3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(12):1592-1599. doi:10.7150/ijms.87254 This issue Cite
Research Paper
Tumor necrosis factor-alpha mediates the negative association between telomere length and kidney dysfunction
Department of Endocrinology, Key Laboratory of Endocrinology, Ministry of Health, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, China.
# These authors contributed equally to this work and share first authorship.
Received 2023-6-17; Accepted 2023-9-8; Published 2023-9-25
Abstract
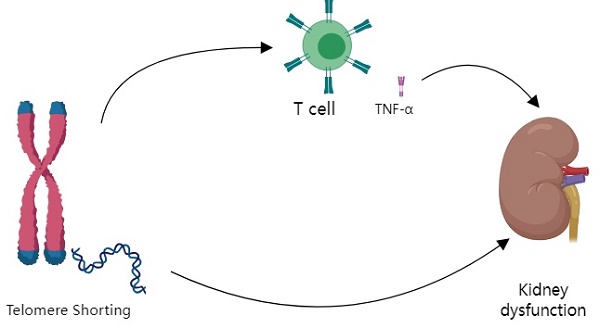
Aim/hypothesis: The relationship between peripheral blood leukocyte telomere length (LTL) and kidney dysfunction, especially in people with hypertension, remains unclear. No clinical study has explored the role of inflammation and oxidative stress in the relationship between LTL and kidney dysfunction. Therefore, we examined the relationship between baseline LTL and albuminuria progression and/or rapid renal function decline in Chinese patients with or without hypertension and investigated whether inflammation and oxidative stress played a mediating role in this relationship.
Methods: We conducted a prospective study including 262 patients in a 7-year follow-up period from 2014 to 2021. Data on LTL, inflammation, oxidative markers, renal function, and urine protein levels were assessed. Kidney dysfunction was defined as either albuminuria progression, rapid decline in renal function, or the composite endpoint (albuminuria progression and rapid decline in renal function). Logistic regression and simple mediation models were used for the analysis.
Results: In this cohort (mean age, 54.3±9.7 years; follow-up period, 5.9±1.1 years), 42(16.0%), 21(8.0%), and 59(22.5%) patients developed albuminuria progression, rapid eGFR decline, and the composite endpoint of kidney dysfunction, respectively. Logistic regression analysis showed that each standard deviation decrease of baseline LTL and the lower quartile (Q) of baseline LTL were significantly correlated with an increased risk of rapid decline in renal function (OR=1.83 [95% CI 1.07, 3.27] per 1SD, P=0.03; OR=7.57 [95% CI 1.25, 145.88] for Q1 vs. Q4, P for trend=0.031); and the composite endpoint of kidney dysfunction (OR=1.37 [95% CI 0.97, 1.96] per 1SD, borderline positive P=0.072; OR=2.96[95% CI 1.15, 8.2] for Q1 vs. Q4, P for trend=0.036). The mediating analysis showed that tumor necrosis factor (TNF)-a partly mediated the relationship between LTL and rapid decline in renal function (direct effect: β=0.046 95%CI [0.006, 0.090],P=0.02; indirect effect: β=0.013 95%CI [0.003, 0.020]), and the mediating proportion was 22.4%.In subgroup analyses, LTL was inversely associated with rapid decline in renal function or the composite endpoint of kidney dysfunction only in patients with hypertension (OR=49.07[3.72,211.12] vs.1.32[0.69,2.58] per 1SD, P for interaction=0.045;OR=3.10 [1.48, 7.52] vs.1.08[0.92,1.63] per 1SD, P for interaction=0.036).
Conclusion: Baseline LTL could independently predict kidney dysfunction at follow-up, especially in participants with hypertension. TNF-a partially mediated the negative association between LTL and kidney dysfunction.
Keywords: Leukocyte Telomere Length, Renal Dysfunction, Albuminuria, Renal Function Decline, Hypertension, TNF-α
Introduction
Telomeres are tandem repeats of the eukaryotic chromosomal terminal DNA sequence TTAGGG. Leukocyte telomere length (LTL) progressively shortens with each cell division, and telomeres attrition is a hallmark of cellular aging. Additionally, LTL has been associated with cardiovascular diseases, diabetes, cancer, and chronic kidney disease (CKD) [1].
LTL is associated with CKD and end-stage renal disease (ESKD) development, and albuminuria progression [2-6]. A Russian study showed a significant relationship between albuminuria levels and telomere length [7]. However, the association between LTL and the declining estimated glomerular filtration rate (eGFR) remains inconclusive. In recent years, genomic and epigenetic studies have found that telomere-related genes and pathways may be a novel biomarkers or therapeutic targets for kidney disease. to find can treat DKD [8]. Claire Hill et al. also found that differential methylation of telomere-related genes is associated with kidney disease in individuals with type 1 diabetes [9]. Olutope Arinola Akinnibosun et al. put forward that preserving telomere length as a potential therapy for CKD [10].
However, our previous study did not find the relationship between LTL and eGFR [11]. A study in the United States showed that decreased kidney function was associated with shorter telomere length at baseline and faster telomere shortening. However, these associations are entirely explained by advancing age [12]. The rate of eGFR decline is a useful indicator of kidney dysfunction; and an annual eGFR decline of 3.3% or more, defined as 'rapid decline,' has been associated with a higher risk of developing ESKD [13]. Prospective studies have reported a high incidence of a rapid decline in eGFR in patients with diabetes with short telomeres [14]. However, in another prospective study during 8 years of follow-up, buccal telomere length was not associated with the changing rate of eGFR [15].
The most common causes of CKD are poorly controlled diabetes and hypertension [16]. However, the relationship between LTL and kidney dysfunction has mostly been studied in general populations and participants with either diabetes, heart failure, or cardiovascular risk [3, 17-19]. Hypertension causes small renal artery sclerosis, interstitial inflammatory fibrosis, and tubular atrophy or loss, thereby resulting in renal damage or decreased renal function [20]. A meta-analysis of 16 studies showed that patients with hypertension and prehypertension have an increased risk of decreased eGFR [21]. However, the relationship between LTL and kidney dysfunction, a leading cause of CKD, has not been studied in participants with hypertension.
Moreover, the mechanisms underlying the association between LTL and kidney dysfunction remain unclear. Some animal studies have shown that chronic inflammation and oxidative stress play important roles in LTL shortening as well as kidney dysfunction [22-24]. Telomere dysfunction is associated with impaired mitochondrial biogenesis and function, increased reactive oxygen species and inflammatory immune response, leading to kidney dysfunction [25, 26]. However, previous clinical studies that reported the relationship between LTL and kidney dysfunction failed to explore the role of inflammation and oxidative stress because of the lack of data on these indices [5, 19]. Therefore, it is necessary to examine these markers to study the role of inflammation and oxidative stress between LTL and kidney dysfunction.
The purpose of our study was to examine the relationship between baseline LTL and albuminuria progression and/or rapid renal function decline during follow-up in patients with or without hypertension in rural Chinese communities and to investigate whether inflammation plays a mediating role in this relationship.
Methods
Study population
The current prospective study, which included a Chinese cohort from the suburb of Changping, Beijing, was conducted between March 2014 and July 2021, during a mean follow-up of 5.97±1.16 years. The research protocol and consent procedures were approved by the Ethics Committee of the Peking Union Medical College Hospital. All the participants provided written informed consent. After excluding participants who lacked baseline LTL results, albuminuria results, serum creatinine results, and had a baseline urine albumin-creatinine ratio [ACR] ≥300 mg/g Cr or eGFR <60 mL/min/1.73 m2, 262 participants were included in this study.
Demographic data collected at baseline included age; sex; history of use of anti-glycemic, antihypertensive, and anti-lipid drugs; and history of diabetes, hypertension, and hyperlipidemia. Anthropometric data, including height, weight, waist circumference (WC), hip circumference, systolic blood pressure (SBP), and diastolic blood pressure (DBP), were collected. The formula for calculating the body mass index (BMI) was height (in meter)/weight (in kilogram) squared. Overweight and obesity were defined as 24 kg/m2 ≤BMI <28 kg/m2 and BMI ≥ 28 kg/m2, respectively.
Urine and blood samples were collected from participants during follow-up to assess albuminuria status and renal function progression. According to the World Health Organization criteria, normal glucose tolerance (NGT) was identified using the 75-g oral glucose tolerance test: fasting plasma glucose (FPG) <6.1 mmol/L and 2-hour post-load plasma glucose (2h-PG) <7.8 mmol/L. Pre-diabetes was indicated by impaired fasting glucose measured as 6.1 mmol/L ≤ FPG <7.0 mmol/L and 2h-PG <7.8 mmol/L, impaired glucose tolerance (IGT) measured as 7.8 ≤2h-PG <11.1 mmol/L and FPG <6.1 mmol/L, or both impaired fasting glucose and IGT. Diabetes was indicated by FPG ≥7.0 mmol/L or 2h-PG ≥11.1 mmol/L.
Calculations of eGFR and ACR
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, based on the measurement of serum creatinine and anthropometric data, such as age and sex. ACR was calculated based on urine microalbumin and urine creatinine levels. The equations are as follows:
eGFR=142 × min (standardized Scr/K,1) α × max (standardized Scr/K,1)-1.200 × 0.9938Age × 1.012 [if female] mL/min/1.73 m2
where K=0.7 (female) or 0.9 (male), α= -0.241 (female) or -0.302 (male), and ACR=urinary albumin/urinary creatinine (mg/g Cr).
Measurement of LTL
LTL assays were performed using the blood samples collected at baseline. The details of the LTL measurements are described in our previous publication [27]. LTL was determined, as the ratio of the telomere repeat copy number to the single copy number (T/S ratio), using a monochrome multiplex quantitative polymerase chain reaction protocol. z scores of log transformed LTL were calculated.
Assessment of oxidative stress and inflammatory markers
Oxidative stress and inflammatory marker levels were measured using the blood samples collected at baseline. TNF-α, interleukine-6(IL-6), 8-hydroxy-2 deoxyguanosine(8-OHDG); superoxide dismutase (SOD); glutathione reductase (GR) concentrations were determined using an ELISA kit (Cloud-Clone Corp, Houston, USA) by the Beijing Institute of Biotechnology.
Definition of hypertension
Hypertension was defined either as having been diagnosed with hypertension, taking antihypertensive drugs, or SBP ≥140 mmHg and DBP ≥90 mmHg at rest.
Definition of the outcomes
Kidney dysfunction was defined according to the following: (1) A rapid decline in eGFR: A rapid decline in eGFR was defined as decline in eGFR of ≥3.3% per year [28, 29]. Percentage change per year of eGFR was calculated as follows: a total of 262 participants with at least two eGFR measurements during follow-up were included in the calculation of the eGFR slope. Linear mixed-effects regression was used to calculate the eGFR slope for each individual, which was then re-expressed as the percentage change per year of eGFR. (2) Albuminuria progression was defined as the development of either microalbuminuria or macroalbuminuria from normal albuminuria at baseline, or macroalbuminuria from microalbuminuria at baseline. (3) The composite endpoint of kidney dysfunction:a rapid decline in eGFR and/or albuminuria progression.
Statistical analysis
Participants were divided into quartiles (Q) based on their baseline LTL levels. Continuous variables with normal distribution are expressed as mean ± standard deviations (SDs), whereas non-normally distributed variables are presented as median and interquartile range. Categorical variables are expressed as numbers with corresponding percentages. Comparisons between groups were performed using a one-way analysis of variance for continuous variables and the χ2 test for categorical variables. Bonferroni correction was used for post-hoc comparisons.
The associations between LTL and the outcomes were analyzed using logistic regression analysis, with odds ratios (OR) and associated 95% confidence intervals (CIs) computed for quartiles of baseline LTL and one SD change in baseline LTL. For the computation of ORs for one SD decrease in baseline LTL, a minus z-score for log transformed LTL was calculated for each participant. Four models were generated: Model 1: not adjusted. Model 2: adjusted for age, sex. Model 3: adjusted for Model 2 +BMI, HbA1c, SBP, DBP, LDL-C, TG, UA. Model 4: adjusted for Model 3+ baseline ACR,baseline eGFR.
To explore whether oxidative stress and inflammatory markers mediated the effect of LTL on kidney dysfunction, R package(mediation) was used for mediation analysis, and all mediation models were adjusted for age, sex, BMI, HbA1c, SBP, DBP, LDL-C, TG, UA, baseline ACR and baseline eGFR.
A two-sided P<0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corporation, Armonk, NY, USA) and R for Windows, version 4.2.3.
Results
Baseline characteristics of the study populations
The baseline characteristics of participants are shown in Table 1. Participants in the lower LTL quartiles had a lower eGFR, higher TNF-a and higher SOD than those in the other quartiles (P<0.05). However, age, sex, BMI, WHR, SBP, DBP, FPG, HbA1C TC, TG, HDL-C, LDL-C, IL-6, 8-OHdG, and GR did not differ between the four groups (P>0.05). Additionally, the percentages of participants with microalbuminuria, diabetes, hypertension, hyperlipidemia, hyperuricemia, and obesity were not significant different between groups (all P>0.05).
Associations of baseline LTL with the risk of albuminuria progression and/or rapid decline in renal function
During the follow-up period, 42 (16.0%) participants developed albuminuria progression,21 (8.0%) participants had a rapid decline in renal function,and 59 (22.5%) participants reached the composite endpoint of kidney dysfunction (albuminuria progression and /or rapid decline in renal function). Associations of baseline LTL with the risk of albuminuria progression and/or rapid decline in renal function are shown in Table 2. After adjusting for traditional risk factors, LTL was significantly inversely correlated with rapid decline in renal function and the composite endpoint of kidney dysfunction, but not with albuminuria progression.
Baseline characteristics of participants by LTL quartiles.
| Total(n=262) | Q1 (n=65) | Q2 (n=66) | Q3 (n=65) | Q4 (n=66) | P | |
|---|---|---|---|---|---|---|
| Age, years | 54.3±9.7 | 54.1±10.1 | 54.2±10.3 | 54.5±9.5 | 54.4±9.1 | 0.998 |
| Male, n(%) | 78(29.8) | 21(32.3) | 19 (28.8) | 21 (32.3) | 17 (25.8) | 0.816 |
| BMI, kg/m2 | 26.2±4.1 | 26.1±4.4 | 26.0±4.3 | 26.3±4.0 | 26.5±4.0 | 0.878 |
| WHR | 0.95±0.14 | 0.95±0.02 | 0.95±0.02 | 0.94±0.02 | 0.98±0.27 | 0.454 |
| SBP, mmHg | 128.3±19.6 | 129.8±18.9 | 126.7±15.0 | 129.0±21.0 | 127.9±22.9 | 0.822 |
| DBP, mmHg | 76.2±10.2 | 78.0±9.2 | 75.3±11.0 | 76.8±9.5 | 74.9±11.0 | 0.294 |
| FPG, mmol/L | 6.6±2.0 | 6.6±2.4 | 6.4±1.4 | 7.0±1.9 | 6.5±2.2 | 0.397 |
| HbA1c, % | 5.9±1.1 | 6.0±1.3 | 5.8±0.8 | 6.1±1.2 | 5.7±0.9 | 0.134 |
| TC, mmol/L | 5.5±1.1 | 5.5±1.0 | 5.5±1.2 | 5.6±0.9 | 5.5±1.1 | 0.958 |
| TG, mmol/L | 1.4(1.0,2.1) | 1.4 (1.1, 2.1) | 1.3 (0.9, 2.4) | 1.5 (1.1, 1.9) | 1.5 (1.0, 2.2) | 0.807 |
| HDL-C, mmol/L | 1.3±0.4 | 1.4±0.7 | 1.3±0.3 | 1.3±0.2 | 1.3±0.3 | 0.337 |
| LDL-C, mmol/L | 2.9±0.7 | 2.9±0.7 | 2.8±0.9 | 2.9±0.7 | 2.9±0.7 | 0.948 |
| eGFR, mL/min/1.73 m2 | 96.9±14.3 | 93.3±15.8 | 101.0±13.6 | 97.5±12.2 | 95.7±14.4 | 0.015* |
| ACR, mg/g Cr | 11.7(6.9,23.5) | 8.9(5.4,16.7) | 13.6 (6.9, 30.1) | 12.3 (6.9, 25.9) | 11.9 (7.6, 18.4) | 0.139 |
| Microalbuminuria, n (%) | 48(18.3) | 10 (15.4) | 17 (25.8) | 13 (20.0) | 8 (12.1) | 0.201 |
| Diabetes, n (%) | 158(60.3) | 33 (50.8) | 40 (60.6) | 46 (70.8) | 39 (59.1) | 0.173 |
| Hypertension, n (%) | 81(30.9) | 22 (33.8) | 19 (28.8) | 21 (32.3) | 19 (28.8) | 0.896 |
| Hyperlipidemia, n (%) | 164(62.6) | 38 (58.5) | 40 (60.6) | 42 (64.6) | 44 (66.7) | 0.730 |
| Hyperuricemia, n (%) | 26(9.9) | 8 (12.3) | 9 (13.6) | 5 (7.7) | 4 (6.1) | 0.416 |
| Obesity, n (%) | 77(29.4) | 21 (32.3) | 20 (30.3) | 15 (23.1) | 21 (31.8) | 0.651 |
| TNF-a, pmol/L | 24.1±10.8 | 28.3±9.0 | 26.3±11.8 | 22.5±10.4 | 19.2±9.5 | <0.001* |
| IL-6, pg/mL | 3.2(2.0,4.6) | 3.2 (1.9,4.5) | 3.4(2.6,4.4) | 2.8(1.8,4.1) | 3.5(2.0,6.1) | 0.096 |
| 8OHdG, pg/mL | 38.6(20.7,58.3) | 37.3(22.6,56.3) | 38.6(16.6,65.1) | 35.1(20.7,53.4) | 43.8(26.6,64.4) | 0.473 |
| SOD, U/mL | 60.6±18.6 | 64.5±16.5 | 65.0±19.4 | 58.6±16.0 | 54.4±20.6 | 0.002* |
| GR, U/L | 6.4(4.8,9.6) | 6.4(4.8, 9.6) | 6.4(4.8, 9.6) | 6.4(3.2,9.6) | 6.4(3.2, 9.6) | 0.813 |
| Lg(LTL), Z score | 0.00±1.00 | -1.23±0.54 | -0.32±0.15 | 0.29±0.20 | 1.26±0.59 | <0.001* |
Data are presented as mean±standard deviation or median (Q1, Q3) for skewed variables or proportion of participants (%), as appropriate. *Statistically significant
LTL, leukocyte telomere length; Q, quartile; BMI, body mass index; WHR, waist-to-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; ACR, urine albumin-creatinine ratio; TNF-a, tumor necrosis factor-a; IL-6, interleukine-6; 8-OHdG, 8-hydroxy-2 deoxyguanosine; SOD, superoxide dismutase; GR, glutathione reductase
The association between baseline LTL and the risk of albuminuria progression and/or rapid decline in renal function.
| Albuminuria progression(n=262) | Rapid decline in renal function(n=262) | Composite endpoint(n=262) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P for trend | OR (95% CI) | P for trend | OR (95% CI) | P for trend | ||||||||||
| Model | Per 1SD | Q1 vs. Q4 | Q2 vs. Q4 | Q3 vs. Q4 | Q3 vs. Q4 | Q1 vs. Q4 | Q2 vs.Q4 | Q3 vs. Q4 | Per 1SD | Q1 vs. Q4 | Q2 vs.Q4 | Q3 vs. Q4 | |||
| 1 | 1.21 (0.85,1.74) | 2.17 (0.82,6.20) | 1.14 (0.38,3.46) | 1.44 (0.50,4.31) | 0.173 | 1.76 (0.67,4.84) | 10.76 (1.92,201.84) | 8.87 (1.55,167.27) | 3.32 (0.41,68.34) | 0.004 | 1.42 (1.04,1.97) | 3.12 (1.28,8.24) | 2.10 (0.83,5.64) | 1.76 (0.67,4.84) | 0.013 |
| 2 | 1.23 (0.85,1.78) | 2.42 (0.90,7.03) | 1.21 (0.40,3.71) | 1.57 (0.54,4.78) | 0.128 | 1.89 (0.71,5.27) | 11.33 (2.00,213.55) | 9.00 (1.56,170.44) | 3.32 (0.40,68.53) | 0.004 | 1.45 (1.05,2.02) | 3.46 (1.40,9.26) | 2.24 (0.88,6.06) | 1.89 (0.71,5.27) | 0.008 |
| 3 | 1.19 (0.81,1.74) | 2.21 (0.79,6.65) | 1.09 (0.34,3.46) | 1.61 (0.54,4.99) | 0.211 | 1.80 (0.66,5.08) | 7.72 (1.28, 148.75) | 6.73 (1.10,130.39) | 3.20 (0.38,67.13) | 0.029 | 1.34 (0.96,1.89) | 2.86 (1.12,7.83) | 1.89 (0.72,5.26) | 1.80 (0.66,5.08) | 0.036 |
| 4 | 1.20 (0.82,1.79) | 2.31 (0.81,7.03) | 0.93 (0.29,3.03) | 1.51 (0.50,4.73) | 0.204 | 1.71 (0.62,4.87) | 7.57 (1.25, 145.88) | 5.93 (0.92,117.02) | 3.02 (0.35,63.64) | 0.031 | 1.37 (0.97,1.96) | 2.96 (1.15,8.20) | 1.61 (0.60,4.56) | 1.71 (0.62,4.87) | 0.036 |
Model 1: not adjusted. Model 2: adjusted for age, sex. Model 3: adjusted for Model 2 +BMI,HbA1c,SBP,DBP,LDL-C,TG,UA. Model 4: adjusted for Model 3+ baseline ACR,baseline eGFR. LTL, leukocyte telomere length; Q, quartile; OR, odds ratio; CI confidence interval. Bold font indicates statistical differences.
With each 1 SD reduction in LTL, the risk of rapid decline in renal function increased by 83% (OR=1.83 [95% CI 1.07, 3.27]; P=0.03) (Table 2). When LTL was modeled as quartiles, the ORs of rapid decline in renal function increased with LTL quartiles decreasing; and the OR was 7.57 for Q1 versus Q4 of LTL in the fully adjusted Model 4 ([95% CI 1.25, 145.88], P for trend=0.031) (Table 2).
With each 1 SD decline in LTL, the risk of composite endpoint of kidney dysfunction increased by 37% (OR=1.37 [95% CI 0.97, 1.96]; borderline positive P=0.072), and the OR was 2.96 for Q1 versus Q4 of LTL ([95% CI 1.15, 8.2], P for trend=0.036) (Tables 2).
Mediation models of the association among inflammation and oxidative stress indicators, LTL, and kidney outcome
We further tested whether inflammation and oxidative stress indicators mediated the association between LTL and rapid decline in renal function or the composite endpoint of kidney dysfunction.
In the mediation analysis, LTL was found to have both significant direct and indirect effects on rapid decline in renal function (direct effect: β=0.046 95%CI [0.006, 0.090)]; indirect effect: β=0.013 95%CI [0.003, 0.020]), whereas TNF-α partly mediated this negative effect of LTL on rapid decline in renal function and the proportion was 22.4% (Table 3). Mediation analysis between LTL and the composite endpoint of kidney dysfunction did not reach a significant level (Table 3). Moreover, we also tested other inflammation and oxidative stress indicators including IL-6, 8-OHdG, SOD and GR, and did not find these indicators played a mediating role in the associations (Supplementary Table 1).
Associations of baseline LTL with the risk of albuminuria progression and/ or rapid decline in renal function in participants with or without hypertension
Among 262 participants, 81 had hypertension. Subgroup analysis showed that the negative associations between LTL and rapid decline in renal function or the composite endpoint of kidney dysfunction were only significant in participants with hypertension after adjusting for confounding factors (OR=49.07 [95%CI 3.72, 211.12], P for interaction=0.045; OR=3.10 [95%CI 1.48, 7.52], P for interaction=0.036) (Table 4). The associations between LTL and albuminuria progression was not significant neither in participants with hypertension or without hypertension.
Mediation models of the association among TNF-a, LTL, and kidney outcome.
| Rapid decline in renal function | Composite endpoint(n=59) | |||
|---|---|---|---|---|
| Estimate (95%CI) | p value | Estimate (95%CI) | p value | |
| All participants | ||||
| Total effect | 0.059 (0.019,0.110) | <0.001 | 0.058 (0.008,0.110) | <0.001 |
| Indirect effect | 0.013 (0.003,0.020) | 0.040 | 0.009 (-0.003,0.020) | 0.200 |
| Direct effect | 0.046 (0.006,0.090) | <0.001 | 0.048 (-0.004,0.100) | 0.080 |
| Prop. Mediated | 0.224 (0.040,0.680) | 0.040 | 0.181 (-0.045,1.570) | 0.200 |
| Participants with hypertension | ||||
| Total effect | -0.002 (-0.164,0.200) | 0.960 | 0.147 (0.047,0.230) | <0.001 |
| Indirect effect | -0.001 (-0.080,0.050) | 0.800 | 0.005 (-0.023,0.030) | 0.640 |
| Direct effect | -0.001 (-0.116,0.160) | 0.920 | 0.141 (0.050,0.230) | <0.001 |
| Prop. Mediated | 0.206 (0.000,0.540) | 0.040 | 0.030 (-0.135,0.330) | 0.640 |
Models were adjusted for age, sex,BMI,HbA1c,SBP,DBP,LDL-C,TG,UA,baseline ACR and baseline eGFR. Bold font indicates statistical differences.
The association between per 1 SD decrease of baseline LTL and the risk of albuminuria progression and/or rapid decline in renal function in participants with or without hypertension
| Albuminuria progression(n=262) | Rapid decline in renal function(n=262) | Composite endpoint(n=262) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI), P | P for interaction | OR (95% CI), P | P for interaction | OR (95% CI), P | P for interaction | ||||
| Model | With hypertension | Without hypertension | With hypertension | Without hypertension | With hypertension | Without hypertension | |||
| 1 | 1.85(0.97,3.89) 0.075 | 0.99(0.65,1.54) 0.995 | 0.131 | 5.49(1.91,23.39) 0.006 | 1.44(0.82,2.61) 0.206 | 0.051 | 2.77(1.45,6.04) 0.004 | 1.11(0.76,1.62) 0.580 | 0.024 |
| 2 | 1.90(0.96,4.12) 0.076 | 1.02(0.66,1.60) 0.908 | 0.164 | 7.50(2.30,40.46) 0.004 | 1.44(0.82,2.60) 0.209 | 0.037 | 2.99(1.52,6.73) 0.003 | 1.13(0.78,1.66) 0.516 | 0.026 |
| 3 | 2.06(0.98,4.77) 0.066 | 1.01(0.65,1.60) 0.937 | 0.172 | 11.27(2.33,182.38) 0.017 | 1.33(0.73,2.45) 0.349 | 0.047 | 2.87(1.42,6.55) 0.005 | 1.06(0.72,1.57) 0.754 | 0.034 |
| 4 | 2.00(0.92,4.76) 0.090 | 1.02(0.65,1.65) 0.905 | 0.185 | 49.07(3.72,211.12) 0.041 | 1.32(0.69,2.58) 0.391 | 0.045 | 3.10(1.48,7.52) 0.005 | 1.08(0.72,1.63) 0.691 | 0.036 |
Model 1: not adjusted. Model 2: adjusted for age, sex. Model 3: adjusted for Model 2 +BMI,HbA1c,SBP,DBP,LDL-C,TG,UA. Model 4: adjusted for Model 3+ baseline ACR,baseline eGFR. LTL, leukocyte telomere length; OR, odds ratio; CI confidence interval. Bold font indicates statistical differences.
In addition, we also conducted mediation analysis among TNF-a, LTL and rapid decline in renal function or the composite endpoint of kidney dysfunction in participants with hypertension, and no mediating effects were found by TNF-a (Table 3).
Discussion
In this prospective study of 262 adults in a rural Chinese community, we demonstrated an independent negative association between baseline LTL and kidney dysfunction (including rapid decline in renal function and the composite endpoint of kidney dysfunction) during follow-up. Additionally, we showed that TNF-α partly mediated the association between LTL and rapid renal function decline. Lastly, we found a negative association between baseline LTL and kidney dysfunction (rapid decline in renal function and the composite endpoint of kidney dysfunction), which remained significant only in participants with hypertension in the subgroup analysis.
This study posed three central questions: 1) whether there was an association between LTL and kidney dysfunction; 2) whether oxidative stress and inflammation played a mediating role in the association between LTL and kidney dysfunction; and 3) whether the association between baseline LTL and kidney dysfunction was affected by hypertension status.
For the first question, we found a negative association between LTL and the composite endpoint of kidney dysfunction, which is consistent with previous studies. A prospective study including 132 individuals with type 1 diabetes and another study including 691 individuals with type 2 diabetes showed that telomere length is associated with albuminuria progression [6, 30]. In addition, an American study with 889 participants (a third of whom had diabetes) and another study with 4085 participants with type 2 diabetes showed that LTL was negatively associated with CKD progression (defined as advanced renal failure or requiring renal replacement therapy [dialysis treatment or kidney transplantation]) [3, 14]. Furthermore, we also found a negative relationship between LTL and rapid decline in renal function, a finding that was not always consistent with that of previous studies. The Mild to Moderate Kidney Disease (MMKD) study including 166 patients with CKD at a median follow-up of 4.5 years found that LTL was shorter in patients who exhibited doubling of baseline serum creatinine levels [30]. Another study including 3964 participants at a follow-up of 8 years found that participants with shorter buccal telomere lengths were more likely to have a normal-to-impaired kidney function trajectory [15]. Recently, a large and long-term follow-up study of 4085 Chinese individuals with type 2 diabetes showed robust results, indicating that shorter LTL at baseline is associated with a rapid decline in eGFR (>4% per year) during 14 years follow-up [3], which directly supports our results. Similarly, However, a study including 151 patients with type 1 diabetes and a follow-up of 11.1 years did not find any association between telomere length and the rate of decline in eGFR [15]. Similarly, another study found that telomere length was not associated with the rate of change in eGFR over a follow-up of 8 years in healthy individuals [31]. However, other studies have found that telomere length is associated with decreased eGFR and lower mortality in patients with CKD, and telomere therapy may be useful for the treatment of CKD [10, 32].
Regarding the second study question, our result suggests that LTL shortening increases the risk of rapid decline in renal function, in which TNF-a plays a mediating role. The relationship between telomeres and inflammation is complex. Inflammation can lead to telomeres dysfunction and cause telomeres shortening [33, 34], whereas telomeres shortening can cause an inflammatory response [35]. In in vitro experiments, the cells with the shortest telomere exhibited the highest levels of TNF-a expression [36]. Animal studies have also shown that TNF-a levels are increased in animals with shorter telomeres [37]. An in vitro experiment revealed that short telomeres can mediate additional downstream activation of ZBP1(S), via TERRA, to form ZBP1 filaments on the mitochondria, thereby promoting the inflammatory cycle [38]. In contrast, other animal experiments have shown that inflammation and oxidative stress significantly contribute to renal damage associated with hypertension, and cytokines, such as interferon-γ, TNF-a, and IL-17, affect Na+/H+ exchangers in the kidney [22]. In addition, blocking TNF-a using a murine anti-TNF-a antibody confers kidney protection compared to that in a vehicle-treated Ins2Akita (with diabetes) mice [24]. Thus, we hypothesized that the adverse effect of telomeres shortening on mitochondrial function might worsen inflammation, increase TNF-a levels, and accelerate kidney dysfunction. Our study indicates that TNF-a might mediate the negative relationship between LTL and kidney dysfunction from a clinical perspective. Further studies are required to clarify the details of these mechanisms, for example, epigenetic analysis. Epigenetics has previously been used to highlight a correlation between telomere length and decreased kidney function [39]. Kidney function is reduced in CKD patients with shorter telomere length [4].
Regarding the last question, we found a more prominent association between shorter LTL and the risk of kidney dysfunction in participants with hypertension than that in participants without hypertension. Hypertension increases the risk of decreased eGFR, blood pressure is an important predictor of early renal function decline [40, 41], and oxidative stress and inflammation might play important roles in these outcomes [42]. Although no studies have reported such results, a previous study showed that the relationship between LTL and CKD progression is stronger in smokers and those with diabetes who are more likely to have higher inflammatory and oxidative stress statuses [30]. Thus, together with the previous study, our results suggest that renal function may not respond to LTL per se, but to the risk factors inflammation and oxidative stress, which are influenced by LTL shortening. LTL could be a useful predictor of renal dysfunction in patients with hypertension. In the future, Mendelian Randomisation may be helpful to explain the greater relationship between LTL and kidney dysfunction in hypertensive patients.
Our study has several strengths. First, our analysis focused on the relationship between LTL and kidney dysfunction in participants with hypertension, for which there are few related studies. Second, we analyzed the role of inflammatory stress in the LTL-CKD link and found that TNF-a mediates the effect between LTL and kidney dysfunction. However, our study has some drawbacks. This study included a small sample size and could not draw causal conclusions because of the study design. Future studies will expand the sample size and extend the follow-up period.
Conclusion
LTL was negatively associated with the kidney dysfunction especially rapid decline in renal function during follow-up. TNF-a partially mediates the association between LTL and rapid decline in renal function. The association between LTL and kidney dysfunction is more pronounced in patients with hypertension. Therefore, LTL may be an important clinical predictor of kidney dysfunction in patients with hypertension. Future studies should be conducted using a larger population to better understand the role of LTL in changes in kidney function.
Supplementary Material
Supplementary figures and table.
Acknowledgements
Funding
This research was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS2021-I2M-1-002) and National Natural Science Foundation of China (Grant No. 82100947).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
The experiments complied with the current laws of China, including ethical approval for the clinical study. All phases of the study complied with the Ethical Principles for Medical Research Involving Human Subjects as expressed in the Declaration of Helsinki.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Cheng F, Carroll L, Joglekar MV, Januszewski AS, Wong KK, Hardikar AA. et al. Diabetes, metabolic disease, and telomere length. The Lancet Diabetes & Endocrinology. 2021;9:117-26
2. Sun Q, Liu J, Cheng G, Dai M, Liu J, Qi Z. et al. The telomerase gene polymorphisms, but not telomere length, increase susceptibility to primary glomerulonephritis/end stage renal diseases in females. J Transl Med. 2020;18:184
3. Cheng F, Luk AO, Wu H, Tam CHT, Lim CKP, Fan B. et al. Relative leucocyte telomere length is associated with incident end-stage kidney disease and rapid decline of kidney function in type 2 diabetes: analysis from the Hong Kong Diabetes Register. Diabetologia. 2022;65:375-86
4. Park S, Lee S, Kim Y, Cho S, Kim K, Kim YC. et al. A Mendelian randomization study found causal linkage between telomere attrition and chronic kidney disease. Kidney Int. 2021;100:1063-70
5. Tentolouris N, Nzietchueng R, Cattan V, Poitevin G, Lacolley P, Papazafiropoulou A. et al. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30:2909-15
6. Fyhrquist F, Tiitu A, Saijonmaa O, Forsblom C, Groop PH. Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. Journal of internal medicine. 2010;267:278-86
7. Pykhtina VS, Strazhesko ID, Tkacheva ON, Akasheva DU, Dudinskaya EN, Vygodin VA. et al. Association of renal function, telomere length and markers of chronic inflammation for patients without chronic kidney and cardiovascular diseases. Advances in gerontology = Uspekhi gerontologii. 2016;29:79-85
8. Hill C, Duffy S, Coulter T, Maxwell AP, McKnight AJ. Harnessing Genomic Analysis to Explore the Role of Telomeres in the Pathogenesis and Progression of Diabetic Kidney Disease. Genes. 2023 14
9. Hill C, Duffy S, Kettyle LM, McGlynn L, Sandholm N, Salem RM. et al. Differential Methylation of Telomere-Related Genes Is Associated with Kidney Disease in Individuals with Type 1 Diabetes. Genes. 2023 14
10. Akinnibosun OA, Maier MC, Eales J, Tomaszewski M, Charchar FJ. Telomere therapy for chronic kidney disease. Epigenomics. 2022;14:1039-54
11. Ma C, He S, Li P, Zhang H, Li W, Li Y. Negative Association between Caloric Intake and Estimated Glomerular Filtration Rate in a Chinese Population: Mediation Models Involving Mitochondrial Function. Gerontology. 2020;66:439-46
12. Bansal N, Whooley MA, Regan M, McCulloch CE, Ix JH, Epel E. et al. Association between kidney function and telomere length: the heart and soul study. American journal of nephrology. 2012;36:405-11
13. Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH. et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353-61
14. Raschenberger J, Kollerits B, Ritchie J, Lane B, Kalra PA, Ritz E. et al. Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Sci Rep. 2015;5:11887
15. Westbrook A, Zhang R, Shi M, Razavi AC, Huang Z, Chen J. et al. Association Between Baseline Buccal Telomere Length and Progression of Kidney Function: The Health and Retirement Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2022;77:471-6
16. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of internal medicine. 2013;158:825-30
17. Mazidi M, Rezaie P, Covic A, Malyszko J, Rysz J, Kengne AP. et al. Telomere attrition, kidney function, and prevalent chronic kidney disease in the United States. Oncotarget. 2017;8:80175-81
18. Eguchi K, Honig LS, Lee JH, Hoshide S, Kario K. Short telomere length is associated with renal impairment in Japanese subjects with cardiovascular risk. PLoS One. 2017;12:e0176138
19. van der Harst P, Wong LS, de Boer RA, Brouilette SW, van der Steege G, Voors AA. et al. Possible association between telomere length and renal dysfunction in patients with chronic heart failure. The American journal of cardiology. 2008;102:207-10
20. Huang M, Matsushita K, Sang Y, Ballew SH, Astor BC, Coresh J. Association of kidney function and albuminuria with prevalent and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65:58-66
21. Garofalo C, Borrelli S, Pacilio M, Minutolo R, Chiodini P, De Nicola L. et al. Hypertension and Prehypertension and Prediction of Development of Decreased Estimated GFR in the General Population: A Meta-analysis of Cohort Studies. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;67:89-97
22. Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64:1208-13
23. Maekawa T, Liu B, Nakai D, Yoshida K, Nakamura KI, Yasukawa M. et al. ATF7 mediates TNF-α-induced telomere shortening. Nucleic acids research. 2018;46:4487-504
24. Awad AS, You H, Gao T, Cooper TK, Nedospasov SA, Vacher J. et al. Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int. 2015;88:722-33
25. Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359-65
26. Nassour J, Aguiar LG, Correia A, Schmidt TT, Mainz L, Przetocka S. et al. Telomere-to-mitochondria signalling by ZBP1 mediates replicative crisis. Nature. 2023;614:767-73
27. Zhou M, Zhu L, Cui X, Feng L, Zhao X, He S. et al. Influence of diet on leukocyte telomere length, markers of inflammation and oxidative stress in individuals with varied glucose tolerance: a Chinese population study. Nutr J. 2016;15:39
28. Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A. et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33:1337-43
29. Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH. et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014;37:226-34
30. Gurung RL, M Y, Liu S, Liu JJ, Lim SC. Short Leukocyte Telomere Length Predicts Albuminuria Progression in Individuals With Type 2 Diabetes. Kidney Int Rep. 2018;3:592-601
31. Astrup AS, Tarnow L, Jorsal A, Lajer M, Nzietchueng R, Benetos A. et al. Telomere length predicts all-cause mortality in patients with type 1 diabetes. Diabetologia. 2010;53:45-8
32. Fazzini F, Lamina C, Raschenberger J, Schultheiss UT, Kotsis F, Schönherr S. et al. Results from the German Chronic Kidney Disease (GCKD) study support association of relative telomere length with mortality in a large cohort of patients with moderate chronic kidney disease. Kidney Int. 2020;98:488-97
33. Saretzki G, Murphy MP, von Zglinicki T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging cell. 2003;2:141-3
34. Saretzki G. Telomerase, mitochondria and oxidative stress. Experimental gerontology. 2009;44:485-92
35. Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual review of pathology. 2010;5:99-118
36. Ong SM, Hadadi E, Dang TM, Yeap WH, Tan CT, Ng TP. et al. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell death & disease. 2018;9:266
37. Lex K, Maia Gil M, Lopes-Bastos B, Figueira M, Marzullo M, Giannetti K. et al. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:15066-74
38. Nassour J, Aguiar LG, Correia A, Schmidt TT, Mainz L, Przetocka S. et al. Telomere-to-mitochondria signalling by ZBP1 mediates replicative crisis. Nature. 2023
39. Gurung RL, Dorajoo R, M Y, Wang L, Liu S, Liu JJ. et al. Association of leukocyte telomere length with chronic kidney disease in East Asians with type 2 diabetes: a Mendelian randomization study. Clinical kidney journal. 2021;14:2371-6
40. Fan F, Qi L, Jia J, Xu X, Liu Y, Yang Y. et al. Noninvasive Central Systolic Blood Pressure Is More Strongly Related to Kidney Function Decline Than Peripheral Systolic Blood Pressure in a Chinese Community-Based Population. Hypertension (Dallas, Tex: 1979). 2016;67:1166-72
41. Chang TI, Lim H, Park CH, Rhee CM, Moradi H, Kalantar-Zadeh K. et al. Associations of Systolic Blood Pressure With Incident CKD G3-G5: A Cohort Study of South Korean Adults. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2020;76:224-32
42. Verma MK, Jaiswal A, Sharma P, Kumar P, Singh AN. Oxidative stress and biomarker of TNF-α, MDA and FRAP in hypertension. Journal of medicine and life. 2019;12:253-9
Author contact
![]() Corresponding author: Yuxiu Li, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Shuaifuyuan No.1, Dongcheng District, Beijing, 100730, China; Email: liyuxiucom.cn
Corresponding author: Yuxiu Li, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Shuaifuyuan No.1, Dongcheng District, Beijing, 100730, China; Email: liyuxiucom.cn

 Global reach, higher impact
Global reach, higher impact