3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(1):27-36. doi:10.7150/ijms.76817 This issue Cite
Review
Novel Insights into Prokineticin 1 Role in Pregnancy-related Diseases
1. Shanghai Changning Maternity & Infant Health Hospital, China.
2. The Department of Obstetrics, Shanghai Obstetrics and Gynecology Hospital of Fudan University, Shanghai 200080, China.
3. NHC Key Lab of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Hospital of Obstetrics and Gynecology, Fudan University, Shanghai 200080, China.
4. Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Hospital of Obstetrics and Gynecology, Fudan University, Shanghai 200080, China.
5. Laboratory for Reproductive Immunology, Institute of Obstetrics and Gynecology, Hospital of Obstetrics and Gynecology, Fudan University, Shanghai 200080, China.
Received 2023-7-5; Accepted 2023-9-26; Published 2024-1-1
Abstract
Prokineticin 1 (PROK1) is a secreted protein involved in a range of physiological activities such as cell proliferation, migration, angiogenesis, and neuronal cell proliferation. Emerging evidences show that PROK1/PROK receptors (PROKRs) are expressed by trophoblasts, and decidual stroma cells at the maternal-fetal interface. PROK1 plays a critical role in successful pregnancy establishment by regulating the decidualization, implantation and placental development. Dysregulation of prokineticin signaling has been described in certain pathological states associated with pregnancy, including pre-eclampsia, recurrent miscarriage and fetal growth restriction. In this review, the expression and pleiotropic roles of PROK1 under physiological and pathological pregnancy conditions are discussed.
Keywords: prokineticin1, maternal-fetal interface, angiogenesis, recurrent miscarriage, preeclampsia, fetal growth restriction
1. Introduction
Prokineticin family include two members, prokineticin 1 (PROK1; also called EG-VEGF) and prokineticin 2 (PROK2; also called Bv8). The Human PROK1 gene maps to the regions of chromosome 1p13.1 and is composed of three exons with no alternative splicing product. The mature PROK1 protein is formulated of 86 amino acids and its relative molecular weight is 8.6 kDa [1]. The Human PROK2 gene maps to chromosome 3p21.1 [2] and encodes a mature protein of 81 amino acid [1]. PROKs are highly conserved in varies species, characterized by a conserved N-terminal sequence (AVITGA), which is essential for the correct binding of receptors [3].
These two secreted proteins act through two G protein-coupled receptors, prokineticin receptor 1 (PROKR1) and prokineticin receptor 2 (PROKR2) [4]. PROKR1 is mainly involved in cell proliferation and angiogenesis, while PROKR2 is involved in the vital vascular endothelial permeability process. Receptors couple to Gq [5], Gi [6] and Gs [7] protein. Prokineticin receptors (PROKRs) mediate the intracellular calcium mobilization, phosphorylation of p44/p42 mitogen-activated protein kinase, serine/threonine kinase Akt and cyclic AMP (cAMP) accumulation, respectively [7]. Additionally, PROKRs can either stimulate or inhibit the accumulation of cyclic AMP (cAMP) via Gs or Gi proteins [7, 8], respectively. Moreover, PROKRs have been shown to act through Gi protein to stimulate mitogen-activated protein kinase (MAPK) [6].
The PROK1 and PROK2 proteins are expressed in multiple organs and tissues, such as brain, heart, digestive tract, bone marrow, ovary, testis, decidua, and placenta. It was found that prokineticins showed dynamic expression throughout the physiological process in many organs, including circadian rhythm [9], menstrual cycle [10] and pregnancy [11]. Additionally, prokineticins are involved in the cancer formation[12].
The expression of prokineticin 1 in the reproductive system plays an important role in the development of the placenta. Prokineticin 1 could affect the growth and development of the placenta, including the processes of implantation [13], invasion and angiogenesis [11]. Whereas the prokineticin 2 mainly affected in the central nervous system and could cause idiopathic hypogonadotropic hypogonadism by abnormal expression. The function of PROK2 in pregnancy-related disease is unclear and needed further study. In pregnancy-related diseases, PROK1 is related to preeclampsia [14], recurrent miscarriage [15], intrauterine growth restriction [14], premature birth [16] and other diseases. In this article, we are going to discuss the role of PROK1 in normal pregnancy and pregnant-related diseases.
2. Biological functions of the prokineticin1
Through constant study, researchers have identified many physiological functions of PROK1. The involvement of PROK1 in diverse physiological activities further demonstrates its important research value.
2.1 Prokineticin1 in ovary
Ovary is one of the organs where PROK1 protein highly expressed. Researchers discovered that PROK1 was expressed in luteal steroidogenic cells of human ovaries. Luteal endothelial cells (LEC) expressed high levels of both PROKR1 and PROKR2 protein. PROK1 protein enhanced proliferation of luteal endothelial cells by increasing [3H]-thymidine binding, MAPK activation and c-jun/fos mRNA expression. The expression of PROKR2 protein is increased in LEC under some stress conditions,such as tumor necrosis factor α (TNFα) and chemical hypoxia,while the PROKR1 protein expression level is unchanged [17]. Exogenous PROK1 protein can inhibit LEC apoptosis [17].
This implies that PROK1 may exert an anti-apoptotic effect on LEC through PROKR2. In addition, PROK1 increased VEGF mRNA expression in bovine luteinizing steroid-producing cells via PROKR1 [17]. These findings suggest that PROK1 plays an important role in luteal function by promoting LEC proliferation and anti-apoptotic effects [18]. Researchers compared PROK1 expression in 13 pairs of human polycystic ovary syndrome (PCOS) and normal ovary samples. PROK1 was highly expressed in theca interna and stroma of PCOS ovaries, suggesting that the angiogenic function of PROK1 may be related to the cyst formation [19]. In another study, researchers transfected rat ovarian granule cells with miR-28-5p mimics, and found that PROK1 protein level is downregulated [20]. MiR-28-5p reverses the promotive effect of PROK1 on cell proliferation and the inhibitory effect on autophagy [20]. PROK1 promoted proliferation and inhibited the apoptosis of rat ovarian granulosa cells through the AKT/mTOR signaling pathway [20].
Additionally, concentration of PROK1 protein in the follicular fluid is relevant to the follicular size and is predictive of the oocyte competence [21]. Follicle Stimulating Hormone and hCG up-regulated PROK1 protein secretion in cumulus cell primary cultures, probably through the cAMP pathway [21]. Successful embryo implantation may be predicted by PROK1 concentration in the follicular fluid and fertilization culture media in conventional in vitro fertilization-embryo transfer [22]. Moreover, expression of PROK1 and PROK2 is correlative to the germ cell development in the human fetal ovary [23]. PROK1 increases ERK phosphorylation and COX2 expression [23].
2.2 Prokineticin1 in gastrointestinal tract
PROK1 protein was originally identified as a promoter of gastrointestinal smooth muscle contraction [24]. PROK1 has a high-affinity site in human ileal smooth muscle [24]. Additionally, PROKR1 mRNA is highly expressed in rat ileum [25]. In rat ileum, PROK1 induces a biphasic contractile response, including an early tetrodotoxin (TTX)-sensitive response and a late TTX-insensitive response [25].
PROK1 mRNA is not expressed in the normal colorectal mucosa, but was detected in all colorectal cancer cell lines [26]. Researchers found that PROK1 increased the microvascular density in colon cancer and promoted cancer cell proliferation [26]. It also increased the liver metastatic ratio [26]. A recent study showed that PROK1 was involved in lymphatic vessel production and participated in lymphatic metastatic pathways [27].
2.3 Prokineticin1 in the bone marrow and blood cells
In human or mouse hematopoietic stem cell cultures, PROK1 increases the number of granulocytic and monocytic colony-forming units [28]. Systemic in vivo exposure to PROK1 significantly increased total leukocytes, neutrophils and monocytes [28]. Moreover, PROK1 could protect multiple myeloma (MM) cells from apoptosis by upregulated Myeloid-cell-leukemia 1 (Mcl-1) [29]. Furthermore, Mcl-1 was upregulated through MAPK, AKT and STAT3 pathway [29].
2.4 Prokineticin1 in neural crest cells
PROKR1 and PROKR2 are expressed in the human enteric neural crest cells (NCCs) [30, 31]. PROK1 protein activates the Akt and MAPK pathways and induces proliferation and differentiation of NCCs via PROKR1 [30]. Neuroblastoma is derived from improperly differentiated neural crest cells. PROK1 induces neuroblastoma cell proliferation by activating the Akt pathway through PROKR1 and PROKR2 [32]. PROKR2 is essential for inhibiting neuroblastoma cell apoptosis [32].
3. The expression of PROK1 at the maternal-fetal interface
3.1 Endometrium
Immunohistochemistry and in situ hybridization analysis showed that PROK1, PROKR1 and PROKR2 were located in a variety of cellular components of the human uterus, including the glandular epithelium, stroma and endothelial cells of the endometrium, as well as in smooth muscle and endothelial cells of the myometrium [33] (Table 1). Immunohistochemistry analysis showed that PROK1 was expressed primarily in the glandular epithelial cells and that its expression was dynamic during the menstrual cycle, and reached a peak in expression during the secretory phase[34]. PROK1 mRNA expression in endometrial cells is controlled by E2 (estrogen) and P4 (progesterone) [34]. Additionally, PROK1 was rarely detected in the endometrial samples from the postmenopausal patients [34]. This suggests that PROK1 expression is highly correlated with steroid hormones.
The expression of PROK1, PROKR1, and PROKR2 at maternal-fetal interface
| Site | Cell | PROK1 | PROKR1 | PROKR2 | Reference |
|---|---|---|---|---|---|
| Endometrium | Glandular epithelial cell | +* | + | Weak | [26] |
| Stromal cell | + | + | - | [26] | |
| Endothelial cell in the endometrium or Myometrium | + | + | Weak | [26] | |
| Smooth muscle cell | + | + | - | [26] | |
| Decidua | Decidual cell | + | + | + | [35] |
| Placenta | Syncytiotrophoblast | + | + | + | [10,37] |
| Cytotrophoblast | + | + | Weak | [10,36] | |
| Extravillous trophoblast | Weak | + | [10] | ||
| Placental microvascular endothelial cells | + | + | [36] | ||
| Placental endothelial cells | - | + | [10] | ||
| Macrophages | + | + | [10,36] |
*: PROK1 is expressed primarily in the glandular epithelial cells.
3.2 Decidua
The researchers found that PROK1 and its receptors PROKR1 and PROKR2 were expressed in decidual cells and play an important role in the process of endometrial decidualization [35]. Expression of PROK1 mRNA in human first-trimester decidua was significantly increased compared to non-pregnant endometrium [35].
3.3 Trophoblasts
The expression of PROK1 mRNA and PROKR1 mRNA peaks in trophoblast cells at 8-10 weeks of gestation [36]. PROK1 protein is localized to the syncytiotrophoblasts [36], expressed minimally in cytotrophoblasts and not expressed in the extravillous trophoblasts [37]. PROKR2 mRNA is not expressed in trophoblasts in the first trimester of pregnancy, except for low levels of expression detected at 8-10 weeks of gestation [37, 38]. Moreover, PROKR1 protein is highly expressed in cytotrophoblasts, placental microvascular endothelial cells and Hofbauer cells, whereas PROKR2 protein is mainly expressed in syncytiotrophoblasts, Hofbauer cells and extravillous trophoblasts [37].
4. Regulation of prokineticin1 expression at maternal-fetal interface
4.1 Hormone
Researchers found that human chorionic gonadotropin (hCG) could induce the expression of PROK1 in a baboon model, human endometrial epithelial cells [39] and first-trimester decidua [39] (Table 2). They showed by dual immunohistochemical analysis that hCG receptors, PROK1 and PROKR1 were present in the glandular epithelial cells of the first trimester decidua [40]. PROK1 and hCG show similar expression pattern during the first trimester of pregnancy. PROK1 expression peaks in trophoblast cells at 8-10 weeks of gestation, while the highest values of hCG are shown at 10-11 weeks of gestation[39]. Human chorionic gonadotropin increases the expression of PROK1, PROKR1 and PROKR2 mRNA and protein in dose- and time-dependent manners[39]. In another study, researchers found that primary endometrial stromal cells decidualization by progesterone and cyclic adenosine monophosphate could be inhibited by PROK1 knockdown[39]. It is reported that hCG regulated PROK1 expression in the ovary [41]. HCG can increase cAMP levels, and cAMP enhances PROK1 promoter expression[39].
Battersby et al. [33] found that during menstrual cycle the expression of PROK1 protein in the endometrium showed periodic changes. The PROK1 protein showed higher expression in the secretory phase of the menstrual cycle than that in the proliferative phase [33]. In endometrial cell culture, PROK1 mRNA was detected in endometrial cells only in the presence of steroids [34]. Using the porcine model of intrauterine infusions of estradiol-17 beta, researchers revealed that it upregulated endometrial expression of PROK1 and PROKR1 mRNA. Moreover, estradiol-17 beta, acting together with prostaglandin E2 (PGE2) increases PROKR1 protein expression in the endometrium [42].
Regulation the expression of PROK1, PROKR1, and PROKR2 at maternal-fetal interface
| Hormone | PROK1 | PROKR1 | PROKR2 | Reference |
|---|---|---|---|---|
| Human chorionic gonadotropin | + | + | + | [39] |
| Progesterone | + | + | [11,26,34] | |
| Estrogen | + | + | [42] | |
| Dihydrotestosterone* | + | [44] | ||
| Insulin | + | [43] | ||
| Hypoxia | + | + | [37] | |
| PPARγ | + | + | + | [47] |
* Only effect with insulin.
Ujvary et al. [43] found that insulin upregulated PROK1 in a dose-dependent manner in decidualizing endometrial stromal cells. Furthermore, dihydrotestosterone is demonstrated to upregulate both of gene and protein expression of PROK1 along with insulin. But dihydrotestosterone alone do not show significant effects at all [44]. On the one hand, insulin interacting with dihydrotestosterone enhances the decidualization, but on the other hand dysregulates the migration of decidual cells and the invasion of trophoblast cells [45].
4.2 Hypoxia
It was reported that hypoxia-inducible factor 1-alpha (HIF1α) response element was identified in the promoter region of PROK1 [46]. Researchers confirmed that PROK1 mRNA and PROKR1 mRNA were upregulated by hypoxia in the human placenta [37]. Additionally, the stimulating effect of insulin on PROK1 gene and protein expression is mediated by HIF1α acting through the phosphatidylinositol 3-kinase (PI3K) signaling pathway [43].
4.3 Peroxisome proliferator-activated receptor-γ
Researchers confirmed that upregulating peroxisome proliferator-activated receptor-γ (PPARγ) increased the PROK1 and its receptors expression in both mRNA and protein level. On the other hand, the PPARγ-/- mice showed low level expression of PROK1 [47]. It is shown that PPARγ plays an important role in the control of trophoblast migration and invasion [48]. It was found that PPARγ upregulated PROK1 and inhibited trophoblast invasion via PROKR2 [47].
5. Role of prokineticin1 in normal pregnancy
5.1 Regulation of trophoblast functions
The depth of invasion of placental trophoblast cells into the uterine wall is quite important in normal pregnancy. Therefore, during the placental development, the proliferation, differentiation and invasion of trophoblast cells are finely regulated. Diverse growth factors and cytokines stimulate differentiation of trophoblast cells to an invasive phenotype, such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), transforming growth factor beta (TGF-β), insulin-like growth factor-II (IGF-II), and interleukin-1 (IL-1) [49].
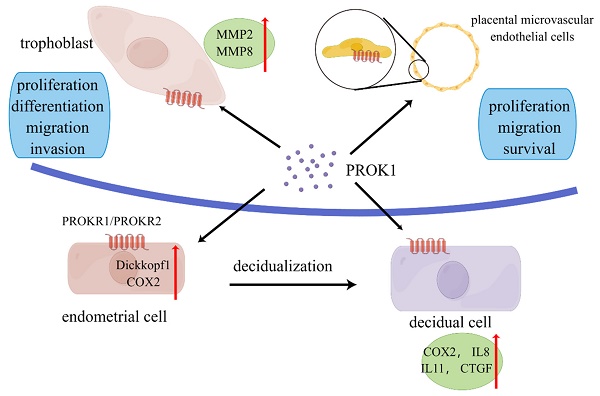
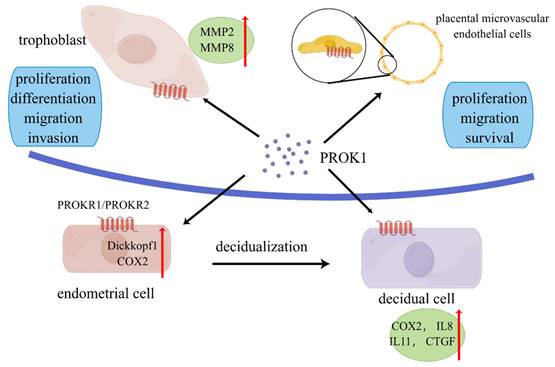
Several studies have confirmed that PROK1 played an important role in trophoblast invasion, affecting trophoblast proliferation, migration, and invasion (Figure 1). Extravillous trophoblastic cells have an important role in the establishment of a successful pregnancy. These cells invade the decidua and spiral arteries to establish the fetal-mother circulation. PROK1 enhanced the invasion of extravillous trophoblastic cells (EVTs) by upregulating the expression of matrix metalloproteinases (MMP) 2 and MMP9 mRNA. And this upregulation was achieved through the receptor PROKR2 [50]. They also found the upregulation of extracellular signal-regulated kinase (ERK) 1/2 after the treatment of high concentration of PROK1 [50]. Researchers found that PROK1 activated ERK1/2 signaling and subsequent upregulation of MMP2 and MMP9 mRNA levels, thereby stimulating invasion of human trophoblast HTR-8/Svneo cells [51]. Therefore, the decrease in PROK1 circulating and placental levels at the end of the first trimester may, with other factors, contribute to EVT invasion and to the establishment of fetal-maternal circulation [52].
Primary cilia are cellular antennae that receive environmental signals and are essential for normal development. PROK1 and its receptors were detected in primary cilia [51]. It was found that inhibition of primary cilia growth could cause decrease of PROK1 and MMP9 expression [51]. In a study published recently, researchers showed that miR-346 and miR-582-3p regulated PROK1-induced trophoblast invasion through repressing MMP 2 and MMP 9[53]. It was shown that miR-346 and miR-582-3p inhibited not only PROK1 expression but also trophoblast invasion and migration in JAR and HTR-8/Svneo cell lines[53]. The PROK1-stimulated cell proliferation was mediated by PI3K/AKT/mTOR, MAPK, and cAMP [13]. PROK1 enhanced adhesion of trophoblast cells to fibronectin and laminin matrices, which are mediated predominantly via leukemia inhibitory factor (LIF) induction [40].
Role of prokineticin1 in the maternal-fetal interface. PROK is secreted mainly by placenta. PROK1 can promote proliferation, differentiation, migration and invasion of trophoblasts through upregulation of MMPs and other pathways. PROK1 acts on placental microvascular endothelium and affects angiogenesis. It acts on endothelial stromal cells and affects decidualization through upregulation of COX2, dickkopf1, etc. PROK1 affects placental implantation through regulation of IL8, IL11 and other cytokines. By Figdraw (www.figdraw.com).
5.2 Effect on endometrium/decidua
Decidualization is a complex process. Endometrial stromal cells are regulated by hormones as well as cytokines and undergo morphological, functional and genetic changes to support embryo implantation and development [54]. PROK1 and its receptors regulate the implantation and decidualization via affecting the expression of implantation-related genes. It has been shown that PROK1 can regulate the expression of many genes, including: LIF, COX-2, IL-6, IL8, and IL-11 [35, 42]. These cytokines are verified to be related to the implantation and decidualization.
Differentiation of endometrial stromal cells into decidua is mediated by PGE2 synthesis through elevation of COX-2 protein [35, 55]. In an experimentation conducted by Sharon et al, the expression of COX-2 protein was significantly increased after the treatment of PROK1 in a human germ cell tumor line. And it was confirmed that PROK1 signaling via PROKR1 induce the expression of COX-2 protein[23]. Another research showed that PROK1 induced COX-2 expression and prostaglandin synthesis in human endometrial cells and first trimester decidua via a Gq coupled pathway [56]. Another study found that PROK1 could upregulate the Dickkocf1 which regulate cells proliferation and decidualization of endometrial stromal cells. It was confirmed through a Gq-calcium-calcineurin-nuclear factor of activated T-cells-mediated pathway [57].
IL-8 is highly expressed in the endometrium and decidua and involves in several processes of endometrial physiology such as angiogenesis, proliferation, chemotaxis, trophoblast invasion and uterine contraction. Researchers found that in endometrial cells PROK1 via PROKR1 could activate the calcineurin/NFAT pathway to induce IL-8 expression [58].
In human endometrial stromal cells, IL-11 has been shown to promote progesterone-induced decidualization, which implies a role for IL-11 in preparing the endometrium for implantation [59]. PROK1-PROKR1 induces the expression of IL-11 in Ishikawa cells and first trimester decidua in a guanine nucleotide-binding protein (G(q/11)), extracellular signal-regulated kinases, Ca2+ and calcineurin-nuclear factor of activated T cells dependent manner through the calcium-calcineurin signaling pathway [60].
Connective tissue growth factor is considered to be up-regulated by PROK1 in the first-trimester decidua[61]. PROK1 regulates the expression of connective tissue growth factor by activating the Gq, PLC, cSrc, EGFR, MAPK/ERK kinase pathway[61].
5.3 Effect on angiogenesis
Several studies have elucidated the angiogenic role of PROK1 in reproductive organs. Study found that PROK1 mainly acted on human placental microvascular endothelial cells (HPEC), and stimulated HPEC's proliferation, migration and survival, while VEGF mainly affected the umbilical vein-derived macrovascular endothelial cells [62]. Compared to VEGF, PROK1 was more effective in stimulating HPEC sprout formation, pseudovascular organization, and it significantly promoted HPEC permeability and paracellular transport [62]. In another study, researchers demonstrated that PROK1 controlled homeobox genes expression in normal human placenta and in placenta from fetal growth restriction (FGR) pregnancies [63]. The homeobox genes expressed in placental microvascular endothelial cells may play a role in the regulation of epithelial-mesenchymal interactions in the placenta and proliferative capacity of placental microvascular endothelial cells [64].
The effects of PROK1 on HPECs suggests that this factor is involved in placental angiogenesis, a process closely related to placental growth and fetal maternal exchanges.
6. Role of prokineticin1 in pathological pregnancy
The dynamic expression of PROK1 throughout pregnancy, its multiple roles in the villi development, and its regulation by hypoxia suggest that this cytokine contributes to the successful pregnancy establishment. Thus, inappropriate expression and/or dysfunction of PROK1 may lead to major complications of pregnancy (Table 3), such as recurrent implantation failure, recurrent pregnancy loss (RPL), ectopic pregnancy (EP), fetal growth restriction (FGR), choriocarcinoma, and pre-eclampsia (PE). These complications are associated with abnormal angiogenesis, as well as trophoblast invasion failures and abnormal placental development.
The role of PROK1 in pregnant related disease
| Disease | Abnormal expression of PROK1 | Functions of PROK1 | Reference |
|---|---|---|---|
| Pre-eclampsia | higher | Inhibit trophoblast invasion | [10,66, 67] |
| Recurrent miscarriage | lower | gene polymorphisms of PROK1 and PROKR1 are related to recurrent miscarriage | [15,70,71] |
| Recurrent implantation failure | higher | unclear | [72] |
| Fetal growth restriction | high | Increase the angiogenesis and proliferation of placental villi | [73] |
6.1 Pre-eclampsia
Pre-eclampsia is the most common hypertensive disease during pregnancy. There are several theories about the pathogenesis of pre-eclampsia. Although the etiology of PE is far from being fully understood, its development is thought to be primarily due to superficial invasion of the maternal decidua and spiral arteries by EVTs [65]. It creates a prolonged hypoxic environment in the placenta, and leads to the release of antiangiogenic factors from the placenta [65]. More specific and earlier markers associated with the disease need to be further investigated. PROK1 satisfies many of the characteristics of an early PE markers. It is abundant in the human placenta during early pregnancy, its expression is elevated by hypoxia, it controls trophoblast invasion, and has specific effects on placental endothelial cells. More importantly, PROK1 inhibit the EVTs which may play a critical role in the cause of PE.
Scientists found that the RNA and protein expression levels of PROK1 were significantly elevated in PE patients compared with age-matched control [10]. The RNA and protein expression of PROK1 in the placenta of PE patients decreases significantly, suggesting that the in-situ expression of PROK1 in the placenta of PE patients may be down regulated at the transcriptional level [66]. Moreover, the RNA and protein expression level of PROKR1 shows no difference between control and PE patients [66].
A group created a new mouse model of gestational hypertension by continuous injection of prokineticin1 [14]. Sustained high levels of ghrelin over the first trimester of pregnancy significantly increased blood pressure and renal impairment in mice [14]. It significantly decreased the thickness of the decidual and junctional zone in the placenta [14]. Moreover, sustained high levels of prokineticin1 in late pregnancy reduced blood pressure in mice compared to mice injected only up to mid-gestation [14].
Both of the RNA and protein expression of PROK1 is upregulated by hypoxia. The promoter regions of the gene contain functional hypoxia response elements that bind to hypoxia-inducible factor 1 (HIF-1) and mediate its expression under hypoxic tension. PROK1 upregulation by hypoxia confirms its role in the first trimester, as placental development and angiogenesis occur in hypoxic environment during this period [67].
The miRNA-200 family is predicted to target the PROK1 5'-untranslated region [68]. Increased miRNA-141 and miRNA-200a inhibit the protein expression of PROK1, matrix metalloproteinase 9 (MMP9) and downstream extracellular signal-regulated kinase (ERK) signaling, thus leading to trophoblast invasion failures [68]. The growth of the primary cilium is inhibited by increases in miR-141 and miR-200a expression [68]. The number of cilia in the human placenta of preeclampsia women was markedly decreased compared to normal placenta [68].
6.2 Recurrent miscarriage
Recurrent miscarriage is related to abnormal blood vessels in the placental bed, suggesting that early disturbance of placental blood vessel development may lead to miscarriage [69].
In 2010, Su et al. [15] studied the genes of 115 women with recurrent miscarriage and 170 women from the control group, and selected 11 single nucleotide polymorphisms from PROK1, PROKR1, and PROKR2 by using genotype analysis. Further using multifactor dimensionality reduction (MDR) method for analysis, they found that 2 SNPs of PROKR1 (rs4627609, rs6731838) and 1 SNP of PROKR2 (rs6053283) are closely related to recurrent miscarriage (P<0.05) [15]. At the same time, the frequency of the C-G and T-A haplotypes of PROKR1 and the A-G-C-G-G haplotypes of PROKR2 were also significantly increased in recurrent miscarriage (P <0.05) [15].
Hence, the gene polymorphism and haplotype of PROK1 receptor are both related to recurrent miscarriage. In a study carried out in 2014, researchers found the PROKR1 (I379V) and PROKR2 (V331M) were nonsynonymous variants showed significant association with recurrent miscarriage [70]. It has been shown that the common variant of V67I may act as a modifier in the PROK1-PROKR system through down-regulation of PROK1 expression [71]. PROK1 (V67I) has been shown to play a role as a modifier gene in the PROK1-PROKR system of human early pregnancy [71].
6.3 Recurrent implantation failure
In a recent study, researchers studied the endometrial samples of 15 women with recurrent implantation failure (RIF) and 15 women from control group. The results showed that PROK1 mRNA levels were 6.09 times higher compared to endometrial samples obtained from women with RIF than in samples obtained from women from control group, whereas PROKR1 mRNA levels were 2.46 times lower in endometrial samples obtained from women with RIF than in samples from women from control group. There was no statistically significant difference between women with RIF and women from control group regarding PROK2 and PROKR2 levels [72]. The results suggested that expression of the PROK1/PROKR1 system could play an important role in implantation.
6.4 Fetal growth restriction
During pregnancy, fetal optimal growth depends on an adequate blood vascular network in the fetal part. Abnormal placental angiogenesis will endanger the supply of nutrients and hormones, which will eventually lead to FGR. Although the exact cause of FGR in not clear yet, several researches shows that the PROK1 and its receptors are highly associated with FGR. Scientists found that in FGR patients in the third trimester of pregnancy, both RNA and protein levels of PROK1 are significantly increased in the placenta [73]. Scientists found that PROK1, PROKR1, and PROKR2 mRNA and protein levels were significantly increased in FGR placentas [73]. PROK1 can increase the angiogenesis of placental villi and the expression of CD31, a marker of endothelial cells in the placental villi. At the same time, PROK1 promotes the proliferation of villi and the anchoring of cytotrophoblast layers, and promotes the survival of placental villi under stress conditions [73]. Therefore, the increase of PROK1, PROKR1 and PROKR2 occurs in FGR as a compensatory mechanism to ensure proper pregnancy progression.
6.5 Choriocarcinoma
It has been suggested that the development and progression of choriocarcinoma is associated with an over-proliferation of the trophoblastic layer, resulting in an increased mass of cells that acquire a migratory and/or aggressive phenotype [74]. Prokineticin 1 is demonstrated to upregulate trophoblast proliferation and invasion [73]. Prokineticin 1 protein and its two receptors are upregulated in the blood and placenta of choriocarcinoma patients [75]. Additionally, in the mouse model of choriocarcinoma, the application of PROKR1 or PROKR2 antagonists to gravid mice delayed tumor progression and increased the duration of pregnancy maintenance [75].
7. Conclusions
During the past 20 years, the prokineticin system has been reported to play a role in a range of physiological functions. Mutations or dysfunction of these factors are also associated with a number of diseases. Targeting prokineticin system may provide treatments for diseases such as cancer, inflammation, pain, and neuropathology. In recent years, more and more concerns have been expressed about their specific role at the maternal-fetal interface. Since the PROK2 is mainly expressed in the central nervous system and PROK1 is widely detected in the steroidogenic organs, researches mainly focused on the PROK1 and further research is needed to reveal the functions of PROK2 in the maternal-fetal interface.
PROK1 is co-regulated by pregnancy-associated hormones, cytokines and other factors. It is highly expressed in embryonic trophoblasts and decidual stromal cells at the maternal-fetal interface, and participates in a series of physiological processes through interaction with its receptors, PROKR1 and PROKR2. PROK1 not only promotes the endometrial decidualization process and enhances uterine receptivity, but also induces migration and invasion of trophoblasts and facilitate embryo implantation and development. Nevertheless, abnormal expression of PROK1 may lead to impaired endometrial decidualization, trophoblastic dysfunction and placental dysplasia, resulting in pregnancy complications such as recurrent miscarriage, PE and FGR, although the exact mechanisms have not been fully clarified. Therefore, normal expression of PROK1 in the first trimester is essential for a successful pregnancy. Regulation of PROK1, PROKR1 and PROKR2 expression may be potential targets for clinical therapy of patients with abnormal pregnancies, although further studies are needed to confirm this.
Abbreviations
PROK1: prokineticin1; PROKR: prokineticin receptor; EG-VEGF: endocrine gland-derived vascular endothelial growth factor; cAMP: cyclic AMP; MAPK: mitogen-activated protein kinase; AKT: proto-oncogene protein c-akt; LEC: luteal endothelial cells; TNFα: tumor necrosis factor α; PCOS: polycystic ovary syndrome; Mcl-1: Myeloid-cell-leukemia 1; MM: multiple myeloma; hCG: human chorionic gonadotropin; PGE2: prostaglandin E2; HIF1α: hypoxia-inducible factor 1-alpha; PI3K: phosphatidylinositol 3-kinase; PPARγ: peroxisome proliferator-activated receptor-γ; EGF: epidermal growth factor; HGF: hepatocyte growth factor; TGF-β: transforming growth factor beta; IGF-II: insulin-like growth factor-II; IL-1: interleukin-1; EVTs: extravillous trophoblastic cells; MMP: matrix metalloproteinases; ERK: extracellular signal-regulated kinase; LIF: leukemia inhibitory factor; G(q/11): guanine nucleotide-binding protein; HPEC: human placental microvascular endothelial cells; RPL: recurrent pregnancy loss; EP: ectopic pregnancy; FGR: fetal growth restriction; PE: pre-eclampsia; RIF: recurrent implantation failure.
Acknowledgements
This study was supported by the Program of National Natural Science Foundation of China (NSFC) (No. 81671484). The Program of National Natural Science Foundation of China (NSFC) (No. 92057119, 31970798), the Program for Zhuoxue of Fudan University (JIF157602), the Support Project for Original Personalized Research of Fudan University (IDF157014/002).
Author contributions
All the authors were involved in writing the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhou QY, Li M, Bullock CM, Knauer DJ, Ehlert FJ. Identification of two prokineticin cDNAs: Recombinant proteins potently contract gastrointestinal smooth muscle. Faseb Journal. 2001;15:A564-A
2. Jilek A, Engel E, Beier D, Lepperdinger G. Murine Bv8 gene maps near a synteny breakpoint of mouse chromosome 6 and human 3p21. Gene. 2000;256:189-95
3. Bullock CM, Li JD, Zhou QY. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Molecular Pharmacology. 2004;65:582-8
4. Cook IH, Evans J, Maldonado-Perez D, Critchley HO, Sales KJ, Jabbour HN. Prokineticin-1 (PROK1) modulates interleukin (IL)-11 expression via prokineticin receptor 1 (PROKR1) and the calcineurin/NFAT signalling pathway. Molecular Human Reproduction. 2010;16:158-69
5. Lin DCH, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. Journal of Biological Chemistry. 2002;277:19276-80
6. Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. Journal of Biological Chemistry. 2002;277:8724-9
7. Chen JC, Kuei C, Sutton S, Wilson S, Yu JX, Kamme F. et al. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Molecular Pharmacology. 2005;67:2070-6
8. Ngan ES, Tam PK. Prokineticin-signaling pathway. Int J Biochem Cell Biol. 2008;40:1679-84
9. Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J. et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405-10
10. Hoffmann P, Saoudi Y, Benharouga M, Graham CH, Schaal JP, Mazouni C. et al. Role of EG-VEGF in human placentation: Physiological and pathological implications. J Cell Mol Med. 2009;13:2224-35
11. Goryszewska E, Kaczynski P, Balboni G, Waclawik A. Prokineticin 1-prokineticin receptor 1 signaling promotes angiogenesis in the porcine endometrium during pregnancy. Biology of Reproduction. 2020;103:654-68
12. Zhao Y, Wu J, Wang X, Jia H, Chen DN, Li JD. Prokineticins and their G protein-coupled receptors in health and disease. Prog Mol Biol Transl Sci. 2019;161:149-79
13. Goryszewska-Szczurek E, Baryla M, Kaczynski P, Waclawik A. Prokineticin 1-prokineticin receptor 1 signaling in trophoblast promotes embryo implantation and placenta development. Scientific Reports. 2021 11
14. Sergent F, Hoffmann P, Brouillet S, Garnier V, Salomon A, Murthi P. et al. Sustained Endocrine Gland-Derived Vascular Endothelial Growth Factor Levels Beyond the First Trimester of Pregnancy Display Phenotypic and Functional Changes Associated With the Pathogenesis of Pregnancy-Induced Hypertension. Hypertension. 2016;68:148 -+
15. Su M-T, Lin S-H, Lee IW, Chen Y-C, Hsu C-C, Pan H-A. et al. Polymorphisms of endocrine gland-derived vascular endothelial growth factor gene and its receptor genes are associated with recurrent pregnancy loss. Human Reproduction. 2010;25:2923-30
16. Erzincan SG, Varol FG, Inan C, Sayin NC. Relationship between second-trimester amniotic fluid levels of Prokineticin-1 and Matrix Metalloproteinase-2 with adverse pregnancy outcome. Placenta. 2018;62:25-7
17. Kisliouk T, Podlovni H, Meidan R. Unique expression and regulatory mechanisms of EG-VEGF/prokineticin-1 and its receptors in the corpus luteum. Ann Anat. 2005;187:529-37
18. Kisliouk T, Podlovni H, Spanel-Borowski K, Ovadia O, Zhou QY, Meidan R. Prokineticins (endocrine gland-derived vascular endothelial growth factor and BV8) in the bovine ovary: expression and role as mitogens and survival factors for corpus luteum-derived endothelial cells. Endocrinology. 2005;146:3950-8
19. Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A. et al. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881-93
20. Meng L, Yang H, Jin C, Quan S. miR-28-5p suppresses cell proliferation and weakens the progression of polycystic ovary syndrome by targeting prokineticin-1. Mol Med Rep. 2019;20:2468-75
21. Alfaidy N, Baron C, Antoine Y, Reynaud D, Traboulsi W, Gueniffey A. et al. Prokineticin 1 is a new biomarker of human oocyte competence: expression and hormonal regulation throughout late folliculogenesis. Biol Reprod. 2019;101:832-41
22. Alfaidy N, Hoffmann P, Gillois P, Gueniffey A, Lebayle C, Garçin H. et al. PROK1 Level in the Follicular Microenvironment: A New Noninvasive Predictive Biomarker of Embryo Implantation. J Clin Endocrinol Metab. 2016;101:435-44
23. Eddie SL, Childs AJ, Kinnell HL, Brown P, Jabbour HN, Anderson RA. Prokineticin Ligands and Receptors Are Expressed in the Human Fetal Ovary and Regulate Germ Cell Expression of COX2. Journal of Clinical Endocrinology & Metabolism. 2015;100:E1197-E205
24. Bullock CM, Li M, Cheng M, Elhert F, Zhou QY. Identification of two prokineticin cDNAs: Recombinant proteins potently contract gastrointestinal smooth muscle. Society for Neuroscience Abstracts. 2001;27:2226
25. Wade PR, Palmer JM, Mabus J, Saunders PR, Prouty S, Chevalier K. et al. Prokineticin-1 evokes secretory and contractile activity in rat small intestine. Neurogastroenterol Motil. 2010;22:e152-61
26. Goi T, Fujioka M, Satoh Y, Tabata S, Koneri K, Nagano H. et al. Angiogenesis and tumor proliferation/metastasis of human colorectal cancer cell line SW620 transfected with endocrine glands-derived-vascular endothelial growth factor, as a new angiogenic factor. Cancer Res. 2004;64:1906-10
27. Naruse T, Goi T, Yamaguchi A. Prokineticin-1 induces normal lymphangiogenic activity and is involved in lymphangiogenesis and lymph node metastasis in colorectal cancer. Oncotarget. 2021;12:1388-97
28. LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci U S A. 2004;101:16813-8
29. Li QF, Zhu HY, Yang YF, Liu J, Xiao FJ, Zhang QW. et al. Prokineticin-1/endocrine gland-derived vascular endothelial growth factor is a survival factor for human multiple myeloma cells. Leuk Lymphoma. 2010;51:1902-12
30. Ngan ES, Lee KY, Sit FY, Poon HC, Chan JK, Sham MH. et al. Prokineticin-1 modulates proliferation and differentiation of enteric neural crest cells. Biochim Biophys Acta. 2007;1773:536-45
31. Ruiz-Ferrer M, Torroglosa A, Núñez-Torres R, de Agustín JC, Antiñolo G, Borrego S. Expression of PROKR1 and PROKR2 in human enteric neural precursor cells and identification of sequence variants suggest a role in HSCR. PLoS One. 2011;6:e23475
32. Ngan ES, Sit FY, Lee K, Miao X, Yuan Z, Wang W. et al. Implications of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 signaling in human neuroblastoma progression. Clin Cancer Res. 2007;13:868-75
33. Battersby S, Critchley HOD, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. Journal of Clinical Endocrinology & Metabolism. 2004;89:2463-9
34. Ngan ESW, Lee KY, Yeung WSB, Ngan HYS, Ng EHY, Ho PC. Endocrine gland-derived vascular endothelial growth factor is expressed in human peri-implantation endometrium, but not in endometrial carcinoma. Endocrinology. 2006;147:88-95
35. Evans J, Catalano RD, Morgan K, Critchley HOD, Millar RP, Jabbour HN. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877-87
36. Denison FC, Battersby S, King AE, Szuber M, Jabbour HN. Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta. Endocrinology. 2008;149:3470-7
37. Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675-84
38. Maldonado-Pérez D, Evans J, Denison F, Millar RP, Jabbour HN. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab. 2007;18:66-72
39. Brouillet S, Hoffmann P, Chauvet S, Salomon A, Chamboredon S, Sergent F. et al. Revisiting the role of hCG: new regulation of the angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci. 2012;69:1537-50
40. Evans J, Catalano RD, Brown P, Sherwin R, Critchley HOD, Fazleabas AT. et al. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. Faseb Journal. 2009;23:2165-75
41. Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA. et al. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427-34
42. Goryszewska E, Kaczynski P, Baryla M, Waclawik A. Pleiotropic role of prokineticin 1 in the porcine endometrium during pregnancy establishment and embryo implantation. Biology of Reproduction. 2021;104:181-96
43. Ujvari D, Jakson I, Oldmark C, Attarha S, Alkasalias T, Salamon D. et al. Prokineticin 1 is up-regulated by insulin in decidualizing human endometrial stromal cells. J Cell Mol Med. 2018;22:163-72
44. Ujvari D, Graells Brugalla C, Hirschberg AL. Dihydrotestosterone potentiates insulin to up-regulate prokineticin-1 in decidualizing human endometrial stromal cells. J Cell Mol Med. 2020;24:3242-5
45. Hirschberg AL, Jakson I, Graells Brugalla C, Salamon D, Ujvari D. Interaction between insulin and androgen signalling in decidualization, cell migration and trophoblast invasion in vitro. J Cell Mol Med. 2021;25:9523-32
46. LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L. et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877-84
47. Garnier V, Traboulsi W, Salomon A, Brouillet S, Fournier T, Winkler C. et al. PPARγ controls pregnancy outcome through activation of EG-VEGF: new insights into the mechanism of placental development. Am J Physiol Endocrinol Metab. 2015;309:E357-69
48. Fournier T, Thérond P, Handschuh K, Tsatsaris V, Evain-Brion D. PPARgamma and early human placental development. Curr Med Chem. 2008;15:3011-24
49. Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol. 1999;128:181-9
50. Tani K, Mitsui T, Mishima S, Ohira A, Maki J, Eto E. et al. EG-VEGF Induces Invasion of a Human Trophoblast Cell Line via PROKR2. Acta Med Okayama. 2021;75:677-84
51. Wang C-Y, Tsai H-L, Syu J-S, Chen T-Y, Su M-T. Primary Cilium-Regulated EG-VEGF Signaling Facilitates Trophoblast Invasion. Journal of Cellular Physiology. 2017;232:1467-77
52. Alfaidy N, Hoffmann P, Boufettal H, Samouh N, Aboussaouira T, Benharouga M. et al. The Multiple Roles of EG-VEGF/PROK1 in Normal and Pathological Placental Angiogenesis. Biomed Research International. 2014. 2014
53. Su MT, Tsai PY, Tsai HL, Chen YC, Kuo PL. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors. 2017;43:210-9
54. Ni N, Li Q. TGFβ superfamily signaling and uterine decidualization. Reprod Biol Endocrinol. 2017;15:84
55. Cheng JG, Stewart CL. Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol Reprod. 2003;68:401-4
56. Evans J, Morgan K, Critchley H, Millar R, Jabbour H. Prokineticin 1 induces cox-2 expression and prostaglandin synthesis in human endometrial cells and first trimester decidua via a GQ coupled pathway. Biology of Reproduction. 2007: 147-.
57. Macdonald LJ, Sales KJ, Grant V, Brown P, Jabbour HN, Catalano RD. Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium. Molecular Human Reproduction. 2011;17:626-36
58. Maldonado-Perez D, Brown P, Morgan K, Millar RP, Thompson EA, Jabbour HN. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochimica Et Biophysica Acta-Molecular Cell Research. 2009;1793:1315-24
59. Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636-43
60. Cook IH, Evans J, Maldonado-Pérez D, Critchley HO, Sales KJ, Jabbour HN. Prokineticin-1 (PROK1) modulates interleukin (IL)-11 expression via prokineticin receptor 1 (PROKR1) and the calcineurin/NFAT signalling pathway. Mol Hum Reprod. 2010;16:158-69
61. Waddell JM, Evans J, Jabbour HN, Denison FC. CTGF expression is up-regulated by PROK1 in early pregnancy and influences HTR-8/Svneo cell adhesion and network formation. Human Reproduction. 2011;26:67-75
62. Brouillet S, Hoffmann P, Benharouga M, Salomon A, Schaal JP, Feige JJ. et al. Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells. Mol Biol Cell. 2010;21:2832-43
63. Murthi P, Brouillet S, Pratt A, Borg A, Kalionis B, Goffin F. et al. An EG-VEGF-Dependent Decrease in Homeobox Gene NKX3.1 Contributes to Cytotrophoblast Dysfunction: A Possible Mechanism in Human Fetal Growth Restriction. Molecular Medicine. 2015;21:645-56
64. Alfaidy N, Brouillet S, Rajaraman G, Kalionis B, Hoffmann P, Barjat T. et al. The Emerging Role of the Prokineticins and Homeobox Genes in the Vascularization of the Placenta: Physiological and Pathological Aspects. Frontiers in Physiology. 2020 11
65. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999-1011
66. Szuber M, Markwitz W, Ropacka M, Breborowicz GH. The possible role of the PK1 and its receptor in the etiology of the preeclampsia. Neuro Endocrinol Lett. 2011;32:563-72
67. Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27-35
68. Wang C-Y, Tsai P-Y, Chen T-Y, Tsai H-L, Kuo P-L, Su M-T. Elevated miR-200a and miR-141 inhibit endocrine gland-derived vascular endothelial growth factor expression and ciliogenesis in preeclampsia. Journal of Physiology-London. 2019;597:3069-83
69. Manolea MM, Gavrilă OA, Popescu FC, Novac L, Mateescu GO. The importance of immunohistochemical evaluation of the vascular changes from the decidua and placenta in recurrent pregnancy loss. Rom J Morphol Embryol. 2012;53:363-8
70. Su M-T, Lin S-H, Chen Y-C, Kuo P-L. Gene-gene interactions and risk of recurrent miscarriages in carriers of endocrine gland-derived vascular endothelial growth factor and prokineticin receptor polymorphisms. Fertility and Sterility. 2014;102:1071-U553
71. Su M-T, Huang J-Y, Tsai H-L, Chen Y-C, Kuo P-L. A Common Variant of PROK1 (V67I) Acts as a Genetic Modifier in Early Human Pregnancy through Down-Regulation of Gene Expression. International Journal of Molecular Sciences. 2016 17
72. Karaer A, Tuncay G, Uysal O, Sevimli TS, Sahin N, Karabulut U. et al. The role of prokineticins in recurrent implantation failure. Journal of Gynecology Obstetrics and Human Reproduction. 2020 49
73. Brouillet S, Murthi P, Hoffmann P, Salomon A, Sergent F, De Mazancourt P. et al. EG-VEGF controls placental growth and survival in normal and pathological pregnancies: case of fetal growth restriction (FGR). Cellular and Molecular Life Sciences. 2013;70:511-25
74. Louwen F, Muschol-Steinmetz C, Reinhard J, Reitter A, Yuan J. A lesson for cancer research: placental microarray gene analysis in preeclampsia. Oncotarget. 2012;3:759-73
75. Traboulsi W, Sergent F, Boufettal H, Brouillet S, Slim R, Hoffmann P. et al. Antagonism of EG-VEGF Receptors as Targeted Therapy for Choriocarcinoma Progression In Vitro and In Vivo. Clin Cancer Res. 2017;23:7130-40
Author contact
![]() Corresponding authors: Ting Peng: qch1868com; Tel: 86-21-33189900; Ming-Qing Li: mqliedu.cn; Tel.: 86-21-33189900.
Corresponding authors: Ting Peng: qch1868com; Tel: 86-21-33189900; Ming-Qing Li: mqliedu.cn; Tel.: 86-21-33189900.

 Global reach, higher impact
Global reach, higher impact