammation, and neurodegeneration [30]. In addition, the mechanical strength of zinc-based stents is insufficient, and alloying also leads to ductility and low strength of the zinc-based alloy [31].
2.2. Degradable polymer scaffold
Polylactic acid (PLA) is widely used in many biomedical applications owing to its biocompatibility, biodegradability, and nontoxic degradation products, especially in the fields of biodegradable stents and drug-carrying coatings. PLA, or polylactide, is a thermoplastic polyester with the main chain formula (C3H4O2)n, which is formed by the dehydration and condensation of lactic acid C(CH3)(OH)HCOOH. PLA has become a popular material; several different forms of polylactide exist: polyl-lactide (PLLA) is the product of the polymerisation of L, L-lactide [32, 33], poly (L-lactide-co-D, L-lactide) (PLD LLA) is used as a PLDLLA/TCP scaffold for bone engineering [34]; poly (lactic acid-co-glycolic acid) (PLGA) is produced by random polymerisation of lactic acid and glycolic acid. PLGA is a functional, high-molecular-weight, degradable organic compound. PLA has good biocompatibility, non-toxicity, good encapsulation, and film-forming properties and is widely used in the pharmaceutical, medical engineering, and modern industrial fields [35, 36].
Biodegradable polymers are currently being studied in several clinical trials, including Absorb BVS (PLLA), Elixir Medical's DESolve (PLLA) stent, ART (PLDA) stent, and REVA's Fantom (PTD-PC) stent. These stents improve allergic reactions, atherosclerosis progression, and impaired vasomotor function after implantation of other stents [37].
However, some challenges exist in research on absorbable polymer scaffolds [38, 39], such as the mechanical properties of the device (thicker strut, lower radial strength, higher fracture sensitivity), longer than expected absorption time during absorption, stent removal due to uncovered strut discontinuity, demanding implantation procedures, high stent delivery failure rates, and stent expansion and misalignment. The incidence of acute and late stent thrombosis has increased [40].
Absorbable stents provide hope for treating vascular diseases by reducing the duration of oral dual antibody drugs and solving chronic inflammation caused by the long-term retention of stents in the body. However, metal scaffolds and polymer-absorbable scaffolds have mechanical properties such as degradation of metal scaffolds, corrosion rate, and radial strength of absorbable polymers. Therefore, new materials and designs are required to address this problem.
3. Optimisation of absorbable stent
3.1. Optimisation of metal support
Researchers have attempted to improve the performance of metal scaffolds by exploring new alloys, surface modifications, and manufacturing strategies.
The main preclinical research of stent in arteriosclerosis
| Materials | Coating | Drugs | Outcome | |
|---|---|---|---|---|
| [105] | 316L stainless steel | Hyaluronic acid and chitosan | ACS14 | Inhibit platelet adhesion and activation Inhibition of smooth muscle cells and macrophages proliferation Reduced inflammation at the site of intervention and promoted the formation of new blood vessels |
| [115] | 316L stainless steel | PLCL | Atorvastatin Fenofibrate | Excellent biocompatibility No inflammatory reaction. |
| [104] | 316L stainless steel | zein (the active layer) and cross-linked alginate (the sacrificial layer) | rutin | Sustained drug release High biocompatibility |
| [116] | 316L stainless steel | PLLA | SZ-21、VEGF、RAPA | Reendothelialization and inhibition of thrombosis, inflammation, and intrastent restenosis |
| [85] | 316L stainless steel | PGMA | Hep/NONOates nanoparticle | Endothelial cell regeneration Anticoagulant activity |
| [86] | 316L stainless steel | EGCG | Pivastatin calcium | Reactive oxygen species |
| [117] | 316L stainless steel | Avidin | biotin-modified endothelial cells | Promotes cell bonding to scaffold struts Reduce intrastent restenosis |
| [118] | 316L stainless steel | PDA-HD | Multifunctional coating of flavonoids baicalin | Anti-ISR, anti-inflammation Promote endothelialization |
| [119] | cobalt-chromium alloy | Polylysine layer and hyaluronic acid-dopamine conjugate | NO | Optimize the release rate and therapeutic dose of NO |
| [120] | cobalt-chromium alloy | Hyaluronic acid/chitosan | Binding siRNA nanocomplexes | Good blood compatibility |
| [96] | cobalt-chromium alloy | silicone nanofilament (SiNf) | CD146- Antibody | Promote reendothelialization Preventive restenosis |
| [121] | Mg-Zn alloy | TiO2 | TiO2 nanocoating | Stimulate endothelial cell adhesion and proliferation Inhibit the release of harmful products from zinc-magnesium coated scaffolds |
| [122] | Mg-Zn alloy | MF2-PA-PLGA | Rare-earth free | Complete biodegradation No foreign body residue Promote reendothelialization |
| [123] | Fe base | PDLLA | Sirolimus | Promote reendothelialization Reasonable corrosion |
| [124] | Zn base | Mixed coating of polycarbonate, tannic acid and copper ions | Copper | Corrosion resistance Reduces inflammation Promote endothelial cell adhesion and proliferation |
| [125] | PLLA | PLLA | 4-octyl itaconate (OI) | Decreased inflammation Inhibition of SMC proliferation Endothelial regeneration integrity |
| [98] | PLLA | PLGA | Rapamycin, VEGF | Promotes endothelial regeneration Reduce intrastent restenosis |
| [126] | PLLA | PCL-PEG-PCL, PCEC | miR-22 | Reduce inflammation Phenotypic transformation of SMC was low IRS suppression |
| [106] | PU | zein | ZnO nanoparticles | Cytocompatibility Anticoagulant reaction Antibiosis |
3.1.1. Alloying
Alloying can increase the mechanical strength, plasticity, and corrosion resistance of metal scaffolds [41, 42]. In addition, rare earth elements can significantly improve the mechanical properties and degradation behaviour of biodegradable metals, such as (Y, Nd, Ho, Dy, and Gd) [43]. Some alloying elements have been demonstrated to improve the degradation behavior of magnesium alloys, such as zinc, aluminum, manganese, calcium, lithium, strontium, and tin [44-46]. However, to improve the degradation rate and biocompatibility of iron-based scaffolds, additives should have lower electrochemical potential or be more valuable than iron [18]. For example, palladium or platinum can improve the mechanical properties, increase the corrosion rate of iron, and stabilise iron in the austenitic form [47]. Iron-gold and iron-silver alloys have faster degradation rates without increased cytotoxicity, platelet adhesion, or thrombogenic effects [48]. Iron nitride exhibits a high degradation rate and good mechanical properties [49]. The mechanical strength of zinc is insufficient for stent implantation; however, the zinc-based scaffold is optimised for its degradation rate and biocompatible, mainly through alloying, to improve tensile strength [31]. For example, copper, magnesium, calcium, and strontium can further improve the mechanical properties and degradation of zinc-aluminum alloys [50]. Zinc-silver alloys can reduce stent-associated infections and adjust mechanical strength by adjusting the silver content [51].
3.1.2. Surface modification
Micro-arc oxidation, phosphating treatment, electrodeposition, and basic heat treatment can change the surface chemistry and metallurgical microstructure of magnesium-based scaffolds and improve the degradation behaviour of magnesium scaffolds [52]. Using plasma immersion ion implantation and deposition, Fe-O films can be constructed to cover iron-based scaffolds, thereby improving the biocompatibility and mechanical activation of platelets [53]. Surface modifications can help improve the corrosion rate of iron-based scaffolders, such as lithography and electron beam evaporation of platinum disks, sandblasting, phosphating, alkaline heating, micro-arc oxidation, and electrodeposition [54]. However, these technologies need to be explored further.
3.1.3. Improving the manufacturing strategy
New manufacturing strategies to achieve grain refinement can also improve the mechanical properties and degradation behaviour of magnesium-based scaffolds. For example, AZ31 exhibits a lower degradation rate due to grain refinement from mechanical processing [55]. The small ZM21 has a higher mechanical strength [56]. In addition, 3D printing technology, particularly selective laser melting, can be used to process magnesium alloys to optimise the machine structure and better control corrosion [57, 58]. Equal-channel angular pressure can produce nanocrystalline iron, which inhibits VSCM proliferation but promotes ECs growth [59]. Owing to micrograin and microstructural defects, electroforming processes increase the degradation rate of iron-based scaffolds, resulting in the increased release of iron [60]. Simultaneously, iron-based scaffolds produced by powder metallurgy (PM) have faster corrosion rates because PM creates more pores [61]. Several new manufacturing methods, including inkjet 3D printing and power spraying of cold air [62], may also improve the degradation rate of iron-based scaffolds. In addition, the mechanical properties of cast zinc alloys can be further improved by grain refinement induced by deformation heat treatment [63]. Severe plastic deformation techniques may alter the mechanical properties of zinc alloys [64].
3.2. Optimisation of polymer scaffolds
Various methods have proved effective for strengthening absorbable polymer scaffolds, including fabrication techniques, geometric parameter optimisation, and scaffold thickness enhancement [65]. High molecular weight polymers can increase the entanglement and length of covalently bonded molecular chains, thereby improving the fracture strain and wear resistance of scaffolds [66]. Increasing the crystallinity of semi-crystalline polymers can increase the hardness and heat or chemical resistance of BDPS [67]. Modifying the molecular structure by controlling the internal structure of the polymer chain orientation can improve the mechanical strength of the scaffold [68]. Simultaneously, changing the geometric parameters can improve the radial strength of the support. For example, the IGaki-Tamai stent has a thick strut in the shape of a zigzag spiral coil; therefore, it has high vascular coverage [69]. Biodegradable, nontoxic lignocellulosic fibres from renewable resources such as wood have been studied as potential augments for biodegradable polymers because of their high strength and more economical performance than traditional synthetic fibers [70-73]. In addition, fabricating absorbable polymer scaffolds with CO2 lasers or adopting new sliding lock mechanisms can improve the mechanical properties of scaffolds [74]. The shape memory PCLAU combined with Fe3O4 nanoparticles can provide sufficient strength for stent implantation [75]. The biocompatibility of BDPS can be improved by plasma surface treatment and the use of high-molecular-weight PLLA [69]. In addition, the degradation of BDPS can be improved by changing the crystallinity, molecular weight, and hydrophilicity of the polymer [76-78].
4. Stent coating
Coating technologies for biomaterials include metal-metal coating, chemical vapour deposition, ion beam assisted deposition, atomic layer deposition, and pulsed laser deposition [79-81]. Using these technologies, targeted drugs, bio-based coatings, polymer coatings, and inorganic coatings can be delivered to the target location. Polymer coatings and inorganic coatings can also be used to produce porous coatings.
4.1. Drug coating
In recent years, research has been increasingly conducted on new drugs for drug-eluting stents. Currently, drug-eluting stents commonly used in clinics mainly use drugs such as rapamycin, paclitaxel, sirolimus, and everolimus to inhibit endothelial and smooth muscle proliferation, and their short-term therapeutic effects have been confirmed. However, the incidence of long-term stent thrombosis and restenosis remains a challenge [82-84]. Therefore, scientists are constantly exploring new drugs and drug combinations to optimise drug coatings. Heparin induces and accelerates endothelial cell regeneration and maintains anticoagulant activity [85]. Statin drug-eluting stents eliminate atherosclerotic plaque [86]. They induce autophagy at atherosclerotic sites and exert anti-inflammatory effects [87]. In addition to synthetic drugs, gene mediators are also of interest because they integrate smoothly during physiological regulation. Nitric oxide (NO) is an endogenous gas signalling molecule that regulates vasodilation, controls smooth muscle cell proliferation, inhibits platelet aggregation, and has antibacterial and anti-inflammatory functions [88]. Researchers have prepared a catalyst on the scaffold [89] that catalyses the release of NO to improve anticoagulation and prevent scaffold restenosis. H2S is another gas-signalling molecule that plays an important role in maintaining cerebrovascular homeostasis and protecting and regulating the central nervous system. H2S promotes angiogenesis and anti-inflammatory mediators [90-93]. The aspirin derivative ACS14 and its metabolite ADTOH are potential H2S donors [94]. ACS14 releases H2S while maintaining the antithrombotic effects of aspirin.
4.2. Bio-based coatings
Special biological material-based scaffold coatings are desirable. They allow endothelial cells on scaffold surfaces to proliferate, differentiate, release, and grow, inhibit thrombosis and neointimal hyperplasia, and alleviate restenosis. Endoglin antiboil-coated scaffolds significantly reduced restenosis by enhancing reendothelialisation in pig models [95]. Coating with anti-CD146 antibody (Ab) -fixed silicon nanofilaments for the efficient and specific capture of late rather than early EPCs demonstrated an approximately two-fold increase in endothelial coverage [96]. In addition, endothelial progenitor cell-capture scaffolds with surface-immobilised antibodies have demonstrated clinically significant improvements in endothelialisation. However, most current antibody-based scaffold surface modification strategies rely on antibody adsorption or direct coupling via amino or carboxyl groups, which results in poor control of the antibody surface concentration and/or molecular orientation and eventual cell capture bioavailability. Cell capture is enhanced by the covalent transplantation of protein G polypeptides to immobilise IgG antibodies [97]. The effect of angiogenic factors (VEGF) on endothelial regeneration and the prevention of restenosis after stenting is expected, especially when combined with a drug-eluting coating, which exhibits significant endothelial regeneration and maintains a very low level of intrastent restenosis [98]. In addition, cell coating is promising, and stents coated with VEGF/HGF-secreting UCB-MSCs reduce the restenosis side effects of cardiac stent implantation and improve reendothelialisation [99].
4.3. Polymer coating
Polymer materials have been used as stent coatings with or without drug elution with mixed success rates. These materials include polyethylene, polyurethane, polyvinyl ester, and polylactide. They can be used as nanomaterials and drug carriers to control drug release rates [14, 100-103]. However, frequently used biodegradable synthetic polymers such as PLGA and PLLA produce acidic degradation products that cause local inflammation and delay tissue healing due to local acidification [104, 105]. Naturally derived biopolymers, such as zein (from corn) and alginate (from seaweed), have been used to replace synthetic biodegradable polymers with less inflammation in long-term applications [106].
4.4. Inorganic coatings
Several inorganic materials can potentially improve the performance of implant surfaces. The inorganic materials used to manufacture scaffold coatings include oxides, nitrides, silicides and carbides, precious metals, hydroxyapatite-based materials, and diamond and diamond-like carbon [107-110]. Titanium oxide-based coatings are the most promising inorganic materials for cardiovascular stents. The stainless steel bioactive scaffold Titan2 (Hexacath, Paris, France), coated with plasma-enhanced titanium vapour deposition in a nitrogen-oxygen mixed atmosphere, inhibits platelet aggregation, minimises fibrin deposition, reduces inflammation, and promotes healing. In recent clinical trials [111-113], the new generation of titanium NO-coated stents, TiOxNy and TITAX-AMI, have been proven safe, successfully reduced in-stent restenosis, and marketed. NO is one of the most important molecules in biological systems and plays a key role in pathophysiology and disease by promoting endothelialisation and activating endothelial cell growth. This has led to the development of novel therapeutic strategies and NO donors [114].
5. Outlook
Given the high mortality rates worldwide from cardiovascular and cerebrovascular diseases caused by arteriosclerosis and the potential of stent technology, researchers and clinicians are focused on developing new materials, methods, and solutions to improve clinical outcomes for the available types of stents. The goal is patient safety and to achieve a higher success rate for cardiovascular therapy. Developing and optimising new categories of scaffolds, including membrane-coated and bioabsorbable scaffolds, can achieve the desired release of bioactive agents for adhesion, cell differentiation, and tissue development with appropriate physicochemical properties and degradation rates. Stents for personalised treatment are expected to become available in the near future.
6. Conclusion
Cardiovascular and cerebrovascular diseases caused by atherosclerosis have high mortality rates in developed countries. Interventional stenting is the most common treatment for atherosclerosis. This method involves less trauma, less risk, and faster recovery, and is clinically effective. Shortcomings exist in the current study of stents; however, the continuous exploration of new stent materials and the optimisation of structural design, the continuous development of reasonable drug release and biotechnology, the realisation of targeted therapy for arteriosclerosis, and the concept of intervention-free implantation are needed.
Abbreviations
PLA: Polylactic acid; PLLA: polyl-lactide; PLDLLA: poly (L-lactide-co-D, L-lactide); PLGA: poly (lactic acid-co-glycolic acid); PM: powder metallurgy; NO: nitric oxide.
Acknowledgements
Author Contributions
Zhuyuan Yu developed the concept of the project and wrote the manuscript. Ying Song, Bingwei Li, Hao Chen were involved in the manuscript writing, including discussion of content and writing, and editing of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Li L, Liu S, Tan J, Wei L, Wu D, Gao S. et al. Recent advance in treatment of atherosclerosis: Key targets and plaque-positioned delivery strategies. Journal of tissue engineering. 2022;13:20417314221088509
2. Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R. et al. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Archives of medical research. 2015;46:328-38
3. Sidelnikov E, Dornstauder E, Jacob C, Maas C, Pinto L, Leidl R. et al. Healthcare resource utilization and costs of cardiovascular events in patients with atherosclerotic cardiovascular disease in Germany - results of a claims database study. Journal of medical economics. 2022;25:1199-206
4. Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. The New England journal of medicine. 2013;368:254-65
5. Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. The New England journal of medicine. 1987;316:701-6
6. Cockerill I, See CW, Young ML, Wang Y, Zhu D. Designing Better Cardiovascular Stent Materials - A Learning Curve. Advanced functional materials. 2021 31
7. Rezvan A, Ni CW, Alberts-Grill N, Jo H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxidants & redox signaling. 2011;15:1433-48
8. Urban P, De Benedetti E. Thrombosis: the last frontier of coronary stenting? Lancet (London, England). 2007;369:619-21
9. Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. Journal of the American College of Cardiology. 2010;56:1897-907
10. Haben C, Park WM, Bena JF, Parodi FE, Lyden SP. Improving midterm results justify the continued use of bare-metal stents for endovascular therapy for chronic mesenteric ischemia. Journal of vascular surgery. 2020;71:111-20
11. Chen Y, Gao P, Huang L, Tan X, Zhou N, Yang T. et al. A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent. Nature communications. 2021;12:7079
12. Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovascular interventions. 2014;7:1081-92
13. Tsai ML, Hsieh MJ, Chen CC, Chang SH, Wang CY, Chen DY. et al. Comparison of 9-Month Angiographic Follow-Up and Long-Term Clinical Outcomes of Biodegradable Polymer Drug-Eluting Stents and Second-Generation Durable Polymer Drug-Eluting Stents in Patients Undergoing Single Coronary Artery Stenting. Acta Cardiologica Sinica. 2020;36:97-104
14. Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart (British Cardiac Society). 2003;89:651-6
15. Scarcello E, Lobysheva I, Bouzin C, Jacques PJ, Lison D, Dessy C. Endothelial dysfunction induced by hydroxyl radicals - the hidden face of biodegradable Fe-based materials for coronary stents. Materials science & engineering C, Materials for biological applications. 2020;112:110938
16. Zhu D, Cockerill I, Su Y, Zhang Z, Fu J, Lee KW. et al. Mechanical Strength, Biodegradation, and in Vitro and in Vivo Biocompatibility of Zn Biomaterials. ACS applied materials & interfaces. 2019;11:6809-19
17. Guillory RJ 2nd, Sikora-Jasinska M, Drelich JW, Goldman J. In Vitro Corrosion and in Vivo Response to Zinc Implants with Electropolished and Anodized Surfaces. ACS applied materials & interfaces. 2019;11:19884-93
18. Seitz JM, Durisin M, Goldman J, Drelich JW. Recent advances in biodegradable metals for medical sutures: a critical review. Advanced healthcare materials. 2015;4:1915-36
19. Loffredo S, Paternoster C, Giguère N, Barucca G, Vedani M, Mantovani D. The addition of silver affects the deformation mechanism of a twinning-induced plasticity steel: Potential for thinner degradable stents. Acta biomaterialia. 2019;98:103-13
20. Fu J, Su Y, Qin YX, Zheng Y, Wang Y, Zhu D. Evolution of metallic cardiovascular stent materials: A comparative study among stainless steel, magnesium and zinc. Biomaterials. 2020;230:119641
21. Li Y, Ji CX, Mei LH, Qiang JW, Ju S. Oral administration of trace element magnesium significantly improving the cognition and locomotion in hepatic encephalopathy rats. Scientific reports. 2017;7:1817
22. Zhang ZQ, Yang YX, Li JA, Zeng RC, Guan SK. Advances in coatings on magnesium alloys for cardiovascular stents - A review. Bioactive materials. 2021;6:4729-57
23. Zhang D, Cai Z, Liao N, Lan S, Wu M, Sun H. et al. pH/hypoxia programmable triggered cancer photo-chemotherapy based on a semiconducting polymer dot hybridized mesoporous silica framework. Chemical science. 2018;9:7390-9
24. Niu J, Tang Z, Huang H, Pei J, Zhang H, Yuan G. et al. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Materials science & engineering C, Materials for biological applications. 2016;69:407-13
25. Bowen PK, Drelich J, Goldman J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Advanced materials (Deerfield Beach, Fla). 2013;25:2577-82
26. Li N, Zheng Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. Journal of Materials Science & Technology. 2013;29:489-502
27. Lin W, Qin L, Qi H, Zhang D, Zhang G, Gao R. et al. Long-term in vivo corrosion behavior, biocompatibility and bioresorption mechanism of a bioresorbable nitrided iron scaffold. Acta biomaterialia. 2017;54:454-68
28. Bian D, Qin L, Lin W, Shen D, Qi H, Shi X. et al. Magnetic resonance (MR) safety and compatibility of a novel iron bioresorbable scaffold. Bioactive materials. 2020;5:260-74
29. Wang Z, Yang B, Chen X, Zhou Q, Li H, Chen S. et al. Nobiletin Regulates ROS/ADMA/DDAHII/eNOS/NO Pathway and Alleviates Vascular Endothelium Injury by Iron Overload. Biological trace element research. 2020;198:87-97
30. Lu Q, Harris VA, Rafikov R, Sun X, Kumar S, Black SM. Nitric oxide induces hypoxia ischemic injury in the neonatal brain via the disruption of neuronal iron metabolism. Redox biology. 2015;6:112-21
31. Chen C, Yue R, Zhang J, Huang H, Niu J, Yuan G. Biodegradable Zn-1.5Cu-1.5Ag alloy with anti-aging ability and strain hardening behavior for cardiovascular stents. Materials science & engineering C, Materials for biological applications. 2020;116:111172
32. Li G, Zhao M, Xu F, Yang B, Li X, Meng X. et al. Synthesis and Biological Application of Polylactic Acid. Molecules (Basel, Switzerland). 2020 25
33. Singhvi MS, Zinjarde SS, Gokhale DV. Polylactic acid: synthesis and biomedical applications. Journal of applied microbiology. 2019;127:1612-26
34. Thavornyutikarn B, Chantarapanich N, Sitthiseripratip K, Thouas GA, Chen Q. Bone tissue engineering scaffolding: computer-aided scaffolding techniques. Progress in biomaterials. 2014;3:61-102
35. Haude M, Erbel R, Erne P, Verheye S, Degen H, Böse D. et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet (London, England). 2013;381:836-44
36. Haude M, Erbel R, Erne P, Verheye S, Degen H, Vermeersch P. et al. Safety and performance of the DRug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de novo coronary lesions: 3-year results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2016;12:e160-6
37. Toong DWY, Toh HW, Ng JCK, Wong PEH, Leo HL, Venkatraman S. et al. Bioresorbable Polymeric Scaffold in Cardiovascular Applications. International journal of molecular sciences. 2020 21
38. Serruys PW, Onuma Y, Ormiston JA, de Bruyne B, Regar E, Dudek D. et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. 2010;122:2301-12
39. Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS. et al. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORB III Trial. Journal of the American College of Cardiology. 2017;70:2852-62
40. Caiazzo G, Kilic ID, Fabris E, Serdoz R, Mattesini A, Foin N. et al. Absorb bioresorbable vascular scaffold: What have we learned after 5 years of clinical experience? International journal of cardiology. 2015;201:129-36
41. Ding Z-Y, Cui L-Y, Zeng R-C, Zhao Y-B, Guan S-K, Xu D-K. et al. Exfoliation corrosion of extruded Mg-Li-Ca alloy. Journal of Materials Science & Technology. 2018;34:1550-7
42. Harawaza K, Cousins B, Roach P, Fernandez A. Modification of the surface nanotopography of implant devices: A translational perspective. Materials today Bio. 2021;12:100152
43. Li H, Wang P, Lin G, Huang J. The role of rare earth elements in biodegradable metals: A review. Acta biomaterialia. 2021;129:33-42
44. Wang HX, Guan SK, Wang X, Ren CX, Wang LG. In vitro degradation and mechanical integrity of Mg-Zn-Ca alloy coated with Ca-deficient hydroxyapatite by the pulse electrodeposition process. Acta biomaterialia. 2010;6:1743-8
45. Baek S-M, Lee S-Y, Kim JC, Kwon J, Jung H, Lee S. et al. Role of trace additions of Mn and Y in improving the corrosion resistance of Mg-3Al-1Zn alloy. Corrosion Science. 2021;178:108998
46. Zhou SL, Liu W, Fu SC. Constitutive modelling of LZ91 magnesium-lithium alloy sheet by uniaxial tension loading tests. Materials Today: Proceedings. 2020;33:1787-91
47. Allenstein U, Ma Y, Arabi-Hashemi A, Zink M, Mayr SG. Fe-Pd based ferromagnetic shape memory actuators for medical applications: Biocompatibility, effect of surface roughness and protein coatings. Acta biomaterialia. 2013;9:5845-53
48. Huang T, Cheng Y, Zheng Y. In vitro studies on silver implanted pure iron by metal vapor vacuum arc technique. Colloids and surfaces B, Biointerfaces. 2016;142:20-9
49. Lin W, Zhang G, Cao P, Zhang D, Zheng Y, Wu R. et al. Cytotoxicity and its test methodology for a bioabsorbable nitrided iron stent. Journal of biomedical materials research Part B, Applied biomaterials. 2015;103:764-76
50. Farabi E, Sharp JA, Vahid A, Fabijanic DM, Barnett MR, Gallo SC. Development of high strength and ductile Zn-Al-Li alloys for potential use in bioresorbable medical devices. Materials science & engineering C, Materials for biological applications. 2021;122:111897
51. Wątroba M, Bednarczyk W, Kawałko J, Bała P. Fine-tuning of mechanical properties in a Zn-Ag-Mg alloy via cold plastic deformation process and post-deformation annealing. Bioactive materials. 2021;6:3424-36
52. Chen C, Chen J, Wu W, Shi Y, Jin L, Petrini L. et al. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials. 2019;221:119414
53. Cisternas M, Bhuyan H, Retamal MJ, Casanova-Morales N, Favre M, Volkmann UG. et al. Study of nitrogen implantation in Ti surface using plasma immersion ion implantation & deposition technique as biocompatible substrate for artificial membranes. Materials science & engineering C, Materials for biological applications. 2020;113:111002
54. Fromel M, Li M, Pester CW. Surface Engineering with Polymer Brush Photolithography. Macromolecular rapid communications. 2020;41:e2000177
55. Zhu J, Zhang X, Niu J, Shi Y, Zhu Z, Dai D. et al. Biosafety and efficacy evaluation of a biodegradable magnesium-based drug-eluting stent in porcine coronary artery. Scientific reports. 2021;11:7330
56. Wang W, Wu H, Zan R, Sun Y, Blawert C, Zhang S. et al. Microstructure controls the corrosion behavior of a lean biodegradable Mg-2Zn alloy. Acta biomaterialia. 2020;107:349-61
57. Liu S, Guo H. Balling Behavior of Selective Laser Melting (SLM) Magnesium Alloy. Materials (Basel, Switzerland). 2020 13
58. Qin Y, Wen P, Guo H, Xia D, Zheng Y, Jauer L. et al. Additive manufacturing of biodegradable metals: Current research status and future perspectives. Acta biomaterialia. 2019;98:3-22
59. Nie FL, Zheng YF, Wei SC, Hu C, Yang G. In vitro corrosion, cytotoxicity and hemocompatibility of bulk nanocrystalline pure iron. Biomedical materials (Bristol, England). 2010;5:065015
60. Moravej M, Prima F, Fiset M, Mantovani D. Electroformed iron as new biomaterial for degradable stents: development process and structure-properties relationship. Acta biomaterialia. 2010;6:1726-35
61. Paim TC, Wermuth DP, Bertaco I, Zanatelli C, Naasani LIS, Slaviero M. et al. Evaluation of in vitro and in vivo biocompatibility of iron produced by powder metallurgy. Materials science & engineering C, Materials for biological applications. 2020;115:111129
62. Chou DT, Wells D, Hong D, Lee B, Kuhn H, Kumta PN. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta biomaterialia. 2013;9:8593-603
63. Sikora-Jasinska M, Mostaed E, Mostaed A, Beanland R, Mantovani D, Vedani M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed ZnAg alloys for degradable implant applications. Materials science & engineering C, Materials for biological applications. 2017;77:1170-81
64. Mostaed E, Sikora-Jasinska M, Drelich JW, Vedani M. Zinc-based alloys for degradable vascular stent applications. Acta biomaterialia. 2018;71:1-23
65. Acharya G, Lee CH, Lee Y. Optimization of cardiovascular stent against restenosis: factorial design-based statistical analysis of polymer coating conditions. PloS one. 2012;7:e43100
66. Biswas A, Singh AP, Rana D, Aswal VK, Maiti P. Biodegradable toughened nanohybrid shape memory polymer for smart biomedical applications. Nanoscale. 2018;10:9917-34
67. Polak-Kraśna K, Abaei AR, Shirazi RN, Parle E, Carroll O, Ronan W. et al. Physical and mechanical degradation behaviour of semi-crystalline PLLA for bioresorbable stent applications. Journal of the mechanical behavior of biomedical materials. 2021;118:104409
68. Papkov D, Delpouve N, Delbreilh L, Araujo S, Stockdale T, Mamedov S. et al. Quantifying Polymer Chain Orientation in Strong and Tough Nanofibers with Low Crystallinity: Toward Next Generation Nanostructured Superfibers. ACS nano. 2019;13:4893-927
69. Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S. et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399-404
70. Im SH, Jung Y, Kim SH. Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta biomaterialia. 2017;60:3-22
71. Bledzki AK, Gassan J. Composites reinforced with cellulose based fibres. Progress in Polymer Science. 1999;24:221-74
72. Eichhorn SJ, Baillie CA, Zafeiropoulos N, Mwaikambo LY, Ansell MP, Dufresne A. et al. Review: Current international research into cellulosic fibres and composites. Journal of Materials Science. 2001;36:2107-31
73. Zimmermann T, Pöhler E, Geiger T. Cellulose Fibrils for Polymer Reinforcement. 2004; 6: 754-61.
74. Wang Q, Fang G, Zhao Y, Wang G, Cai T. Computational and experimental investigation into mechanical performances of Poly-L-Lactide Acid (PLLA) coronary stents. Journal of the mechanical behavior of biomedical materials. 2017;65:415-27
75. Gu S-Y, Chang K, Jin S-P. A dual-induced self-expandable stent based on biodegradable shape memory polyurethane nanocomposites (PCLAU/Fe3O4) triggered around body temperature. 2018; 135: 45686.
76. Omagari K, Ueda K, Zhijing Z, Higashi K, Inoue M, Fukami T. et al. Mechanistic study of preparation of drug/polymer/surfactant ternary hot extrudates to obtain small and stable drug nanocrystal suspensions. International journal of pharmaceutics. 2020;591:120003
77. Cong S, Creamer A, Fei Z, Hillman SAJ, Rapley C, Nelson J. et al. Tunable Control of the Hydrophilicity and Wettability of Conjugated Polymers by a Postpolymerization Modification Approach. Macromolecular bioscience. 2020;20:e2000087
78. Bekale L, Agudelo D, Tajmir-Riahi HA. Effect of polymer molecular weight on chitosan-protein interaction. Colloids and surfaces B, Biointerfaces. 2015;125:309-17
79. Koch CF, Johnson S, Kumar D, Jelinek M, Chrisey DB, Doraiswamy A. et al. Pulsed laser deposition of hydroxyapatite thin films. Materials Science and Engineering: C. 2007;27:484-94
80. Yang J, Jiao Y, Cui FZ, Lee I-S, Yin Q-s, Zhang YJS. et al. Modification of degradation behavior of magnesium alloy by IBAD coating of calcium phosphate. 2008; 202: 5733-6.
81. Wank JR, George SM, Weimer AW. Coating Fine Nickel Particles with Al2O3 Utilizing an Atomic Layer Deposition-Fluidized Bed Reactor (ALD-FBR). 2004; 87: 762-5.
82. Puranik AS, Dawson ER, Peppas NA. Recent advances in drug eluting stents. International journal of pharmaceutics. 2013;441:665-79
83. Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. The New England journal of medicine. 2007;356:1009-19
84. Sheiban I, Villata G, Bollati M, Sillano D, Lotrionte M, Biondi-Zoccai G. Next-generation drug-eluting stents in coronary artery disease: focus on everolimus-eluting stent (Xience V). Vascular health and risk management. 2008;4:31-8
85. Zhu T, Zhou M, Gao W, Fang D, Liu Z, Wu G. et al. Coronary Stents Decorated by Heparin/NONOate Nanoparticles for Anticoagulant and Endothelialized Effects. Langmuir: the ACS journal of surfaces and colloids. 2020;36:2901-10
86. Wang K, Shang T, Zhang L, Zhou L, Liu C, Fu Y. et al. Application of a Reactive Oxygen Species-Responsive Drug-Eluting Coating for Surface Modification of Vascular Stents. ACS applied materials & interfaces. 2021;13:35431-43
87. Han F, Xiao QQ, Peng S, Che XY, Jiang LS, Shao Q. et al. Atorvastatin ameliorates LPS-induced inflammatory response by autophagy via AKT/mTOR signaling pathway. Journal of cellular biochemistry. 2018;119:1604-15
88. Carpenter AW, Schoenfisch MH. Nitric oxide release: part II. Therapeutic applications. Chemical Society reviews. 2012;41:3742-52
89. Zhao Q, Fan Y, Zhang Y, Liu J, Li W, Weng Y. Copper-Based SURMOFs for Nitric Oxide Generation: Hemocompatibility, Vascular Cell Growth, and Tissue Response. ACS applied materials & interfaces. 2019;11:7872-83
90. Narne P, Pandey V, Phanithi PB. Role of Nitric Oxide and Hydrogen Sulfide in Ischemic Stroke and the Emergent Epigenetic Underpinnings. Molecular neurobiology. 2019;56:1749-69
91. Karimi SA, Hosseinmardi N, Janahmadi M, Sayyah M, Hajisoltani R. The protective effect of hydrogen sulfide (H(2)S) on traumatic brain injury (TBI) induced memory deficits in rats. Brain research bulletin. 2017;134:177-82
92. Lin WC, Huang CC, Lin SJ, Li MJ, Chang Y, Lin YJ. et al. In situ depot comprising phase-change materials that can sustainably release a gasotransmitter H(2)S to treat diabetic wounds. Biomaterials. 2017;145:1-8
93. Lin WC, Pan WY, Liu CK, Huang WX, Song HL, Chang KS. et al. In situ self-spray coating system that can uniformly disperse a poorly water-soluble H(2)S donor on the colorectal surface to treat inflammatory bowel diseases. Biomaterials. 2018;182:289-98
94. Sparatore A, Perrino E, Tazzari V, Giustarini D, Rossi R, Rossoni G. et al. Pharmacological profile of a novel H(2)S-releasing aspirin. Free radical biology & medicine. 2009;46:586-92
95. Cui S, Liu JH, Song XT, Ma GL, Du BJ, Lv SZ. et al. A novel stent coated with antibodies to endoglin inhibits neointimal formation of porcine coronary arteries. BioMed research international. 2014;2014:428619
96. Park KS, Kang SN, Kim DH, Kim HB, Im KS, Park W. et al. Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis. Acta biomaterialia. 2020;111:91-101
97. Boulanger MD, Level HA, Elkhodiry MA, Bashth OS, Chevallier P, Laroche G. et al. Bioaffinity-based surface immobilization of antibodies to capture endothelial colony-forming cells. PloS one. 2022;17:e0269316
98. Wang J, Xue Y, Liu J, Hu M, Zhang H, Ren K. et al. Hierarchical Capillary Coating to Biofunctionlize Drug-Eluting Stent for Improving Endothelium Regeneration. Research (Washington, DC). 2020;2020:1458090
99. Chang HK, Kim PH, Kim DW, Cho HM, Jeong MJ, Kim DH. et al. Coronary stents with inducible VEGF/HGF-secreting UCB-MSCs reduced restenosis and increased re-endothelialization in a swine model. Experimental & molecular medicine. 2018;50:1-14
100. Bourantas CV, Papafaklis MI, Kotsia A, Farooq V, Muramatsu T, Gomez-Lara J. et al. Effect of the endothelial shear stress patterns on neointimal proliferation following drug-eluting bioresorbable vascular scaffold implantation: an optical coherence tomography study. JACC Cardiovascular interventions. 2014;7:315-24
101. Neamtu I, Chiriac AP, Diaconu A, Nita LE, Balan V, Nistor MT. Current concepts on cardiovascular stent devices. Mini reviews in medicinal chemistry. 2014;14:505-36
102. Charpentier E, Barna A, Guillevin L, Juliard JM. Fully bioresorbable drug-eluting coronary scaffolds: A review. Archives of cardiovascular diseases. 2015;108:385-97
103. Jurgeleit T, Quandt E, Zamponi C. Magnetron Sputtering as a Fabrication Method for a Biodegradable Fe32Mn Alloy. Materials (Basel, Switzerland). 2017 10
104. Lenzuni M, Suarato G, Miele D, Carzino R, Ruggeri M, Bertorelli R. et al. Development of biodegradable zein-based bilayer coatings for drug-eluting stents. RSC advances. 2021;11:24345-58
105. Lu B, Han X, Zhao A, Luo D, Maitz MF, Wang H. et al. Intelligent H2S release coating for regulating vascular remodeling. Bioactive materials. 2021;6:1040-50
106. Wang HJ, Hao MF, Wang G, Peng H, Wahid F, Yang Y. et al. Zein nanospheres assisting inorganic and organic drug combination to overcome stent implantation-induced thrombosis and infection. The Science of the total environment. 2023;873:162438
107. Beshchasna N, Ho AYK, Saqib M, Kraśkiewicz H, Wasyluk Ł, Kuzmin O. et al. Surface evaluation of titanium oxynitride coatings used for developing layered cardiovascular stents. Materials science & engineering C, Materials for biological applications. 2019;99:405-16
108. Castellino M, Stolojan V, Virga A, Rovere M, Cabiale K, Galloni MR. et al. Chemico-physical characterisation and in vivo biocompatibility assessment of DLC-coated coronary stents. Analytical and bioanalytical chemistry. 2013;405:321-9
109. Kalnins U, Erglis A, Dinne I, Kumsars I, Jegere S. Clinical outcomes of silicon carbide coated stents in patients with coronary artery disease. Medical science monitor: international medical journal of experimental and clinical research. 2002;8:Pi16-20
110. Unverdorben M, Sattler K, Degenhardt R, Fries R, Abt B, Wagner E. et al. Comparison of a silicon carbide coated stent versus a noncoated stent in humans: the Tenax- versus Nir-Stent Study (TENISS). Journal of interventional cardiology. 2003;16:325-33
111. Karjalainen PP, Ylitalo A, Niemelä M, Kervinen K, Mäkikallio T, Pietilä M. et al. Two-year follow-up after percutaneous coronary intervention with titanium-nitride-oxide-coated stents versus paclitaxel-eluting stents in acute myocardial infarction. Annals of medicine. 2009;41:599-607
112. Karjalainen PP, Annala AP, Ylitalo A, Vahlberg T, Airaksinen KE. Long-term clinical outcome with titanium-nitride-oxide-coated stents and paclitaxel-eluting stents for coronary revascularization in an unselected population. International journal of cardiology. 2010;144:42-6
113. Karjalainen PP, Ylitalo A, Niemelä M, Kervinen K, Mäkikallio T, Pietili M. et al. Titanium-nitride-oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction: a 12-months follow-up report from the TITAX AMI trial. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2008;4:234-41
114. Sobrevia L, Ooi L, Ryan S, Steinert JR. Nitric Oxide: A Regulator of Cellular Function in Health and Disease. Oxid Med Cell Longev. 2016;2016:9782346
115. Roopmani P, Satheesh S, Raj DC, Krishnan UM. Development of Dual Drug Eluting Cardiovascular Stent with Ultrathin Flexible Poly(l-lactide-co-caprolactone) Coating. ACS biomaterials science & engineering. 2019;5:2899-915
116. Hu T, Lin S, Du R, Fu M, Rao Q, Yin T. et al. Design, preparation and performance of a novel drug-eluting stent with multiple layer coatings. Biomaterials science. 2017;5:1845-57
117. Alferiev IS, Hooshdaran B, Pressly BB, Zoltick PW, Stachelek SJ, Chorny M. et al. Intraprocedural endothelial cell seeding of arterial stents via biotin/avidin targeting mitigates in-stent restenosis. Scientific reports. 2022;12:19212
118. Liu L, Lan X, Chen X, Dai S, Wang Z, Zhao A. et al. Multi-functional plant flavonoids regulate pathological microenvironments for vascular stent surface engineering. Acta biomaterialia. 2023;157:655-69
119. Elnaggar MA, Seo SH, Gobaa S, Lim KS, Bae IH, Jeong MH. et al. Nitric Oxide Releasing Coronary Stent: A New Approach Using Layer-by-Layer Coating and Liposomal Encapsulation. Small (Weinheim an der Bergstrasse, Germany). 2016;12:6012-23
120. Hossfeld S, Nolte A, Hartmann H, Recke M, Schaller M, Walker T. et al. Bioactive coronary stent coating based on layer-by-layer technology for siRNA release. Acta biomaterialia. 2013;9:6741-52
121. Yang F, Chang R, Webster TJ. Atomic Layer Deposition Coating of TiO(2) Nano-Thin Films on Magnesium-Zinc Alloys to Enhance Cytocompatibility for Bioresorbable Vascular Stents. International journal of nanomedicine. 2019;14:9955-70
122. Dou Z, Chen S, Wang J, Xia L, Maitz MF, Tu Q. et al. A "built-up" composite film with synergistic functionalities on Mg-2Zn-1Mn bioresorbable stents improves corrosion control effects and biocompatibility. Bioactive materials. 2023;25:223-38
123. Zheng JF, Qiu H, Tian Y, Hu XY, Luo T, Wu C. et al. Preclinical Evaluation of a Novel Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold in Porcine Coronary Artery at 6 Months. JACC Cardiovascular interventions. 2019;12:245-55
124. Pan K, Zhang W, Shi H, Dai M, Yang Z, Chen M. et al. Facile fabrication of biodegradable endothelium-mimicking coatings on bioabsorbable zinc-alloy stents by one-step electrophoretic deposition. Journal of materials chemistry B. 2022;10:3083-96
125. Qian HL, Chen SY, Jia F, Huang WP, Wang J, Ren KF. et al. "Spongy skin" as a robust strategy to deliver 4-octyl itaconate for conducting dual-regulation against in-stent restenosis. Biomaterials. 2023;296:122069
126. Wang J, Qian HL, Chen SY, Huang WP, Huang DN, Hao HY. et al. miR-22 eluting cardiovascular stent based on a self-healable spongy coating inhibits in-stent restenosis. Bioactive materials. 2021;6:4686-96
Author contact
![]() Corresponding author: Zhuyuan Yu; Email: 1113826630com.
Corresponding author: Zhuyuan Yu; Email: 1113826630com.

 Global reach, higher impact
Global reach, higher impact