3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(12):1513-1526. doi:10.7150/ijms.87995 This issue Cite
Research Paper
Effects of maternal toxic substance consumption during breastfeeding on lactic acid bacteria abundance and nutritional content
1. Universidad de Guadalajara. University Center of Exact Sciences and Engineering. Division of Basic Sciences, Department of Pharmacology. Laboratory of Industrial Microbiology Research. Guadalajara, Jalisco, México.
2. Universidad de Guadalajara. University Health Sciences Centre. Department of Human Reproduction Clinics, Child Growth and Development, Pediatrics Specialty. Guadalajara, Jalisco, México.
3. O.P.D. Hospital Civil de Guadalajara "Fray Antonio Alcalde". Division of Pediatrics, Neonatology Service. Guadalajara, Jalisco, México.
Received 2023-7-11; Accepted 2023-9-1; Published 2023-9-11
Abstract
Breast milk is widely recognized as the primary source of nourishment for newborns, making it an unparalleled and indispensable provider of essential nutrients, microbiological components, immunological factors, and energy content. To investigate this further, a cohort comprising 254 breastfeeding women participated in interviews, and milk samples were aseptically collected for subsequent analysis involving bromatological, microbiological, and clinical analysis. The investigation focused on the identification of specific microorganisms in breast milk and their susceptibility to the exposure of toxic substances and controlled medications. Notably, this study places particular emphasis on the significant decline in lactic acid bacteria observed in breast milk when influenced by substances such as cocaine, cannabis, crystal, and morphine. These detrimental agents have been found to adversely affect the growth of microorganisms within breast milk. On the contrary, the outcomes of this study indicate that the utilization of toxic substances does not exert a noteworthy impact on the nutritional quality of breast milk.
Keywords: breastfeeding, toxic substances, newborn, microbiota, inhibition
Introduction
Breast milk, is widely recognized as the primary source of nutrition for newborns during their first six months of life, makes it a unique and irreplaceable food as it provides all the necessary nutritional, immunological, microbiological, and energy contents for the optimal development [1]. However, specific circumstances may prevent a mother from directly breastfeeding her child [2]. It is known that there are very few cases where breastfeeding should be immediately interrupted, as this decision should be the last resort that healthcare professionals should turn to [3]. These factors include a mother having an infectious disease that poses a risk to the health of the newborn, such as mothers infected with untreated HIV, hepatitis B or hepatitis C [4], active tuberculosis, advanced-stage cancer, untreated syphilis, and any other disease that can be transmitted through an untreated perinatal infection [5]. Currently, the World Health Organization (WHO) recommends continuing breastfeeding for all HIV-positive mothers who are undergoing treatment with Antiretroviral Therapy (ART) [6] and whose viral load is < 50 copies/mL, as it has been demonstrated that the efficiency of ART carries a transmission risk less than 2% compared to when breastfeeding is discontinued [7].
The consumption of toxic substances (illicit drugs and control medications) by the mother jeopardizes exclusive breastfeeding and the health of the newborn, causing a significant global health problem. [8]. This makes it necessary to suspend this practice, as these compounds can cause neonatal withdrawal syndrome [9]. The consumption of such substances, including nicotine, cocaine (COC), alcohol, amphetamines (AMP), cannabis (THC), phencyclidine, methamphetamines (M-AMP), opioids (OPI) and barbiturates (BAR), which can cross the enteromammary barrier due to their lipid affinity and reach the milk in viable form, causing various systemic alterations in the newborn [10]. These alterations include excitability, disorders in the central nervous system, vomiting, muscular paralysis, tachycardia, respiratory failure, and death [11, 12].
One of the most consumed harmful substances during lactation is alcohol. Due to its social consumption and the unknown minimum safe consumption level for breastfeeding, it is recommended not to consume it [13]. Regular alcohol consumption can inhibit prolactin, decrease milk production by 10 - 25 %, and block the release of oxytocin [14, 15]. This compound can be transferred in small amounts to breast milk, changing its organoleptic characteristics such as taste and smell. Metabolically, newborns are incapable of oxidising alcohol, having negative effects on their behavior, sleep patterns, psychomotor development, and in the worst-case scenario, future kidney problems [16, 17].
Tobacco consumption during breastfeeding is another important factor to consider. Its consumption can change organoleptic properties, causing breast refusal [9]. The complications that arise include the inhibition of prolactin release, low milk production, and interference with ejection of breast milk [16]. Nicotine, which is the main component of tobacco, can be present in breast milk as cotinine and cause adverse effects such as inadequate weight gains and more frequent colic in the infant [18]. Additionally, passive exposure to tobacco smoke increases the risk of sudden infant death syndrome, respiratory infections, cough, and asthma [17]. However, due to the significant benefits that breast milk provides to the newborn, it should not be restricted, and alternative pharmacological treatments should be considered to reduce the mother's use of these substances as much as possible [18, 19].
Due to the importance of breastfeeding for the newborn's nutrition, the WHO, United Nations Children's Fund (UNICEF), the American Academy of Pediatrics (AAP), and the Spanish Association of Pediatrics (AEP) recommend evaluating and promoting breastfeeding according to these criteria, as the benefits that breastfeeding provides to the child will be reflected both in the short and long term [12, 20]. Breast milk acts as a vehicle for probiotics microorganisms [21], which are responsible for colonizing the newborn's gut, helping to strengthen their immune system and improve their digestive processes by protecting the intestinal lumen [22]. For these reasons, it is considered the newborn's first vaccine. Additionally, breast milk provides all the necessary nutritional and energy content, including proteins, lipids, carbohydrates, oligosaccharides, vitamins, and minerals [1, 23].
The breast milk microbiota represents a fundamental factor for newborns, which is why the importance of exclusive breastfeeding for the first 6 months of life and continuing it for at least two years in combination with complementary feeding [23, 24]. It is important to mention that in México, there is no established control standard or uniform criteria for values that can be considered satisfactory in unprocessed breast milk. However, according to international standards in the United Kingdom, bacterial growth should not exceed 1 X 105 CFU/mL before pasteurization [25]. Similarly, the Human Milk Banking Association of North America (HMBANA) and the European Milk Banking Association (EMBA) have issued a statement warning about the dangers of inappropriate use and consumption of breast milk [26, 27], which should not contain pathogenic bacteria, or no more than 1 X 104 CFU/mL [28].
Breastfeeding should ensure that newborns receive safe and nutritionally optimal feeding according to their needs and requirements. In this study, we investigated how the use of toxic substances, as well as the consumption of alcohol, tobacco, and coffee, affect the nutritional quality and the presence of lactic acid bacteria (LAB) in breast milk.
Materials and Methods
Raw material
Breast milk samples were collected based on donations from lactating women after the fifteenth day postpartum (mature milk), who were admitted to the obstetrics service of the Hospital Civil “Fray Antonio Alcalde” (HCFAA) in the city of Guadalajara, México. These samples were collected following the health control criteria of the Department of Health through the Pediatrics Division, Neonatology Service of the HCFAA. These samples were collected from both clinically healthy mothers and those with positive toxicology screening, such as COC, THC, AMP, M-AMP, OPI, and BAR, as well as mother's self-reported consuming alcohol and tobacco during lactation. The standards for toxic substances were selected according to the frequency of consumption and discarding only those patients whose milk production was less than 3 mL per breast, since this amount is insufficient for the analyses.
The study included a total of 254 lactating mothers who were categorized into groups based on their consumption of different substances. These groups including a control group of 151 clinically healthy mothers (evaluated through clinical analysis by the central laboratory of HCFAA). Additionally, specific groups were formed for mothers with positive toxicology screenings with a total of 25 patients, as well as those who consumed alcohol (32 patients), tobacco (29 patients), and coffee (17 patients).
Donor mothers selection for each group was accomplished using convenience non-probabilistic sampling.
Donor mothers were informed about the study and the signing of informed consent and confidentiality letters was carried out in accordance with the provisions of the general health law for health research [29], title 2nd, chapter I, article 17, section I, minimal risk research, always taking care of the patient's integrity and considering the ethical criteria established by the research ethics committee of the HCFAA in Guadalajara, México (approval reference number HCG/CEI-0907/22 and research registration 141/22 approved on June 8th, 2022), as well as the safeguarding of patients personal information, in accordance with the federal law on protection of personal data of sensitive nature, the protection of sensitive personal data[30].
Analysis of the nutritional properties of breast milk
A nutritional content analysis was performed using a LACTOSCAN SA MILK AN-ALYZER® [31] ultrasound equipment adjusted to breast milk samples (LCD display - 4 lines x 16 characters), which analyzes the content of fat, protein, lactose, and total carbohydrates in g/dL.
Quantification and isolation of lactic acid bacteria present in breast milk from mothers consuming toxic substances
The quantification of LAB in breast milk was carried out using plate counting techniques on selective agar for Lactobacillus (agar MRS) [32], grown under anaerobic conditions at 37°C for 24 to 48 hours. Their subsequent identification was done using proteomic tests by the MALDI-TOF mass spectrometry method, which involves the specific and rapid identification of microorganisms by analyzing proteins through a library of a specific mass spectrum for the genus and species, providing results from a culture in less than 24 hours and at a lower cost compared to other molecular biology methods [33].
Identification of toxic substances presence in breast milk through qualitative analysis
Descriptive surveys, containing both open-ended and closed-ended questions, were conducted with enrolled donor mothers to gather verbal information regarding their use of toxic substances and the frequency of their usage. Subsequently, collected milk samples underwent qualitative analysis for the identification of substances of abuse using the SureStep™ Drug Screen Card I kit from Diagnóstica Internacional [34], which is based on lateral flow immunochromatography and is a one-step in vitro test. For comparison purposes, this kit was evaluated by intentionally contaminating breast milk with the following standards: pentobarbital, fentanyl, midazolam, buprenorphine, morphine, benzodiazepines for controlled medications, and COC, THC, ecstasy, lysergic acid diethylamide (LSD), crystal for toxic substances (the concentrations used are presented in Table 1). The standards were obtained through an approved distributor for research in accordance with the General Health Law and Article 479 of the current penal code in México [35, 36]. Mothers with positive results in their milk samples were subsequently confirmed with urine toxicology tests.
In vitro evaluation of the effect of toxic substances and control medications in microorganisms isolated from breast milk
Different microorganisms were identified in breast milk, such as Lacticasibacillus rhamnosus, Limosilactobacillus reuteri, Lactobacillus lactis, Lacticasibacillus casei, Lacticasibacillus paracasei, Levilactobacillus brevis, Bacillus cereus, Bacillus subtidis, Staphylococcus lentus, Staphylococcus haemolyticus, and Enterococcus faecalis. Their resistance capacity to various toxic substances and controlled medications was evaluated on MRS and Mueller-Hinton [37] agar through surface extension culture. Wells containing controlled doses of the substances mentioned in Figure 5, relative to the lethal dose in humans, were placed on the agar. Subsequently, they were incubated at 37°C for 24 to 48 hours, and the inhibition zones produced by the different substances were measured.
Statistical analysis
Differences in the nutritional content of milk samples, microbiological content, and maternal toxic substances use were assessed according to the data distribution with the Shapiro-Wilk test. Parametric data were analyzed using Tukey's one-way ANOVA with Honest Significant Difference (HSD) [38]. Non-parametric distributions were evaluated with Kruskal-Wallis one-way ANOVA with Mann-Whitney-Wilcox procedure and post hoc multiple comparisons tests with Bonferroni significance correction [39]. Statistical and graphical analyses were performed with R and Statgraphics 19® software [40, 41]. As well as Microsoft Excel for database processing [42].
Results
Factors that may interfere with the process of exclusive breastfeeding
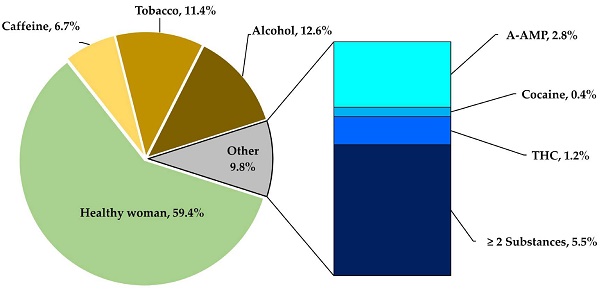
This study considered a total donor mothers of 254 aged 14 to 46 years, (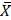 = 26, Mode = 23). The women underwent evaluation tests, including open and conditioned questionnaires, clinical analyses, toxicological tests, and nutritional analyses, to be classified into one of five groups using a non-probabilistic convenience model. The groups included a control group of healthy donor mothers, which was compared to groups of donor mothers who consumed toxic substances and had positive toxicological tests for COC, AMP, M-AMP, OPI and THC. As well as groups consumer's alcohol, tobacco, and coffee, all during the lactation period. These categories correspond to the factors that compromises a successful lactation, as shown in Figure 1A, which displays the frequency with which these substances were used by the mothers.
= 26, Mode = 23). The women underwent evaluation tests, including open and conditioned questionnaires, clinical analyses, toxicological tests, and nutritional analyses, to be classified into one of five groups using a non-probabilistic convenience model. The groups included a control group of healthy donor mothers, which was compared to groups of donor mothers who consumed toxic substances and had positive toxicological tests for COC, AMP, M-AMP, OPI and THC. As well as groups consumer's alcohol, tobacco, and coffee, all during the lactation period. These categories correspond to the factors that compromises a successful lactation, as shown in Figure 1A, which displays the frequency with which these substances were used by the mothers.
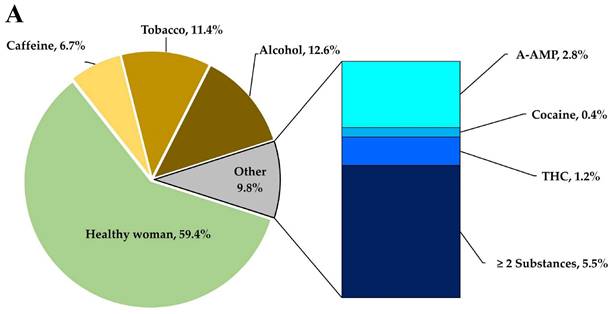
Patterns of substance consumption in relation to gestational age. (A) Shows the frequency of toxic substances use during the lactation period. (B) Depicts the variation in gestational age in relation to the consumption of substances of abuse (**** p < 0.0001; *** p < 0.001; ns, non-significative).
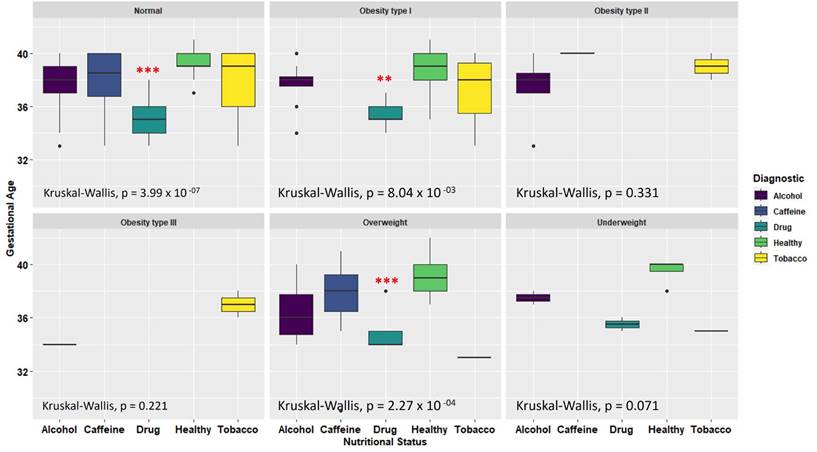
Relationship of maternal nutritional status and toxic substance consumption with the reduction of gestational age (Kruskal-Wallis test, ** p = 0.01, *** p = 0.001).
In the others' section of Figure 1A, which corresponds to 9.8 % of toxic substances cases, it was found that 2.8 % of mothers were ingesting methamphetamines, 0.4 % were inhaling cocaine, and 1.2 % were smoking marijuana. It is worth noting that around 5.5 % of mothers were using two or three types of substances used simultaneously.
Another important data point related to toxic substances use is the gestational age of the newborns, which can be observed in Figure 1B. The figure depicts a clear relationship between toxic substances during pregnancy and a reduction in gestational age. Typically, pregnancies for healthy mothers last between 38 to 40 weeks, with a mean of 39.17 ± 1.20 weeks in this cohort. However, donor mothers who consume toxic substances have a significant reduction in gestational age, with a mean of 35.8 ± 1.4 weeks (p < 0.0001). This decrease can lead to an optimal gestational age reduction of up to one month and an increased risk of premature birth.
Regarding the groups of mothers consuming alcohol (p = < 0.0001) and tobacco (p = < 0.001), there is a significant difference (p = < 0.0001) in gestational age compared to healthy mothers. The statistical analysis demonstrated no significant (ns) difference in gestational age between mothers consuming caffeine and healthy mothers.
To compare the results regarding the reduction in gestational age among mothers who were consuming a toxic substance and to determine if this reduction was not attributable to the mothers low weight or nutritional status. A statistical analysis was conducted using the nutritional status according to the Body Mass Index (BMI). A Kruskal-Wallis test resulted in a non-significant contribution to the double factor analysis (p = 0.2072). Notably, regardless of the nutritional status in which the mothers were situated, the consumption of any toxic substance led to a decrease in gestational age, as observable in Figure 2. These findings suggest that the reduction in gestational age among the donor mothers is not related to the nutritional status, but rather to the consumption of toxic substances.
Effect of toxic substances consumption on the nutritional properties of breast milk
This study investigated variations in the nutrient composition of breast milk in relation to the use of toxic substances in donors during lactation. Donors were divided into groups as described in section (Raw material). Analysis of the protein content of breast milk (Figure 3A) revealed a standard of 1.39 ± 0.6 g/dL, with no significant differences found between the control group (clinically healthy) and the group of individuals who consume toxic substances (p = 0.002). However, a difference was observed between healthy mothers and alcohol users, although it was not statistically significant. Total lipids in breast milk (Figure 3B) had a standard of 3.58 ± 0.5 g/dL, with no significant differences found between groups (p = 0.042).
Macronutrients available in breast milk of mothers who use toxic substances. (A) The protein content present in breast milk was compared between the groups of mothers who use toxic substances, alcohol, tobacco, and caffeine, against the values of healthy mothers (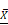 = 1.48 g/dL); (B) total lipid content in breast milk of the five groups,
= 1.48 g/dL); (B) total lipid content in breast milk of the five groups, 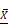 = 3.60 g/dL for healthy mothers; (C) lactose content,
= 3.60 g/dL for healthy mothers; (C) lactose content, 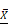 = 8.16 g/dL for healthy mothers; (D) energy content,
= 8.16 g/dL for healthy mothers; (D) energy content,  = 86.30 Kcal/dL for healthy mothers.
= 86.30 Kcal/dL for healthy mothers.
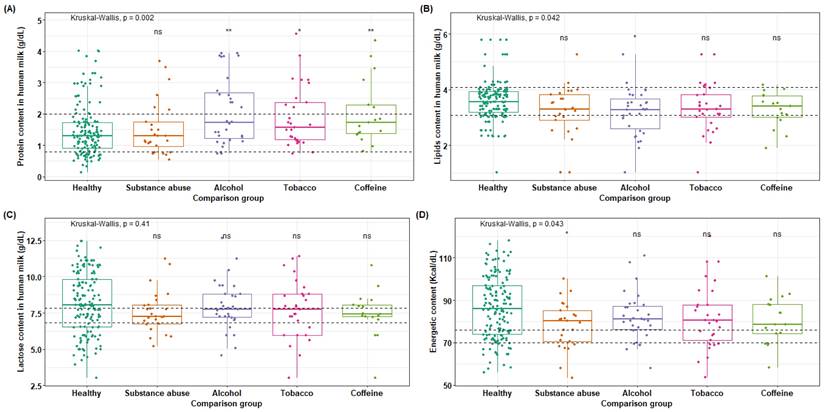
Regarding lactose values (Figure 3C), a slight increase in lactose content is observed in healthy mothers compared to the four groups within the standard range of 7.34 ± 0.5 g/dL, although no significant difference was reached (p = 0.41). However, the alcohol and tobacco groups have means that fall within the upper limit of the standard value. In (Figure 3D), the energy content in Kcal per 100 mL is shown, where all groups exceed the maximum standard limit of 70-76 Kcal/dL. However, a slight significant difference is only observed between the groups of donors who are consumers of toxic substances and clinical healthy donors, while no significant difference is found with the other groups (p = 0.043).
The amounts of saturated and unsaturated fats were also evaluated, but these did not show statistically significant variations. This indicates, that although there are variations in the nutrients of the milk, the impact is minimal and does not compromise the nutritional quality of the milk for proper feeding. Nevertheless, doctors should evaluate this situation regarding the risk of toxic substances ingestion to the newborn through breast milk.
Identification of traces of toxic substances in breast milk
After collecting milk samples from donor mothers who are consumers of toxic substances, confirmed through toxicological tests in urine, the milk was evaluated for the presence of these substances. To do this, corresponding control samples were first performed with the concentrations of substances shown in Table 1, in order to evaluate the efficiency of the kit that determines the presence of COC, THC, OPI, AMP, and M-AMP. The mentioned concentrations were calculated according to the information provided by the international immunodiagnostics and the Substance Abuse Mental Health Services Administration (SAMHSA) of the USA. Controlled drugs and toxic substances were handled according to the provisions of Chapter VII, Article 479 of the General Health Law, in its latest reform of May 16th, 2022, regarding the maximum allowable dose guidance [29, 43, 44].
Once the sensitivity of the toxic substances test kits was validated in both intentionally contaminated urine samples and breast milk samples, analyses were performed on milk samples from donor who are consumers of toxic substances. Out of the 254 milk samples collected, it was identified that 9.8% of the samples tested positive for toxic substances, as shown in Figure 1, while all samples tested negative for amphetamines and opioids.
Quantification of microorganisms in breast milk of toxic substances consumers
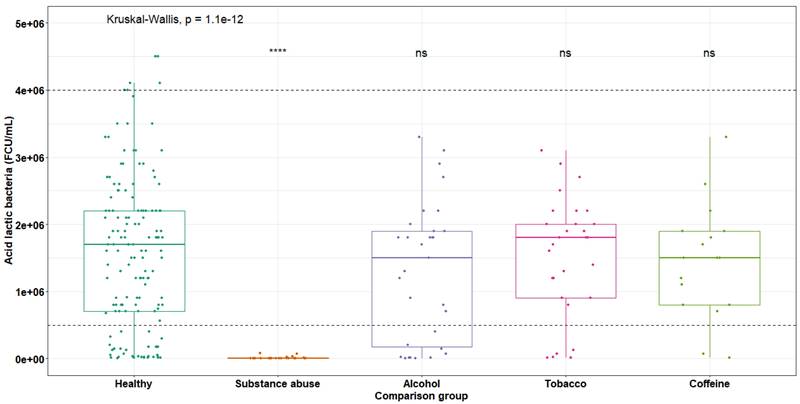
Once the milk samples were collected from mothers consuming different harmful substances, the cultivation and quantification of LAB on MRS agar was carried out for each group previously mentioned. Figure 5 shows the variations in CFU/mL, where it can be observed that the group of donors who are consumers of toxic substances is the most affected. While established parameters for normal mothers range from 1 x 104 to 1 x 106 CFU/mL, for this group of consumers of toxic substances, the mean is 9.59 x 103 CFU/mL, indicating a decrease of one logarithm relative to the minimum limit. The statistical analysis using Kruskal-Wallis test, demonstrating a significant difference in the decrease of LAB with respect to healthy mothers (p < 0.001).
In mothers who habitually consumed alcohol ( = 1.29 x 106), tobacco (
= 1.29 x 106), tobacco ( = 1.53 x 106) and coffee (
= 1.53 x 106) and coffee ( = 1.45 x 106), no significant decrease was observed. This is because these groups exhibited a normal distribution within normal ranges.
= 1.45 x 106), no significant decrease was observed. This is because these groups exhibited a normal distribution within normal ranges.
Inhibition of microbial growth in breast milk upon exposure to toxic substances and controlled medications
To assess the effect of toxic substances and controlled medications on microbial growth in breast milk, diverse microorganisms were isolated and identified from samples of milk from healthy mothers. This included BAL, saprophytic, and some pathogenic bacteria. Subsequently, the microorganisms were exposed to specific concentrations of substances relative to the lethal dose in humans per kilogram of body weight: pentobarbital 0.28 mg [46], fentanyl 0.04 mg [47], midazolam 0.10 mg [48], buprenorphine 0.32 mg [49], morphine 0.60 mg [50], COC 1.20 mg [51], THC 10 mg [52], ecstasy 5 mg [53], LSD 0.60 mg [54], and crystal 0.20 mg [55], as shown in Figure 5.
Concentration of controlled drugs and toxic substances for the validation of the Surestep Drug Screen Card I Kit.
| Toxic substances | Concentration (µg/ 10 ml) | Urine test (POS/NEG) | Breastmilk test (POS/NEG) |
|---|---|---|---|
| Control | 0 | NEG | NEG |
| Controlled drugs | |||
| Pentobarbital (BAR) | 3 | POS | POS |
| Fentanyl (OPI) | 3 | POS | POS |
| Midazolam (OPI) | 3 | POS | POS |
| Buprenorphine (BZD) | 0.1 | POS | POS |
| Morphine (OPI) | 3 | POS | POS |
| Buenzodiacephine (BZD) | 3 | POS | POS |
| Toxic substances | |||
| Cocaine (COC) | 25 | POS | POS |
| Marihuana (THC) | 15 | POS | POS |
| Ecstasy (M-AMP) | 10 | POS | POS |
| Lysergid (LSD) | 10 | POS | POS |
| Crystal (M-AMP) | 10 | POS | POS |
The abbreviation POS in rows three and four signifies that the qualitative test yielded a POSITIVE result for that concentration of the toxic substances or controlled drug.
The abbreviation NEG in rows three and four signifies that the qualitative test yielded a NEGATIVE result for that concentration of the toxic substances or controlled drug.
The concentrations used for the substances were determined according to the minimum detectable values of the kit used [45].
Variations in LAB content in breast milk of toxic substances consuming mothers (maximum standard range 4.0 x 106, minimum 5.0 x 105 CFU/mL), average for healthy donors 1.64 x 106 CFU/mL
Inhibitory effects of toxic substances and controlled medications on microbial growth (The red dashed line represents the average value of the antibiotic control used for the inhibitions, which measures 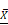 = 9 ± 1.56 mm in diameter).
= 9 ± 1.56 mm in diameter).
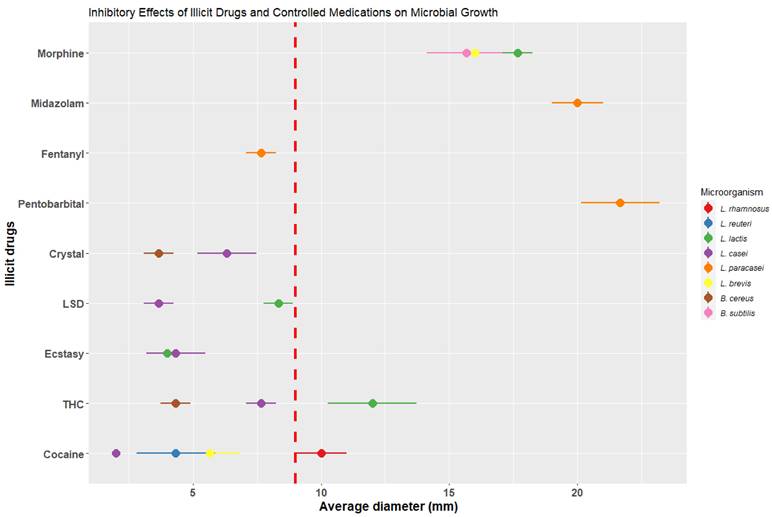
Once each of the microorganisms was incubated with the corresponding exposure to each substance and validated in triplicate, it was observed that 58.33 % of the microorganisms were affected by some of the toxic substances and 41.66 % by some of the controlled drugs, preventing their microbial growth in the presence of the substances. As for lactic acid bacteria such as L. rhamnosus and L. reuteri, their growth was affected in the presence of COC, crystal, and THC, resulting in inhibitions ranging from 3.67 ± 0.58 to 5.67 ± 1.15 mm in diameter. However, the inhibition caused by toxic substances was lower compared to the control (using a degradation-resistant antibiotic such as carbencillin at a concentration of 0.1 mg/disk [56]), which presented an average diameter of 9 ± 1.56 mm. The species L. brevis was inhibited when exposed to all 5 types of toxic substances, being most affected by crystal with a diameter of 6.33 ± 1.15 mm and THC with a diameter of 7.67 ± 0.58 mm.
Regarding the controlled medications, L. reuteri exhibited an inhibition of 16 ± 1 mm in diameter, surpassing the average value of the control. L. casei was most affected by midazolam (20 ± 1 mm), fentanyl (7.67 ± 0.58), and pentobarbital (21.67 ± 1.53 mm), exceeding the control with carbencillin (Figure 5). It is important to highlight that the microorganisms L. lactis, L. casei, and S. haemolyticus did not experience inhibition in their growth in the presence of toxic substances and controlled medications, which indicates a resistance to such substances.
These bacterial inhibition assays allow us to observe the direct effect of toxic substances and controlled drugs on the cultivable microbiota of healthy mothers' milk. As a result of these analyses, a decrease in the number of bacteria was observed, which was reflected in the plate emptying counts.
When comparing these results with the quantification of cultivable microorganisms for LAB, counts below the established minimum limits of up to 2 Log were observed in milk samples from consuming donor mothers of toxic substances, with values between 1 x 103 and 1 x 104 CFU/mL, with an average of 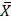 = 9.59 x 103 ± 3.21 x 102 CFU/mL, compared to a standard value of
= 9.59 x 103 ± 3.21 x 102 CFU/mL, compared to a standard value of  = 1.4 x 106 ± 1.0 x 106 CFU/mL for healthy mothers.
= 1.4 x 106 ± 1.0 x 106 CFU/mL for healthy mothers.
Discussion
Relationship between toxic substance and gestational age
After analyzing the data regarding the 5 groups and interpreting the statistical analyses with respect to toxic substances use and the gestation weeks period, it was identified that mothers who mainly consumed cocaine, methamphetamines, and marijuana had a lower number of gestational weeks (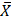 = 35.8) compared to healthy mothers, demonstrating a significant difference between the two groups. The National Institute on Drug Abuse of the United States that drug use during pregnancy and lactation increases the risk of spontaneous abortions, as well as causing migraines, seizures, and high blood pressure that can directly affect the fetus [43]. In addition, smoking marijuana or tobacco during the gestational period increases the risk of stillbirth by 1.8 to 2.8 times [57, 58].
= 35.8) compared to healthy mothers, demonstrating a significant difference between the two groups. The National Institute on Drug Abuse of the United States that drug use during pregnancy and lactation increases the risk of spontaneous abortions, as well as causing migraines, seizures, and high blood pressure that can directly affect the fetus [43]. In addition, smoking marijuana or tobacco during the gestational period increases the risk of stillbirth by 1.8 to 2.8 times [57, 58].
Evaluation of the quality and safety of breast milk from toxic substances consuming donors
In the analysis of the nutritional content of milk from mothers who consume toxic substances, it is observed that although there is a slight variation in nutrient levels, such as protein, between mothers who consume alcohol and those who do not, these differences are not statistically significant (p = 1.1 e-12) and would not significantly impact infant nutrition.
However, the real risk for the infant lies in the concentration of toxic substances that may be present in the milk and thus enter their organism. Our analyses identified traces of drugs in some milk samples, as mentioned by Lawrence et al. in 2016, that the consumption of toxic substances presents a high risk for the newborns, since most drugs can reach the alveolar cells of the breasts in free form and are available in the milk. The drugs with the highest receptors in the breasts are phenytoin, salicylate, and diazepam [59].
Philip O. Anderson also mentions in his article published in June 2022 that in patients with diseases during lactation such as arthritis that require pharmacological treatment with controlled medications such as opioids, they should be evaluated and considered as the last line to use them [60]. Anderson suggests that although the concentrations of these controlled medications in the milk are lower than those used for neonatal analgesia, alternative medications should be chosen. In 2012, Saguer reported a case of cocaine poisoning in a three-month-old baby in Colombia, who was admitted to the emergency unit due to seizures caused by cocaine [61]. Furthermore, studies conducted by Pediatrics in 2018 revealed the presence of cannabis metabolites in breast milk, confirming the transmission of various toxic substances through breastfeeding [20].
Alcohol, tobacco, and their relationship with breast milk
Regarding the results obtained from breast milk analyzed from mothers who consume alcohol and tobacco, we observed that the nutritional values with respect to the total content of lipids and carbohydrates remain unchanged, except for the protein content where a slight increase is observed, but it remains not significant. Authors such as Acosta in 2020 observed that mothers who consume alcohol and tobacco had premature abandonment of breastfeeding, on average only breastfeeding for two months [62]. The American Academy of Pediatrics notes in its latest update in July 2020 that alcohol consumption does not have a safe dose during breastfeeding and decreases milk production, posing a potential risk to the infant's health [63].
Rowe et al. in 2013 reported data from a study titled "The transfer of alcohol to breast milk," which evaluated 12 women who ingested 0.3 g/kg of ethanol and found that the mean maximum concentration of ethanol in milk was 320 mg/L evaluated in the 4 hours after ingestion [46]. The study also found that the ingestion of 1.5 g/kg significantly reduced milk production and that constant consumption of 21 drinks daily caused psychomotor delay in infants [65].
Regarding the concentration of nicotine and cotinine with an average consumption of 17 cigarettes per day, it was observed that the concentrations in milk decreased by 50 % to 66 %, equivalent to 25.2 mg/kg/d present in breast milk [64, 65]. Our study identified that out of a total of 254 women, 30, equivalent to 11.4 % of the total, smoked an average of one pack of cigarettes per day (20 cigarettes), which increases the concentration of nicotine in the breast milk they produce, as reported by Rowe [64, 66, 67].
The physicochemical results for these groups, for both proteins, lipids, and carbohydrates, although showing slight variations, were statistically non-significant, and the nutrients were within the established ranges for healthy women. In November 2022, Philip O. Anderson discussed alcohol consumption disorder during lactation as a primarily psychological issue for both mother and child's health. As a result, treatment with medications such as naltrexone, baclofen, gabapentin, ondansetron, and topiramate is recommended as they are unlikely to harm the infant [68].
Effects of toxic substances on microorganisms isolated from breast milk
Research conducted by López M. in 2017 and Amezcua in 2018 demonstrated that mothers who were under the influence of toxic substances showed a microbiological count by plate pouring on MRS agar of 1 x 104 or 1 x 105 in normal mothers [69, 70], whereas in mothers who were under the influence of toxic substances, the counts decreased significantly to 1 x 102 [71]. When we subjected the microorganisms isolated from breast milk to growth in the presence of different toxic substances, it was shown that some microorganisms did indeed die, or their growth was inhibited either by exposure to these compounds.
In 2012, Arroyo et al. from the National Institute of Perinatology Isidro Espinosa de los Reyes conducted a study on 57 lactating mothers who were consumers of toxic substances such as COC, THC, OPI, AMP, and benzodiazepines [72]. The study demonstrated the presence of these substances in breast milk samples, confirming the feasibility of drug transmission in milk. In 2020, the Organization of Teratology Information Specialists mentioned that cocaine can pass directly into milk in any of its forms, posing a serious risk to the child's health, causing irritability, seizures, and even death [73]. The Fourth Trimester Project at the University of North Carolina in 2022 states that the consumption of marijuana during lactation causes the main component of this THC, to be stored in body fat and remain there for up to 30 days, thus demonstrating that the milk of mothers who consume these toxic substances contains high concentrations of THC [75]. The information provided by these authors and research institutions reinforces the results obtained by our research group regarding the positivity of toxicological screenings in breast milk.
Regarding the relationship between the presence of these drugs of abuse and their relationship with microbial growth Ramos (2012) demonstrated that the leaf extract of Erythroxylum coca has an inhibitory effect on the growth of the bacteria ATCC Porphyromonas gingivalis as this leaf contains alkaloids related to cocaine [76]. In 2019, a study related to the effect of the cannabinoid system demonstrated that acute stress in mice, which were administered certain concentrations of this substance, increased the expression of the intestinal endocannabinoid system degradation enzyme [77].
With this information provided by these authors in in vitro experiments with different types of substances, demonstrates the direct relationship shown by our research group on the decrease of LAB in breast milk samples from mothers who consumed these substances. Once certain microorganisms were isolated from the milk and exposed to concentrations of and controlled medications in relation to the lethal dose in humans per Kg of body weight, it was observed that L. reuteri, L. casei, and L. rhamnosus were affected in their growth. These microorganisms were exposed to cocaine, methamphetamine, marijuana, morphine, pentobarbital, fentanyl, and midazolam, and their growth was found to be negatively impacted.
However, this inhibition not only occurred in LAB, but also, in other groups of microorganisms, including pathogenic and saprophytic bacteria, in which controlled drugs, LSD, ecstasy, and cocaine were the substances that generated the most inhibition. Thus, suggesting that the constant use and consumption of toxic substances and controlled medications during lactation could have a detrimental effect on the proper growth of microbiota naturally present in milk. Within our study, the toxic substances most commonly used by donor in breastfeeding stage were crystal (M-AMP) in first place, followed by THC and COC. Most of them not only consumed a single substance but even had mixtures of these three toxic substances at the same time, and to a lesser extent, they consumed ecstasy, LSD and fentanyl.
Conclusions
Result of study states the importance of monitoring lactating mothers who use toxic substances, as they often deny their consumption during prenatal care and lactation, putting the newborn health at risk. In studies conducted on breast milk samples from donor mothers consuming toxic substances, qualitative analysis identified the presence of cocaine, methamphetamines (primarily crystal meth), and marijuana. However, this analysis was limited to observing the presence or absence of the toxic substances. It has been shown that although the consumption of these substances does not have a significant effect on the nutritional content of breast milk, it does present a potential risk to the development of the milk's own microbiota. It mainly affects the growth of acid lactobacilli when substances such as morphine, midazolam, and pentobarbital are used, which have a greater bacterial inhibition compared to the control with antibiotics. In addition, the use of toxic substances such as crystal meth, THC, and COC affects the growth of Lactobacillus and is more frequently consumed in our population.
With the results obtained, it highlights a social problem that affects the health of newborns breastfed with milk from donor mothers consuming toxic substances. For these reasons, it is necessary to implement preventive measures and support in hospitals during pregnancy and lactation, as well as offer pharmacological alternatives to donors with addiction issues, avoiding breastfeeding for their children until they can safely reintegrate. Otherwise, this habit can have negative consequences for the newborn health, inadequate microbial colonization in the intestine, and generate complications in adulthood. These advancements aim to ensure that healthcare personnel identify the risks that arise when a lactating mother experiences addiction problems, enabling immediate action to guarantee health and improve the quality of life for future generations.
Abbreviations
AAP: American Academy of Pediatrics; AEP: Asociación Española de Pediatria; AMP: amphetamines; BAR: barbiturates; CFU: colony forming units; COC: cocaine; d/dL: grams per deciliter; d: day; EMBA: European Milk Banking Association; g: grams; HCFAA: Hospital Civil "Fray Antoni Alcalde"; HMBANA: Human Milk Banking Association of North America; HSD: Honest Significant Difference; Kg: kilograms; LAB: lactic acid bacteria; LSD: lysergic acid diethylamide; M-AMP: methamphetamines; mL: milliliters; NEG: negative; OPI: opioids; POS: positive; SAMHSA: Substance Abuse Mental Health Services Administration; THC: cannabis; UNICEF: United Nations Children's Fund; WHO: World Health Organization; 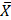 : arithmetic mean.
: arithmetic mean.
Acknowledgements
We would like to thank the entire breastfeeding research team at CUCEI/UDG and the support from the Hospital Civil "Fray Antonio Alcalde" in Guadalajara, as well as the unconditional support from the BBy Company under the supervision of Dr. Vansh Langer. Special thanks to L.N. Miriam Montaño Perales for her support in the lactation and neonatology, L.N. Nadia Guerrero for her contributions in the neonatal intensive care unit, and Dr. Mario Alemán Duarte for his support with graphical analysis. And to the entire team of nutrition interns, nurses, and medical interns.
Informed Consent Statement
This study was also approved by the Ethical Research Committee 08 June 2022, with registration number HCG/CEI-0907/22 and research registration 141/22.
Author Contributions
Amezcua López: Conceptualization, Software, Formal analysis, Investigation, Data curation, Writing-original draft, Writing-review and editing, Visualization. Garcia Morales: Methodology, Validation, Formal analysis, Investigation, Writing-original draft, Visualization. Pérez-Rulfo Ibarra: Formal analysis, Resources, Data curation, Writing-review and editing, Funding acquisition. Solis Pacheco: Conceptualization, Software, Investigation, Resources, Writing-original draft, Visualization, Project administration. Aguilar Uscanga: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing-original draft, Writing-review and editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. National Academies of Sciences, Engineering, Medicine; Health, Medicine Division; Food, Nutrition Board. Nutrition During Pregnancy and Lactation: Exploring New Evidence: Proceedings of a Workshop. Harrison M, editor. Washington (DC): National Academies Press (US). 2020:7-21 DOI: 10.17226/25841
2. Centers for Disease Control and Prevention. Contraindications to breastfeeding or feeding expressed breast milk to infants. 2023 May 31 [cited. 2023 Sep. 6]. https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/contraindications-to-breastfeeding.html
3. Cook KJ, Larson KL. Breastworks: Breastfeeding practices among women with substance use disorder. Appl Nurs. 2019;47(1):41-45 DOI: 10.1016/j.apnr.2019.04.006
4. MacVicar S, Humphrey T, Forbes-McKay KE. Breastfeeding and the substance-exposed mother and baby. Birth. 2018;45(4):450-458 DOI: 10.1111/birt.12338
5. Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually transmitted infections treatment guidelines, 2021. Recommendations and Reports: Morbidity and Mortality Weekly Report. 2021;70(4):1-187 DOI: 10.15585/mmwr.rr7004a1
6. Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America; Pediatric Infectious Diseases Society; HHS Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission— A Working Group of the Office of AIDS Research Advisory Council (OARAC). Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States. 2023 Jan 31; 31-51. https://www.ncbi.nlm.nih.gov/books/NBK586310/.
7. Lolekha R, Chokephaibulkit K, Phanuphak N, Chaithongwongwatthana S, Kiertiburanakul S, Chetchotisakd P, Boonsuk S. Thai national guidelines for the prevention of mother-to-child transmission of human immunodeficiency virus 2017. Asian Biomed. Res Rev News. 2017;11(2):145-159 DOI: 10.5372/1905-7415.1102.547
8. Redecilla Ferreiro S, Moráis López A, Moreno Villares JM, On behalf of the Committee on Nutrition and Breastfeeding of the Spanish Paediatric Association. Recommendations from the Committee on Nutrition and Breastfeeding of the Spanish Paediatric Association on vegetarian diets. [Position paper on vegetarian diets in infants and children. Committee on Nutrition and Breastfeeding of the Spanish Paediatric Association]. Anales de Pediatría. 2020;92(5):306.e1-306.e6 DOI: 10.1016/j.anpedi.2019.10.013
9. Issany A, Hore M, Singh L, Israel J, Kocher MG, Wen X. Reciprocal Associations Between Maternal Smoking Cessation and Breastfeeding. Breastfeeding Medicine. 2022;17(3):226-232 DOI: 10.1089/bfm.2021.0199
10. Zaballos M. Intoxicaciones por drogas ilícitas: cocaína, heroína, marihuana. Universidad Complutense de Madrid. 2011 https://www.ucm.es/data/cont/docs/107-2014-03-18-T%2023%20INTOXICACIONES%20POR%20cocaina,%20opiaceos%20y%20marihuana%202.pdf
11. Ares S, Arena J, Díaz N. La importancia de la nutrición materna durante la lactancia, ¿necesitan las madres lactantes suplementos nutricionales? Anales de Pediatría. 2015(84):301-356 DOI: 10.1016/j.anpedi.2015.07.024
12. Department of Child and Adolescent Health and Development. Anti-infective drugs. En: World Health Organization, editor. BREASTFEEDING AND MATERNAL MEDICATION Recommendations for Drugs in the Eleventh WHO Model List of Essential Drugs. 3 United Nations Plaza New York, NY 10017 USA: Nutrition Section. 2003 p. 9-14
13. Ministerio de Sanidad. Límites de Consumo de Bajo Riesgo de Alcohol. SANIDAD. 2020 https://socidrogalcohol.org/wp-content/Pdf/publicaciones/alcohol/documentos/Limites_Consumo_Bajo_Riesgo_Alcohol_Actualizacion.pdf
14. Han AA, Buerger AN, Allen H, Vincent M, Thornton SA, Unice KM, Maier A, Quiñones-Rivera A. Assessment of ethanol exposure from hand sanitizer use and potential for developmental toxicity in nursing infants. J Appl Toxicol. 2022;42(9):1424-1442 DOI: 10.1002/jat.4284
15. Rocha BO, Machado MP, Bastos LL, Barbosa Silva L, Santos AP, Santos LC, Ferrarez Bouzada MC. Risk Factors for Delayed Onset of Lactogenesis II Among Primiparous Mothers from a Brazilian Baby-Friendly Hospital. J Hum Lact. 2020;36(1):146-156 DOI: 10.1177/0890334419835174
16. Skelton K, Benjamin-Neelon SE, Kelly C, Young-Wolff K. Management of Cannabis Use in Breastfeeding Women: The Untapped Potential of International Board-Certified Lactation Consultants. Breastfeed Med. 2020;15:117-120 DOI: 10.1089/bfm.2019.0272
17. Gibson L, Porter M. Drinking or smoking while breastfeeding and later developmental health outcomes in children. BMC Res Notes. 2020;13(1):232. DOI: 10.1186/s13104-020-05072-8
18. Baraona LK, Lovelace D, Daniels JL, McDaniel L. Tobacco Harms, Nicotine Pharmacology, and Pharmacologic Tobacco Cessation Interventions for Women. J Midwifery Womens Health. 2017;62(3):253-269 DOI: 10.1111/jmwh.12616
19. del Carmen Ruíz Alcocer M, editor. síndrome de dificultad respiratoria. En: PAC: Neonatología 4, Alimentación en el recién nacido. Águilas y Seijas, Lomas de Chapultepec, 11000, México, DF.: a Revista Mexicana de Pediatría. 2016 p. 22-8
20. American Academy of Pediatrics. Alcohol & Breast Milk. from HealthyChildren.org. 2020 https://www.healthychildren.org/English/ages-stages/baby/breastfeeding/Pages/Alcohol-Breast-Milk.aspx#:~:text=Alcohol%20passes%20through%20your%20breast,avoiding%20drinking%20alcohol%20while%20breastfeeding
21. Hernández J. Microbiota y leche humana, una amistad indispensable [Internet]. Medscape. 2017 https://espanol.medscape.com/verarticulo/5901743
22. Guala A, Boscardini L, Visentin R, Angellotti P, Grugni L, Barbaglia M, Chapin E, Castelli E, Finale E. Skin-to-Skin Contact in Cesarean Birth and Duration of Breastfeeding: A Cohort Study. ScientificWorldJournal. 2017;2017:1940756. DOI: 10.1155/2017/1940756
23. Cuadros-Mendoza CA, Vichido-Luna MÁ, Montijo-Barrios E, Zárate-Mondragón F, Cadena-León JF, Cervantes-Bustamante R, Toro-Monjáraz E, Ramírez-Mayans JA. Actualidades en alimentación complementaria. Acta Pediatr Mex. 2017;38(3):182. DOI: 10.18233/apm38no3pp182-2011390
24. National Institutes of Health (NIH). Substance Use While Pregnant and Breastfeeding. U.S. Department of Health and Human. 2020 Services website https://nida.nih.gov/publications/research-reports/substance-use-in-women/substance-use-while-pregnant-breastfeeding
25. González-Rodríguez RI, Jiménez-Escobar I, Gutiérrez-Castrellón P. Microbiota de la leche humana y su impacto en la salud humana. Gac Med Mex. 2020;156(Supl 3):S58-S66 DOI: 10.24875/GMM.M20000439
26. European Milk Bank Association. Joint EMBA and HMBANA statement on milk sharing has been released. España: EMBA. 2021 Services website: https://europeanmilkbanking.com/joint-emba-and-hmbana-statement-on-milk-sharing-has-been-released/
27. EMBA. EMBA International Milk Banking Congress 2017 [Internet]. European Milk Bank Association. 2017 [cited 4 de octubre de 2023]. Services website: https://europeanmilkbanking.com/emba-international-milk-banking-congress-october-5th-and-6th-2017/
28. Kontopodi E, Hettinga K, Stahl B, van Goudoever J. B, & van Elburg, R. M. (2022). Food Chemistry. Testing the effects of processing on donor human milk: Analytical methods. Food Chem. 2021;373(Pt A):131413. DOI: 10.1016/j.foodchem.2021.131413
29. General Health Law. 1984. Latest Amendment published in the Official Gazette of the Federation on 24-03-2023. https://www.diputados.gob.mx/LeyesBiblio/pdf/LGS.pdf.
30. FEDERAL LAW FOR THE PROTECTION OF PERSONAL DATA HELD BY INDIVIDUALS. 2010. Official Gazette of the Federation on July 5. 2010 https://www.diputados.gob.mx/LeyesBiblio/pdf/LFPDPPP.pdf
31. Milkotronic Ltd. Operation manual, LACTOSCAN SA MILK ANALYZER LCD display - 4 lines x 16 characters. Bulgaria. 2019 Services website: https://lactoscan.com/editor/ufo/manuals/SA/Lactoscan_SA_Eng.pdf
32. Microkit, P. M.R.S. AGAR. Microkit: Medios de Cultivo. 2016; Services website: https://www.medioscultivo.com/1832-2/
33. R. S. Espectrometría de masas MALDI-TOF en el diagnóstico microbiológico. RSI - Revista Sanitaria de Investigación. 2021 Services website: https://revistasanitariadeinvestigacion.com/espectrometria-de-masas-maldi-tof-en-el-diagnostico-microbiologico/
34. Diagnóstica Internacional. Surestep Drug Screen Card I. 2022; https://diagnosticainternacional.com.mx/products/surestep-drug-screen-card-i.
35. Federal National Criminal Procedure Code. 1397. National Execution of Penalties Law published in the Official Gazette of the Federation on 16-06-2016. Article 195 Latest amendment published in the Official Gazette of the Federation. 08-05-2023. https://www.diputados.gob.mx/LeyesBiblio/pdf_mov/Codigo_Penal_Federal.pdf.
36. General Health Law. (1984). CHAPTER VII - Crimes Against Health in the Form of Drug Dealing. Article 479, Latest amendment published in the Official Gazette of the Federation on 24-03-2023. https://mexico.justia.com/federales/leyes/ley-general-de-salud/titulo-decimo-octavo/capitulo-vii/.
37. Previsto U. BD Mueller Hinton II agar BD Mueller Hinton II agar 150 mm BD Mueller Hinton II agar, square. 2017; Services website: www.bd.com. https://www.bd.com/resource.aspx?IDX=8774.
38. Zaiontz C. Tukey HSD (Honestly Significant Difference) [Internet]. Real Statistics Resources. 2018 Disponible en: https://real-statistics.com/one-way-analysis-of-variance-anova/unplanned-comparisons/tukey-hsd/
39. Soetewey A. Kruskal-Wallis test, or the nonparametric version of the ANOVA [Internet]. Stats and R. 2022 [cited 5 de invierno de 2023]; Services website: https://statsandr.com/blog/kruskal-wallis-test-nonparametric-version-anova
40. RStudio Team. Integrated Development for R. RStudio, PBC. 2022 Services website: https://www.rstudio.com/categories/integrated-development-environment/
41. Statgraphics Technologies, In. Centurion 19 Product Details: Powerful statistical software package. Statgraphics.com. 2020 [cited 26 de mayo de 2023]; Services website: https://www.statgraphics.com/centurion-19
42. Giessen S, Nakanishi H. Excel total: Für Beruf, Schule, Studium & Zuhause. 1a ed. Franzis. 2006
43. DrugFacts. Substance Use in Women [Internet]. National Institute on Drug Abuse. 2020 [cited el 5 de verano de 2023]; Services website: https://nida.nih.gov/es/publicaciones/drugfacts/el-consumo-de-sustancias-en-las-mujeres
44. National Institute of Child Health, Human Development [NICHD]. Fumar tabaco y consumir drogas durante el embarazo puede duplicar el riesgo de muerte fetal. 2013 [cited el 7 de abril de 2023]; Services website: https://espanol.nichd.nih.gov/noticias/prensa/121113-consumir-drogas-muerte-feta.
45. INTERNATIONAL IMMUNO-DIAGNOSTICS. SURESTEP DRUG SCREEN CARD I. 2022; https://es.scribd.com/document/602016947/IID-1027-Surestep-Drug-Screen-Card-I-DOA5-510524.
46. De D, Cuantitativa Y. FICHA TÉCNICA (RESUMEN DE CARACTERÍSTICAS DEL PRODUCTO) 1. DENOMINACIÓN DEL MEDICAMENTO VETERINARIO EUTANAX 200 mg/ml solución inyectable. Aemps.es. 2016 Services website: https://cimavet.aemps.es/cimavet/pdfs/es/ft/555+ESP/FT_555+ESP.pdf
47. Espejel C. Fentanilo: ¿Por qué es tan peligroso consumir este opioide, 100 veces más potente que la morfina? El Financiero. 2022 Services website: https://www.elfinanciero.com.mx/salud/2022/10/16/fentanilo-por-que-es-tan-peligroso-consumir-este-opioide-100-veces-mas-potente-que-la-morfina/
48. FICHA TECNICA MIDAZOLAM ACCORD 5 mg/ml SOLUCION INYECTABLE Y PARA PERFUSION EFG. (2016). Aemps.es. Services website: https://cima.aemps.es/cima/dochtml/ft/72016/FT_72016.html.
49. Roncero C, Sáez-Francàs N, Castells X, Casas M. Eficacia y manejo clínico de la buprenorfina. Trastornos adictivos. 2008;10(2):77-87 DOI: 10.1016/s1575-0973(08)74549-5
50. de Medicamentos y Productos Sanitarios A. E. PROSPECTO MORFINA KALCEKS 10 MG/ML SOLUCION INYECTABLE EFG. 2022 Services website: https://cima.aemps.es/cima/dochtml/p/82883/P_82883.html
51. Nogué Xarau S, Picón Cabrera M, Mestre Roca G, de la Devesa RCR. Urgencias en usuarios de cocaína. Med Integral. 2002;39(6):249-259 Services website: https://www.elsevier.es/es-revista-medicina-integral-63-articulo-urgencias-usuarios-cocaina-13029968
52. Najjar R. El Canabis (marihuana) [Internet]. DrugFacts. 2020 [citado el 4 de primavera de 2023]. Services website: https://nida.nih.gov/es/publicaciones/drugfacts/el-canabis-marihuana#:~:text=%C2%BFEs%20posible%20sufrir%20una%20sobredosis,una%20sobredosis%20de%20marihuana%20solamente
53. Gov H. D. Abuso de la MDMA (éxtasis). Nih.gov. Services website: https://nida.nih.gov/sites/default/files/1182-abuso-de-la-mdma-xtasis_0.pdf.
54. LSD - Energy Control. Energy Control. 2017 (2020, agosto 9); Services website: https://energycontrol.org/sustancias/lsd/.
55. Praktyczna. Anfetaminas. Empedium. 2021 Services website: https://empendium.com/manualmibe/compendio/chapter/B34.II.20.8
56. Suárez C, Gudiol F. Beta-lactam Antibiotics. Infect Dis Clin Microbiol. 2009;27(2):116-129 DOI: 10.1016/j.eimc.2008.12.001
57. National Institute of Child Health, Human Development [NICHD]. Fumar tabaco y consumir drogas durante el embarazo puede duplicar el riesgo de muerte fetal. 2013 [Recuperado el 7 de abril de 2023]; de Services website: https://espanol.nichd.nih.gov/noticias/prensa/121113-consumir-drogas-muerte-fetal.
58. Chinchilla Araya T, Durán Monge M. del P. Efectos fetales y posnatales del tabaquismo durante el embarazo. Medicina legal de Costa Rica. 2019 36(2), 68-75. Services website: https://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-00152019000200068. On-line version ISSN 2215-5287Print version ISSN 1409-0015
59. Lawrence RA. Chapter 13 - Transmission of Infectious Diseases Through Breast Milk and Breastfeeding. En: Lawrence RA, Lawrence RM, eds. Breastfeeding: A Guide for the Medical Profession. Philadelphia: Eighth. 2011 pp. 406-473. DOI: 10.1016/B978-1-4377-0788-5.10013-6
60. Anderson PO. Drug Treatment of Osteoarthritis During Breastfeeding. Breastfeed Med. 2022;17(6):472-474 PMID: 35687117. DOI: 10.1089/bfm.2022.0119
61. ACCIÓN 13 Noticias. Bebé se intoxica con cocaína al amamantarse. Servicio Independiente de Noticias para Colombia y el mundo. Resumen de Noticias Martes. 2012 [cited May 23/2023]; Services website: https://www.accion13.org.co/BebeSeIntoxicaConCocainaAlAmamantarseLasVictimasDeLaDroga.htm
62. Acosta DV, Lugo Rodriguez G, Domenech MG, Vera de Molinas Z, Maidana de Larrosa G, Samaniego Silva L. Interrupción de la lactancia materna relacionada con el consumo de medicamentos. Ars Pharm. 2020;61(2):97-103 DOI: 10.30827/ars.v61i2.9391
63. American Academy of Pediatrics. Alcohol & Breast Milk. 2020 [cited Aud 14/2023]; Services website: https://www.healthychildren.org/English/ages-stages/baby/breastfeeding/Pages/Alcohol-Breast-Milk.aspx.
64. Rowe H, Baker T, Hale TW. Maternal medication, drug use, and breastfeeding. Pediatr Clin North Am. 2013;60(1):275-294 DOI: 10.1016/j.pcl.2012.10.009
65. Gaxiola-Robles R, Zenteno-Savín T, Labrada-Martagón V, Celis de la Rosa AdJ, Acosta Vargas B, Méndez-Rodríguez LC. Mercury concentration in breast milk of women from Northwest Mexico; possible association with diet, tobacco and other maternal factors. Nutr Hosp. 2013;28(3):934-942 DOI: 10.3305/nh.2013.28.3.6447
66. Reece-Stremtan S, Marinelli KA, The Academy of Breastfeeding Medicine (Eds.). ABM Clinical Protocol #21: Guidelines for Breastfeeding and Substance Use or Substance Use Disorder, Revised 2015. Breastfeed Med. 2015 10(3)
67. Baby Friendly Initiative; UNICEF UK. Co-sleeping guide for health professionals. 2016; Services website: https://www.unicef.org.uk/babyfriendly/baby-friendly-resources/sleep-and-night-time-resources/co-sleeping-and-sids/.
68. Anderson PO. Drugs for Treating Alcohol Use Disorder During Lactation. Breastfeed Med. 2022;17(11):872-874 PMID: 36378820. DOI: 10.1089/bfm.2022.0241
69. López Mincitar M, Angulo Castellanos E, García Morales E, Amezcua López JA. Microbiota en leche de madres de recién nacidos sanos y con diagnóstico de sepsis atendidos en neonatología del Hospital Civil de Guadalajara "Fray Antonio Alcalde" [Tesis de especialidad]. Universidad de Guadalajara. 2018 Guadalajara, Jalisco, México
70. Amezcua López JA, García Morales E, Solís Pacheco JR, Gutiérrez Padilla JA, Minia Zepeda Morales AS, Angulo Castellanos E, López Mincitar M, Flores Arévalo KF, Rodríguez Arreola A, Aguilar Uscanga BR. Influence of the diet of Mexican women on the nutritional quality and the presence of beneficial microorganisms in human milk. Nutr Hosp. 2019;36(4):786-791 DOI: 10.20960/nh.02477
71. Department of Child and Adolescent Health and Development. Analgesics, antipyretics, nonsteroidal anti-inflammatory drugs, drugs used to treat gout and disease-modifying agents used in rheumatic disorders. En: UNICEF, editor. BREASTFEEDING AND MATERNAL MEDICATION, G AND MATERNAL MEDICATION Recommendations for Drugs in the Eleventh WHO Model List of Essential Drugs. UNICEF. 2002 pp. 6-7. Services website: https://apps.who.int/iris/bitstream/handle/10665/62435/55732.pdf?sequence=1&isAllowed=y
72. Arroyo-Cabrales LM, Canseco-Herrera M, Castillo-Romero MG, Belmont-Gómez A. Addicted mothers: determination of drug levels and evaluation of growth and development of their children in the first six months of life. Perinatol Reprod Hum. 2012;26(3):180-186 Services website: https://www.scielo.org.mx/scielo.php?pid=S0187-53372012000300003&script=sci_abstract, de https://www.scielo.org.mx/pdf/prh/v26n3/v26n3a3.pdf
73. The Organization of Teratology Information Specialists. Cocaine. MotherToBaby. Servicios en línea: https://mothertobaby.org/fact-sheets/cocaine-pregnancy/. 2020
74. Office of Disease Prevention, Health Promotion, Office of the Assistant Secretary for Health, Office of the Secretary, U.S. Department of Health and Human Services. Eat Healthy While Breastfeeding: Quick Tips [Internet]. Pregnancy. 2023 [cited July 13/2023]. Disponible en: https://health.gov/myhealthfinder/pregnancy/nutrition-and-physical-activity/eat-healthy-while-breastfeeding-quick-tips
75. OASH (Office of Disease Prevention and Health Promotion). Tips: How to eat a healthy diet while breastfeeding. 2020 [cited July 29/2023]; Services website: https://health.gov/espanol/myhealthfinder/embarazo/nutricion-actividad-fisica/alimentate-saludablemente-mientras-amamantas-consejos-rapidos.
76. Ramos-Clemente Armando W. Actividad antibacteriana del extracto de Erythroxylum coca sobre Porphyromonas Gingivalis, estudio in vitro. 2012 [cited February 18/2023]; Services website: https://core.ac.uk/download/pdf/323347868.pdf.
77. Jiménez M. Efecto del sistema cannabinoide endógeno sobre la función de barrera intestinal. [España]: Universidad Complutense de Madrid. 2019
Author Biography
Jesús Alonso Amezcua López: Food and Biotechnology Engineer from the Universidad de Guadalajara, México. Final-year student of the Doctorate in Science in Biotechnological Processes at the University Center for Exact Sciences and Engineering at the Universidad de Guadalajara. Professor in the Nutrition degree program and the Food Engineering degree program. Activist and collaborator in the Comprehensive University Breastfeeding Program, and APROLAM. Researcher in the field of hospital nutrition with a focus on maternal and child nutrition, specializing in neonatal feeding. Winner of the Latin American Outstanding Student Award 2023 in the "Innovative Idea" category.
Elisa García Morales: Medical Doctor, Surgeon, and Obstetrician from the Universidad de Guadalajara, México. Specialist in Pediatrics from the same university. Sub-specialization in Neonatology from the Universidad Nacional Autónoma de Mexico of Mexico. Master's degree in human nutrition, with a focus on maternal and child health, and a Doctorate in Clinical Research from the Universidad de Guadalajara, Mexico. Member of the Mexican Council of Certification in Pediatrics, A.C. and the College of Pediatrics of Jalisco, A.C. Specialist Physician "C" since 1998 to date in the Neonatology Service, Pediatric Division, Hospital Civil de Guadalajara "Fray Antonio Alcalde." Candidate for membership in the National System of Researchers. Full professor of the Neonatology specialty and Associate Professor "B" at the School of Medicine, Bachelor's Degree in Medicine, Department of Human Reproduction, Child Growth and Development, University Center for Health Sciences, Universidad de Guadalajara.
Daniel Pérez-Rulfo Ibarra: Medical Doctor, Surgeon, and Obstetrician from the Universidad de Guadalajara, México. Specialist in Pediatrics from the Universidad Nacional Autónoma de México. Sub-specialization in Pediatric Neurology from the Instituto Nacional de Pediatría. Master's degree in Family Sciences from Universidad Anáhuac from México, and a Doctorate in Administration from the Instituto Universitario Hispano Mexicano. Member of the Mexican Council of Certification in Pediatrics, A.C. Member of the National System of Researchers (SNI) level 1, and member of the Mexican Council of Neurology, A.C. Professor at the Universidad de Guadalajara, at the University Center for Health Sciences, in the Department of Human Reproduction, Growth, and Development.
Josué Raymundo Solís Pacheco: Doctor of Science in Biotechnology, INSA-Toulouse, France. Diploma of in-depth studies "Master," INSA-Toulouse, France. Biochemical Engineer in Food, Technological Institute of Tehuacán, Puebla, México. Full-Time Research Professor B at the Universidad de Guadalajara. Member of the National System of Researchers, Level I. Professor with PRODEP profile. Reviewer for scientific articles in international journals such as Food and Bioprocess Technology, Food Analytical Methods, Journal of Advances in Microbiology, Food Biotechnology, Journal of Food Process. Additionally, reviewer and evaluator of CONACYT and PEI projects.
Blanca Rosa Aguilar Uscanga: Doctor of Science in Biotechnology, graduate of the Institut National des Sciences Appliquées in Toulouse, France. Master of Food Science and Biochemical Engineer specializing in Food. Currently a full-time Research Professor at the University Center for Exact Sciences and Engineering at Universidad de Guadalajara in México. Member of the National System of Researchers (SNI) with a level 2 designation. Honorary Associate Professor at INRS-Institute Armand Frappier in Canada. Evaluator for CONACYT, PRODEP, and reviewer for scientific articles in various international journals. Currently serving as the President of the International Food Sciences Congress of the Common Ground International Network for Nutritional Studies. The research areas she focuses on include Food Biotechnology, development of functional foods such as human milk, probiotics production, microbial metabolites, and food safety.
![]() Corresponding author: blanca.aguilarudg.mx.
Corresponding author: blanca.aguilarudg.mx.

 Global reach, higher impact
Global reach, higher impact