Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(10):1272-1281. doi:10.7150/ijms.86738 This issue Cite
Research Paper
A functional evaluation of anti-fatigue and exercise performance improvement following vitamin B complex supplementation in healthy humans, a randomized double-blind trial
1. Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan City 333325, Taiwan.
2. Graduate Institute of Applied Science and Engineering, Fu-Jen Catholic University, New Taipei City, 242062, Taiwan.
3. Tajen University, Pingtung 907101, Taiwan.
Received 2023-6-2; Accepted 2023-8-9; Published 2023-8-15
Abstract
B vitamins play a crucial role in maintaining fundamental cellular functions and various essential metabolic pathways in the body. Although they do not directly provide energy, each B vitamin acts as a cofactor in energy metabolism processes. Based on the evidence presented above, we hypothesized that a 28-day supplementation of vitamin B would enhance physical performance and reduce physical fatigue. The objective of this study was to evaluate the anti-fatigue effect of vitamin B supplementation, specifically vitamin B1, B2, B6, and B12, and its potential to improve exercise performance. We employed a randomized double-blind crossover design with a 28-day supplementation period. Sixteen male and sixteen female subjects, aged 20-30 years, were divided into two groups: the placebo group (n=16, equal gender distribution) and the Ex PLUS® group (n=16, equal gender distribution). The participants received either placebo or Ex PLUS® (one tablet per day) for 28 consecutive days. Following the intervention, there was a 14-day wash-out period during which the subjects did not receive any further interventions. After supplementation with Ex PLUS®, we found a significant increase in the running time by 1.26-fold (p < 0.05) to exhaustion compared to that before supplementation and that in the placebo group. In addition, the Ex PLUS® supplementation group presented significantly reduced blood lactate and blood ammonia concentrations during exercise and at rest after exercise compared with placebo (p < 0.05). In conclusion, 28 consecutive days of vitamin B complex (Ex PLUS®) supplementation significantly improved exercise endurance performance and reduced exercise fatigue biochemical metabolites in not athletes. In addition, it does not cause adverse effects in humans when taken at appropriate doses.
Keywords: B vitamins, exercise performance, antifatigue, endurance, health
Introduction
Fatigue, defined as the inability to maintain power output and strength, is a symptom or comorbidity of neurological disorders [1]. An important feature of fatigue is the "feeling of exhaustion," the mental or physical exhaustion that occurs when the demands of the brain or muscles cannot be met [2]. Among them, physiological fatigue is further divided into central fatigue and peripheral fatigue, which are mainly caused by excessive physical load, insufficient rest, and mental stress [3]. A previous study found that more than 50% of people feel fatigued, and more than one-third of them explicitly believe that they are affected by fatigue, which severely reduces their quality of life and productivity [4]. However, it is also a physiological mechanism of self-protection after the body reaches a certain level of activity, which can prevent the occurrence of life-threatening excessive functional failure [5]. During exercise, with increasing exercise time or intensity, a large amount of energy is consumed. If the original supply cannot be maintained continuously, the glycogen in the liver and muscle will be broken down into glucose, then further metabolized to meet higher energy needs [6]. When metabolites such as lactic acid, blood ammonia, and blood urea nitrogen accumulate too much, resulting in an imbalance of pH and osmotic pressure, energy supply cannot be maintained, resulting in muscle fatigue and reduced exercise performance [7]. Therefore, it is necessary to adopt appropriate strategies to improve or delay the generation of fatigue, among which a strategy through diet or nutritional supplementation is one of the most direct methods [8].
In addition to specific efficacy properties, many foods contain essential nutrients, including vitamins and minerals, which play an important role in maintaining essential cellular functions and various essential metabolic pathways [9], including involvement in energy production, DNA synthesis, oxygen transport, and metabolism [2]. Among them, the vitamins B are a group of eight water-soluble vitamins, all of which, except folic acid, are involved in at least one step of the intracellular energy production system [10]. Vitamin B acts as a coenzyme in metabolic and anabolic enzymatic reactions and is a cofactor for many essential enzymes involved in RNA and DNA biosynthesis [11]. An adequate supply of each vitamin B is necessary for the proper functioning of the energy production system, but each vitamin B has different pathways involved in energy metabolism. A shortage of any of these vitamins can limit the rate of energy production and can have serious metabolic and health consequences [12].
Vitamin B1 (thiamine) is rapidly absorbed by the small intestine into three phosphorylated forms: thiamine monophosphate (TMP), thiamine pyrophosphate (TPP), and thiamine triphosphate (TTP) [13]. TPP is involved in protein, lipid, and carbohydrate metabolism to produce dehydrogenase reactions, resulting in the decarboxylation of pyruvate and branched-chain amino acids (BCAA) to form acetyl-CoA [14]. Vitamin B2 (riboflavin) is a component of the coenzymes flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), redox cofactors of several dehydrogenases involved in energy metabolism, redox balance, and other cell regulatory processes [15]. Vitamin B6 (pyridoxine) plays an important role in the metabolism of fatty acids, carbohydrates and amino acids and plays a key role in the energy production of the citric acid cycle [16]. It is involved in amino acid metabolism, one-carbon reactions, glycogenolysis and gluconeogenesis, heme synthesis, and tryptophan formation from niacin, as well as lipid metabolism and hormonal action, and it provides additional glucose when needed2. Vitamin B12 (cobalamin) is a general term for a group of compounds called corrinoids, coenzymes involved in the metabolism of every cell in the human body, especially affecting the synthesis and regulation of DNA but also affecting fatty acid metabolism and amino acid metabolism [16, 17]. The above four vitamins B (B1, B2, B6, B12) are mainly involved in energy metabolism and utilization, and they are also the main vitamin B types commonly used as dietary supplements [18].
While food intake is the main source of energy, vitamins B act as catalysts, helping to convert energy into better uses to more efficiently supply what the body needs [19]. Therefore, to a certain extent, taking an appropriate amount of vitamin B complex can also be regarded as a sports nutrition supplement, thereby improving exercise performance. However, no prior studies have examined the benefits of vitamin B supplementation on exercise performance alone. Therefore, in this trial, we aimed to evaluate the benefits of vitamin B complex supplementation in enhanced exercise performance, delayed fatigue, and physiological adaptation in humans.
Materials and methods
Vitamin B complex and placebo preparation
The supplement in this study was provided by Prince Pharmaceutical Co. Ltd. (New Taipei City, Taiwan). One vitamin B complex tablet (Ex PLUS®) contains vitamin B1 (33.6 mg), vitamin B2 (10 mg), vitamin B6 (50 mg), vitamin B12 (750 µg), vitamin E (16.8 mg), inositol (20 mg), calcium (18.9 mg), and taurine (20 mg). The placebo had the same color and size but did not contain the above active ingredients.
Participants
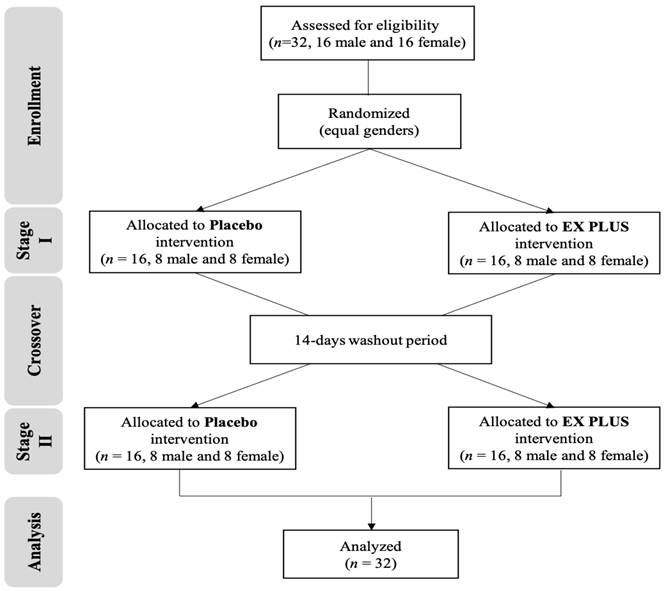
This study was conducted in accordance with the Declaration of Helsinki and was approved and reviewed by the Institutional Review Board of Landseed International Hospital (Taoyuan, Taiwan; LSHIRB number 20-037-A2). The trial is first registered with clinicaltrials.gov as NCT05586295 (09/12/2022). Sixteen male and sixteen female healthy adult non-athletes aged 20-30 were included. Exclusions were made based on smoking, cardiovascular disease, hypertension, body mass index (BMI) >27, metabolic disease, asthma and sports injury (nerve, muscle, bone). After a detailed explanation of all the risks and benefits of the experimental procedure, the consenting participants signed the informed consent form in person before starting the experiment. Subjects were required to cooperate by maintaining a normal diet during the experiment and not taking nutritional supplements such as alcohol or vitamin-B-related products. The participant flow chart was shown on Figure 1, and subjects' basic information as shown on Table 1.
Subjects' basic information.
| Characteristics | Placebo in stage I | Ex PLUS® in stage I |
|---|---|---|
| Age (years) | 21.3±1.2 a | 21.3±1.2 a |
| Height (cm) | 168.8±8.9 a | 168.8±8.9 a |
| Weight (kg) | 62.4±7.6 a | 63.5±7.2 a |
| VO2max (mL/kg/min) | 44.5±5.1 a | 44.3±5.0 a |
Data are presented as mean ± SD. The same superscript letters (a) indicate no significant difference between groups at p > 0.05.
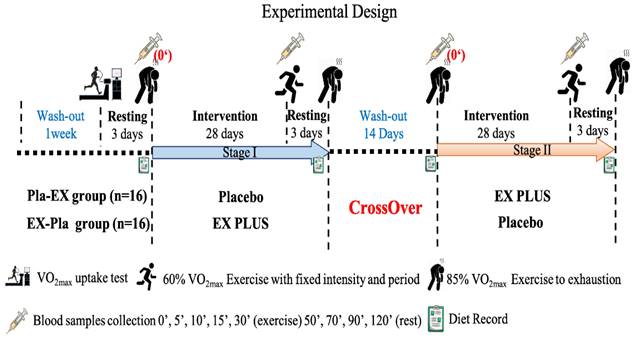
Experimental design
Based on previous testing periods of animal and human B-vitamin supplementation, a duration of 28 consecutive days was chosen as the supplementation period [14, 20]. For this trial, we used a randomized double-blind crossover design, the blinding was assisted by Prince Pharmaceutical Co., Ltd. (New Taipei City, Taiwan), and the blinding was lifted after the experiment was completed. Subjects were randomly assigned to a placebo or Ex PLUS® group (n=16/group, equal genders) by random selection and supplemented with placebo or Ex PLUS® (one tablet per day) for 28 consecutive days. Following the intervention, there was a 14-day wash-out period during which subjects received no further intervention. This was followed by a second 28-day replacement therapy intervention cycle (subjects who received placebo during the first cycle received Ex PLUS® and vice versa). All subjects were supplemented only during the experiment and did not participate in any exercise training.
Before each phase of intervention, we measured the subjects' body composition, common blood biochemical parameters, and exercise tolerance. After consecutive 28 days of supplementation, we re-measured the subjects' exercise fatigue biochemical values, exercise endurance performance, and body composition. In addition, we asked all subjects to record their diet before and after the intervention, and a professional nutritionist analyzed the daily nutrient intake to ensure that the relevant sports performance was not affected by diet (Figure 2).
Participant flow chart.
Experimental design.
Maximal oxygen uptake (VO2max)
VO2max was used as a reference for exercise fatigue detection and exercise endurance intensity setting. We performed VO2max measurements according to Bruce's protocol and as previously described [21, 22], using a treadmill (Pulsar, h/p/cosmos, Nussdorf-Traunstein, Germany) and an automatic breathing analyzer (Vmax 29c, Sensor Medics, Yorba Linda, CA, USA), and monitored heart rate (HR) using a polar heart rate device (Polar Electro Oy, Kempele, Finland). The treadmill started at 7.2 km/h and increased by 1.8 km/h every 2 minutes until exhaustion. When the HR reached the maximum (maximum heart rate = 220-age), the respiratory exchange rate exceeded 1.10 (the volume ratio of carbon dioxide produced to oxygen consumed, VCO2/VO2), and the rating of perceived exertion (RPE) reached 10, a subject was determined to be exhausted. We then averaged the three highest VO2max values in the test for the subjects to obtain VO2max values.
Endurance performance test and exercise fatigue-related indicators
According to the heart rate and speed recorded during the maximum oxygen uptake test, a regression calculation is performed to obtain the heart rate and speed corresponding to 60% and 85% of the maximum oxygen uptake, and the speed is adjusted according to the heart rate value. The detailed formula for intensity adjustment was based on a previous study [23]. Before the formal test, according to each subject's personal 60% VO2max intensity, after a 5-minute warm-up on the treadmill, we set the intensity to a maximum of 85% VO2max to start the test. Subjects were instructed to run to exhaustion on a treadmill, and the time was recorded as their individual endurance performance. We monitored the subjects' physical condition every 2 minutes by heart rate.
On the other hand, to assess changes in indicators of fatigue, all subjects were asked to fast for at least 8 hours before the test. On the test day, we set a personal 60% VO2max intensity for each subject to run for 30 minutes and rest for 90 minutes, during which blood was collected at baseline; at exercise periods 5 (E5), 10 (E10), 15 (E15), and 30 (E30) minutes; and at 20 (R20), 40 (R40), 60 (R60), and 90 (R90) minutes of rest after the 30 minutes of exercise. All collected blood was centrifuged to obtain serum and analyzed for fatigue indicators, including lactate, blood ammonia (NH3), glucose, and creatine kinase (CK), using a Hitachi 7060 autoanalyzer (Hitachi 7060, Tokyo, Japan).
Body composition
Subjects underwent body composition measurements before and after each phase of the intervention. All subjects were required to fast for 8 hours before the measurement. During the test, all subjects stood on the bottom electrode with arms extended at a 30° angle to the torso, held the induction handle with both hands, and did not move or speak. Measurements were performed within 60 s at 1, 5, 50, 260, 500, and 1000 kHz using a bioelectrical impedance analyzer (BIA) (InBody 570, In-body, Seoul, Korea) with the multi-frequency principle.
Clinical biochemistry and hematology analysis
Blood was collected from each subject before each phase of supplementation for clinical biochemical and hematological analysis to confirm the basic biochemical indicators and health status. All the subjects were asked to fast for 8 hours the night before blood was drawn. After blood collection, the serum was obtained by centrifugation, and aspartate transaminase (AST), alanine aminotransferase (ALT), albumin (ALB), blood urea nitrogen (BUN), creatinine (CREA), uric acid (UA), total protein (TP), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) indicators were measured using an automatic analyzer (Hitachi 7060, Hitachi, Tokyo, Japan). In addition, the complete blood count (CBC) profiles were also analyzed (MindrayBC-2800 Vet, Shenzhen, China).
Statistical analysis
All data are expressed as the mean ± SD. Statistical analyses were performed in IBM SPSS Statistics ver. 24.1 (IBM Co., Armonk, NY, USA). Differences within groups before and after the intervention were analyzed using a Bonferroni-adjusted paired t-test. Intergroup differences were analyzed using Student's unpaired t-test. For non-parametric data, including fat mass and ratio of body composition change, the Mann-Whitney U test was used for comparison. Time to exhaustion was analyzed via two-way repeated-measures ANOVA with post hoc Bonferroni test. Differences were considered statistically significant at p < 0.05. We used the Harvard calculator (http://hedwig.mgh.harvard.edu/sample_size/size.html) to calculate the required sample size for the clinical trial. We assumed a 0.05 significance level, a power of 0.8, and a standard deviation of the difference within 0.73. From these assumptions, at least 32 patients were required for this two-treatment crossover study.
Results
Subjects' dietary analysis and Body composition changes
In order to ensure that subjects' exercise performance and fatigue biochemical values were not affected by differences in dietary intake and energy, 3 days dietary record analysis was performed before and after Ex PLUS® supplementation. As shown in Table 2, there were no significant differences in carbohydrate, protein, or fat intake between the placebo and Ex PLUS® groups before and after the intervention, and there were no significant changes within either group after the intervention. On the other hand, body composition can also be affected by dietary changes, which, in turn, can affect exercise performance. The Ex PLUS® supplementation did not cause changes in body composition, nor was there a significant difference between the two groups (Table 2).
Effects of Ex PLUS® supplementation on biochemical parameters and hematology
Table 3 shows the routine serum biochemical indicators and hematological data of the placebo and Ex PLUS® groups before and after the intervention. The results showed that all subjects participated in this trial in a healthy state, and there was no damage to various indicators in the blood after the intervention.
Effects of Ex PLUS® supplementation on fatigue biochemical parameters during exercise and rest
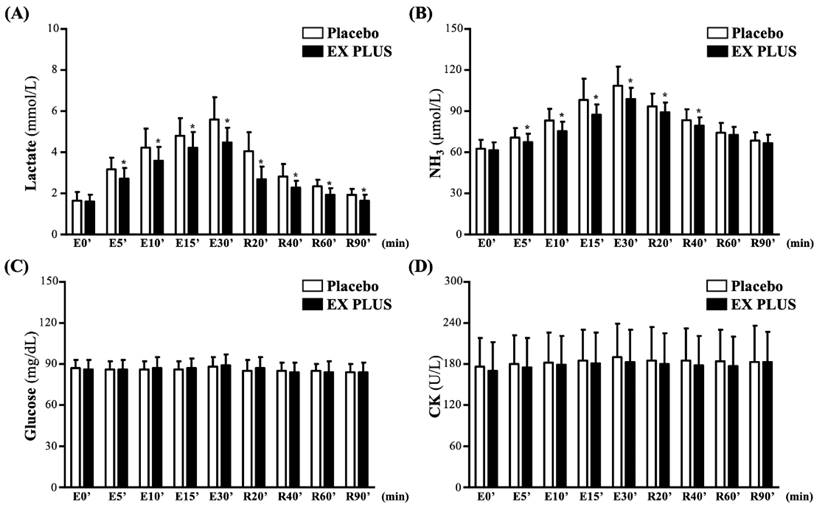
Both lactate and NH3 levels gradually increased during exercise and gradually decreased after rest and recovery. As shown in Figure 3A, compared to the placebo group, the Ex PLUS® group presented a significantly reduced level of lactate production from exercise at 5 minutes until 30 minutes after exercise, with accelerated recovery from rest at 20 minutes until test completion (p < 0.05). This was similar to the NH3 levels, with the Ex PLUS® group presenting a significantly reduced NH3 production level from 5 minutes to 30 minutes after exercise and accelerated recovery from rest at 20 and 40 minutes compared to the placebo group (p < 0.05) (Figure 3B). However, during recovery from exercise and rest, the glucose level and CK activity did not fluctuate significantly, and there was no significant difference in them between the two groups (Figure 3C and 3D).
Subjects' dietary intake and body composition.
| Characteristics | Items | Before | After | ||
|---|---|---|---|---|---|
| Placebo | Ex PLUS® | Placebo | Ex PLUS® | ||
| Dietary intake | Carbohydrate (g/day) | 172±27 a | 171±19 a | 169±24 a | 167±18 a |
| Protein (g/day) | 71±19 a | 70±14 a | 69±18 a | 70±17 a | |
| Fat (g/day) | 75±16 a | 74±8 a | 77±13 a | 76±8 a | |
| Total Calories (kcal/day) | 1654±272 a | 1628±140 a | 1648±257 a | 1627±130 a | |
| Body composition | Body weight (kg) | 62.4±7.6 a | 63.5±7.2 a | 62.3±7.9 a | 62.9±7.1 a |
| BMI (kg/m2) | 21.9±1.9 a | 22.3±2.0 a | 21.9±1.6 a | 22.1±1.6 a | |
| Muscle mass (kg) | 28.3±5.3 a | 28.4±5.3 a | 28.2±5.4 a | 28.2±5.2 a | |
| Fat mass (%) | 19.4±6.7 a | 20.6±6.9 a | 19.4±6.8 a | 20.5±7.0 a | |
Data are presented as mean ± SD. The same superscript letters (a) indicate no significant difference between groups at p > 0.05. BMI, body mass index.
Biochemical analysis and blood count profiles of the subjects before and after the intervention.
| Characteristics | Items | Before | After | ||
|---|---|---|---|---|---|
| Placebo | Ex PLUS® | Placebo | Ex PLUS® | ||
| Biochemistry parameters | AST (U/L) | 24±5 a | 23±5 a | 24±4 a | 23±5 a |
| ALT (U/L) | 14±4 a | 14±3 a | 14±4 a | 14±3 a | |
| ALB (g/dL) | 5.15±0.33 a | 5.15±0.30 a | 5.20±0.24 a | 5.17±0.30 a | |
| TC (mg/dL) | 191±20 a | 193±25 a | 193±20 a | 193±25 a | |
| TG (mg/dL) | 54±14 a | 57±16 a | 57±13 a | 57±16 a | |
| HDL (mg/dL) | 67.6±6.2 a | 67.2±6.2 a | 68.3±5.8 a | 67.5±5.5 a | |
| LDL (mg/dL) | 94.6±12.6 a | 95.2±12.6 a | 95.6±12.4 a | 95.6±12.2 a | |
| BUN (mg/dL) | 15.7±2.6 a | 15.3±2.7 a | 15.8±2.4 a | 15.4±2.8 a | |
| CREA (g/dL) | 1.10±0.11 a | 1.08±0.12 a | 1.11±0.10 a | 1.09±0.12 a | |
| UA (mg/dL) | 5.2±1.0 a | 5.2±1.1 a | 5.3±1.0 a | 5.2±1.1 a | |
| Glucose (mg/dL) | 81±6 a | 83±7 a | 82±6 a | 83±7 a | |
| CBC | WBCs (103/mcL) | 5.9±0.9 a | 5.9±0.8 a | 6.1±0.8 a | 6.2±0.8 a |
| Neutrophils (%) | 54.7±5.3 a | 54.1±6.7 a | 54.5±6.2 a | 53.9±8.9 a | |
| Lymphocytes (%) | 34.2±4.4 a | 34.7±6.6 a | 35.0±5.1 a | 35.3±8.7 a | |
| Monocytes (%) | 7.5±1.4 a | 7.3±1.4 a | 7.0±1.7 a | 7.0±1.5 a | |
| Eosinophils (%) | 3.0±1.8 a | 3.0±2.2 a | 3.2±2.0 a | 2.7±1.9 a | |
| Basophils (%) | 0.7±0.2 a | 0.8±0.4 a | 0.7±0.3 a | 0.8±0.4 a | |
| Platelets (103/mcL) | 260±33 a | 262±36 a | 251±44 a | 255±39 a | |
Data are shown as the mean ± SD. Statistical significance between the Placebo and Ex PLUS® groups at the same collection time points was analyzed using Student's unpaired t-test. The same superscript letter (a) indicates no significant differences among the groups at p > 0.05. AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CREA, creatine; UA, uric acid; TP, total protein; TC, total cholesterol; TG, triacylglycerol. HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, white blood cell.
Effects of Ex PLUS® supplementation on (A) lactate, (B) NH3, (C) CK, and (D) glucose serum levels during and after exercise. Data are shown as the mean ± SD. *Significant difference compared to the placebo group (p<0.05).
Effects of Ex PLUS® supplementation on endurance performance
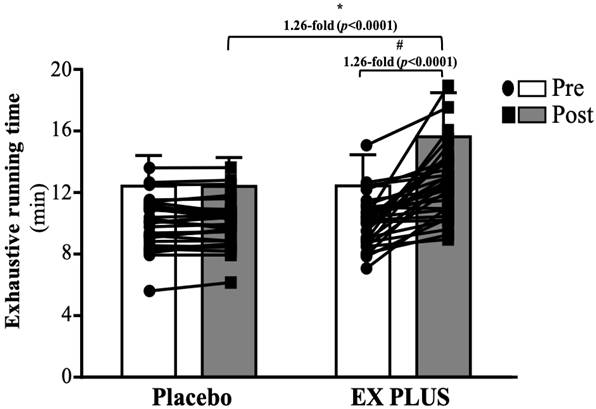
Before intervention, the exhaustion test times were 12.57 ± 2.03 and 12.59 ± 2.06 (min) for the placebo and Ex PLUS® groups, respectively. There was no significant difference between the two groups. After 28 days of intervention, the times to exhaustion were 12.56 ± 1.90 and 15.82 ± 1.91 (min) for the placebo and Ex PLUS® groups, respectively. The Ex PLUS® group showed a significant 1.26-fold improvement in time to exhaustion with regard to both baseline measurements (p < 0.0001) and the placebo group (p < 0.0001) (Figure 4).
Effects of Ex PLUS® supplementation on the time to exhaustion. Data are shown as the mean ± SD, n = 32 subjects/group. *Significant difference compared to the placebo group (p<0.05). #Significant difference (p<0.05) compared with the pretest in the same group.
Discussion
In humans, nutrients and energy are primarily used by the body through food intake. Although the accompanying vitamins or minerals do not act as a primary energy source, they play a vital role in energy metabolism. In the current study, we found that consecutive 28 days of Ex PLUS® supplementation improved exercise endurance performance and reduced fatigue metabolites, such as lactate or blood ammonia in not athletes. In addition, supplementation in appropriate doses did not cause adverse effects on the human body.
B vitamins are involved in the regulation of energy metabolism and in the synthesis and degradation of carbohydrates, proteins, and fats [24]. Moderate supplementation has potential synergistic effects on improving physical activity, energy metabolism, and exercise performance [25]. The accumulation of lactate and ammonia during exercise is generally believed to increase hydrogen ions and acidosis in muscles and is considered a major cause of muscle fatigue [26]. Thiamine is primarily stored in muscles, and when the intracellular concentration of thiamine decreases, it reduces the activation of enzymes that lead to ATP biosynthesis; this causes fatigue, which, in turn, reduces exercise performance [27]. However, an increase in serum thiamine is associated with a decrease in pyruvate and lactate levels [28]. A past animal study observed a decrease in lactate production following thiamine administration [29]. In addition, in trials of three crossover treatments (placebo, training, and thiamine), both training and thiamine intervention reduced blood lactate concentrations in athletes during exercise [30]. This might be due to the fact that thiamine not only is a coenzyme of pyruvate dehydrogenase, which stimulates pyruvate, but also can be converted to acetyl-CoA by the decarboxylation of BCAA and α-keto acids, which then enter the citric acid cycle to generate NADH and FADH2 and then generate ATP through the electron transport chain to provide the energy required for movement [2, 31, 32]. In our previous animal experiments, we found that 4 weeks of thiamine tetrahydrofurfuryl disulfide supplementation significantly improved the endurance performance of mice, showing a dose-dependent effect [13]. The current study also confirmed that vitamin B complex supplementation in humans could reduce the rate of increase in lactate during fixed-intensity exercise and had the effect of accelerating clearance (Figure 3A). On the other hand, riboflavin is composed of two coenzymes (FAD and FMN) and is an important component involved in the energy metabolism of carbohydrates, fats, and proteins and in electron transfer in steroid hormone production [32]. Among them, FAD is involved in further energy production through the β-oxidation of fatty acids and through the oxidative decarboxylation of pyruvate to produce acetyl-CoA from the catabolism of glucose and branched-chain amino acids [9].
Among other B vitamins, vitamin B6, folic acid, and vitamin B12 all contribute to the metabolism of homocysteine and are involved in the metabolism of proteins and amino acids, which, in turn, play a role in important pathways used during physical activity [33]. Among them, folic acid and vitamin B12 are also key nutrients for damaged cell and tissue repair as coenzymes for deoxyribonucleic acid (DNA) synthesis, erythrocyte synthesis, amino acid metabolism, and the decomposition of odd fatty acid chains, respectively [34]. In addition, vitamin B12 acts as a cofactor to convert amino acids to propionyl-CoA, while fatty acids are oxidized to acetyl-CoA and propionyl-CoA. After entering the tricarboxylic acid (TCA) cycle, propionyl-CoA is carboxylated to methylmalonyl-CoA and finally converted to succinyl-CoA by methylmalonyl-CoA mutase to generate energy [35]. Pyridoxal 5'-phosphate (PLP) is the biologically active form of vitamin B6 in the human body, mainly involved in the metabolism of proteins and amino acids, and is a cofactor for transaminases, decarboxylases, and other enzymes for the metabolic transformation of amino acids and nitrogenous compounds [25, 36, 37]. Ammonia has long been recognized as a central and peripheral factor in the onset of exercise-induced fatigue, and its level increases with exercise intensity and duration [38, 39]. Despite many theories, there have been few studies on B vitamins reducing blood ammonia levels after exercise. In a past study, administration of BCAAs supplemented with vitamin B6 for 8 weeks in taekwondo athletes was found to reduce post-exercise NH3 concentrations [40]. Although our study did not include the addition of protein supplements, vitamin B complex supplementation alone significantly reduced the increase in blood ammonia levels during exercise (Figure 3B). However, the lower increase in NH3 concentration after exercise may be associated with lower ATP consumption and slower rates of glycolysis and glycogenolysis [41]. Another way that vitamin B6 produces energy is by participating in glycogenolysis and gluconeogenesis during exercise [42]. During exercise, the process of gluconeogenesis involves the breakdown of amino acids to provide energy to muscles and the conversion of lactate to glucose in the liver, and several enzymes are involved in this metabolically driven conversion [43]. This may be one of the factors that improved exercise endurance performance in this study. Additionally, a past study reported on eight physically active subjects who performed two tests on a bicycle ergometer at 60% and 85% of VO2max for 30 minutes and 20 minutes, respectively. The results found no significant difference in blood glucose concentration [44] which was similar to our findings (Figure 3D), but the related mechanism still needs to be further explored.
In the current study, we demonstrated that consecutive 28 days of vitamin B complex supplementation significantly improved exercise endurance performance. In addition to helping the metabolism of nutrients to produce energy through the B vitamins, other elements may be involved. Taurine is an important free amino acid that, although not fully involved in protein synthesis, has multiple physiological effects, including regulation of osmotic pressure, membrane stability and calcium kinetics, as well as the enhancement of systemic anti-inflammatory responses and total antioxidant capacity [45, 46]. These properties can directly boost physical performance and reduce muscle fatigue. In addition, other studies have shown that taurine enhances exercise endurance performance by enhancing the gluconeogenesis of amino acids (AAs) in the liver and levels of glycerol in the blood, promoting lipid metabolism, and improving hepatic glucose storage and energy [47]. On the other hand, taurine deficiency increases the NADH/NAD+ ratio, inhibits pyruvate dehydrogenase activity, and causes pyruvate deficiency, thereby significantly reducing glucose oxidation and ATP biosynthesis [48], which may affect CK levels in blood and muscle [45]. A past study showed that acute taurine supplementation (approximately 1 g taken 5 times daily before and after exercise) during endurance training was effective in reducing blood lactate levels during endurance training [49]. However, our dose was only 2% of that used in the past study, so the reduction in lactate concentrations in the current study may not be entirely the benefit of taurine supplementation. Furthermore, CK activity did not change significantly in this study, which may be due to the fact that CK activity usually increases gradually 2 hours after exercise and peaks at 24-72 hours, which may also be affected by exercise intensity [50]. These may be factors that contributed to the relative flattening of CK activity in the blood during and after exercise in this study (Figure 3C). Unlike the B vitamins, vitamin E acts as a fat-soluble and chain-breaking antioxidant that prevents the progression of lipid peroxidative chain reactions and maintains the integrity of polyunsaturated fatty acids in cell membranes [51]. Although vitamin E plays a key role in protecting the central nervous system from free radical damage, there is still a polar debate about whether vitamin E protects against post-exercise injury [52]. Additionally, vitamin E was not found to interact with B vitamins in an earlier study [53]. Therefore, it is necessary to further explore whether the combination of vitamin E and B vitamins has a better synergistic effect on exercise performance in the future.
In the current study, we demonstrated that B-complex vitamin supplementation (Ex PLUS®) has the effect of improving exercise endurance performance and reducing exercise fatigue. However, there are still some limitations in the research process, including the following: 1: There are congenital differences in physique or physiological metabolism between men and women. To more clearly explore the efficacy of placebo and Ex PLUS® in improving exercise performance and reducing fatigue, a gender-excluded statistical analysis was performed. 2: In this study, subjects were required to provide blood samples at different time points during exercise and at rest after exercise. After analyzing the indicators related to exercise fatigue, there was no extra blood to analyze the concentrations of various B vitamins in the blood. 3: The importance of glycogen storage for exercise endurance performance is well known. However, in human trials, muscle puncture is required to detect glycogen levels in muscle. This may involve regulations in medical practice and the need for subjects to recover over an extended period of time, thus excluding glycogen testing from this study.
Conclusions
In conclusion, in the current study, we demonstrated that 28 consecutive days of supplementation with Ex PLUS® (with a vitamin B complex, taurine, and vitamin E) increased exercise endurance performance, reduced post-exercise fatigue metabolite production, and accelerated recovery in not athletes. In addition, adequate supplementation does not cause any adverse damage or burden to the human body.
Acknowledgements
The authors are grateful to the graduate students at the Sport Nutrition Laboratory, National Taiwan Sport University, for their technical assistance in conducting the analysis experiments.
Funding
This study was supported by Prince Pharmaceutical Co. Ltd. and the Ministry of Science and Technology of Taiwan (application type: academia-industry collaboration project). The grant number is MOST-109-2622-H-179-001.
Authors' contributions
Mon-Chien Lee, Sih-Yu Shen and Chi-Chang Huang designed the study. Mon-Chien Lee, Chin-Shan Ho and Chi-Chang Huang carried out the experiments. Mon-Chien Lee, Sih-Yu Shen and Chin-Shan Ho analyzed the data and interpreted the results. Mon-Chien Lee and Chi-Chang Huang wrote the manuscript.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organization; 2020
2. Xu Y, Lian Y, Li J, Zhang Y, Liu Y, Wang X. et al. KangPiLao decoction modulates cognitive and emotional disorders in rats with central fatigue through the GABA/Glu pathway. Front Pharmacol. 2022;13:939169. doi: 10.3389/fphar.2022.939169
3. Tardy AL, Pouteau E, Marquez D, Yilmaz C, Scholey A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients. 2020;12(1):228. doi: 10.3390/nu12010228
4. Yamamoto T, Azechi H, Board M. Essential role of excessive tryptophan and its neurometabolites in fatigue. Can J Neurol Sci. 2012;39(1):40-47 doi: 10.1017/s031716710001266x
5. Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol. 2017;13(11):662-675 doi: 10.1038/nrneurol.2017.117
6. Yang Y, Wang H, Zhang M, Shi M, Yang C, Ni Q, Wang Q, Li J, Wang X, Zhang C, Li Z. Safety and antifatigue effect of Korean Red Ginseng capsule: A randomized, double-blind and placebo-controlled clinical trial. J Ginseng Res. 2022;46(4):543-549 doi: 10.1016/j.jgr.2021.09.001
7. Gonzalez JT, Fuchs CJ, Betts JA, van Loon LJ. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am J Physiol Endocrinol Metab. 2016;311(3):E543-553 doi: 10.1152/ajpendo.00232.2016
8. Westerblad H, Allen DG, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci. 2002;17:17-21 doi: 10.1152/physiologyonline.2002.17.1.17
9. Lee MC, Hsu YJ, Ho HH, Hsieh SH, Kuo YW, Sung HC, Huang CC. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms. 2020;8(4):545. doi: 10.3390/microorganisms8040545
10. Alagawany M, Elnesr SS, Farag MR, Tiwari R, Yatoo MI, Karthik K, Michalak I, Dhama K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health - a comprehensive review. Vet Q. 2020;41(1):1-29 doi: 10.1080/01652176.2020.1857887
11. Kennedy DO. B Vitamins and the Brain: Mechanisms, Dose and Efficacy-A Review. Nutrients. 2016;8(2):68. doi: 10.3390/nu8020068
12. Hanna M, Jaqua E, Nguyen V, Clay J. B Vitamins: Functions and Uses in Medicine. Perm J. 2022;26(2):89-97 doi: 10.7812/TPP/21.204
13. Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007;35(3):277-289 doi: 10.1177/147323000703500301
14. Huang WC, Huang HY, Hsu YJ, Su WH, Shen SY, Lee MC. et al. The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement. Nutrients. 2018;10(7):851. doi: 10.3390/nu10070851
15. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine Deficiency: An Important Consideration in Critically Ill Patients. Am J Med Sci. 2018;356(4):382-390 doi: 10.1016/j.amjms.2018.06.015
16. Leone P, Tolomeo M, Piancone E, Puzzovio PG, De Giorgi C, Indiveri C. et al. Mimicking human riboflavin responsive neuromuscular disorders by silencing flad-1 gene in C. elegans: Alteration of vitamin transport and cholinergic transmission. IUBMB Life. 2022;74(7):672-683 doi: 10.1002/iub.2553
17. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, its Panel on Folate, Other B Vitamins, Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US). 1998
18. Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW. et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040. doi: 10.1038/nrdp.2017.40
19. Wu Y, Li S, Wang W, Zhang D. Associations of dietary vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12 and folate equivalent intakes with metabolic syndrome. Int J Food Sci Nutr. 2020;71(6):738-749 doi: 10.1080/09637486.2020.1719390
20. White DJ, Cox KH, Peters R, Pipingas A, Scholey AB. Effects of Four-Week Supplementation with a Multi-Vitamin/Mineral Preparation on Mood and Blood Biomarkers in Young Adults: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients. 2015; 7(11):9005-9017.
21. Ford TC, Downey LA, Simpson T, McPhee G, Oliver C, Stough C. The Effect of a High-Dose Vitamin B Multivitamin Supplement on the Relationship between Brain Metabolism and Blood Biomarkers of Oxidative Stress: A Randomized Control Trial. Nutrients. 2018;10(12):1860. doi: 10.3390/nu10121860
22. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85(4):546-562 doi: 10.1016/0002-8703(73)90502-4
23. Huang WC, Lee MC, Lee CC, Ng KS, Hsu YJ, Tsai TY. et al. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients. 2019;11(11):2836. doi: 10.3390/nu11112836
24. Chen Y, Michalak M, Agellon LB. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J Biol Med. 2018;91(2):95-103
25. Kim YN, Hwang JH, Cho YO. The effects of exercise training and acute exercise duration on plasma folate and vitamin B12. Nutr Res Pract. 2016;10(2):161-166 doi: 10.4162/nrp.2016.10.2.161
26. Baker JS, McCormick MC, Robergs RA. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J Nutr Metab. 2010;2010:905612. doi: 10.1155/2010/905612
27. Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017; 49(10):e384. doi: 10.1038/emm. 2017 194
28. Nozaki S, Mizuma H, Tanaka M, Jin G, Tahara T, Mizuno K. et al. Thiamine tetrahydrofurfuryl disulfide improves energy metabolism and physical performance during physical-fatigue loading in rats. Nutr Res. 2009;29(12):867-872 doi: 10.1016/j.nutres.2009.10.007
29. Falder S, Silla R, Phillips M, Rea S, Gurfinkel R, Baur E. et al. Thiamine supplementation increases serum thiamine and reduces pyruvate and lactate levels in burn patients. Burns. 2010;36(2):261-269 doi: 10.1016/j.burns.2009.04.012
30. Park DH, Gubler CJ. Studies on the physiological functions of thiamine, V. Effects of thiamine deprivation and thiamine antagonists on blood pyruvate and lactate levels and activity of lactate dehydrogenase and its isozymes in blood and tissues. Biochim Biophys Acta. 1969;177(3):537-543
31. Choi SK, Baek SH, Choi SW. The effects of endurance training and thiamine supplementation on anti-fatigue during exercise. J Exerc Nutrition Biochem. 2013;17(4):189-198 doi: 10.5717/jenb.2013.17.4.189
32. Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163(1-2):94-112 doi: 10.1016/j.cbi.2006.04.014
33. Sato A, Sato S, Omori G, Koshinaka K. Effects of Thiamin Restriction on Exercise-Associated Glycogen Metabolism and AMPK Activation Level in Skeletal Muscle. Nutrients. 2022;14(3):710. doi: 10.3390/nu14030710
34. Woolf K, Hahn NL, Christensen MM, Carlson-Phillips A, Hansen CM. Nutrition Assessment of B-Vitamins in Highly Active and Sedentary Women. Nutrients. 2017; 9(4):329. doi: 10.3390/nu9040329.
35. Woolf K, Manore MM. B-vitamins and exercise: does exercise alter requirements? Int J Sport Nutr Exerc Metab. 2006;16(5):453-484 doi: 10.1123/ijsnem.16.5.453
36. Takahashi-Iñiguez T, García-Hernandez E, Arreguín-Espinosa R, Flores ME. Role of vitamin B12 on methylmalonyl-CoA mutase activity. J Zhejiang Univ Sci B. 2012;13(6):423-437 doi: 10.1631/jzus.B1100329
37. Wolinsky I, Driskell JA. Sports nutrition: vitamins and trace elements. CRC Press. 2005
38. Wagenmakers AJ, Coakley JH, Edwards RH. Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle's disease. Int J Sports Med. 1990;11(Suppl 2):S101-13 doi: 10.1055/s-2007-1024861
39. Chen S, Minegishi Y, Hasumura T, Shimotoyodome A, Ota N. Involvement of ammonia metabolism in the improvement of endurance performance by tea catechins in mice. Sci Rep. 2020;10(1):6065. doi: 10.1038/s41598-020-63139-9
40. Durkalec-Michalski K, Kusy K, Główka N, Zieliński J. The effect of multi-ingredient intra- versus extra-cellular buffering supplementation combined with branched-chain amino acids and creatine on exercise-induced ammonia blood concentration and aerobic capacity in taekwondo athletes. J Int Soc Sports Nutr. 2021;18(1):48. doi: 10.1186/s12970-021-00451-3
41. Włodarczyk M, Kusy K, Słomińska E, Krasiński Z, Zieliński J. Changes in Blood Concentration of Adenosine Triphosphate Metabolism Biomarkers During Incremental Exercise in Highly Trained Athletes of Different Sport Specializations. J Strength Cond Res. 2019;33(5):1192-1200 doi: 10.1519/JSC.0000000000003133
42. Manore MM. Vitamin B6 and exercise. Int J Sport Nutr. 1994;4(2):89-103 doi: 10.1123/ijsn.4.2.89
43. Okada M, Ishikawa K, Watanabe K. Effect of vitamin B6 deficiency on glycogen metabolism in the skeletal muscle, heart, and liver of rats. J Nutr Sci Vitaminol (Tokyo). 1991;37(4):349-357 doi: 10.3177/jnsv.37.349
44. Crozier PG, Cordain L, Sampson DA. Exercise-induced changes in plasma vitamin B-6 concentrations do not vary with exercise intensity. Am J Clin Nutr. 1994;60(4):552-558 doi: 10.1093/ajcn/60.4.552
45. Schaffer S, Kim HW. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol Ther (Seoul). 2018;26(3):225-241 doi: 10.4062/biomolther.2017.251
46. De Luca A, Pierno S, Camerino DC. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1
47. Ishikura K, Miyazaki T, Ra SG, Endo S, Nakamura Y, Matsuzaka T. et al. Effect of taurine supplementation on the alterations in amino Acid content in skeletal muscle with exercise in rat. J Sports Sci Med. 2011;10(2):306-314
48. Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids. 2016;48(2):549-558 doi: 10.1007/s00726-015-2110-2
49. Kowsari E, Moosavi ZA, Rahimi A, Faramarzi M, Haghighi MM. The effect of short-term taurine amino acid supplement on neuromuscular fatigue, serum lactate level and choice reaction time after maximal athletic performance. J Res Medi Dental Sci. 2018;6(1):358-364
50. Heckel Z, Atlasz T, Tékus É, Kőszegi T, Laczkó J, Váczi M. Monitoring exercise-induced muscle damage indicators and myoelectric activity during two weeks of knee extensor exercise training in young and old men. PLoS One. 2019;14(11):e0224866. doi: 10.1371/journal.pone.0224866
51. Brigelius-Flohé R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76(4):703-716
52. Ricciarelli R, Argellati F, Pronzato MA, Domenicotti C. Vitamin E and neurodegenerative diseases. Mol Aspects Med. 2007;28(5-6):591-606 doi: 10.1016/j.mam.2007.01.004
53. Nadiger HA, Krishnan R, Radhaiah G. Studies on interactions of vitamin E with thiamine, niacin and vitamin B12. Clin Chim Acta. 1981;116(1):9-16 doi: 10.1016/0009-8981(81)90163-7
Author contact
![]() Corresponding author: Chi-Chang Huang, Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan City, Taiwan; and Tajen University, Pingtung, Taiwan. E-mail: john5523edu.tw
Corresponding author: Chi-Chang Huang, Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan City, Taiwan; and Tajen University, Pingtung, Taiwan. E-mail: john5523edu.tw

 Global reach, higher impact
Global reach, higher impact