3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(7):858-869. doi:10.7150/ijms.82008 This issue Cite
Review
Current Role and Future Perspectives of Immunotherapy and Circulating Factors in Treatment of Biliary Tract Cancers
1. Division of General and Hepatobiliary Surgery, Department of Surgical Sciences, Dentistry, Gynecology and Pediatrics, University of Verona, University Hospital G.B. Rossi, Verona, Italy.
2. Digestive Molecular Clinical Oncology Research Unit, Section of Medical Oncology, University of Verona, University Hospital G.B. Rossi, Verona, Italy.
Received 2022-12-20; Accepted 2023-4-7; Published 2023-5-11
Abstract
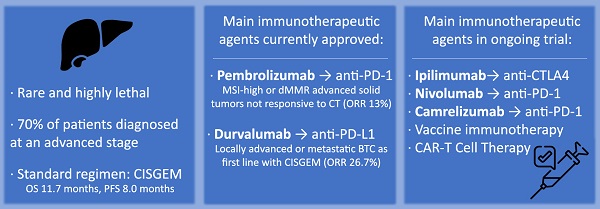
Biliary tract cancers (BTCs) are a heterogenous group of malignancies arising from the epithelial cells of the biliary tree and the gallbladder. They are often locally advanced or already metastatic at the time of the diagnosis and therefore prognosis remains dismal. Unfortunately, the management of BTCs has been limited by resistance and consequent low response rate to cytotoxic systemic therapy. New therapeutic approaches are needed to improve the survival outcomes for these patients. Immunotherapy, one of the newest therapeutic options, is changing the approach to the oncological treatment. Immune checkpoint inhibitors are by far the most promising group of immunotherapeutic agents: they work by blocking the tumor-induced inhibition of the immune cellular response. Immunotherapy in BTCs is currently approved as second-line treatment for patients whose tumors have a peculiar molecular profile, such as high levels of microsatellites instability, PD-L1 overexpression, or high levels of tumor mutational burden. However, emerging data from ongoing clinical trials seem to suggest that durable responses can be achieved in other subsets of patients.
The BTCs are characterized by a highly desmoplastic microenvironment that fuels the growth of cancer tissue, but tissue biopsies are often difficult to obtain or not feasible in BTCs. Recent studies have hence proposed to use liquid biopsy approaches to search the blood circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA) to use as biomarkers in BTCs. So far studies are insufficient to promote their use in clinical management, however trials are still in progress with promising preliminary results. Analysis of blood samples for ctDNA to research possible tumor-specific genetic or epigenetic alterations that could be linked to treatment response or prognosis was already feasible. Although there are still few data available, ctDNA analysis in BTC is fast, non-invasive, and could also represent a way to diagnose BTC earlier and monitor tumor response to chemotherapy. The prognostic capabilities of soluble factors in BTC are not yet precisely determined and more studies are needed. In this review, we will discuss the different approaches to immunotherapy and tumor circulating factors, the progress that has been made so far, and the possible future developments.
Keywords: biliary tract cancers, cholangiocarcinoma, immunotherapy, chemotherapy, checkpoint inhibitors, circulating factors, target therapy
Introduction
Biliary tract cancers (BTC) are rare and highly lethal epithelial cell malignancies that arise from the biliary duct cells. They represent the second most common primary liver malignancy after hepatocellular carcinoma, accounting for 15% of all primary liver tumors and 3% of gastrointestinal cancers (1). Incidence amounts to 10,000 new cases/year in Europe (0.5 to 3 cases per 100,000 people) and 12,000 new cases/year in the United States (1.6 cases per 100,000 people) (2,3). Incidence is higher in Asia, with 5.7 to 85 cases per 100,000 people (4,5). The main risk factors for BTCs are chronic viral infections (hepatitis virus B and hepatitis virus C), cirrhosis or non-alcoholic fatty liver disease, obesity, alcohol and tobacco consumption, diabetes, sclerosing cholangitis and liver fluke infections in endemic areas (6,7). Biliary tract cancers could be classified according to their anatomical site of origin in intrahepatic (iCCA), perihilar (pCCA), distal (dCCA) cholangiocarcinoma and gallbladder cancer (GBC). There are consistent reports of an increasing worldwide incidence of iCCA and a decreasing or stable incidence of extra hepatic CCA (both pCCA and dCCA) (8-14). Nowadays, surgery remains the only potential cure for these cancers, but post-surgery tumor relapses are common (15-17) resulting in poor prognosis. About 70% of patients are diagnosed at an advanced stage due to a lack of specific symptoms and screening protocols (18,19); for late-stage disease not suitable for radical resection, chemotherapy is a cornerstone for the treatment (20). The combination of cisplatin and gemcitabine [CISGEM regimen] is the standard first-line therapy for this kind of patients, with a median overall survival (OS) and a progression-free survival (PFS) of 11.7 and 8.0 months, respectively (21). The second-line chemotherapy, according to the ABC-06 phase 3 trial, is the combination of fluorouracil and oxaliplatin (FOLFOX regimen) (22); beyond second line there is no validated standard treatment. New therapeutic approaches are clearly needed to improve the survival outcomes for these patients. The main molecular pathways that characterize BTCs are the JAK/STAT signaling pathway, the FGF and Ras/BRaf/MEK/ERK pathway, the EGFR and HER2 signaling pathway, and many others. Farmacological therapies that target these altered signaling pathways are being tested in alternative to standard systemic therapy regimens as a way to improve the grim chanches of survival of patients with advanced BTCs. The main ongoing trials of these targeted therapies are summarized in Table 1 23).
The immunotherapy revolution
Immunotherapy has changed the paradigm of cancer's treatment, leading the patient's immune system to attack cancer cells as though they were foreign invaders (24). Immune checkpoint inhibitors (ICIs) are up to now the most promising group of immunotherapeutic agents: they act by blocking the tumor-induced inhibition of the immune cellular response. Immune checkpoints are molecules that normally ensure self-tolerance, preventing the immune system from attacking cells indiscriminately. Moreover, ICIs are triggered when the receptor proteins on the surface of T cells recognize and bind to ligand mate proteins on other cells, such as tumor cells. These proteins are called immune checkpoint proteins. When these molecules connect with their partners proteins, they send an “off” signal to the T lymphocytes. This prevents the body's immune system from destroying cancer. Immune checkpoint inhibitors block checkpoint proteins from binding with their partner proteins. In this way there is no transmission of the “off” signal, so T cells are enabled to kill cancer (25).
Main ongoing trials for targeted therapy in BTC.
| Study name | Phase | Drug / Target | Setting |
|---|---|---|---|
| FIGHT-302 (NCT03656536)111 | 3 | Pemigatinib / FGFR2 | Unresectable or metastatic BTC with FGFR2 rearrangement who did not underwent prior systemic therapy. |
| PORCUPINE 2 (NCT04907851)112 | 2 | Denosumab / RANKL RXC004 / Porcupine | PDAC and BTC which have progressed after first line of SoC systemic therapy. |
| NCT04211168113 | 2 | Lenvatinib / VEGFR | Histologically confirmed advanced BTC who progressed after first line systemic therapy. |
| FIDES-01 (NCT03230318)114 | 2 | Derazantinib / FGFR2 | Histologically confirmed advanced BTC with confirmed FGFR2 fusion status, who progressed after at least one regimen of systemic therapy. |
| My Pathway (NCT02091141)115 | 2 | Trastuzumab / HER2 Pertuzumab / HER2 | Histologically confirmed metastatic solid tumors who have already received standard first-line systemic treatment and have HER2 overexpression or amplification. |
| NCT04238715116 | 2 | E7090 / FGFR2 | Histologically confirmed unresectable iCCC or phCCC, with centrally confirmed FGFR2 gene fusion by FISH, who received at least one prior line of systemic therapy. |
BTC: biliary tract cancer, PDAC: pancreatic ductal adenocarcinoma, SoC: Standard of care, iCCC: intrahepatic cholangiocarcinoma, phCCC: peri-hilar cholangiocarcinoma, FISH: fluorescent in-situ hybridization, FGFR2: fibroblast growth factor receptor 2, RANKL: Receptor Activator of Nuclear Factor κ B ligand, VEGFR: vascular endothelial growth factor receptors, HER2: human epidermal growth factor receptor 2.
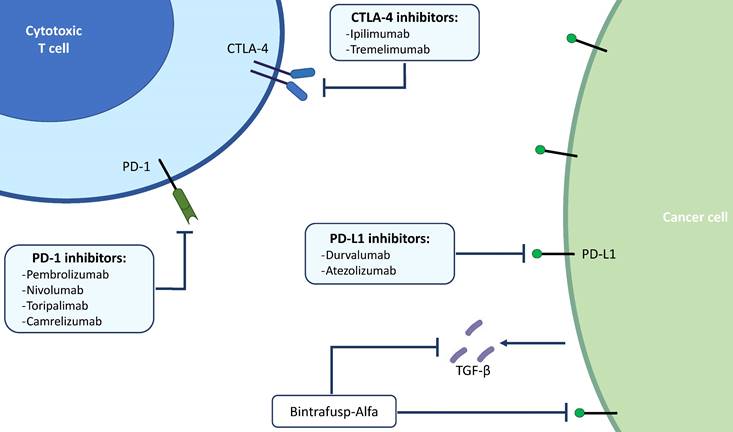
The first ICI to be developed was Ipilimumab, an immune antibody that binds to CTLA4. When T cells meet an antigen-presenting cell (APC), two signals are required to stimulate T cell proliferation: the major histocompatibility complex (MHC) on APCs and the binding of CD28 on the T cell to the APC. The simultaneous activation of these signals induces proliferation of T cells and activates the expression of CTLA-4. As CTLA-4 accumulates in the T cell, it outperforms CD28 and disables T cell proliferation. By targeting CTLA-4, Ipilimumab shuts down one of the inhibitory pathways that block effective anti-tumor responses.
The other class of ICIs targets programmed cell death protein 1(PD-1) on T cells and its ligand (PD-L1) on the APCs. In particular, PD-1 downregulates T cell activation upon binding to PD-L1 on APCs. Blocking PD-1 or PD-L1 can, therefore, enhance the T cell response. The first anti-PD-1 therapies came into the clinic in 2014, with approval of Pembrolizumab and Nivolumab (26).
The other major class of immunotherapy is chimeric antigen receptor (CAR) T cells. In this therapy, T cells are removed from a patient and genetically engineered to express a CAR derived from a tumor-specific antigen; the CAR-expressing T cells, and their cytotoxic functions, are thereby specifiable directed toward tumor cells. The first CAR T cell therapy to be approved was Kymriah (24).
The ICIs are now approved for a variety of solid tumors including non-small cell lung cancer, melanoma, cutaneous squamous cell carcinoma, urothelial carcinoma, cervical cancer, Hodgkin's lymphoma, head and neck squamous cell carcinoma, Merkel cell carcinoma, renal cell carcinoma, MSI-H or dMMR colorectal cancer, and hepatocellular carcinoma (27).
The reduced immunogenicity of HCC along with the immunosuppressive tumor microenvironment of the liver has hampered the development and implementation of immunotherapy (28,29). Current immunotherapies for liver cancer include ICIs, the adoptive transfer of immune cells, bio specific antibodies, vaccines, and oncolytic viruses. The ICIs against PD-1 and CTLA-4 have demonstrated utility in HCC. In advanced CCA and GBC, PD-1 ICIs have resulted in antitumor responses, but only in a minority of select patients. Since the time ICIs were developed, there have been an increased percentage of patients eligible for this kind of therapy, from 1.5% in 2011 to 43.6% in 2018. Even the percentage of patients with cancer estimated to respond to checkpoint inhibitor drugs increased from 0.1% to 12.5% (30).
State of the art and future developments of immunotherapy in BTCs
Immune checkpoint inhibitors
Nowadays, the most widely studied therapeutic target for cancer immunotherapy is programmed death-ligand 1 (PD-L1) (Figure 1). It has been already reported how solid tumors with high level of microsatellite instability (also called “MSI-high”) have favorable responses to PD-L1 blockade immunotherapy (31,32). Pembrolizumab, which binds and blocks PD-1, is approved for many different solid tumors with microsatellite instability. The KEYNOTE-158 (phase 2) and KEYNOTE-028 (phase 1b) studies have evaluated the safety and efficacy of Pembrolizumab in advanced BTC: the monoclonal antibody showed manageable toxicity and durable antitumor activity in 6% to 13% of patients with advanced BTC, regardless of PD-L1 expression (33). Published results of the pivotal clinical trials that have tested immunotherapeutic drugs in BTCs are summarized in Table 2.
Main targets of immunotherapy in BTCs. BTCs; biliary tract cancers; PD-1, programmed death 1; PD-L1, programmed death ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; TGF-β, transforming growth factor beta.
Published results of main clinical trials for immunotherapy in BTC.
| Study name | Phase | Drug / Target | Setting | Outcomes |
|---|---|---|---|---|
| KEYNOTE-02833 | 1b | Pembrolizumab / PD-1 | Histologically confirmed advanced BTC, with disease progression after ≥1 prior standard therapy. | ORR 13% PFS 1.8 months OS 5.7 months |
| KEYNOTE-15833 | 2 | Pembrolizumab / PD-1 | Previously treated unresectable or metastatic MSI-H/dMMR non-colorectal cancer, including advanced BTC. | ORR 5.8% PFS 2 months OS 7.4 months |
| NCT0282991837 | 2 | Nivolumab / PD-1 | Histologically confirmed advanced refractory BTC undergoing treatment with 1-3 lines of systemic therapy. | ORR 22% PFS 3.68 months |
| CA209-53840 | 2 | Nivolumab / PD-1 Ipilimumab / CTLA4 | Unresectable or metastatic rare cancers, including advanced BTC. | ORR 23% PFS 2.9 months OS 5.7 months |
| TOPAZ-1 (NCT03875235)44 | 3 | GEMCIS + Durvalumab / PD-L1 | Chemotherapy-naïve patients with advanced BTC. | ORR 26.7% |
| INTR@PID BTC047 (NCT03833661)45 | 2 | Bintrafusp-alfa / PD-L1:TGF-β | Second-line treatment in patients with advanced or metastatic BTC who have failed or are intolerant to first-line platinum-based chemotherapy. | ORR 10.1% PFS 1.8 months OS 7.6 months |
| NCT01938612118 | 1 | Durvalumab / PD-L1 Tremelimumab / CTLA4 | Second line treatment for advanced or metastatic solid tumors. | ORR 10.8% OS 10.1 months |
| JapicCTI-153098119 | 1 | GEMCIS + Nivolumab / PD-1 | First line treatment of unresectable BTC. | ORR 37% PFS 4.2 months |
| NCT03311789120 | 2 | GEMCIS + Nivolumab / PD-1 | First line treatment of unresectable BTC. | ORR 55.6% PFS 6.1 months OS 8.5 months |
| NCT0186916654 | 1 | CART / EGFR | EGFR‑positive (>50%) advanced unresectable, relapsed, or metastatic BTC. | PFS 4 months |
| NCT0193584355 | 1 | CART / HER2 | HER2-positive (>50%) advanced unresectable, relapsed, or metastatic BTC and pancreatic cancers. | ORR 55% PFS 4.8 months |
BTC: biliary tract cancer, ICIs: immune checkpoint inhibitors, PD-1: programmed cell death protein 1, PD-L1: programmed death-ligand 1, CTLA-4: cytotoxic T-lymphocyte antigen 4, GEMCIS: gemcitabine + cisplatin chemotherapy, ORR: Overall response rate, PFS: progression free survival, OS: overall survival, MSI-H: high microsatellite instability, dMMR: mismatch repair deficiency, CAR-T: chimeric antigen receptor T cells, EGFR: epidermal growth factor receptor, HER2: human epidermal growth factor receptor 2, TGF-β: Transforming growth factor beta.
Thanks to these studies, Pembrolizumab was the first immunotherapeutic agent with an on-label indication for the treatment of BTC. It was approved by the FDA for treatment of a variety of advanced solid tumors, including BTC, that were MSI-high or mismatch repair deficient (dMMR), were not responsive to first line chemotherapy and had no satisfactory alternative treatment options. This was the first approval of a so-called “tissue-agnostic” anticancer treatment, which is based on the cancer's genetic and molecular features without regard to the cancer type or where the cancer started in the body (34). Unfortunately, the majority of BTCs are not MSI-high or dMMR. In fact, MSI-high tumors account for approximately 2% of BTCs, and not all MSI-high tumors respond favorably to ICI. Therefore, novel biomarkers are needed to screen for patients with BTC that could benefit from ICI therapy with PD-L1 blockade (35).
Nivolumab, another PD-L1 blocker, has also been tested both alone and in combination with gemcitabine and cisplatin (GEMCIS) in many phase 2 studies, with promising efficacy and manageable safety profile (36,37). In a phase 2 trial, Kim et al. enrolled 46 patients with advanced BTC who had done at least first-line chemotherapy. After the treatment with Nivolumab, the overall response rate (ORR) was 11%, progression free survival (PFS) was 3.7 months and overall survival (OS) was 14.2 months (37). Right now, the current National Comprehensive Cancer Network (NCCN) guidelines recommend Nivolumab as a subsequent-line treatment option for unresectable and metastatic BTC with disease progression, that are not MSI-high nor have high PD-L1 expression (class 2B recommendation) (38,39).
Ipilimumab, an anti-CTLA-4 drug, is currently undergoing clinical trials to test its efficacy on different solid tumors. In the CA209-538 (phase 2) clinical trial the combination of Ipilimumab plus Nivolumab was associated with positive outcomes in patients with advanced BTC. This combination has shown better response rates than single-agent anti-PD-1 therapy, with an objective response rate of 23% among patients with intra-hepatic and gallbladder cholangiocarcinoma. There were no responses in the patients with extrahepatic cholangiocarcinoma. Overall survival was 5.7 months (95% CI, 2.7-11.9 months) (40). Additional trials will be required to determine whether this combined immunotherapy regimen can be superior to single-agent anti-PD-1 therapy in patients with advanced BTC.
The efficacy of immunotherapy in BTC is being tested also as first-line therapy in chemotherapy-naïve patients. The KEYNOTE-966 phase 3 trial (NCT04003636) is currently underway and has enrolled patients with histologically confirmed diagnosis of advanced or unresectable BTC in order to evaluate the overall survival in patients who received GEMCIS + Pembrolizumab vs only GEMCIS therapy. Promising results from this phase 3 KEYNOTE-966 trial were recently announced: the combination of Pembrolizumab and GEMCIS demonstrated a statistically significant improvement in overall survival versus chemotherapy alone in this setting, although conclusive results have yet to be published (41). The TOPAZ-1 trial (NCT03875235), a double-blind placebo-controlled study of Durvalumab (an anti-PD-L1 drug) plus GEMCIS in chemotherapy-naïve patients with advanced BTC has recently ended. Overall survival (hazard ratio [HR] 0.80; 95%CI 0.66-0.97) and progression free survival (hazard ratio [HR] 0.75; 95%CI 0.64-0.89) were significantly improved in the group of patients who received Durvalumab, with manageable safety. The ORR was 26.7% in the group of patients receiving Durvalumab and 18.7% in the placebo arm (42). Based on the result from the TOPAZ-1 trial, Durvalumab is now approved by the Food and Drug Administration (FDA) for the treatment of adult patients with locally advanced or metastatic BTC in combination with GEMCIS chemotherapy. Furthermore, this regimen is now also recommended as first-line treatment in the NCCN guidelines as an option for first-line treatment of patients with unresectable, locally advanced, or metastatic disease (39).
Bintrafusp-alfa is a bifunctional protein composed of a human anti-PD-L1 IgG1 monoclonal antibody fused with two extracellular domains of the transforming growth factor β (TGF-β) receptor II (a TGF-β “trap”). Bintrafusp-alfa showed promising results in patients with BTC whose disease progressed after first-line treatment (43,44). The phase 2 INTR@PID BTC047 study evaluated Bintrafusp-alfa as a monotherapy in the second-line treatment in 159 patients with advanced or metastatic BTC who have failed or are intolerant of first-line platinum-based chemotherapy. The results showed an improved ORR of 10.1% (95% CI: 5.9% to 15.8%) and a manageable safety profile (45). Based on these results, Bintrafusp-alfa is currently under further investigation in patients with BTC, both as monotherapy and in combination with CISGEM. Ongoing trials for new immunotherapeutic agents are summarized in Table 3.
Vaccine immunotherapy
Other types of immunotherapies, such as vaccines and cellular therapy, have been tested during the last years. A vaccine with four immunogenic peptides (HLA-A*2402-restricted epitope peptides, lymphocyte antigen 6 complex locus K, TTK protein kinase, insulin-like growth factor-II mRNA-binding protein 3 and DEP domain containing 1) was attempted by Aruga et al. The same group developed a phase 1 clinical trial of a multiple-peptide vaccination for patients with advanced BTC using three peptides: peptides-cell division cycle associated 1 (CDCA1), cadherin 3 (CDH3) and kinesin family member 20A (KIF20A). In both studies vaccines were well-tolerated but yielded very limited success (46,47). Other groups obtained very similar results using peptides such as MUC-1 (Mucin 1, cell surface associated) and WT1 (Wilms' tumor protein-1), with low to zero toxicity but very poor clinical response (48-50). As of now, the best strategy to develop a vaccine is to use several different antigenic peptides, although which to use seems to rely heavily on the different BTC subtype.
Main ongoing trials for immunotherapy in BTCs.
| Study name | Phase | Drug / Target | Setting |
|---|---|---|---|
| Keynote - 158 (NCT02628067)33 | 2 | Pembrolizumab / PD-1 | Histologically/cytological confirmed incurable advanced BTC, disease progression after ≥1 prior standard therapy, ECOG-PS 0-1, no prior exposure to ICIs. |
| CA 209-538 (NCT02923934)40 | 2 | Ipilimumab / CTLA-4 + Nivolumab / PD-1 | First or second line therapy in neuroendocrine tumors, rare gynaecological tumors and advanced upper GI tumors, including BTC. |
| Keynote-966 (NCT04003636)117 | 3 | GEMCIS + Pembrolizumab /PD-1 | First line therapy for advanced or unresectable BTC. |
| NCT03797326121 | 2 | Lenvatinib / VEGFR + Pembrolizumab / PD-1 | Second line therapy in selected solid tumors, including BTC. |
| NCT04720131122 | 2 | Capecitabine + Camrelizumab / PD-1 + Apatinib / VEGFR2 | First or second line treatment for advanced BTC. |
| NCT04708067123 | 1 | RT + Bintrafusp-alfa/PD-L1:TGF-β | Second line treatment for advanced or metastatic iCCC. |
PD-1: programmed cell death protein 1, BTC: biliary tract cancer, ICIs: immune checkpoint inhibitors, ECOG-PS: eastern cooperative oncology group- performance status, PD-L1: programmed death-ligand 1, CTLA-4: cytotoxic T-lymphocyte antigen 4, GI: gastrointestinal, GEMCIS: gemcitabine + cisplatin chemotherapy, RT: radiation therapy, iCCC: intrahepatic cholangiocarcinoma, TGF-β: Transforming growth factor beta.
CAR T cell theraphy
Chimeric antigen receptor (CAR) T cell therapy has already proven its efficacy and safety in patients with hematological malignancies such as relapsed lymphoblastic leukemia, diffuse large B-cell lymphoma, mantle cell lymphoma and others (51-53). CAR-T cell therapy is now being tested in many different solid tumors. Guo et al. in a phase 1 study evaluated the efficacy and safety of EGFR-specific CAR-T cells in patients with advanced unresectable or relapsed BTC. Of 17 evaluable patients, 1 achieved complete response and 10 achieved stable disease, and median PFS was 4 months (range, 2.5-22 months) (54). Feng et al. carried out a phase 1 clinical trial enrolling patients with HER2-positive advanced biliary tract and pancreatic cancers. The objective response rate was 55% while median PFS was 4.8 months (range, 1.5-8.3 months), showing the safety and feasibility of CAR-T-HER2 immunotherapy (55). Currently CAR-T cell therapy in BTC is still in its early development phase, but it seems to have encouraged overall response rate and manageable safety. Further studies are however needed to assess the long-term efficacy and to establish the correct subset of patients with BTC that would benefit from this kind of therapy.
Innate immune cells therapy
Recent clinical studies have highlighted the possible role of innate immune cells as potential candidates for new immunotherapeutic strategies. Natural killer cells (NK) are especially known for their ability to destroy tumor cells in vitro, determined by the balance of inhibitory and activating receptors on their cell surface. However, it is technically difficult to generate large numbers of NK cells and they show a short life span in vivo. The clinical application of NK cells is carried out by culturing and activating the NK cells isolated from blood of either patient (autologous) or blood donor (allogeneic). While positive clinical outcomes were observed in hematological cancer patients, the transfer of expanded autologous NK cells has not yet shown clear positive clinical responses in solid tumors. Recently, NK cell therapy for cholangiocarcinoma has been successfully done (NCT03358849) with allogeneic NK cell, and further studies are ongoing (NCT03937895). The possibility to engineer CAR-NK cells has attracted much attention, but further studies are needed to assess feasibility and outcomes in patients with BTC (56).
Predictive and prognostic biomarkers in BTCs
Current markers for ICI therapy response
In Table 4 we have summarized current available markers for ICI therapy response in BTCs. Programmed death ligand 1 (PD-L1) is the transmembrane molecule that binds to PD-1 in the process of immune checkpoint inhibition. The ICI drugs like Pembrolizumab bind PD-1 to stop the tumor-induced immune suppression. Thus, it was though that assessing the tumor tissue's expression levels of PD-L1 could hint to patients' response to treatment with ICI (57.58). However, the role of PD-L1 expression in BTC is still to be precisely assessed. As previously stated, the KEYNOTE-158 and KEYNOTE-028 trials in patients with advanced BTC did not found any correlation between PD-L1 levels assessed with immunohistochemistry and response to ICI treatment with Pembrolizumab (33). In a phase 2 trial of Nivolumab in patients with advanced BTC already treated with first line chemotherapy conducted by Kim and colleagues, patients with ≥1% of tumor cells expressing PD-L1 had a statistically significant higher median PFS compared with patients with PD-L1-negative tumor tissue (37). Data regarding assessment of PD-L1 in order to initiate ICI therapy is still controversial. Unfortunately, several methodological issues are at fault in the evaluation of PD-L1, namely the use of different assays, the differences in scoring systems, and the discrepancy between metastatic and primary lesions. These issues could contribute to the discording results reported in the literature (59).
Current available biomarkers for BTCs.
| Biomarkers | Available data |
|---|---|
| PD-L1 | Controversial data: - Keynote 15833 and Keynote 02833: no correlation between PD-L1 levels and response to ICIs; - NCT0282991837: patients with ≥1% of tumor cells expressing PD-L1 had a higher median PFS compared with patients with PD-L1-negative tumor tissue. |
| TMB | No sufficient data, given the low rate of TMB high BTCs: - Keynote 15833: tumors with TMB high showed better efficacy of Pembrolizumab. None of the patients with BTCs were TMB high. |
| MSI | MSI is a good predictor of response to ICIs; however, MSI-high BTCs are rare. - Keynote 15833: Pembrolizumab for treatment of BTC that had MSI or dMMR. |
| EpCAM-enriched CTCs | - Reduzzi et al.85 confirmed the prognostic role of eCTCs on survival in BTCs. |
| V-CTCs | - Han et al86: V-CTC > 50/mL blood is a significant factor affecting survival in patients with BTCs. |
| ctDNA | Potential complementary tool in the clinical practice to detect gene alterations, aiding in screening patients who may benefit from targeted therapies. - Chen et al93: for most genes, the mutation frequencies in ctDNA were similar with those detected in tissue samples. - Csoma et al93: positive correlation between the estimated tumor volume and cfDNA yield; Comparing tissue and LB results, similar tumor variant burden was observed. |
| ncRNA (eg: miRNA) | - Kishimoto et al103: increased level of miR-21 in patients with BTCs, making it a highly sensitive biomarker. |
PD-L1: programmed death-ligand 1, ICIs: immune checkpoint inhibitors, PFS: progression free survival, TMB: tumor mutational burden, MSI: microsatellite instability, dMMR: mismatch repair deficiency, EpCAM: epithelial cell adhesion molecule, CTC: circulating tumor cells, V-CTC: vimentin-positive CTC, ctDNA: circulating tumor DNA, LB: liquid biopsy, cfDNA: circulating free DNA, ncRNA: non coding RNA, miRNA: micro-RNA.
The overall number of somatic mutations in a tumor cell is referred as “tumor mutation burden” (TMB). Given that more mutations a tumor has, the more immunogenic peptides will be generated and displayed on the major histocompatibility complex (MHC) on the tumor cell surface, it is though that tumors with high TMB will be more immunogenic. In fact, therapies with ICI showed at first improved outcomes in tumors with typically high TMB, such as non-small cell lung cancer and melanoma (60).
The gold standard assessment method for TMB is currently whole-exome sequencing (WES). This method has been used in several studies, but it is not feasible in clinical practice because of its high cost and long turnaround time. Therefore, TMB is routinely assessed on tumor-biopsies using panel-based sequencing, but differences in panel size, mutation types, and bioinformatic platforms exist, thus making this process not standardized. Furthermore, WES covers approximately 30 Mb of coding regions, while panel-based sequencing targets different genes, depending on which panel it is used, usually between 0.80 and 2.40 Mb (60,61). In the KEYNOTE-158 trial, tumors with a TMB greater than 10 mutations per megabase (mut/Mb) were considered “TMB-high” and showed better efficacy of the Pembrolizumab immunotherapy. Unfortunately, in this same study, none of the 63 patients with BTC had a TMB greater than 10 mut/Mb (62). In a genomic profiling study of 309 tumor biopsies from patients with BTC, Jain et al. found that only 60 patients had a TMB greater than 6 mut/Mb, of which 9 patients had a TMB greater than 20 mut/Mb (63). In a recent study by Zhang et al. on 24 patients with BTC, 3 patients with TMB-high were treated with ICI and achieved response to therapy (2 partial responses and 1 complete response) (64). Thanks to the KEYNOTE-158 trial Pembrolizumab is currently approved by the FDA for patients with any advanced solid tumor, including BTC, with a TMB greater than 10 mut/Mb. However, while high-TMB has been associated with improved survival in patients receiving ICI in a wide variety of cancer types (65,66), further studies are needed for the validation of its predictive capabilities in clinical practice. Issues remain regarding the optimal TMB cutoff, which could potentially vary between different solid tumors, and the best testing platforms (60). The data produced on TMB used as a marker for ICI therapy in BTC has been so far not conclusive, given the low rates of TMB-high BTC. Thus, further studies are needed to assess its potential predictive value.
Tumors that have a deficiency in the mismatch repair mechanism (mismatch repair deficiency, dMMR) tend to develop many more mutations than other kinds of tumors, therefore the neoantigens generated from these mutations make these tumors more immunogenic. Tumors that have dMMR are generally characterized by microsatellite instability (MSI) (19). As stated before, solid tumors with MSI have showed good response to therapy with ICI (31,32). Thanks to the KEYNOTE-158 trial, the FDA has already approved Pembrolizumab for treatment of a variety of advanced solid tumors, including BTC, that had MSI or dMMR, that were not responsive to first line chemotherapy and had no satisfactory alternative treatment options (33). Despite MSI being a good predictor of good response to immune checkpoint blockade, MSI-high BTC seem to be quite rare. In the patient cohort studied by Goeppert and colleagues the number of patients with high level MSI (MSI-high) BTC was about 2%. Even with this low rate of MSI-h BTC, they suggest testing for MSI especially in young patients showing with an atypical histomorphology (35). The data we have so far is not enough to implement routine MSI screening in BTC patients. Anyway, the evaluation of this biomarker in concert with other ones could provide us with a perfect tool to assess prognosis and predict treatment responses in BTC patients (59).
The role of circulating factors in BTCs
Cholangiocarcinoma is characterized by a highly desmoplastic microenvironment containing stromal cells, especially cancer-associated fibroblast (CAFs), and other immune cells such as tumor-associated macrophages (TAMs), tumor-infiltrating lymphocytes (TILs) that release many cytokines and chemokines that stimulate cancer growth and recruitment of other immune cells (67,68).
Chemokines are a family of small proteins that attract leukocytes and are involved in tumor genesis. For instance, in cholangiocarcinoma, CCL2 produced by CAFs (69), leads to tumor progression, modulating metastasis, angiogenesis and cancer proliferation (70).
Many other soluble factors, including MCP-1, CXCL-12, CXCL-14, PDGF, TGFb, FGF1/2, are responsible for the persistent activation of CAFs; these cells stimulate tumor growth by secreting other soluble factors (71). Chuaysri et al. (72) demonstrated that patients with high levels of CAFs have the worst prognosis. Finally, CAFs contribute to recruit inflammatory cells, maintaining the tumor microenvironment neoangiogenesis (73).
Neoplastic cells release CCL-2 that activates CAFs and TAMs to become Treg, creating an immunosuppressive environment by secreting TGF β and IL-10 (74,75). Tregs overexpress FoxP3, a transcription factor associated with the up-regulation of CTLA-4 on the cell surface; CTLA-4 binds to CD80 expressed by antigen-presenting cells and inhibits cytotoxic T-cell activation, contributing to tumor development (76). FoxP3, a distinctive feature of Tregs, is overexpressed also by CCA cells, thus correlating with lymphatic metastasis and poor survival (77,78). Knockdown of FoxP3 in CCA cells in vitro reduced proliferation and invasiveness, inhibited T cell survival, and reduced IL10 and TGF-β signaling in the tumor microenvironment (78).
Circulating Cells
The analysis of tumor biomarkers isolated from biological fluids, referred as “liquid biopsy”, originated as a way to search for circulating tumor cells (CTCs). These CTCs are shed from the primary tumor site and then circulate through the bloodstream. Studies have searched the possibility to use these CTCs both as a non-invasive way to perform a tumor biopsy and to diagnose the presence of minimal residual disease undetectable by high-resolution imaging technologies. Data from CTCs could also be useful to understand the various mechanisms of drug resistance, tumor progression and metastasis (79). The first step to CTC isolation involves the so-called enrichment process, a selection based on biological or physical characteristics. Immunoselection, the main biological enrichment process, selects CTCs based on the detection by antibodies of specific markers, mostly epithelial cell-adhesion molecules (EpCAM) but also mesenchymal proteins (80,81). Studies on CTCs in BTC are limited to EpCAM-enriched CTCs have shown how CTCs seem to be associated with tumor extent and overall survival in patients with BTC (82-84). In a recent study, Reduzzi et al. confirmed the prognostic role of eCTCs on survival. However, they could not find any association with other kinds of CTCs that expressed different membrane markers (85).
Other protein expressed linked to the epithelial-mesenchymal transition (EMT) have been analysed in CTCs to search for a correlation with cancer progression. In a prospective clinical trial by Han et al. Vimentin-positive CTCs (V-CTCs) were analysed besides eCTCs in a sample of 62 patients, of which 52 had BTC and 10 had benign biliary diseases. In this study, the two groups did not show any statistically significant difference in CTC and V-CTC count. However, a statistically significant difference was found by analysing the V-CTC/total CTC count ratio (VCR). Furthermore, a blood concentration of V-CTC higher than 50/mL was correlated with survival rates, thus pointing V-CTC out as a potential biomarker for early diagnosis and prognosis in BTC (86).
Circulating DNA
Through the so-called “liquid biopsy” it is possible to search for circulating free DNA (cfDNA), circulating tumor DNA (ctDNA) and circulating cell-free RNA (ccfRNA) to scan for possible tumor-specific genetic or epigenetic alterations. This kind of analysis is fast, non-invasive, cost-effective, and could represent a way to achieved early diagnosis of BTC, monitor tumor response to treatment, and detect tumor recurrences in advance. Furthermore, the process can be repeated multiple times to track the tumor's genetic changes over time in a non-invasive way during follow-up. Since the discovery of foetal cfDNA in the maternal bloodstream, cfDNA has become a popular research topic. The majority of cfDNA is released in the blood stream by lysis of normal cells. A small portion of it, called ctDNA, comes directly from primary tumor cells, metastatic cells, or CTCs (87,88). Recent studies have suggested concordance rates between tumor and plasma samples from 80% to 90% (89). In BTC, ctDNA analysis could play an even more important role, since biopsy samples are often inadequate for molecular profiling in CCA and GBC (90). Mody et al. enrolled 124 patients with BTC who underwent ctDNA testing with a 73-gene panel. Excluding variants of unknown significance, alterations were observed in 55% of patients, mainly BRAF mutation, ERBB2 amplification, FGFR2 fusions and mutations, and IDH1 mutations. The technique was shown to be feasible in BTC, however the concordance between ctDNA and tissue biopsy has to be precisely assessed (91). In a phase 2 study, Jensen et al. analysed ctDNA from 24 patients with KRAS-mutated BTC. In 13 of 24 patients the known tumor tissue KRAS alteration was detectable at baseline. Patients with detectable KRAS mutation in ctDNA showed inferior PFS and OS. Furthermore, survival was significantly improved in patients with low level of total plasma DNA at baseline (92). Chen et al. used next generation sequencing of 150 cancer-related genes to detect gene alterations in blood ctDNA from 154 patients with BTC. TP53 was the most frequently altered gene in ctDNA, followed by KRAS and EGFR. For most genes, the mutation frequencies in ctDNA were similar with those detected in tissue samples. This study outlines the role for ctDNA as a potential complementary tool in the clinical practice, aiding to screen patients who may benefit from targeted therapies (93). In a prospective study, Csoma et al. compared 25 tumor biopsy and 25 paired liquid biopsy samples from patients with BTC. Their analysis found a positive significant correlation between estimated tumor volume and the quantity of cfDNA. Pathogenic variations in the genetic material were proven in 68% of patients and presented in some of the most usually affected genes in BTCs such as FGFR2, IDH1, IDH2, KRAS and TP53, and most of them were matched between ctDNA and tissue biopsies (94). The consistency between blood ctDNA and tumor biopsy analysis emerged in the latest years could be the key to a new perspective for BTCs personalized therapy. As of now, however, more studies are needed to link ctDNA to survival outcomes and treatment response.
In BTC, the possibility to search for ctDNA not only in blood but also in the bile has recently emerged as a valid alternative. In a study on 42 patients with BTC there was an 80% mutational concordance between the paired bile ctDNA and tumor biopsy sample, giving the proof-of-concept that ctDNA on the bile of BTC patient could be an effective approach to genetic characterization in this subset of patients (95).
Some studies proposed DNA-methylation patterns as a tool to differentiate patients with CCA from healthy controls in a less invasive way. Assessing DNA-methylation patterns in serum cfDNA, bile cfDNA or biliary brushing samples has been already tested in many trials. Although several genes have been reported to be methylated in BTC, statistical data is not sufficient yet to determine which one is a potential biomarker for cancer detection (96-98).
A study by Qiu et al. identified 3369 common differentially methylated regions (DMRs) in 105 patients with BTC. A lower level of methylation was associated with a remarkably longer overall survival (hazard ratio [HR] = 0.07, p=0.017). Furthermore, BTCs with minimal methylation changes had infiltration of CD8+ lymphocytes, and PD-L1 expression, indicating an inflamed tumor immune microenvironment with PD-L1 expression elicited by immune attack, potentially suggesting better immunotherapy efficacy. More studies are needed to test immunotherapy efficacy combined with demethylation agents (99).
Besides DNA, some studies have highlighted the role of non-DNA molecules as possible biomarkers in BTCs. Non-coding RNA (ncRNA) is a kind of RNA that will not be translated into proteins. It can be detected in the blood, and it has been extensively studied for diagnostic purposes (100). MicroRNA or miRNA is the preferred marker among all the various types of ncRNAs because of its more stable structure, making it a more reliable marker than the other ncRNAs (101,102). A study by Kishimoto et al. aimed at evaluating whether circulating miR-21 could be a potential biomarker for BTC, analysed plasma samples from 94 patients with histologically proven BTC, 23 patients with benign biliary disease, and 50 healthy volunteers. Expression levels of miR-21 were significantly higher at baseline in patients with BTC and decreased significantly after surgery. The use of a combination of plasma miR-21 and CA19-9 levels to differentiate BTC patients from healthy volunteers or patients with benign biliary disease appeared very promising: in this study, miR-21 was a highly sensitive biomarker, while CA19-9 had a high specificity (103). Besides, miR-21 is not a specific biomarker for BTC and has been reported as a biomarker in other types of cancers such as lymphoma, colorectal cancer, oesophageal squamous cell carcinoma and hepatocellular carcinoma (104-107).
Assessing miRNA not only on the blood but also on the bile has also emerged as a valid alternative. In a study by Shigehara et al., bile was sampled from 9 patients with BTC and 9 age-matched patients with choledocholithiasis. The presence and stability of miRNAs on these bile samples was confirmed. Furthermore, differential analysis demonstrated that 10 of the 667 miRNAs analysed were significantly more highly expressed in the malignant group than in the benign group, and the ROC-curve analysis showed that some specific miRNAs, namely miR-9 and miR-145, could be useful diagnostic markers for BTC (108).
“Hot” and “Cold” tumors
It is well known how the heterogeneity of the tumor microenvironment (TME) has a role in determining how well a tumor responds after immunotherapy. Non-T cell-infiltrated tumors, the so-called “cold” tumors, contain very few CD8+ T cells but harbor many immunosuppressive cells such as M2-like TAMs (tumor-associated macrophages), MDSCs (myeloid-derived suppressor cells) and tolerogenic DCs (dendritic cells). These tumors have a subpar response to immunotherapy in contrast with the so-called “hot” tumors, which have a high concentration of CD8+ T cells and express more immune checkpoint molecules and have therefore a good response to immunotherapy. As of now, many studies are designing ways to improve the efficacy of checkpoint inhibitors in “cold” tumors by turning them into “hot” tumors through the activation of a pro-inflammatory process. Combining immune checkpoint inhibitors with targeted therapy or with radiotherapy, traditional systemic therapy, or other agents (such as cell-based therapies, vaccines, cytokines, or colony stimulating factors) could improve the efficacy of immunotherapy and increase drastically the number of patients that could benefit from it (109). Furthermore, many of the biomarkers that have been studied to predict treatment response in BTCs are ultimately immune-related biomarkers (such as PD-L1 expression): therefore, these markers could be unreliable in “cold” tumors. Given this apparent pivotal role of the TME, the careful assessment of the immunological status of the tumor could be used as a marker itself to differentiate between “hot” and “cold” tumors. However, maintaining a strict distinction of “hot” versus “cold” tumors could prove itself really dangerous as it turns an overly complex scenario into a “black or white” categorization (110).
Conclusions
Immunotherapy is changing the approach to the treatment of cancer; so far, immune checkpoint inhibitors are approved as second line therapy in BTCs, for patients with MSI instability, TMB-high and PDL-1 overexpression, not responsive to first-line chemotherapy. However, ongoing studies suggest promising results of ICIs in a wider subset of patients, alone and in combination with standard chemotherapy. Moreover, circulating factors such as tumor DNA, could represent a new tool to achieve early diagnosis of BTC, monitor tumor response to treatment and predict tumor recurrence.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P. et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016May1;13(5):261-80
2. Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D. et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: Results of EUROCARE-5. Eur J Cancer. 2015Oct1;51(15):2169-78
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019Jan1;69(1):7-34
4. Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M. et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020Jun1;126(11):2666-78
5. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB. et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. Journal of Hepatology. 2019Jul1;71(1):104-14
6. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver International. 2019May1;39(S1):19-31
7. Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Journal of Hepatology. 2020Jan1;72(1):95-103
8. Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002Dec1;37(6):806-13
9. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353-7
10. Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004May;24(2):115-25
11. Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472-7
12. Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A. et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48(6):816-20
13. Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010Jul;42(7):490-5
14. Utada M, Ohno Y, Tamaki T, Sobue T, Endo G. Long-term trends in incidence and mortality of intrahepatic and extrahepatic bile duct cancer in Japan. J Epidemiol. 2014;24(3):193-9
15. Yang H, Wang J, Li Z, Yang Y, Yang L, Zhang Y. et al. Risk Factors and Outcomes of Early Relapse After Curative Resection of Intrahepatic Cholangiocarcinoma. Frontiers in Oncology. 2019Sep4;9:854
16. Sulpice L, Rayar M, Boucher E, Pracht M, Meunier B, Boudjema K. Treatment of recurrent intrahepatic cholangiocarcinoma. British Journal of Surgery. 2012Nov6;99(12):1711-7
17. Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I. et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. British Journal of Surgery. 2018May14;105(7):848-56
18. Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver International. 2019May1;39(S1):98-107
19. Boilève A, Hilmi M, Smolenschi C, Ducreux M, Hollebecque A, Malka D. Immunotherapy in Advanced Biliary Tract Cancers. Cancers (Basel). 2021 Mar 29;13(7)
20. Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Annals of Oncology. 2016Sep1;27:v28-37
21. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A. et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. New England Journal of Medicine. 2010Apr8;362(14):1273-81
22. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy
23. Simile MM, Bagella P, Vidili G, Spanu A, Manetti R, Seddaiu MA. et al. Targeted therapies in cholangiocarcinoma: Emerging evidence from clinical trials. Vol. 55, Medicina (Lithuania). MDPI AG. 2019
24. Cable J, Greenbaum B, Pe'er D, Bollard CM, Bruni S, Griffin ME. et al. Frontiers in cancer immunotherapy—a symposium report. Ann N Y Acad Sci. 2021Apr16;1489(1):30-47
25. Immune Checkpoint Inhibitors - National Cancer Institute [Internet]. [cited 2022 Mar 18]. Available from: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors.
26. Sharma P, Allison JP. The future of immune checkpoint therapy. Science (1979). 2015Apr3;348(6230):56-61
27. Perrier A, Didelot A, Laurent-Puig P, Blons H, Garinet S. Epigenetic Mechanisms of Resistance to Immune Checkpoint Inhibitors. Biomolecules 2020, Vol 10, Page 1061. 2020Jul16;10(7):1061
28. Guo X, Shen W. Latest evidence on immunotherapy for cholangiocarcinoma. Oncol Lett. 2020Dec;20(6):381
29. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nature Reviews Gastroenterology and Hepatology. 2021Aug1;18(8):525-43
30. Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2019 May 1;2(5)
31. Sasaki T, Takeda T, Okamoto T, Ozaka M, Sasahira N. Chemotherapy for Biliary Tract Cancer in 2021. J Clin Med. 2021 Jul 2;10(14)
32. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017Jul28;357(6349):409-13
33. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A. et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020Oct15;147(8):2190-8
34. FDA approves pembrolizumab for first-line treatment of MSI-H/dMMR colorectal cancer | FDA [Internet]. [cited 2022 Mar 18]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-first-line-treatment-msi-hdmmr-colorectal-cancer.
35. Goeppert B, Roessler S, Renner M, Singer S, Mehrabi A, Vogel MN. et al. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br J Cancer. 2019Jan8;120(1):109-14
36. Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu J. et al. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. Journal for Immunotherapy of Cancer. 2020Jun1;8(1):367
37. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE. et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020Jun1;6(6):888-94
38. Xu L, Zhou J. Interpretation of NCCN guidelines for hepatobiliary tumors V3 update in 2021. Hepatobiliary Surg Nutr. 2021Oct;10(5):682-5
39. National Comprehensive Cancer Network. NCCN Guidelines for Hepatobiliary Cancer V.5.2022 - [Internet]. [cited 2023 Feb 20]
40. Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M. et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020Sep1;6(9):1405-9
41. Merck's KEYTRUDA® (pembrolizumab) Plus Chemotherapy Significantly Improved Overall Survival Versus Chemotherapy in First-Line Advanced or Unresectable Biliary Tract Cancer in KEYNOTE-966 Trial. [Internet]. [cited. 2023 Feb 17]. Available from: https://www.merck.com/news/mercks-keytruda-pembrolizumab-plus-chemotherapy-significantly-improved-overall-survival-versus-chemotherapy-in-first-line-advanced-or-unresectable-biliary-tract-cancer-in-keynote-966/
42. Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A. et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. Journal of Clinical Oncology. 2022Jan19;40(4_suppl):378-378
43. Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S. et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020 May 26;8(1)
44. Kang YK, Doi T, Kondo S, Chung HC, Muro K, Helwig C. et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in Asian patients with pretreated recurrent or refractory gastric cancer: Preliminary results from a phase I trial. Journal of Clinical Oncology. 2018 Feb 26;36(4)
45. Merck Reports Topline Data for Bintrafusp Alfa as Second-Line Monotherapy Treatment | Merck [Internet]. [cited 2022 Mar 18]. Available from: https://www.merckgroup.com/en/news/bintrafusp-topline-data-biliary-tract-cancer-16-03-2021.html.
46. Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T. et al. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clin Cancer Res. 2013Apr15;19(8):2224-31
47. Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T. et al. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J Transl Med. 2014 12
48. Yamamoto K, Ueno T, Kawaoka T, … SHA, 2005 U. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25:3575-80
49. Lepisto AJ. et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955-64
50. Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y. et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother. 2011Jan;34(1):92-9
51. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT. et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020Apr2;382(14):1331-42
52. Hucks G, Rheingold SR. The journey to CAR T cell therapy: the pediatric and young adult experience with relapsed or refractory B-ALL. Blood Cancer J. 2019 Feb 1;9(2)
53. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP. et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019Jan3;380(1):45-56
54. Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q. et al. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin Cancer Res. 2018Mar15;24(6):1277-86
55. Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H. et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018Oct1;9(10):838-47
56. Sabry M, Lowdell MW. Killers at the crossroads: The use of innate immune cells in adoptive cellular therapy of cancer. Stem Cells Transl Med. 2020Sep1;9(9):974-84
57. Powles T, Walker J, Andrew Williams J, Bellmunt J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat Rev. 2020 Jan 1;82
58. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A. et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37(7):537-46
59. Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers (Basel). 2021Feb1;13(3):1-11
60. Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020Dec1;10(12):1808-25
61. Endris V, Buchhalter I, Allgäuer M, Rempel E, Lier A, Volckmar AL. et al. Measurement of tumor mutational burden (TMB) in routine molecular diagnostics: in silico and real-life analysis of three larger gene panels. Int J Cancer. 2019May1;144(9):2303-12
62. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A. et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. International Journal of Cancer. 2020Oct15;147(8):2190-8
63. Jain A, Shroff RT, Zuo M, Weatherly J, Meric-Bernstam F, Isaacs R. et al. Tumor mutational burden (TMB) and co-existing actionable mutations in biliary tract cancers (BTC). Journal of Clinical Oncology. 2017May30;35(15_suppl):4086-4086
64. Zhang W, Shi J, Wang Y, Zhou H, Zhang Z, Han Z. et al. Next-generation sequencing-guided molecular-targeted therapy and immunotherapy for biliary tract cancers. Cancer Immunol Immunother. 2021Apr1;70(4):1001-14
65. Kim JY, Kronbichler A, Eisenhut M, Hong SH, van der Vliet HJ, Kang J. et al. Tumor Mutational Burden and Efficacy of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019 Nov 1;11(11)
66. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019Feb1;51(2):202-6
67. Rimassa L, Personeni N, Aghemo A, Lleo A. The immune milieu of cholangiocarcinoma: From molecular pathogenesis to precision medicine. Journal of Autoimmunity. 2019Jun1;100:17-26
68. Sirica A, (Baltimore GGH, Md.) U, 2014 U. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology. 2014;59(6):2397-402
69. Lin Y, Li B, Yang X, Cai Q, Liu W, Tian M. et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia. 2019Dec1;21(12):1133-42
70. Yoshimura T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: a foe or ally? Cell Mo Immunology. 2018;15(4):335-45
71. Cadamuro M, Stecca T, Brivio S, … VM… et BA (BBA, 2018 U. The deleterious interplay between tumor epithelia and stroma in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4):1435-43
72. Chuaysri C, Thuwajit P, … APO, 2009 U. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21:957-69
73. Sirica A. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2009;9(1):44-54
74. Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22(4):327-34
75. Vivier E, Ugolini S, Blaise D, … CCNR, 2012 U. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunology. 2012;12(4):239-52
76. Fabris L, Sato K, Alpini G, Hepatology MS, 2021 U. The tumor microenvironment in cholangiocarcinoma progression. Hepatology. 2020Jan1;73(S1):2021
77. Kitano Y, Okabe H, Yamashita Y, Cancer SN… journal of, 2018 U. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. British Journal of Cancer. 2018;118(2):171-80
78. Ma C, Peng C, Lu X, Ding X, Zhang S, Zou X. et al. Downregulation of FOXP3 inhibits invasion and immune escape in cholangiocarcinoma. Biochemical and Biophysical Research Communications. 2015Mar6;458(2):234-9
79. Alix-Panabier̀es C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013Jan;59(1):110-8
80. Rushton AJ, Nteliopoulos G, Shaw JA, Coombes RC. A Review of Circulating Tumour Cell Enrichment Technologies. Cancers (Basel). 2021Mar1;13(5):1-33
81. Yamada T, Matsuda A, Koizumi M, Shinji S, Takahashi G, Iwai T. et al. Liquid Biopsy for the Management of Patients with Colorectal Cancer. Digestion. 2019Dec1;99(1):39-45
82. Ustwani O Al, Iancu D, Yacoub R, Iyer R. Detection of circulating tumor cells in cancers of biliary origin. J Gastrointest Oncol. 2012;3(2):97-104
83. Yang JD, Campion MB, Liu MC, Chaiteerakij R, Giama NH, Ahmed Mohammed H. et al. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology. 2016Jan1;63(1):148-58
84. Backen AC, Lopes A, Wasan H, Palmer DH, Duggan M, Cunningham D. et al. Circulating biomarkers during treatment in patients with advanced biliary tract cancer receiving cediranib in the UK ABC-03 trial. British Journal of Cancer. 2018Jul21;119(1):27-35
85. Reduzzi C, Vismara M, Silvestri M, Celio L, Niger M, Peverelli G. et al. A novel circulating tumor cell subpopulation for treatment monitoring and molecular characterization in biliary tract cancer. Int J Cancer. 2020Jun15;146(12):3495-503
86. Han SY, Park SH, Ko HS, Jang A, Seo H Il, Lee SJ. et al. Vimentin-Positive Circulating Tumor Cells as Diagnostic and Prognostic Biomarkers in Patients with Biliary Tract Cancer. J Clin Med. 2021 Oct 1;10(19)
87. Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017Apr1;17(4):223-38
88. Rizzo A, Ricci A, … STCG&, 2020 U. Circulating tumor DNA in biliary tract cancer: current evidence and future perspectives. cgp.iiarjournals.org.
89. Hao YX, Fu Q, Guo YY, Ye M, Zhao HX, Wang Q. et al. Effectiveness of circulating tumor DNA for detection of KRAS gene mutations in colorectal cancer patients: a meta-analysis. Onco Targets Ther. 2017Feb16;10:945-53
90. Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JSC. et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014 Dec 15;5
91. Mody K, Kasi PM, Yang J, Surapaneni PK, Bekaii-Saab T, Ahn DH. et al. Circulating Tumor DNA Profiling of Advanced Biliary Tract Cancers. JCO Precis Oncol. 2019Dec;3(3):1-9
92. Jensen LH, Andersen RF, Byriel L, Fernebro E, Jakobsen A, Lindebjerg J. et al. Phase II study of gemcitabine, oxaliplatin and capecitabine in patients with KRAS exon 2 mutated biliary tract cancers. Acta Oncol. 2020Mar3;59(3):298-301
93. Chen C, Wang T, Yang M, Song J, Huang M, Bai Y. et al. Genomic Profiling of Blood-Derived Circulating Tumor DNA from Patients with Advanced Biliary Tract Cancer. Pathol Oncol Res. 2021 Oct 15;27
94. Csoma SL, Bedekovics J, Veres G, Árokszállási A, András C, Méhes G. et al. Circulating Cell-Free DNA-Based Comprehensive Molecular Analysis of Biliary Tract Cancers Using Next-Generation Sequencing. Cancers (Basel). 2022 Jan 1;14(1)
95. Han JY, Ahn KS, Kim TS, Kim YH, Cho KB, Shin DW. et al. Liquid Biopsy from Bile-Circulating Tumor DNA in Patients with Biliary Tract Cancer. Cancers (Basel). 2021 Sep 1;13(18)
96. Andresen K, Boberg KM, Vedeld HM, Honne H, Jebsen P, Hektoen M. et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015May1;61(5):1651-9
97. Wasenang W, Chaiyarit P, Proungvitaya S, Limpaiboon T. Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin Epigenetics. 2019 Mar 4;11(1)
98. Yang JD, Yab TC, Taylor WR, Foote PH, Ali HA, Lavu S. et al. Detection of Cholangiocarcinoma by Assay of Methylated DNA Markers in Plasma. Gastroenterology. 2017Apr;152(5):S1041-2
99. Qiu Z, Ji J, Xu Y, Zhu Y, Gao C, Wang G. et al. Common DNA methylation changes in biliary tract cancers identify subtypes with different immune characteristics and clinical outcomes. BMC Med. 2022 Dec 1;20(1)
100. Afonso MB, Rodrigues PM, Simão AL, Castro RE. Circulating microRNAs as Potential Biomarkers in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. J Clin Med. 2016 Mar 3;5(3)
101. Li X, Xiuli Q, Shengliang D, Yumei X, Xinlei R, Gaoyun C. et al. MicroRNAs: potential biomarkers for disease diagnosis. Biomed Mater Eng. 2014;24(6):3917-25
102. Bartel DP. MicroRNAs. Cell. 2004;116:281-97
103. Kishimoto T, Eguchi H, Nagano H, Kobayashi S, Akita H, Hama N. et al. Plasma miR-21 is a novel diagnostic biomarker for biliary tract cancer. Cancer Sci. 2013Dec;104(12):1626-31
104. Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H. et al. Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2011Jun28;105(1):104-11
105. Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S. et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012Jan;56(1):167-75
106. Kanaan Z, Rai SN, Eichenberger MR, Roberts H, Keskey B, Pan J. et al. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012Sep;256(3):544-51
107. Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K. et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008Jun;141(5):672-5
108. Shigehara K, Yokomuro S, Ishibashi O, Mizuguchi Y, Arima Y, Kawahigashi Y. et al. Real-Time PCR-Based Analysis of the Human Bile MicroRNAome Identifies miR-9 as a Potential Diagnostic Biomarker for Biliary Tract Cancer. PLoS ONE. 2011Aug17;6(8):e23584
109. Loeuillard E, Conboy CB, Gores GJ, Rizvi S. Immunobiology of cholangiocarcinoma. JHEP Reports. 2019Oct1;1(4):297-311
110. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumors with combination immunotherapies. Vol. 18, Nature Reviews Drug Discovery. Nature Publishing Group. 2019 p. 197-218
111. Bekaii-Saab TS, Valle JW, van Cutsem E, Rimassa L, Furuse J, Ioka T. et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncology. 2020Oct;16(30):2385-99
112. A Study to Assess RXC004 Efficacy in Advanced Solid Tumours After Progression on Standard of Care (SoC) Therapy (PORCUPINE2) [Internet]. [cited 2022 Oct 23]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04907851.
113. Toripalimab Plus Lenvatinib as Second-line Treatment in Advanced Biliary Tract Cancers [Internet]. [cited 2022 Oct 23]. Available from: https://www.clinicaltrials.gov/ct2/show/record/NCT04211168.
114. Mazzaferro V, El-Rayes BF, Droz dit Busset M, Cotsoglou C, Harris WP, Damjanov N. et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019Jan13;120(2):165-71
115. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B. et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021Sep;22(9):1290-300
116. A Study of E7090 in Participants with Unresectable Advanced or Metastatic Cholangiocarcinoma With Fibroblast Growth Factor Receptor (FGFR) 2 Gene Fusion [Internet]. [cited 2022 Oct 23]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT04238715.
117. Pembrolizumab (MK-3475) Plus Gemcitabine/Cisplatin Versus Placebo Plus Gemcitabine/Cisplatin for First-Line Advanced and/or Unresectable Biliary Tract Carcinoma (BTC) (MK-3475-966/KEYNOTE-966) (KEYNOTE-966) [Internet]. [cited 2022 Oct 23]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT04003636.
118. A Phase I, Open-Label, Multicentre Study to Evaluate the Safety, Tolerability, Pharmacokinetics of MEDI4736 in Patients with Advanced Solid Tumours. [Internet]. [cited. 2023 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT01938612
119. Ueno M. et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. The Lancet Gastroenterology & Hepatology. 2019;4(8):611-621
120. Study of PD-1 Inhibitor in Combination with Gemcitabine/Cisplatin for Advancer BTCs. [Internet]. [cited. 2023 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT03311789
121. Efficacy Safety of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) in Previously Treated Participants with Select Solid Tumors (MK-7902-005/E7080-G000-224/LEAP-005). [Internet]. [cited. 2023 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT03797326
122. Camrelizumab Combined with Apatinib and Capecitabine in Patients With Advanced Unresectable Biliary Tract Cancer. [Internet]. [cited. 2023 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT04720131
123. Hypofractionated Radiation Therapy and Bintrafusp Alfa for the Treatment of Advanced Intrahepatic Cholangiocarcinoma. [Internet]. [cited. 2023 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT04708067
Author contact
![]() Corresponding author: Simone Conci, MD, PhD, Department of Surgery, Division of General and Hepatobiliary Surgery, University of Verona Medical School, G.B. Rossi University Hospital Piazzale L.A. Scuro, 10, Verona 37134, Italy. Tel.: +39-045-8124655; Fax: +39-045-8027426; Email: simone.conciveneto.it.
Corresponding author: Simone Conci, MD, PhD, Department of Surgery, Division of General and Hepatobiliary Surgery, University of Verona Medical School, G.B. Rossi University Hospital Piazzale L.A. Scuro, 10, Verona 37134, Italy. Tel.: +39-045-8124655; Fax: +39-045-8027426; Email: simone.conciveneto.it.

 Global reach, higher impact
Global reach, higher impact