Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(2):200-206. doi:10.7150/ijms.88486 This issue Cite
Research Paper
Apparent diffusion coefficient histogram in the differentiation of benign and malignant testicular tumors
1. Department of Radiology, Viet Duc Hospital, Hanoi, Vietnam.
2. Department of Radiology, Hanoi Medical University, Hanoi, Vietnam.
3. Department of Medical Imaging, Da Nang University of Medical Technology and Pharmacy, Vietnam.
4. Department of Radiology, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam.
# These authors contributed equally to this article as co-first authors.
Received 2023-7-26; Accepted 2023-10-30; Published 2024-1-1
Abstract
Purpose: This retrospective study assessed the value of histogram parameters of the apparent diffusion coefficient (ADC) map (HA) in differentiating between benign and malignant testicular tumors. We compared the diagnostic performance of two different volume-of-interest (VOI) placement methods: VOI 1, the entire tumor; VOI 2, the tumor excluding its cystic, calcified, hemorrhagic, and necrotic portions.
Materials and methods: We retrospectively evaluated 45 patients with testicular tumors examined with scrotal contrast-enhanced magnetic resonance imaging. These patients underwent surgery with the pathological result of seven benign and 39 malignant tumors. We calculated the HA parameters, including mean, median, maximum, minimum, kurtosis, skewness, entropy, standard deviation (SD), mean of positive pixels, and uniformity of positive pixels by the two different VOI segmentation methods. We compared these parameters using the chi-square test, Mann-Whitney U test, and area under the receiver operating characteristic curve (AUC) to determine their optimal cut-off, sensitivity (Se), and specificity (Sp).
Result: This study included 45 patients with 46 testicular lesions (seven benign and 39 malignant tumors), one of which had bilateral testicular seminoma. With the VOI 1 method, benign lesions had significantly lower maximum ADC (p = 0.002), ADC skewness (p = 0.017), and ADC variance (p = 0.000) than malignant lesions. In contrast, their minimum ADC was significantly higher ADC (p = 0.000). With the VOI 2 method, the benign lesions had significantly higher ADC SD (p = 0.048) and maximum ADC (p = 0.015) than malignant lesions. In contrast, their minimum ADC was significantly lower (p = 0.000). With the VOI 1 method, maximum ADC, ADC variance, and ADC skewness performed well in differentiating benign and malignant testicular lesions with cut-offs (Se, Sp, AUC) of 1846.000 (74.4%, 100%, 0.883), 39198.387 (79.5%, 85.7%, 0.868), and 0.893 (48.7%, 100%, 0.758).
Conclusion: The HA parameters showed value in differentiating benign and malignant testicular neoplasms. The entire tumor VOI placement method was preferable to the VOI placement method excluding cystic, calcified, hemorrhagic, and necrotic portions in measuring HA parameters. Using this VOI segmentation, maximum ADC performed best in discriminating benign and malignant testicular lesions, followed by ADC variance and skewness.
Keywords: testicular cancer, benign testicular tumor, malignant testicular tumor, diffusion-weighted imaging, apparent diffusion coefficient maps, histogram analysis
Introduction
Testicular tumor is a rare entity representing approximately 1%-1.5% of tumors in men, but it is the most common tumor in patients aged 15-44 years [1]. While 95% of testicular tumors are malignant, the incidence of benign tumors tends to increase with the usefulness of imaging diagnosis [1,2]. For malignant lesions, total testicular resection with or without chemoradiotherapy, depending on histopathologic results, is considered the optimal treatment [3]. In contrast, for benign lesions, tumor resection with preservation of normal testicular parenchyma helps to maintain endocrine and reproductive functions [1]. Therefore, discrimination between benign and malignant neoplasms plays a pivotal role in treatment management and in avoiding unnecessary overtreatment in benign cases.
While biopsy is considered the gold standard in differentiating benign and malignant tumors, it is an invasive procedure with potential hemorrhage or infection risks and limitations in evaluating the extent of lesions [2]. Ultrasound with a high-frequency linear probe (7-10 MHz) is the initial imaging diagnostic tool in screening testicular pathology, with undeniable advantages such as determining intra- or extra-testicular masses and discriminating tumors from non-tumor lesions such as infections or testicular torsions. However, the value of conventional ultrasound, contrast-enhanced ultrasound, and elastography in differentiating benign and malignant tumors and evaluating the extent of lesions remains controversial [4] [5][6].
Scrotal magnetic resonance imaging (MRI) is an extremely valuable imaging modality for assessing testicular pathologies, including testicular neoplasms, as recommended by the European Society of Urogenital Radiology [7]. This non-invasive tool with multiple sequences and planes, combined with the injection of a contrast agent, plays a vital role in determining tumor components such as fat, calcification, necrosis, hemorrhage, and local extent, thereby aiming at determining the lesion's nature [8].
Diffusion-weighted imaging (DWI) with the quantification of parameters for the apparent diffusion coefficient (ADC) map through region-of-interest (ROI) segmentation is increasingly used to differentiate benign and malignant tumors in many organs, including the testicles. According to Wang et al., ADC value ≤ 0.90 × 10-3 mm2/s suggests a malignant tumor [2]. However, ADC-map-based measurements vary according to ROI location and size [9]. Histogram analysis of ADC maps (HA) is a recent method applied in distinguishing two groups based on statistical indicators using volume-of-interest (VOI) placement to cover the entire tumor. This method has advantages over placing the ROI on a single slice or localized site in determining tumor heterogeneity, including hemorrhage and necrosis (a common finding in malignant tumors), helping to better distinguish between two groups [1]. Therefore, this study evaluated the diagnostic value of HA parameters in discriminating benign and malignant neoplasms using two VOI placement methods, one using the entire tumor and the other excluding its cystic, necrotic, calcified, and hemorrhagic portions.
Methods
Study population
This retrospective study was conducted in the Viet Duc University Hospital Radiology Department between January 2019 and June 2023 and included 45 patients with testicular neoplasms. All research subjects were examined with contrast-enhanced scrotal MRI and then underwent surgery with a pathological result of benign or malignant tumors. The exclusion criteria included patients with a previous history of biopsy or treatment (radiotherapy or operative tumor resection) and any artifacts that affect the quality of MRI images and prevent accurate measurements. This study was performed according to the Declaration of Helsinki. All procedures were conducted according to appropriate laws and regulations. The patient or their legal representative provided informed consent to participate in this study.
Technique
The patients underwent contrast-enhanced scrotal MRI using a 3.0 Tesla (SIGNA Pioneer MR; GE Healthcare, USA) or 1.5 Tesla (Gyroscan and Intera, Philips Healthcare) scan system. The same technique and protocol were used for all patients (Table 1).
Imaging protocol for scrotal MRI using 3.0 Tesla [4] and 1.5 Tesla [10].
| Sequence | Plane | (Tesla) | TR (ms) | TE (ms) | Gap (mm) | Thickness (mm) | FOV | Matrix |
|---|---|---|---|---|---|---|---|---|
| T1W | Axial | 1.5 | 650 | 15 | 0.5 | 3 | 240x270 | 180x256 |
| 3.0 | 500-700 | 8-10 | 3 | 3 | 180x180 | 200-250 x 300-350 | ||
| T2 TSE | Axial Coronal Sagittal | 1.5 | 4000 | 100 | 0.5 | 3 | 240x270 | 180x256 |
| 3.0 | 5000-7000 | 100-120 | 3.3 | 3 | 200x200 | 300-340x200-250 | ||
| DWI | Axial | 1.5 | 4000 | 60 | 8 | 2 | 340x340 | 128x128 |
| 3.0 | 3000 | 100 | 0.5 | 3 | 220x176 | 90x90 | ||
| T1W CE+ | Axial | 1.5 | 650 | 15 | 0.5 | 3 | 240x270 | 180x256 |
| 3.0 | 500-700 | 8-10 | 3.3 | 3 | 180x180 | 240-260x200-220 |
FOV: field of view; T2 TSE: T2-weighted turbo spin-echo; T1W: T1-weighted spin echo; CE+: a single dose of intravenous contrast agent injection (Gadolinium-DTPA [0.2 mmol/kg] at an injection rate of 2.0 mL/s).
DWI was performed on the axial plane with a repetition time (TR) of 4000 ms, echo time (TE) of 60 ms, Flip angle of 90o, slice thickness of 8 mm, field of view (FOV) of 340, matrix of 128×128, and two b values (0 and 1000) [11]
The ADC map assesses the diffusion capacity of intracellular water molecules according to the formula S(b)/S(50) = exp[-(b - 50) ×ADC], where S(b) is the signal intensity at a b-value of 1000 s/mm2, and S(50) is the signal intensity at a b-value of 50 s/mm2.
Image analysis
MRI images were assessed retrospectively, transferred from the Picture Archiving and Communication System workstation to a personal computer, and converted into the DCM format. The VOI measurement was performed with The Medical Imaging Interaction Toolkit software (MITK Workbench v2022.10; Division of Medical Image Computing, German Cancer Research Center, Heidelberg, Germany) by two urogenital radiologists with at least six years of experience and no knowledge of the final pathologic results; disagreements were resolved by discussion.
The boundaries of entire tumors were evaluated using the ADC maps at a b-value of 1000 s/mm2.
We determined the following regions in conventional MRI images [2,7,12]:
1. Tumoral hemorrhagic or calcified portions: hyperintense on T1W.
2. Tumoral cystic degeneration or necrotic portions: significantly hypointense on T1W, hyperintense on T2W, and unenhanced in contrast-enhanced T1W.
3. The normal testicular parenchyma: the contralateral normal testicular parenchyma in the same slide.
We measured HA using two different VOI placement methods:
1. VOI 1: the VOI was manually drawn on each slide of the entire tumor boundary, containing cystic, hemorrhagic, calcified, and necrotic portions.
2. VOI 2: the VOI was manually drawn on each section of the entire tumor, excluding cystic, hemorrhagic, calcified, and necrotic portions.
Then, we calculated the following accumulated ADC parameters: mean, maximum (max), minimum (min), kurtosis, skewness, entropy, standard deviation (SD), mean of positive pixels (MPP), and uniformity of positive pixels (UPP).
Histopathological examination
All histopathological results were classified as benign and malignant tumors according to The World Health Organization 2022 criteria [13].
Statistical analysis
The data were analyzed using SPSS (version 20.0; Chicago, IL, USA) to assess the correlation between HA parameters and the pathological features of testicular neoplasms. Parameters with a normal distribution (p > 0.005) are presented as mean ± SD and compared using the Chi-square or Fisher's exact test. Parameters with a nonnormal distribution (p < 0.005) are presented as the median (25th-75th percentiles) and compared using the Mann-Whitney U test. A p-value of <0.005 was considered statistically significant.
A receiver operating characteristic (ROC) curve was created to determine the optimal cut-off for each parameter by maximizing the sum of sensitivity (Sn) and specificity (Sp) using the Youden index.
Results
The retrospective study included 45 patients with 46 testicular tumors (seven benign and 39 malignant), of which one 61-year-old patient had bilateral testicular seminoma. The mean age was 24.57 ± 11.0 years in the benign group and 36.10 ± 11.72 years in the malignant group; the youngest patients were 7 and 20 years old, respectively, and the oldest patients were 43 and 65 years old, respectively. The age distribution did not differ significantly between the two groups (p = 0.409).
The distribution of benign and malignant testicular tumors is shown in Table 2. The benign tumor group included seven cases, of which four had epidermoid cysts, two had Leydig cells neoplasms, and one had a dermoid cyst. The malignant tumor group included 39 cases, of which 19 had seminoma, 16 had non-seminoma germ cell tumors, and four had lymphomas. The pathological descriptions are summarized in Table 2.
Descriptive statistics for pathological descriptions.
| Benign neoplasm | Number of lesions | Volume mm3 | Malignant neoplasm | Number of lesions | Volume mm3 |
|---|---|---|---|---|---|
| Epidermoid Cyst | 4 | 8229.62 | Seminoma | 19 | 96780.09 |
| Dermoid Cyst | 1 | 1240.93 | Mixed germ cell tumor | 12 | 78834.75 |
| Leydig cells tumor | 2 | 882.78 | Yolk sac tumor | 2 | 40545.76 |
| Teratoma | 2 | 37222.85 | |||
| Lymphoma | 4 | 171898.06 | |||
| Total | 7 | Total | 39 |
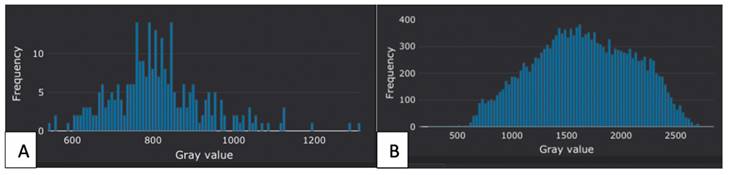
Figure 3 shows a representative histogram of one case in the benign and malignant tumor groups after being processed by the software.
Table 3 compares HA parameters using the two VOI placements. With the VOI 1 method, ADC max (p = 0.002), skewness (p = 0.017), and variance (p = 0.000) were significantly lower in the benign group than in the malignant group, while ADC min (p = 0.000) was significantly higher. With the VOI 2 method, ADC SD (p = 0.048) and max (p = 0.015) were significantly lower in the benign group than in the malignant group, while ADC min (p = 0.000) was significantly higher.
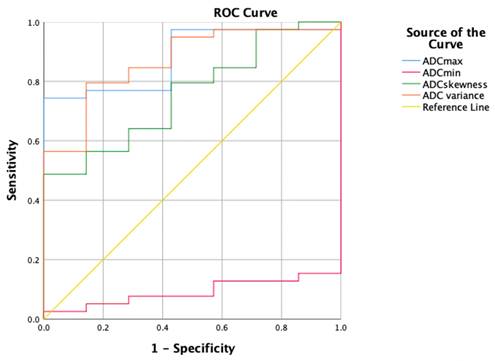
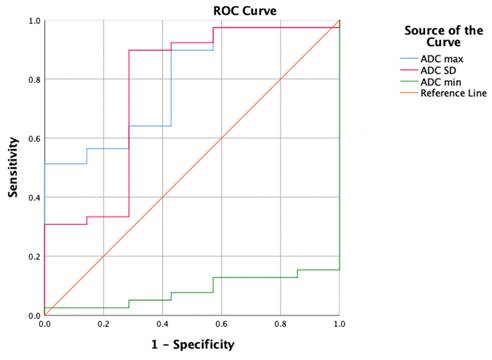
The ROC curves for the HA parameters with the VOI 1 placement method are shown in Table 4 and Figure 4. ADC max had the highest diagnostic ability (area under the ROC curve [AUC] = 0.883), followed by ADC variance (AUC = 0.868), ADC skewness (AUC = 0.758), and ADC min (AUC = 0.092). The optimal cut-off for ADC max was 1846.000, with a Sn of 74.4%, Sp of 100%, and Youden index of 0.744.
The ROC curves for the HA parameters with the VOI 2 placement method are shown in Table 5 and Figure 5. ADC max had the highest diagnostic ability (AUC = 0.791), followed by ADC SD (AUC = 0.769) and ADC min (AUC = 0.084). The optimal cut-off for ADC max was 1693.500, with a Sn of 51.3%, Sp of 100%, and Youden index of 0.513.
Discussion
HA analysis is a quantitative method for evaluating the statistical parameters in each image based on VOI placement to cover the entire tumor. Therefore, the histogram's shape and asymmetry reflect microstructural differences in tumor composition corresponding to histopathological grade, distinguishing benign and malignant tumors [8,9]. Previous studies have shown that placing the VOI so that it covers the entire tumor volume allows for a better assessment than the previous approach of placing an ROI on a specific slice or localized tumor region, often avoiding its cystic, necrotic, and hemorrhagic portions, leading to limitations in reflecting its heterogeneity, especially for malignant tumors that have more necrotic and bleeding portions than benign tumors [8,14]. This study examined 45 patients with 46 testicular tumors (seven benign and 39 malignant) to evaluate the diagnostic value of HA in discriminating benign and malignant neoplasms with two VOI placement methods, one using the entire tumor and the other excluding its cystic, necrotic, calcified, and hemorrhagic portions.
We compared each parameter's AUC value with the two VOI placement methods, finding that those for ADC max and variance using VOI 1 (0.883 and 0.868, respectively) tended to be significantly larger than those of ADC max and SD using VOI 2 (0.791 and 0.769, respectively). Apart from these parameters, ADC skewness also showed a high AUC of 0.758 with VOI 1. Therefore, our results suggest that the VOI 1 placement method likely improved the ability to differentiate between benign and malignant tumors. Our results are similar to those of Pederson et al., which studied a group of malignant testicular tumors consisting of 26 tumors and pointed out the highest value of whole-tumor VOI placement in diagnosing testicular tumors [9].
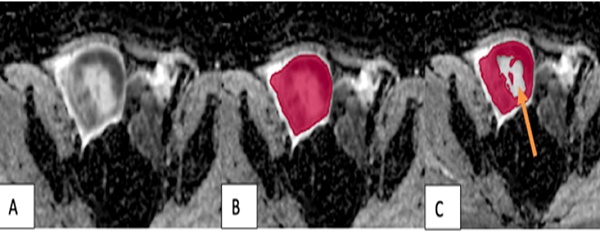
The VOI placement method in malignant tumors. (A) A tumor in the right testicle showed uneven hypointensity on the ADC map. (B) The VOI 1 method (red) covered the entire tumor, including its cystic and necrotic portions. (C) The VOI 2 method excluded the tumor's cystic and necrotic portions (arrow).
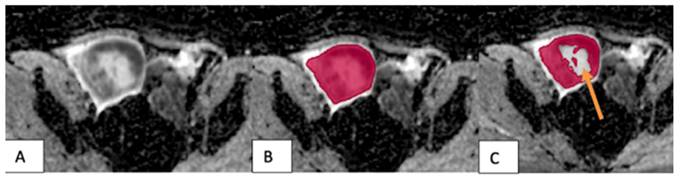
The VOI placement method in benign tumors: (A) A tumor in the left testicle showed slight hypointensity on the ADC map. (B) The VOI 1 method (red) covered the entire tumor, including its cystic and necrotic portions. (C) The VOI 2 method excluded the tumor's cystic and necrotic portions (arrow).

Representative histograms for one case in the benign and malignant tumor groups. (A) A histogram of a 25-year-old man with the histopathological result of an epidermoid cyst. (B) A histogram of a 44-year-old man with seminoma.
Comparison of HA parameters between the two VOI placement methods.
| Parameter | Method | Benign neoplasm | Malignant neoplasm | P value |
|---|---|---|---|---|
| ADC mean | VOI 1 | 917.78±185.03 | 1006.87±302.47 | 0.457 |
| VOI 2 | 878.37±170.79 | 849.96±230.85 | 0.758 | |
| ADC median | VOI 1 | 916.70±190.53 | 947.80±325.51 | 0.808 |
| VOI 2 | 877.94±183.92 | 825.13±242.88 | 0.588 | |
| ADC SD | VOI 1 | 150.23±60.99 | 322.01±157.59 | 0.007 |
| VOI 2 | 146.04±74.68 | 206.10±71.49 | 0.048* | |
| ADC MPP | VOI 1 | 917.78±185.03 | 1008.31±303.46 | 0.451 |
| VOI 2 | 878.37±170.79 | 850.93±230.67 | 0.766 | |
| ADC max | VOI 1 | 1366.57±355.82 | 2318.36±751.62 | 0.002* |
| VOI 2 | 1282.86±325.87 | 1824.49±545.14 | 0.015* | |
| ADC min | VOI 1 | 583.86±64.49 | 249.59±200.80 | 0.000* |
| VOI 2 | 598.14±72.52 | 280.82±200.18 | 0.000* | |
| ADC skewness | VOI 1 | 0.22±0.47 | 0.84±0.63 | 0.017* |
| VOI 2 | 0.39±0.38 | 0.83±0.86 | 0.195 | |
| ADC kurtosis | VOI 1 | 3.37±0.75 | 4.29±1.96 | 0.231 |
| VOI 2 | 3.17±0.98 | 5.15±2.84 | 0.077 | |
| ADC UPP | VOI 1 | 0.05±0.04 | 0.03±0.01 | 0.252 |
| VOI 2 | 0.05±0.04 | 0.03±0.01 | 0.191 | |
| ADC Entropy | VOI 1 | 4.97±1.09 | 5.59±0.43 | 0.189 |
| VOI 2 | 4.70±1.06 | 5.39±0.50 | 0.139 | |
| ADC Uniformity | VOI 1 | 0.05±0.04 | 0.03±0.01 | 0.252 |
| VOI 2 | 0.05±0.04 | 0.03±0.01 | 0.191 | |
| ADC Variance | VOI 1 | 25757.91±20992.12 | 127890.64±126698.45 | 0.000* |
| VOI 2 | 26109.01±25506.31 | 47454.96±32173.35 | 0.104 |
Parameters were tested for normality using the Kolmogorov-Smirnov test with a p-value of >0.05 indicating they followed a normal distribution. Normally distributed parameters are presented as mean ± SD and compared using the Chi-square test.
*: p-value < 0.05.
The ROC curves for the HA parameters using the VOI 1 placement method.
| Parameter | AUC | Cut-off | Sn | Sp | Youden Index |
|---|---|---|---|---|---|
| ADC max | 0.883 | 1846.000 | 74,4 | 100 | 0,744 |
| ADC variance | 0.868 | 39198.387 | 79,5 | 85,7 | 0,652 |
| ADC skewness | 0.758 | 0.893 | 48,7 | 100 | 0,487 |
The ROC curves for the HA parameters with the VOI 2 placement method.
| Parameter | AUC | Cut-off | Sn | Sp | Youden Index |
|---|---|---|---|---|---|
| ADC max | 0.791 | 1693.500 | 51.3 | 100 | 0.513 |
| ADC SD | 0.769 | 130.376 | 89.7 | 71.4 | 0.611 |
Our study's results differ from Fan et al., who used the VOI 1 placement method in 61 testicular lesions. They found that ADC max was not a reliable indicator for distinguishing benign and malignant lesions [1]. In the group of 18 benign lesions in Fan's study, five out of 18 cases (27.78%) were non-tumors (including four cases of inflammation and one case of testicular torsion), which could explain the different ADC max findings between studies [1].
We evaluated HA parameters using the VOI 1 method. ADC max (p = 0.002), ADC (p = 0.017), and variance (p = 0.000) were significantly lower in the benign group than in the malignant group, while ADC min was significantly higher (p = 0.000). Skewness indicates asymmetry, and variance indicates dispersion. There was greater homogeneity in the benign group than in the malignant group, so its histogram was sharper. Therefore, the skewness and variance in the homogeneous group will be lower than in the less homogeneous group. Our results are similar to those of Min et al., who compared a more homogeneous group of seminomas with a less homogeneous group of non-seminomas [15].
In our study, with both VOI placement methods, ADC min was consistently greater in the benign tumor group than in the malignant tumor group. Previous studies also showed that ADC min was significantly lower in the malignant testicular lesion group than in the benign group [1]. However, we found that ADC min was unreliable in discriminating the two groups since its AUC was 0.092 with VOI 1 and 0.084 with VOI 2. This finding differs from Fan et al. [1]. Our study's benign group included four cases with epidermoid cysts (57.14%), a benign tumor with diffusion restriction and a low ADC. Consequently, the ADC min value in our benign group might be closer to our malignant group [16], potentially explaining the difference between our findings and those of Fan et al.
Among the HA parameters, ADC max had the highest diagnostic value in differentially diagnosing benign and malignant tumors with an AUC (cut-off, Sn, Sp) of 0.883 (1846.000, 74.4%, 100%), followed by ADC variance. Our study is the first to note the valuable role of ADC max in distinguishing between benign and malignant tumors. In addition, we encountered one patient with bilateral testicular seminoma in our study. Testicular neoplasm is rare, with tumors in both testicles even rarer, accounting for about 1%-2% of testicular masses diagnosed. Tumors in both testicles in the same patient often share similar histology and pathology; most are seminoma [17].
Our study had certain limitations. Firstly, its sample size was relatively small, which may affect the representativeness of its results. Secondly, its research subjects underwent scrotal MRI with 3.0 Tesla or 1.5 Tesla scan systems. Therefore, future studies should include a larger number of patients examined using the same imaging system. In this study, the VOI was manually drawn on each slide of the entire tumor, so there is a risk of error due to drawing VOI on the benign part and affecting the results, this is the reason why we placed VOI carefully, repeated the calculation at least 3 times to minimize the errors.
The ROC curves for the HA parameters with the VOI 1 placement method.
The ROC curves for the HA parameters with the VOI 2 placement method.
Conclusions
The HA parameters, essentially ADC max showed diagnostic value in differentiating benign and malignant testicular masses. The entire tumor VOI placement method was preferable to the VOI placement method excluding cystic, calcified, hemorrhagic, and necrotic portions in measuring HA parameters. Using this VOI placement method, ADC max performed best in discriminating benign and malignant testicular lesions, followed by ADC variance and skewness.
Acknowledgements
Ethical approval
Hanoi Medical University's institutional review board supported this retrospective study. This study was conducted according to the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Availability of data and material
The datasets generated and/or analysed during the current study are not publicly available due to privacy concerns but are available from the corresponding author on reasonable request.
Orcid ID
Nguyen Minh Duc https://orcid.org/0000-0001-5411-1492.
Author contributions
Study concept and design: Nguyen Dinh Minh, Nguyen Duy Hung, and Nguyen Minh Duc; acquisition of data: Nguyen Dinh Minh, Nguyen Duy Hung, and Nguyen Minh Duc; analysis and interpretation of data: Nguyen Dinh Minh, Nguyen Duy Hung, and Nguyen Minh Duc; drafting of the manuscript: Nguyen Duy Hung and Nguyen Minh Duc; critical revision of the manuscript: Nguyen Duy Hung and Nguyen Minh Duc; study supervision: Nguyen Dinh Minh and Nguyen Duy Hung confirm the authenticity of all the raw data. All authors read and approved final version of this manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fan C, Min X, Feng Z. et al. Discrimination between benign and malignant testicular lesions using volumetric apparent diffusion coefficient histogram analysis. European Journal of Radiology. 2020;126:108939. doi:10.1016/j.ejrad.2020.108939
2. Wang W, Sun Z, Chen Y. et al. Testicular tumors: discriminative value of conventional MRI and diffusion weighted imaging. Medicine. 2021;100(48):e27799. doi:10.1097/MD.0000000000027799
3. Khan O, Protheroe A. Testis cancer. Postgraduate Medical Journal. 2007;83(984):624-632 doi:10.1136/pgmj.2007.057992
4. Liu R, Lei Z, Li A, Jiang Y, Ji J. Differentiation of testicular seminoma and nonseminomatous germ cell tumor on magnetic resonance imaging. Medicine (Baltimore). 2019;98(45):e17937. doi:10.1097/MD.0000000000017937
5. Tenuta M, Sesti F, Bonaventura I. et al. Use of contrast enhanced ultrasound in testicular diseases: A comprehensive review. Andrology. 2021;9(5):1369-1382 doi:10.1111/andr.13057
6. Lai DKH, Cheng ESW, Mao YJ. et al. Sonoelastography for Testicular Tumor Identification: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy. Cancers. 2023;15(15):3770. doi:10.3390/cancers15153770
7. Tsili AC, Sofikitis N, Stiliara E, Argyropoulou MI. MRI of testicular malignancies. Abdom Radiol. 2019;44(3):1070-1082 doi:10.1007/s00261-018-1816-5
8. Tsili AC, Sofikitis N, Pappa O, Bougia CK, Argyropoulou MI. An Overview of the Role of Multiparametric MRI in the Investigation of Testicular Tumors. Cancers (Basel). 2022;14(16):3912. doi:10.3390/cancers14163912
9. Pedersen MRV, Loft MK, Dam C, Rasmussen LÆL, Timm S. Diffusion-Weighted MRI in Patients with Testicular Tumors-Intra- and Interobserver Variability. Curr Oncol. 2022;29(2):837-847 doi:10.3390/curroncol29020071
10. Tsili AC, Tsampoulas C, Giannakopoulos X. et al. MRI in the Histologic Characterization of Testicular Neoplasms. American Journal of Roentgenology. 2007;189(6):W331-W337 doi:10.2214/AJR.07.2267
11. Parker RA, Menias CO, Quazi R. et al. MR Imaging of the Penis and Scrotum. RadioGraphics. 2015;35(4):1033-1050 doi:10.1148/rg.2015140161
12. Tsili AC, Ntorkou A, Astrakas L. et al. Diffusion-weighted magnetic resonance imaging in the characterization of testicular germ cell neoplasms: Effect of ROI methods on apparent diffusion coefficient values and interobserver variability. European Journal of Radiology. 2017;89:1-6 doi:10.1016/j.ejrad.2017.01.017
13. Moch H, Amin MB, Berney DM. et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. European Urology. 2022;82(5):458-468 doi:10.1016/j.eururo.2022.06.016
14. Lambregts DMJ, Beets GL, Maas M. et al. Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol. 2011;21(12):2567-2574 doi:10.1007/s00330-011-2220-5
15. Min X, Feng Z, Wang L. et al. Characterization of testicular germ cell tumors: Whole-lesion histogram analysis of the apparent diffusion coefficient at 3T. European Journal of Radiology. 2018;98:25-31 doi:10.1016/j.ejrad.2017.10.030
16. Balasundaram P, Garg A, Prabhakar A, Joseph Devarajan LS, Gaikwad SB, Khanna G. Evolution of epidermoid cyst into dermoid cyst: Embryological explanation and radiological-pathological correlation. Neuroradiol J. 2019;32(2):92-97 doi:10.1177/1971400918821086
17. De Oliveira PS, De Oliveira TR, Pereira S, Martinho D, Lopes T. Bilateral synchronous testicular seminoma: A rare presentation of a rare disease. Arch Ital Urol Androl. 2018;90(1):68. doi:10.4081/aiua.2018.1.68
Author contact
![]() Corresponding author: Nguyen Minh Duc, MD, Department of Radiology, Pham Ngoc Thach University of Medicine, 2 Duong Quang Trung Ward 12 District 10 Ho Chi Minh City, Vietnam. E-mail: bsnguyenminhducedu.vn; ORCID ID: 0000-0001-5411-1492.
Corresponding author: Nguyen Minh Duc, MD, Department of Radiology, Pham Ngoc Thach University of Medicine, 2 Duong Quang Trung Ward 12 District 10 Ho Chi Minh City, Vietnam. E-mail: bsnguyenminhducedu.vn; ORCID ID: 0000-0001-5411-1492.

 Global reach, higher impact
Global reach, higher impact