ISSN: 1449-1907International Journal of Medical Sciences
Int J Med Sci 2024; 21(2):376-395. doi:10.7150/ijms.92131 This issue Cite
Research Paper
Altered Gut Microbiota as a Potential Risk Factor for Coronary Artery Disease in Diabetes: A Two-Sample Bi-Directional Mendelian Randomization Study
1. Department of Cardiovascular Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
2. Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
3. Department of Surgical Oncology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
4. Department of Endocrinology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
5. Department of Cardiothoracic Surgery, Shenshan Medical Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Shanwei, China.
6. Department of Cardiology, Chinese PLA General Hospital, Beijing, China.
7. Department of Anesthesiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
* These authors contributed equally to this work and share first authorship.
# These authors share last authorship.
Abstract
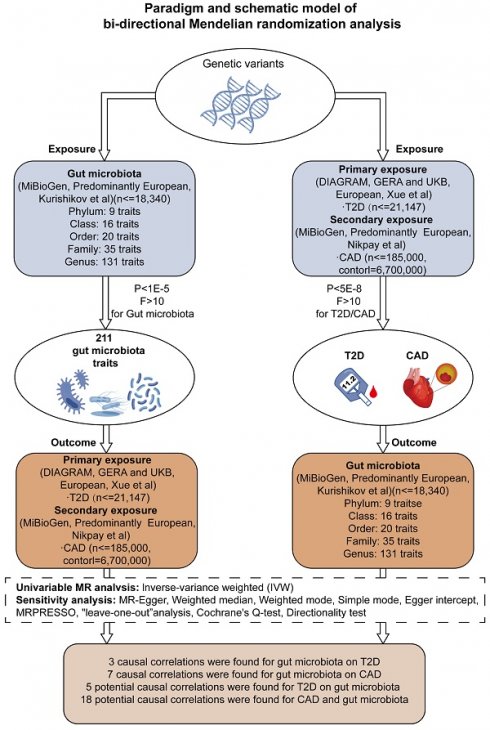
The current body of research points to a notable correlation between an imbalance in gut microbiota and the development of type 2 diabetes mellitus (T2D) as well as its consequential ailment, coronary artery disease (CAD). The complexities underlying the association, especially in the context of diabetic coronary artery disease (DCAD), are not yet fully understood, and the causal links require further clarification. In this study, a bidirectional Mendelian randomization (MR) methodology was utilized to explore the causal relationships between gut microbiota, T2D, and CAD. By analyzing data from the DIAGRAM, GERA, UKB, FHS, and mibioGen cohorts and examining GWAS databases, we sought to uncover genetic variants linked to T2D, CAD, and variations in gut microbiota and metabolites, aiming to shed light on the potential mechanisms connecting gut microbiota with DCAD. Our investigation uncovered a marked causal link between the presence of Oxalobacter formigenes and an increased incidence of both T2D and CAD. Specifically, a ten-unit genetic predisposition towards T2D was found to be associated with a 6.1% higher probability of an increase in the Oxalobacteraceae family's presence (β = 0.061, 95% CI = 0.002-0.119). In a parallel finding, an augmented presence of Oxalobacter was related to an 8.2% heightened genetic likelihood of CAD (β = 0.082, 95% CI = 0.026-0.137). This evidence indicates a critical pathway by which T2D can potentially raise the risk of CAD via alterations in gut microbiota. Additionally, our analyses reveal a connection between CAD risk and Methanobacteria, thus providing fresh perspectives on the roles of TMAO and carnitine in the etiology of CAD. The data also suggest a direct causal relationship between increased levels of certain metabolites — proline, lysophosphatidylcholine, asparagine, and salicylurate — and the prevalence of both T2D and CAD. Sensitivity assessments reinforce the notion that changes in Oxalobacter formigenes could pose a risk for DCAD. There is also evidence to suggest that DCAD may, in turn, affect the gut microbiota's makeup. Notably, a surge in serum TMAO levels in individuals with CAD, coinciding with a reduced presence of methanogens, has been identified as a potentially significant factor for future examination.
Keywords: coronary artery disease, type 2 diabetes, causality, gut microbiota, metabolites, Mendelian randomization

 Global reach, higher impact
Global reach, higher impact