3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(13):1722-1731. doi:10.7150/ijms.86832 This issue Cite
Review
Extracellular Vesicles in Sepsis: Pathogenic Roles, Organ Damage, and Therapeutic Implications
1. Department of Emergency, Xijing Hospital, Air Force Medical University, Xi'an, China.
2. University College London, London, UK.
Received 2023-6-5; Accepted 2023-10-5; Published 2023-10-16
Abstract
Despite significant advances in anti-infective treatment and organ function support technology in recent years, the mortality rate of sepsis remains high. In addition to the high costs of sepsis treatment, the increasing consumption of medical resources also aggravates economic pressure and social burden. Extracellular vesicles (EVs) are membrane vesicles released from different types of activated or apoptotic cells to mediate intercellular communication, which can be detected in both human and animal body fluids. A growing body of researches suggest that EVs play an important role in the pathogenesis of sepsis. In this review, we summarize the predominant roles of EVs in various pathological processes during sepsis and its related organ dysfunction.
Keywords: sepsis, extracellular vesicles, inflammation, exosomes, organ dysfunction
Introduction
Sepsis is a syndrome of systemic inflammatory response caused by the invasion of pathogenic microorganisms such as bacteria into the organism. Every year, 50 million people suffer from sepsis and 11 million die as a result [1]. Sepsis may cause shock, multiple organ failure, and even death if not detected or treated quickly. The Global Sepsis Alliance reports that the number of patients hospitalized for sepsis has doubled over the past 10 years [2]. Sepsis is a high heterogeneous disease, and the heterogeneity of sepsis at the individual patient level has hindered progress in the field beyond current treatment standards. In addition, sepsis has a rapid onset, symptoms similar to other disorders, and no specialized tests, so rapid diagnosis and treatment of sepsis is essential. Most sepsis deaths can be avoided with rapid diagnosis and treatment. Early treatment of sepsis not only improves the patient prognosis, but also reduces the hospital stay, which is cost-effective and resource-conserving.
EVs are a kind of membranous small vesicles released by cells to the extracellular matrix, with a particle size distribution range from 30 nm to 1 μm, which play a key role in intercellular communication and body regulation through signaling molecules such as proteins and lipids on the membrane, as well as neurotransmitters, enzymes, hormones and nucleic acids wrapped in the membrane [3-5]. The composition of EVs cargoes is complex, containing hundreds to thousands of different proteins, unique lipids, some DNA, and numerous small non-coding RNAs. There is evidence that EVs are involved in many physiopathological processes, including cellular homeostasis, infection transmission, cancer development, and cardiovascular disease [6-9]. Over the past two decades, a large number of original studies investigating sepsis EVs have been published [10-13]. In this review, we mainly introduce the changes of EVs cargoes in sepsis and EV functions in the sepsis-related organ dysfunction.
Cargoes of EVs in sepsis
There is a positive correlation between the number of EVs and the severity of sepsis when sepsis is present or when bacteria irritate the body [14]. EVs carrying altered proteins have been found in the fluids of patients with sepsis, which may contribute to the progression of the disease [15]. Acute-phase reactive proteins and immunoglobulins, which are involved in the inflammatory response, are upregulated in the early stages of sepsis [16]. A variety of cell types, including activated macrophages, monocytes, and neutrophils, generate EVs with altered protein profiles [17]. In serum of sepsis mice, a number of cytokines and chemokines are specifically encapsulated in exosomes, and exosome inhibitor GW4869 can reduce exosome formation and inflammatory cytokine release significantly [18]. There is evidence that cytokines and chemokines in exosomes are different from serum-free cytokines and chemokines in that they may have a role in lymphocyte differentiation and proliferation [19]. Exosomes derived from macrophages stimulated by LPS also produced high levels of cytokines. According to recent studies, EVs released by different types of cells carry molecular patterns associated with damage, including high mobility group box 1 protein, histones, and extracellular cold-induced RNA-binding protein [19].
It is important for nucleic acid transport to use EVs, which protect nucleic acids from degradation by nucleases and keep them stable. In sepsis, EVs carry a variety of nucleic acids, including mRNA, microRNA, long noncoding RNA (lncRNA), and circRNA. In patients with sepsis, EVs express higher amounts of mRNA involved in antioxidant defense and oxidative stress [20]. The microRNA expression profile of EVs is altered in sepsis and may be associated with the risk, severity, and prognosis of sepsis [21, 22]. MicroRNAs play a role in sepsis via a variety of pathways, including immunomodulation, microvascular dysfunction, and organ dysfunction [5]. In addition, lncRNA, and circRNA were also altered. Studies showed that EVs carrying the lncRNA NEAT1 in sepsis have been found to aggravate sepsis-related encephalopathy, and lncRNA-p21 could inhibit LPS-induced lung cells injury [23, 24]. Serum exosomes from patients suffering from sepsis were up-regulated with hsa_circRNA_104484 and hsa_circRNA_104670, suggesting that they could be used as diagnostic markers for the disease [25].
EVs and inflammation in sepsis
EVs originating from diverse cellular sources have been substantiated to exert significant roles in various biological processes. In the course of sepsis pathogenesis, pathogens (such as bacteria, viruses, or fungi) or their toxins induce systemic infection [26]. These pathogens or their toxins stimulate the immune system, triggering the generation of abundant inflammatory cells and mediators, which disseminate throughout the body via the circulatory system, consequently provoking Systemic Inflammatory Response Syndrome (SIRS) and leading to organ failure and death [27, 28].
Gram-positive and Gram-negative bacteria, as the most prevalent infectious agents in sepsis, can produce EVs carrying bacterial endotoxins and transmitting bacterial proteins [29], which enter the septic patient's fluid circulation [30]. During Gram-negative bacterial infection, outer membrane vesicles (OMVs) serve as crucial facilitators for the entry of LPS and caspase-11 activation into the cytoplasm [31]. OMVs bearing specific antigens, dependent on TLR2 or TLR4, induce activation of B cells and CD4(+) T cells, resulting in the activation of adaptive immunity [32, 33]. Concurrently, through various pathways, including the NF-κB signaling cascade, innate and adaptive immune responses in sepsis are activated [34], thereby inducing systemic inflammation [35-37].
During sepsis, host-derived EVs are predominantly produced by immune cells, such as platelets and innate immune cells [3]. It is generally believed that EVs originating from immune cells exacerbate the onset of sepsis, as they carry higher levels of damage-associated molecular patterns (DAMPs) and cytokines [38]. They can activate various pattern recognition receptors (PRRs) and signaling pathways to induce pro-inflammatory responses [39, 40], such as the release of pro-inflammatory cytokines IL-12, IL-15, IL-17, and IFN-γ, promoting macrophage proliferation and M1 polarization, ultimately leading to a "cytokine storm" [41, 42] (Table 1). In addition to activating innate immune responses, EVs in sepsis also induce Th1/Th2 cell differentiation, enhance T lymphocyte proliferation and migration during the course of sepsis, and activate adaptive immune responses [41], further mediating inflammation. Inhibition of the exosome generation process has been shown to reduce the inflammatory response and significantly improve survival rates in sepsis [43]. Recent research has demonstrated that modifying miRNA within extracellularly generated exosomes can suppress the cytokine storm in sepsis and inhibit its development [44]. Interestingly, some studies have shown that certain EVs may suppress inflammation in septic patients, for example, EVs containing α-2-macroglobulin secreted by neutrophils contribute to bacterial clearance and alleviate inflammation [45]. Moreover, immature dendritic cell-derived EVs mitigate acute systemic inflammatory responses in sepsis by enhancing apoptotic cell clearance [46]. Overall, EVs can balance pro-inflammatory responses and immune suppression.
The cytokines within exosomes in sepsis participate in immune regulation and peak timing [19]
| Cytokines | Function | Mechanism | Peak time in EVs | Peak time in Serum |
|---|---|---|---|---|
| IL-1β[47, 48] | Pro-inflammatory | Induces inflammation and stimulates immune responses. | 12h | 24-48h |
| IL-2[49] | Pro-inflammatory | Stimulates T-cell proliferation and differentiation. | 2-12h | 12h |
| IL-6[50] | Pro-inflammatory | Induces acute phase response and B-cell differentiation. | 2h | 2-12h |
| IL-12[51] | Pro-inflammatory | Promotes Th1 cell differentiation, stimulates NK cells. | 24h | 12-24h |
| IL-15[52] | Pro-inflammatory | Supports NK cell survival, stimulates T-cell proliferation. | 2h | 12h |
| IL-17[53] | Pro-inflammatory | Induces pro-inflammatory responses, promotes neutrophil recruitment. | 24h | 12h |
| TNF-α[48] | Pro-inflammatory | Regulates immune cells, induces inflammatory response. | 2h | 2h |
| IFN-γ[54] | Pro-inflammatory | Activates macrophages, promotes Th1 cell differentiation. | 12-24h | 12h |
| IL-4[55] | Anti-inflammatory | Promotes Th2 cell differentiation, B-cell activation. | 24h | 24h |
| IL-5[55] | Anti-inflammatory | Induces eosinophil activation and differentiation. | 24h | 12h |
| IL-10[55] | Anti-inflammatory | Inhibits inflammatory cytokines, regulates immune response. | 24h | 2-12h |
| CCL2[56] | Chemotactic factor | Chemotactic for monocytes, memory T cells. | 12h | 2-12h |
| CCL3[57] | Chemotactic factor | Chemotactic for NK cells, monocytes, dendritic cells. | 12h | 2h |
| CCL5[58, 59] | Chemotactic factor | Chemotactic for T cells, eosinophils, basophils. | 24h | 12h |
| CXCL9[60, 61] | Chemotactic factor | Chemotactic for T cells, promotes Th1 response. | 24h | 12h |
| CXCL10[60, 61] | Chemotactic factor | Chemotactic for T cells, NK cells, promotes Th1 response. | 24h | 12h |
However, there is a scarcity of research on the role of EVs in the immunosuppressive mechanisms of sepsis. Unveiling their functional mechanisms will provide insights for immune-based therapeutic approaches for sepsis.
EVs and cardiovascular function in sepsis
A close relationship exists between sepsis and cardiovascular dysfunction. Arterial hypotension is the most common feature of cardiovascular dysfunction in septic patients, primarily due to factors such as reduced blood volume, decreased vascular tone, and myocardial suppression [62]. These factors lead to a decline in left and right ventricular ejection fractions, potentially causing severe consequences. Patients with sepsis-associated cardiovascular dysfunction often exhibit poor tolerance to fluid administration, which is related to decreased central venous oxygen saturation (ScvO2) [63, 64]. Adequate fluid resuscitation to increase blood volume and improve microcirculation is crucial for maintaining tissue oxygenation; however, achieving this goal may be difficult for septic patients due to cardiovascular dysfunction.
EVs may play a critical role in cardiovascular dysfunction during sepsis. In vitro experiments have demonstrated that OMVs from a uropathogenic Escherichia coli strain induce irregular calcium oscillations with reduced frequency in cardiomyocytes. Following intraperitoneal injection of sterile OMVs, OMVs can be detected within the heart. Troponin T levels in the blood significantly increase, while echocardiography reveals increased heart wall thickness and heart rate [65].
During the progression of sepsis, the assembly of the TXNIP-NLRP3 complex in monocytes can be embedded into CD63+ exosomes, transported from circulating monocytes to local macrophages, and promote the cleavage of inactive IL-1β and IL-18 in macrophages, exacerbating cardiovascular inflammation and myocardial dysfunction [66]. Moreover, hsa-miR-1262 in sepsis-derived EVs may interact with SLC2A1, thereby inhibiting glycolytic activity and promoting cardiomyocyte apoptosis [67]. Platelet-derived exosomes from septic patients generate nitric oxide (NO), which contributes to sepsis-induced myocardial dysfunction and vascular damage [68]. One study demonstrated that exosomes from septic patients induced endothelial cell and apoptotic cell vascular smooth muscle, a result of increased exosomal NADPH activity [69]. Likewise, another study showed that platelet-derived exosome production in sepsis may be regulated by NO and bacterial components, promoting the generation of reactive oxygen species, peroxynitrite, caspase-3 activation, and vascular endothelial cell apoptosis, ultimately causing vascular dysfunction in sepsis [70].
EVs and coagulation dysfunction in sepsis
Most sepsis patients exhibit coagulation dysfunction, which varies in severity. The most severe coagulation disorder in sepsis is disseminated intravascular coagulation (DIC), characterized by systemic thrombus formation and bleeding [71]. During the development of sepsis, EVs display procoagulant tissue factors and phosphatidylserine on their surfaces, thereby modulating inflammatory responses and coagulation [72]. Tissue factor is a cell surface receptor for coagulation factors VII/VIIIa and plays a crucial role in initiating the extrinsic coagulation pathway. Escherichia coli OMVs induce coagulation in a TLR4-dependent manner [73], and increase TF activity within the organism via the caspase-11-GSDMD pathway [74], further promoting a hypercoagulable state in sepsis [75]. Under normal physiological conditions, tissue factor is not expressed within cells; however, during bacterial infections, tissue factor appears in the blood to inhibit bacterial dissemination and limit the impact of microbes on the host [76, 77]. Nevertheless, excessive coagulation activation can lead to impaired tissue circulation, endothelial dysfunction, and organ damage [62, 78].
Increased circulating EVs derived from endothelial cells, platelets, red blood cells, and white blood cells are associated with coagulation activation, tissue factor release, inflammation, and ROS production, leading to small intravascular thrombi and endothelial damage [79-81]. Studies have shown that EVs isolated from plasma during sepsis impair erythrocyte deformability [82]. As tissue factor is primarily expressed by activated monocytes in the body, EVs transporting tissue factor are considered to be mainly released from activated monocytes [83], forming tissue factor- and CD13-positive EVs that promote inflammation and coagulation. The proportion of tissue factor- and CD13-positive EVs increases with symptom severity. Other studies have also demonstrated a strong positive correlation between procoagulant tissue factor activity in EVs and the severity of sepsis [84], and reported that the content of procoagulant tissue factor in circulating EVs is associated with the onset of DIC [77], and the formation of microthrombi, activation of the coagulation and fibrinolytic systems, and activation of the complement system occur, thereby activating white blood cells and endothelial cells to promote the release of pro-inflammatory mediators, further intensifying the onset of inflammation [85, 86], and further leading to coagulation dysfunction. Additionally, platelet-, leukocyte-, and endothelial cell-derived EVs containing phosphatidylserine on their surfaces can promote coagulation activity during sepsis; however, their procoagulant roles in sepsis remain unclear [87, 88]. Leukocyte-derived EVs inhibit endothelial nitric oxide synthase activation, enhance inducible nitric oxide synthase (iNOS) expression in vivo, induce systemic vasodilation, and reduce mean arterial pressure in septic infectious shock [89].
EVs and acute lung injury in sepsis
Sepsis frequently leads to pulmonary inflammation, further progressing to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [90], causing irreversible lung damage primarily characterized by diffuse alveolar damage, hypoxemia, and respiratory distress [91]. Studies suggest that lung injury may stem from direct lung injury caused by epithelial damage and indirect lung injury induced by endothelial cell damage [92, 93]. During the development of sepsis-induced lung injury, cytokines mediate the aggregation and infiltration of numerous immune cells in lung tissue, activating a positive feedback loop for the inflammatory response, ultimately culminating in a cytokine storm and disrupting the alveolar-capillary endothelial barrier structure, allowing neutrophils and macrophages to infiltrate the alveoli [94, 95].
In sepsis-induced ALI and ARDS, both bronchoalveolar lavage fluid (BALF) and circulating EVs exhibit upregulation in quantity [96, 97]. EVs within BALF are predominantly secreted by alveolar macrophages [98], and the activation of alveolar macrophages, as initiators of innate immune system activation, can induce inflammatory responses. Concurrently, research reveals that damaged pulmonary epithelial and endothelial cells release EVs containing various miRNAs (miR-221 and miR-320a), cytokines, and caspase-3, potentially activating alveolar macrophages to release multiple pro-inflammatory mediators [99-102], promoting macrophage EV release and stimulating TNF-α expression [103]. Similarly, following LPS injection in mice, alveolar macrophage EV release rapidly increases within one hour [104]. Furthermore, EVs released by activated alveolar macrophages can activate and recruit resting macrophages, subsequently activating the NLRP3 inflammasome and intensifying the inflammatory response caused by sepsis [105, 106]. In addition to EVs derived from alveolar macrophages, monocyte EVs may induce pulmonary endothelial cell damage and mediate pyroptosis through cleaved GSDMD and active caspase 1 [107, 108], while endothelial cell damage and the release of nitrated S1PR3-containing EVs could exacerbate pulmonary inflammation [109]. Moreover, various circulating cell-derived EV contents, including miR-145, miR-210-3p, and miR1-3p, can impact pulmonary inflammation [110, 111] and endothelial barrier dysfunction [112] via different cellular signaling pathways, although the source of these plasma EVs requires further investigation.
The viral infection caused by SARS-CoV-2 can lead to symptoms similar to those of sepsis caused by bacterial infections. [113]. Similar to bacterial sepsis, SARS-CoV-2 infection can cause pulmonary inflammation, alveolar damage, gas exchange impairment, and shock [114], although the distinction lies in SARS-CoV-2 directly invading the lungs to cause injury. Several inflammatory factors, such as IL-6, TNFα, IL-1β, and granulocyte-colony stimulating factor, may play pivotal roles in acute lung injury caused by SARS-CoV-2 [115-117]. Research indicates that EVs exhibit elevated immune and vascular-related markers in patients with moderate-to-severe SARS-CoV-2 infection [118]. Additionally, pulmonary EVs may carry ACE2, allowing SARS-CoV-2's spike protein to enter target cells via binding to the ACE2 on EVs [119], thereby playing a role in the pathogenesis of the disease.
EVs and acute kidney injury (AKI) in sepsis
In septic patients, renal dysfunction is a common and severe complication. Elevated serum urea (or blood urea nitrogen) and creatinine are common in sepsis-associated renal dysfunction, and even mild increases in creatinine concentration are associated with poorer prognosis in critically ill patients [120, 121]. Acute kidney injury (AKI) is a serious renal dysfunction, clinically characterized by oliguria (reduced urine output), usually secondary to sepsis-induced infectious shock and hypovolemia [122].
EVs from various cellular sources may have regulatory effects on sepsis-mediated acute kidney injury, possibly related to the multiple non-coding RNAs carried by the EVs (Table 2). The increased number of platelet-derived EVs is negatively correlated with AKI biomarkers blood urea nitrogen and creatinine concentrations [123]. Adipose-derived mesenchymal stem cell (MSC)-derived EVs significantly suppress renal oxidative stress and inflammatory response [124], and further research suggests that the protective effect of adipose-derived MSC-derived EVs may be mediated through the SIRT1 signaling pathway [125]. Another study demonstrated that exogenous umbilical cord MSC-derived EVs can inhibit the NF-κB signaling pathway and attenuate renal inflammatory infiltration through miR-146b [126]. EVs derived from endothelial progenitor cells may, through their encapsulated miR-382-3p, target the E3 ubiquitin-protein ligase (BTRC), thereby ameliorating the IκBα/NF-κB axis and inhibiting immune responses in multiple organs, including the kidney [126]. Additionally, studies revealed that endothelial progenitor cell-derived EVs deliver miR-21 to regulate RUNX1, thereby reducing oxidative stress, inflammation, and apoptosis levels in renal tubular epithelial cells [127]. Multiple studies have shown that non-coding RNAs in EVs released by renal tubular epithelial cells during AKI may mediate macrophage polarization towards a pro-inflammatory M1 phenotype [128-130]. The polarization state of macrophages may also affect the effects of their released EVs on renal tubular epithelial cells; M1 macrophage-derived EVs promote renal epithelial cell apoptosis, while M2 macrophage-derived EVs carry miR-93-5p, which suppresses renal epithelial cell pyroptosis and alleviates AKI by regulating TXNIP [131]. Therefore, the communication and dialogue between macrophages and renal tubules through EVs may be a critical factor in acute kidney injury in sepsis. Interestingly, in sepsis induced by CLP, Limb myotubes might exert remote ischemic preconditioning on the kidney due to hypoxia. Through HIF-1α dependent upregulation of miR-21, by targeting PDCD4/NF-κB and PTEN/AKT pathways in renal tubular epithelial cells, thereby exerting anti-inflammatory and anti-apoptotic effects [132]. However, in addition to the direct regulatory effects on renal cells, EVs-induced coagulation dysfunction in sepsis also promotes thrombus formation in the renal microcirculation [3, 133], leading to disordered intrarenal perfusion and medullary hypoxia [134].
EVs and neurological dysfunction in sepsis
The impact of sepsis on the brain primarily manifests as acute and long-term neurological dysfunction, including sepsis-associated encephalopathy and cognitive impairment [136]. The pathogenesis mainly involves the interplay of systemic inflammation, blood-brain barrier dysfunction, neuroinflammation, microcirculatory dysfunction, and cerebral dysfunction [137, 138]. Following LPS stimulation, choroid plexus epithelial cells secrete EVs containing inflammatory proteins and miRNAs (miR-146a and miR-155), which are absorbed by astrocytes and microglia via cerebrospinal fluid and transmit inflammatory information, thereby affecting the central nervous system [139].
The Role of Non-Coding RNAs Carried by EVs in Sepsis-Induced Acute Kidney Injury
| miRNA | Sources | Function | Mechanism |
|---|---|---|---|
| miR-146b[126] | mesenchymal stem cell | Attenuation of renal inflammatory infiltration | Inhibition of the NF-kB Signaling Pathway |
| miR-93-5p[131] | M2 Macrophages | Inhibition of renal epithelial cell pyroptosis | Inhibition of the TXNIP-NLRP3 axis |
| miR-21[127, 132] | Limb myotubes, Endothelial Progenitor Cells | Inhibition of renal epithelial cell Inflammation and Apoptosis | Inhibition of the RUNX1, PDCD4/NF-κB and PTEN/AKT Signaling Pathway |
| miR-382-3p[135] | Endothelial Progenitor Cells | Attenuation of renal inflammation | Inhibition of BTRC to restrain NF-kB Signaling Pathway |
In the cecal ligation and puncture (CLP) rat model, blood-brain barrier damage and increased reactive oxygen species (ROS) levels promote ferroptosis, during which the expression of serum exosomal NEAT1 is upregulated, potentially regulating miR-9-5p/TFRC and GOT1 axis through a competing endogenous RNA mechanism to promote neuronal ferroptosis in rats, thus exacerbating sepsis-associated encephalopathy [140].
EVs and liver injury in sepsis
Although there are no overt structural abnormalities in the liver and biliary system in septic patients, alterations in liver function remain common. Liver dysfunction is mainly characterized by elevated bilirubin or transaminase levels [141]. Liver injury may lead to changes in the clearance rate of bacteria or LPS and result in the release of pro-inflammatory cytokines, further exacerbating the symptoms of sepsis [142]. Studies have found that macrophages release high-mobility group box 1 (HMGB1) via EVs as a DAMP, mediating cytotoxicity and leading to cell death and tissue injury. The interaction between HMGB1 and the receptor for advanced glycation end-products is involved in loading HMGB1 into EVs. Through transferrin-mediated endocytosis, these EVs transfer HMGB1 to target cells, activating the NLRP3 inflammasome, and consequently resulting in hepatocyte pyroptosis [143, 144].
Role of EVs in sepsis.
MCS-derived EVs, the other side?
While EVs derived from bacteria and immune cells may precipitate various complications in sepsis, those originating from mesenchymal stem cells (MSCs) have been found to play a pivotal role in different experimental models of acute tissue injury during the process of sepsis, which are largely attributed to the paracrine actions of MSC-EVs. These exosomes can either interact with receptors on the surface of target cells or fuse with them, releasing their contents into the cell and subsequently altering the function of the recipient cell [145].
In cardiovascular system, MSC-derived EVs have been identified that may contribute to this regulatory effect, including carrying PTEN-induced putative kinase 1, which ameliorates mitochondrial dysfunction in cardiomyocytes [146]. Additionally, several MSC-carried miRNAs, such as miR-233 [147] and miRNA-141 [148], may be involved in cardioprotective effects. In the kidneys, MSC-derived EVs have been shown to provide renal protection by inhibiting oxidative stress, cell apoptosis, and fibrosis, and promoting autophagy [127, 149]. They also achieve immune regulation by inducing the anti-inflammatory and immunosuppressive effects of M2 macrophages and regulatory Tregs [150], and by modulating NK cells [151, 152]. This mechanism may also be attributed to the broad gene regulatory effects of their miRNA contents, as evidenced by a wealth of preclinical studies [127, 149, 153, 154]. Similarly, the administration of MSC-derived EVs to patients with acute lung injury caused by sepsis in clinical settings has shown comparable protective effects. Intravenous injection of MSC-derived EVs can increase the rate of alveolar fluid clearance, reduce pulmonary protein permeability, enhance antibacterial activity [155], and significantly improve acute lung injury patient's survival rates caused by COVID-19 and Epidemic Influenza A [156, 157]. Nevertheless, further confirmation is needed to validate the protective role of MSC-derived exosomes on different organs in sepsis. In addition, despite the demonstrated protective effects of MSC-derived EVs on various tissue injuries, the effectiveness and consistency of MSC-derived EVs functions still require further evaluation for clinical treatment.
Conclusions
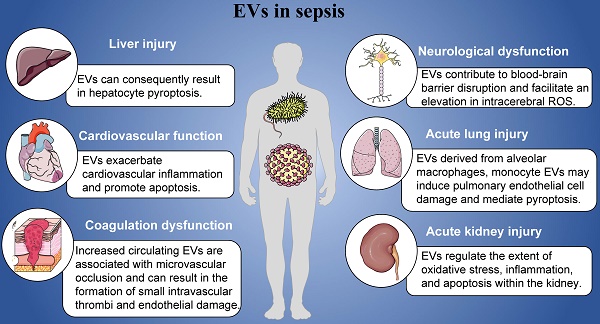
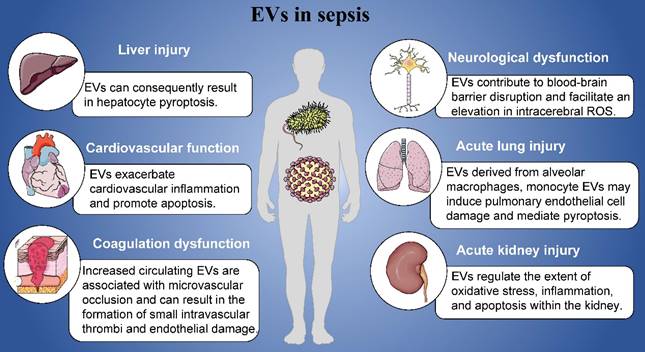
In this review, we mainly introduced the roles of EVs in the pathogenesis of sepsis including cardiovascular function, coagulation dysfunction, acute lung injury, acute kidney injury, neurological dysfunction and liver injury (Figure 1). Sepsis manifests as a pleiotropic process in which exosomes carry a multitude of proinflammatory molecules, activate cellular signaling, and cause multiple organ malfunctions. Based on the contents of EVs, EVs might play specific roles in the organ damages and other complications induced by sepsis. Furthermore, we have detailed the mechanisms by which EVs exert their influence in this process. At the same time, it has also been pointed out that EVs derived from mesenchymal stem cells may play a positive effect in different organ of patients with sepsis, that is, they can significantly inhibit acute organ injury caused by sepsis. Studying its regulatory mechanism in sepsis can provide a theoretical basis for future diagnosis, treatment strategies, and vaccine prevention.
Acknowledgements
Funding
This study was funded by the Military Major Project (BWS21J002).
Availability of data and materials
The current study was based on the results of relevant published studies.
Author contributions
NA, PZ and WY contributed to writing and editing of this review. CZ revised this review. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P. et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. American Journal of Respiratory and Critical Care Medicine. 2016;193:259-72
2. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Medicine. 2021;47:1181-247
3. Raeven P, Zipperle J, Drechsler S. Extracellular Vesicles as Markers and Mediators in Sepsis. Theranostics. 2018;8:3348-65
4. Burgelman M, Vandendriessche C, Vandenbroucke RE. Extracellular Vesicles: A Double-Edged Sword in Sepsis. Pharmaceuticals. 2021 14
5. Tian C, Wang K, Zhao M, Cong S, Di X, Li R. Extracellular vesicles participate in the pathogenesis of sepsis. Frontiers in Cellular and Infection Microbiology. 2022 12
6. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:640 -+
7. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836-48
8. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nature Reviews Clinical Oncology. 2018;15:617-38
9. Yang Q, Liu J, Wu B, Wang X, Jiang Y, Zhu D. Role of extracellular vesicles in osteosarcoma. Int J Med Sci. 2022;19:1216-26
10. Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K. et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. Embo Molecular Medicine. 2016;8:1162-83
11. Appiah MG, Park EJ, Darkwah S, Kawamoto E, Akama Y, Gaowa A. et al. Intestinal Epithelium-Derived Luminally Released Extracellular Vesicles in Sepsis Exhibit the Ability to Suppress TNF-alpha and IL-17A Expression in Mucosal Inflammation. International Journal of Molecular Sciences. 2020 21
12. Hong X, Wang J, Li S, Zhao Z, Feng Z. MicroRNA-375-3p in endothelial progenitor cells-derived extracellular vesicles relieves myocardial injury in septic rats via BRD4-mediated PI3K/ AKT signaling pathway. International Immunopharmacology. 2021 96
13. Kawamoto E, Masui-Ito A, Eguchi A, Soe ZY, Prajuabjinda O, Darkwah S. et al. INTEGRIN AND PD-1 LIGAND EXPRESSION ON CIRCULATING EXTRACELLULAR VESICLES IN SYSTEMIC INFLAMMATORY RESPONSE SYNDROME AND SEPSIS. Shock. 2019;52:13-22
14. Dakhlallah DA, Wisler J, Gencheva M, Brown CM, Leatherman ER, Singh K. et al. Circulating extracellular vesicle content reveals de novo DNA methyltransferase expression as a molecular method to predict septic shock. Journal of extracellular vesicles. 2019;8:1669881 -
15. Morris DC, Jaehne AK, Chopp M, Zhang Z, Poisson L, Chen Y. et al. Proteomic Profiles of Exosomes of Septic Patients Presenting to the Emergency Department Compared to Healthy Controls. Journal of Clinical Medicine. 2020 9
16. Xu Y, Ku X, Wu C, Cai C, Tang J, Yan W. Exosomal proteome analysis of human plasma to monitor sepsis progression. Biochemical and Biophysical Research Communications. 2018;499:856-61
17. Wisler JR, Singh K, McCarty AR, Abouhashem ASE, Christman JW, Sen CK. Proteomic Pathway Analysis of Monocyte-Derived Exosomes during Surgical Sepsis Identifies Immunoregulatory Functions. Surgical Infections. 2020;21:101-11
18. Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J. et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2015;1852:2362-71
19. Gao K, Jin J, Huang C, Li J, Luo H, Li L. et al. Exosomes Derived From Septic Mouse Serum Modulate Immune Responses via Exosome-Associated Cytokines. Frontiers in Immunology. 2019 10
20. Real JM, Pinto Ferreira LR, Esteves GH, Koyama FC, Salles Dias MV, Bezerra-Neto JE. et al. Exosomes from patients with septic shock convey miRNAs related to inflammation and cell cycle regulation: new signaling pathways in sepsis? Critical Care. 2018 22
21. Hermann S, Brandes F, Kirchner B, Buschmann D, Borrmann M, Klein M. et al. Diagnostic potential of circulating cell-free microRNAs for community-acquired pneumonia and pneumonia-related sepsis. Journal of Cellular and Molecular Medicine. 2020;24:12054-64
22. Reithmair M, Buschmann D, Maerte M, Kirchner B, Hagl D, Kaufmann I. et al. Cellular and extracellular miRNAs are blood-compartment-specific diagnostic targets in sepsis. Journal of Cellular and Molecular Medicine. 2017;21:2403-11
23. Wei X-b, Jiang W-q, Zeng J-h, Huang L-q, Ding H-g, Jing Y-w. et al. Exosome-Derived lncRNA NEAT1 Exacerbates Sepsis-Associated Encephalopathy by Promoting Ferroptosis Through Regulating miR-9-5p/TFRC and GOT1 Axis. Molecular Neurobiology. 2022;59:1954-69
24. Sui X, Liu W, Liu Z. Exosomal lncRNA-p21 derived from mesenchymal stem cells protects epithelial cells during LPS-induced acute lung injury by sponging miR-181. Acta Biochimica Et Biophysica Sinica. 2021;53:748-57
25. Tian C, Liu J, Di X, Cong S, Zhao M, Wang K. Exosomal hsa_circRNA_104484 and hsa_circRNA_104670 may serve as potential novel biomarkers and therapeutic targets for sepsis. Scientific Reports. 2021 11
26. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-10
27. Huang M, Cai S, Su J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int J Mol Sci. 2019 20
28. Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34:129-36
29. Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O'Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5:e1000382
30. Namork E, Brandtzaeg P. Fatal meningococcal septicaemia with "blebbing" meningococcus. Lancet. 2002;360:1741
31. Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD. et al. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell. 2016;165:1106-19
32. Aung KM, Sjostrom AE, von Pawel-Rammingen U, Riesbeck K, Uhlin BE, Wai SN. Naturally Occurring IgG Antibodies Provide Innate Protection against Vibrio cholerae Bacteremia by Recognition of the Outer Membrane Protein U. J Innate Immun. 2016;8:269-83
33. Shah B, Sullivan CJ, Lonergan NE, Stanley S, Soult MC, Britt LD. Circulating bacterial membrane vesicles cause sepsis in rats. Shock. 2012;37:621-8
34. Soult MC, Lonergan NE, Shah B, Kim WK, Britt LD, Sullivan CJ. Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. J Surg Res. 2013;184:458-66
35. Park KS, Choi KH, Kim YS, Hong BS, Kim OY, Kim JH. et al. Outer membrane vesicles derived from Escherichia coli induce systemic inflammatory response syndrome. PLoS One. 2010;5:e11334
36. Finethy R, Luoma S, Orench-Rivera N, Feeley EM, Haldar AK, Yamamoto M. et al. Inflammasome Activation by Bacterial Outer Membrane Vesicles Requires Guanylate Binding Proteins. mBio. 2017 8
37. Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692-701
38. Tian C, Wang K, Zhao M, Cong S, Di X, Li R. Extracellular vesicles participate in the pathogenesis of sepsis. Front Cell Infect Microbiol. 2022;12:1018692
39. Nair RR, Mazza D, Brambilla F, Gorzanelli A, Agresti A, Bianchi ME. LPS-Challenged Macrophages Release Microvesicles Coated With Histones. Front Immunol. 2018;9:1463
40. Murao A, Tan C, Jha A, Wang P, Aziz M. Exosome-Mediated eCIRP Release From Macrophages to Induce Inflammation in Sepsis. Front Pharmacol. 2021;12:791648
41. Gao K, Jin J, Huang C, Li J, Luo H, Li L. et al. Exosomes Derived From Septic Mouse Serum Modulate Immune Responses via Exosome-Associated Cytokines. Front Immunol. 2019;10:1560
42. Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci Rep. 2018;8:8973
43. Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J. et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015;1852:2362-71
44. Li Y, Zhang H, Chen C, Qiao K, Li Z, Han J. et al. Biomimetic Immunosuppressive Exosomes that Inhibit Cytokine Storms Contribute to the Alleviation of Sepsis. Adv Mater. 2022;34:e2108476
45. Dalli J, Norling LV, Montero-Melendez T, Federici Canova D, Lashin H, Pavlov AM. et al. Microparticle alpha-2-macroglobulin enhances pro-resolving responses and promotes survival in sepsis. EMBO Mol Med. 2014;6:27-42
46. Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y. et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected]. J Immunol. 2009;183:5983-90
47. Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661
48. Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1beta and TNF-alpha in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660
49. Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR. et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114-8
50. Unver N, McAllister F. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018;41:10-7
51. Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722-8
52. Wang X, Zhao XY. Transcription Factors Associated With IL-15 Cytokine Signaling During NK Cell Development. Front Immunol. 2021;12:610789
53. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. 2023;23:38-54
54. Cope A, Le Friec G, Cardone J, Kemper C. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol. 2011;32:278-86
55. Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R. et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984-1010
56. Zhu S, Liu M, Bennett S, Wang Z, Pfleger KDG, Xu J. The molecular structure and role of CCL2 (MCP-1) and C-C chemokine receptor CCR2 in skeletal biology and diseases. J Cell Physiol. 2021;236:7211-22
57. Uyangaa E, Kim JH, Patil AM, Choi JY, Kim SB, Eo SK. Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6Chi Monocytes and NK Cells via CCL2-CCL3 Cascade. PLoS Pathog. 2015;11:e1005256
58. Zeng Z, Lan T, Wei Y, Wei X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022;9:12-27
59. Marques RE, Guabiraba R, Russo RC, Teixeira MM. Targeting CCL5 in inflammation. Expert Opin Ther Targets. 2013;17:1439-60
60. Muller M, Carter S, Hofer MJ, Campbell IL. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity-a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 2010;36:368-87
61. Koper OM, Kaminska J, Sawicki K, Kemona H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv Clin Exp Med. 2018;27:849-56
62. Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14:417-27
63. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. Jama. 2010;303:739-46
64. Lanspa MJ, Pittman JE, Hirshberg EL, Wilson EL, Olsen T, Brown SM. et al. Association of left ventricular longitudinal strain with central venous oxygen saturation and serum lactate in patients with early severe sepsis and septic shock. Critical care (London, England). 2015;19:304
65. Svennerholm K, Park KS, Wikstrom J, Lasser C, Crescitelli R, Shelke GV. et al. Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction. Sci Rep. 2017;7:17434
66. Wang L, Zhao H, Xu H, Liu X, Chen X, Peng Q. et al. Targeting the TXNIP-NLRP3 interaction with PSSM1443 to suppress inflammation in sepsis-induced myocardial dysfunction. J Cell Physiol. 2021;236:4625-39
67. Sun F, Geng H, Sun Y, Feng W, Tian T, Ye L. et al. Exosomes derived from the blood of patients with sepsis regulate apoptosis and aerobic glycolysis in human myocardial cells via the hsa-miR-1262/SLC2A1 signaling pathway. Mol Med Rep. 2022 25
68. Azevedo LC, Janiszewski M, Pontieri V, Pedro Mde A, Bassi E, Tucci PJ. et al. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care. 2007;11:R120
69. Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit Care Med. 2004;32:818-25
70. Gambim MH, do Carmo Ade O, Marti L, Verissimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107
71. Levi M, van der Poll T. Coagulation and sepsis. Thrombosis research. 2017;149:38-44
72. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139-62
73. Wang E, Liu Y, Qiu X, Tang Y, Wang H, Xiao X. et al. Bacteria-released outer membrane vesicles promote disseminated intravascular coagulation. Thromb Res. 2019;178:26-33
74. Peng Y, Gao M, Liu Y, Qiu X, Cheng X, Yang X. et al. Bacterial outer membrane vesicles induce disseminated intravascular coagulation through the caspase-11-gasdermin D pathway. Thromb Res. 2020;196:159-66
75. Soult MC, Dobrydneva Y, Wahab KH, Britt LD, Sullivan CJ. Outer membrane vesicles alter inflammation and coagulation mediators. J Surg Res. 2014;192:134-42
76. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16:231-41
77. Kleinjan A, Boing AN, Sturk A, Nieuwland R. Microparticles in vascular disorders: how tissue factor-exposing vesicles contribute to pathology and physiology. Thromb Res. 2012;130(Suppl 1):S71-3
78. Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W. et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103:1342-7
79. Boisrame-Helms J, Delabranche X, Degirmenci SE, Zobairi F, Berger A, Meyer G. et al. Pharmacological modulation of procoagulant microparticles improves haemodynamic dysfunction during septic shock in rats. Thromb Haemost. 2014;111:154-64
80. Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp RG. et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930-5
81. Meziani F, Delabranche X, Asfar P, Toti F. Bench-to-bedside review: circulating microparticles-a new player in sepsis? Critical care (London, England). 2010;14:236
82. Subramani K, Raju SP, Chu X, Warren M, Pandya CD, Hoda N. et al. Effect of plasma-derived extracellular vesicles on erythrocyte deformability in polymicrobial sepsis. Int Immunopharmacol. 2018;65:244-7
83. Matsumoto H, Yamakawa K, Ogura H, Koh T, Matsumoto N, Shimazu T. Clinical Significance of Tissue Factor and CD13 Double-Positive Microparticles in Sirs Patients with Trauma and Severe Sepsis. Shock. 2017;47:409-15
84. Woei AJFJ, van der Starre WE, Tesselaar ME, Garcia Rodriguez P, van Nieuwkoop C, Bertina RM. et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res. 2014;133:799-803
85. Iba T, Umemura Y, Wada H, Levy JH. Roles of Coagulation Abnormalities and Microthrombosis in Sepsis: Pathophysiology, Diagnosis, and Treatment. Arch Med Res. 2021;52:788-97
86. Giustozzi M, Ehrlinder H, Bongiovanni D, Borovac JA, Guerreiro RA, Gasecka A. et al. Coagulopathy and sepsis: Pathophysiology, clinical manifestations and treatment. Blood Rev. 2021;50:100864
87. Zhang Y, Meng H, Ma R, He Z, Wu X, Cao M. et al. Circulating Microparticles, Blood Cells, and Endothelium Induce Procoagulant Activity in Sepsis through Phosphatidylserine Exposure. Shock. 2016;45:299-307
88. Tripisciano C, Weiss R, Eichhorn T, Spittler A, Heuser T, Fischer MB. et al. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci Rep. 2017;7:6522
89. Mortaza S, Martinez MC, Baron-Menguy C, Burban M, de la Bourdonnaye M, Fizanne L. et al. Detrimental hemodynamic and inflammatory effects of microparticles originating from septic rats. Crit Care Med. 2009;37:2045-50
90. Huppert LA, Matthay MA, Ware LB. Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med. 2019;40:31-9
91. Kumar V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front Immunol. 2020;11:1722
92. Englert JA, Bobba C, Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight. 2019 4
93. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A. et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788-800
94. Lee C, Choi WJ. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch Pharm Res. 2021;44:99-116
95. Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W. et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11:316-29
96. Letsiou E, Sammani S, Zhang W, Zhou T, Quijada H, Moreno-Vinasco L. et al. Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. Am J Respir Cell Mol Biol. 2015;52:193-204
97. Li H, Meng X, Liang X, Gao Y, Cai S. Administration of microparticles from blood of the lipopolysaccharide-treated rats serves to induce pathologic changes of acute respiratory distress syndrome. Exp Biol Med (Maywood). 2015;240:1735-41
98. Ye C, Li H, Bao M, Zhuo R, Jiang G, Wang W. Alveolar macrophage - derived exosomes modulate severity and outcome of acute lung injury. Aging (Albany NY). 2020;12:6120-8
99. Lee H, Abston E, Zhang D, Rai A, Jin Y. Extracellular Vesicle: An Emerging Mediator of Intercellular Crosstalk in Lung Inflammation and Injury. Front Immunol. 2018;9:924
100. Moon HG, Cao Y, Yang J, Lee JH, Choi HS, Jin Y. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 2015;6:e2016
101. Lanyu Z, Feilong H. Emerging role of extracellular vesicles in lung injury and inflammation. Biomed Pharmacother. 2019;113:108748
102. Lee H, Zhang D, Zhu Z, Dela Cruz CS, Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6:35250
103. Li ZG, Scott MJ, Brzoska T, Sundd P, Li YH, Billiar TR. et al. Lung epithelial cell-derived IL-25 negatively regulates LPS-induced exosome release from macrophages. Mil Med Res. 2018;5:24
104. Soni S, Wilson MR, O'Dea KP, Yoshida M, Katbeh U, Woods SJ. et al. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. 2016;71:1020-9
105. Lee H, Zhang D, Laskin DL, Jin Y. Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation. J Immunol. 2018;201:1500-9
106. Zhang D, Lee H, Wang X, Groot M, Sharma L, Dela Cruz CS. et al. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax. 2019;74:865-74
107. Mitra S, Wewers MD, Sarkar A. Mononuclear Phagocyte-Derived Microparticulate Caspase-1 Induces Pulmonary Vascular Endothelial Cell Injury. PLoS One. 2015;10:e0145607
108. Mitra S, Exline M, Habyarimana F, Gavrilin MA, Baker PJ, Masters SL. et al. Microparticulate Caspase 1 Regulates Gasdermin D and Pulmonary Vascular Endothelial Cell Injury. Am J Respir Cell Mol Biol. 2018;59:56-64
109. Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, Sammani S. et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol. 2012;47:628-36
110. Li G, Wang B, Ding X, Zhang X, Tang J, Lin H. Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp Mol Med. 2021;53:1180-91
111. Cao X, Zhang C, Zhang X, Chen Y, Zhang H. MiR-145 negatively regulates TGFBR2 signaling responsible for sepsis-induced acute lung injury. Biomed Pharmacother. 2019;111:852-8
112. Gao M, Yu T, Liu D, Shi Y, Yang P, Zhang J. et al. Sepsis plasma-derived exosomal miR-1-3p induces endothelial cell dysfunction by targeting SERP1. Clin Sci (Lond). 2021;135:347-65
113. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782-93
114. Li H, Liu L, Zhang D, Xu J, Dai H, Tang N. et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-20
115. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506
116. Liu J, Li S, Liu J, Liang B, Wang X, Wang H. et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763
117. Remy KE, Mazer M, Striker DA, Ellebedy AH, Walton AH, Unsinger J. et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020 5
118. Aharon A, Dangot A, Kinaani F, Zavaro M, Bannon L, Bar-Lev T. et al. Extracellular Vesicles of COVID-19 Patients Reflect Inflammation, Thrombogenicity, and Disease Severity. Int J Mol Sci. 2023 24
119. Xia X, Yuan P, Liu Y, Wang Y, Cao W, Zheng JC. Emerging roles of extracellular vesicles in COVID-19, a double-edged sword? Immunology. 2021;163:416-30
120. Poston JT, Koyner JL. Sepsis associated acute kidney injury. Bmj. 2019;364:k4891
121. Kuwabara S, Goggins E, Okusa MD. The Pathophysiology of Sepsis-Associated AKI. Clin J Am Soc Nephrol. 2022;17:1050-69
122. Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819-25
123. Tokes-Fuzesi M, Woth G, Ernyey B, Vermes I, Muhl D, Bogar L. et al. Microparticles and acute renal dysfunction in septic patients. J Crit Care. 2013;28:141-7
124. Chang CL, Sung PH, Chen KH, Shao PL, Yang CC, Cheng BC. et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am J Transl Res. 2018;10:1053-70
125. Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719
126. Zhang R, Zhu Y, Li Y, Liu W, Yin L, Yin S. et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol Lett. 2020;42:669-79
127. Zhang Y, Huang H, Liu W, Liu S, Wang XY, Diao ZL. et al. Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death Dis. 2021;12:335
128. Li ZL, Lv LL, Tang TT, Wang B, Feng Y, Zhou LT. et al. HIF-1alpha inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388-404
129. Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL. et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J Am Soc Nephrol. 2018;29:919-35
130. Lv LL, Feng Y, Wu M, Wang B, Li ZL, Zhong X. et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020;27:210-26
131. Juan CX, Mao Y, Cao Q, Chen Y, Zhou LB, Li S. et al. Exosome-mediated pyroptosis of miR-93-TXNIP-NLRP3 leads to functional difference between M1 and M2 macrophages in sepsis-induced acute kidney injury. J Cell Mol Med. 2021;25:4786-99
132. Pan T, Jia P, Chen N, Fang Y, Liang Y, Guo M. et al. Delayed Remote Ischemic Preconditioning ConfersRenoprotection against Septic Acute Kidney Injury via Exosomal miR-21. Theranostics. 2019;9:405-23
133. Iba T, Ogura H. Role of extracellular vesicles in the development of sepsis-induced coagulopathy. J Intensive Care. 2018;6:68
134. Ma S, Evans RG, Iguchi N, Tare M, Parkington HC, Bellomo R. et al. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation. 2019;26:e12483
135. Liu Y, Luo T, Li H, Zhao X, Zhou M, Cheng M. Protective effect of endothelial progenitor cell-derived exosomal microRNA-382-3p on sepsis-induced organ damage and immune suppression in mice. Am J Transl Res. 2022;14:6856-73
136. Carter BL, Underwood J. Sepsis and the brain: a review for acute and general physicians. Clin Med (Lond). 2022;22:392-5
137. Pan S, Lv Z, Wang R, Shu H, Yuan S, Yu Y. et al. Sepsis-Induced Brain Dysfunction: Pathogenesis, Diagnosis, and Treatment. Oxid Med Cell Longev. 2022;2022:1328729
138. Sekino N, Selim M, Shehadah A. Sepsis-associated brain injury: underlying mechanisms and potential therapeutic strategies for acute and long-term cognitive impairments. J Neuroinflammation. 2022;19:101
139. Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K. et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med. 2016;8:1162-83
140. Wei XB, Jiang WQ, Zeng JH, Huang LQ, Ding HG, Jing YW. et al. Exosome-Derived lncRNA NEAT1 Exacerbates Sepsis-Associated Encephalopathy by Promoting Ferroptosis Through Regulating miR-9-5p/TFRC and GOT1 Axis. Mol Neurobiol. 2022;59:1954-69
141. Kim TS, Choi DH. Liver Dysfunction in Sepsis. Korean J Gastroenterol. 2020;75:182-7
142. Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33:498-510
143. Wang G, Jin S, Ling X, Li Y, Hu Y, Zhang Y. et al. Proteomic Profiling of LPS-Induced Macrophage-Derived Exosomes Indicates Their Involvement in Acute Liver Injury. Proteomics. 2019;19:e1800274
144. Wang G, Jin S, Huang W, Li Y, Wang J, Ling X. et al. LPS-induced macrophage HMGB1-loaded extracellular vesicles trigger hepatocyte pyroptosis by activating the NLRP3 inflammasome. Cell Death Discov. 2021;7:337
145. Cantaluppi V, Biancone L, Quercia A, Deregibus MC, Segoloni G, Camussi G. Rationale of mesenchymal stem cell therapy in kidney injury. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013;61:300-9
146. Zhou Q, Xie M, Zhu J, Yi Q, Tan B, Li Y. et al. PINK1 contained in huMSC-derived exosomes prevents cardiomyocyte mitochondrial calcium overload in sepsis via recovery of mitochondrial Ca(2+) efflux. Stem Cell Res Ther. 2021;12:269
147. Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K. et al. Exosomal miR-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci Rep. 2015;5:13721
148. Pei Y, Xie S, Li J, Jia B. Bone marrow-mesenchymal stem cell-derived exosomal microRNA-141 targets PTEN and activates beta-catenin to alleviate myocardial injury in septic mice. Immunopharmacol Immunotoxicol. 2021;43:584-93
149. Wang X, Wang Y, Kong M, Yang J. MiR-22-3p suppresses sepsis-induced acute kidney injury by targeting PTEN. Bioscience reports. 2020 40
150. Zou X, Gu D, Zhang G, Zhong L, Cheng Z, Liu G. et al. NK Cell Regulatory Property is Involved in the Protective Role of MSC-Derived Extracellular Vesicles in Renal Ischemic Reperfusion Injury. Human gene therapy. 2016;27:926-35
151. Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clinical practice. 2014;127:75-80
152. Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D. et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516-25
153. He Z, Wang H, Yue L. Endothelial progenitor cells-secreted extracellular vesicles containing microRNA-93-5p confer protection against sepsis-induced acute kidney injury via the KDM6B/H3K27me3/TNF-α axis. Experimental cell research. 2020;395:112173
154. Li H, Zhang X, Wang P, Zhou X, Liang H, Li C. Knockdown of circ-FANCA alleviates LPS-induced HK2 cell injury via targeting miR-93-5p/OXSR1 axis in septic acute kidney injury. Diabetol Metab Syndr. 2021;13:7
155. Park J, Kim S, Lim H, Liu A, Hu S, Lee J. et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74:43-50
156. Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA. et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem cells translational medicine. 2021;10:1279-87
157. Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X. et al. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering (Beijing, China). 2020;6:1153-61
Author contact
![]() Corresponding authors: Wen Yin, xjyyywedu.cn. Peng Zhao 147594244com.
Corresponding authors: Wen Yin, xjyyywedu.cn. Peng Zhao 147594244com.

 Global reach, higher impact
Global reach, higher impact