3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(6):818-835. doi:10.7150/ijms.82419 This issue Cite
Review
Regulatory mechanisms and clinical applications of tumor-driven exosomal circRNAs in cancers
Department of Gastric Surgery, Cancer Hospital of China Medical University/Liaoning Cancer Hospital, Shenyang, Liaoning, China
Received 2023-1-6; Accepted 2023-3-9; Published 2023-5-8
Abstract
Malignant tumors seriously affect people's survival and prognosis. Exosomes, as vesicle structures widely existing in human tissues and body fluids, are involved in cell-to-cell transmission. Tumor-derived exosomes were secreted from tumors and involved in the development of carcinogenesis. Circular RNA (circRNA), a novel member of endogenous noncoding RNAs, is widespread in human and play a vital role in many physiological or pathological processes. Tumor-driven exosomal circRNAs are often involved in tumorigenesis and development including the proliferation, invasion, migration and chemo-or-radiotherapy sensitivity of tumor cell by multiple regulatory mechanisms. In this review, we will elaborate the roles and functions of tumor-driven exosomal circRNAs in cancers which may be used as potential cancer biomarkers and novel therapeutic targets.
Keywords: cancer, tumor-driven exosomes, exosomal circRNAs, mechanisms, clinical application
Introduction
Cancer is a major public health problem worldwide. GLOBOCAN 2020 suggested that there were 19,292,789 cancer cases and 9,958,133 cancer deaths globally, which indicated the burden of cancer incidence and mortality was rapidly growing worldwide [1]. China is a country with a large population. Despite the rapid economic development and continuous improvement of people's living standards, the proportion of advanced tumors is high and the prognosis is poor [2]. At present, it is believed that primary prevention is a particularly effective way to control cancer. In-depth exploration of the mechanism of cancer occurrence and development will provide more ideas and means for primary prevention of cancer [3].
In the early years, exosomes were then speculated to be a way for cells to excrete waste [4]. However, with the deepening of research, the role of exosomes had been gradually paid more attentions to by researchers. Studies have shown that exosomes were involved in many biological processes including immune response, antigen presentation, cell differentiation, tumor growth and invasion [5]. Exosomes were small membrane-bound extracellular vesicles with a diameter ranging from 40-160 nm, which could be detected in various bodily fluids and carry a wide repertoire of cellular components from their parental cells, including proteins, lipids, DNAs, mRNAs, and noncoding RNAs (ncRNAs) [6-8]. Tumor-derived exosomes were secreted from tumors and involved in the development of tumor outgrowth. Exosomes from malignant cells could act on various recipient cells and eventually induced the growth, metastasis, angiogenesis, drug resistance or immune escape of adjacent tumor cells or normal recipient cells. Some tumor-derived exosomes had also been used as ideal diagnostic biomarkers and therapeutic targets for clinical application [9]. More excitingly, using exosomes as naturally derived transport devices for the treatment in cancers is also being actively explored [8].
Circular RNAs (circRNAs), a class of endogenous noncoding RNA (ncRNA) that were produced by a non-canonical splicing event, were covalently closed, endogenous biomolecules in eukaryotes with tissue-specific and cell-specific expression patterns [10, 11]. Their covalently closed ring structure made circRNAs have a high stability protecting these molecules from exonuclease-mediated degradation. Consistent with the fate of exosomes initially discovered which were regarded as ineffective products produced by metabolic processes in organisms, the biological functions of circRNAs in organisms had received little attention from researchers [12]. In recent years, thousands of circRNAs in eukaryotes had been found with high-throughput RNA sequencing (RNA-seq) and circRNA-specific bioinformatics algorithms [13, 14]. Shortly afterwards, many studies on circRNAs had been carried out. To date, by means of important non-coding functions including miRNA sponge and interacting with proteins, circRNAs had been implicated to participate in several human diseases, including cancers [15]. Numerous studies have shown that circRNAs were often enriched in exosomes from cancers and played a regulatory role between tumor cells and tumor microenvironment (TME).
In this review, we will describe recent progress in our understanding of the regulatory mechanisms and biology functions of tumor-derived exosomal circRNAs which may provide more extensive content for the research and application of exosomal circRNAs in cancers.
Tumor-driven exosomal circRNAs and malignant phenotypes
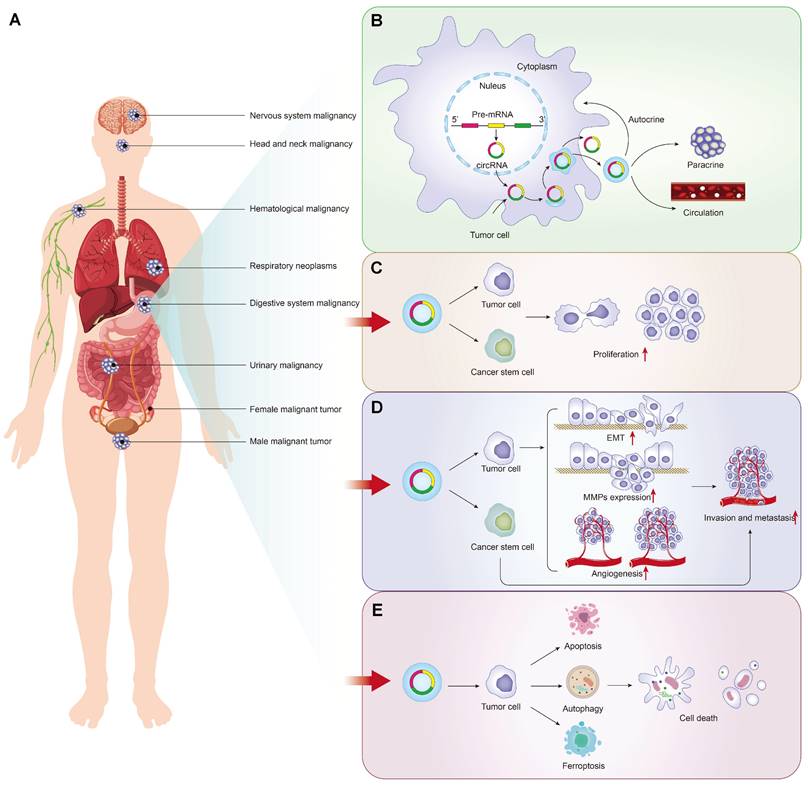
Self-sufficiency in growth signals, insensitivity to growth-inhibitory (antigrowth) signals, evasion of programmed cell death (apoptosis), limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis were the six hallmarks of malignant cells [16]. Uncontrolled proliferation, metastasis and programmed death of tumor cells play a vital role in cancer phenotypes. As shown in Figure 1A/1B, tumor-driven exosomal circRNAs are also involved in several malignant phenotypes described above. Detailed information of all tumor-driven exosome circRNAs retrieved in this review were shown in supplementary Table 1[17-125].
Tumor-driven exosomal circRNAs and proliferation
Independence on growth signaling is apparent in many cancers. Tumor-driven exosomal circRNAs make tumor cells to generate many of their own growth signals by inducing tumor cells to be blocked at G1/G0 phase. We observed the same phenomenon induced by tumor-driven exosomal circRNAs in many cancers. Cancer cells transfected with circRNAs exhibited the reduced cell viability and colony formation ability in vitro. In vivo,these tumor-driven exo-circRNAs also enhanced tumor volume and weight in nude mice (Table 1[18-21, 25-29, 31, 33, 37-39, 41, 42, 44-46, 49, 50, 53-56, 60-64, 66-70, 75, 78-82, 84-86, 88, 90, 92, 93, 95, 97-99, 101-104, 106, 108, 111, 119, 120, 122]).
In addition to the effect of tumor-driven exosomal circRNAs on cell cycle, circRNAs can also regulate cyclin protein expression to regulate proliferation. For example, in HCC and esophageal cancer, exosomal circRNA_100284[33] and circ-SFMBT2[67] regulated the cell cycle via up-regulating cyclin-D1 and increased proliferation and oncogenic capacity. Ki67 is a nuclear antigen closely related to tumor cell proliferation and its expression is mainly concentrated in M phase of cell cycle [126]. The abnormal expression of some tumor-driven exosomal circRNAs directly reflects the proliferation rate and proliferation activity of malignant tumor cells. In NLCSC, exo-circSHKBP1 induced cell proliferation by increasing the Ki67 protein levels [88].
Some circRNA-related special mechanisms that regulated tumor cell proliferation were also identified in the literature searching. Cancer stem-like cells were defined as a small population of tumor cells which were able to self-renew and generate the neoplasm by unlimitedly proliferating [127]. In colon cancer, breast cancer and malignant pleural mesothelioma cancer, circ-ABCC1[36], circHIF1A [99] and circPLK1[92] induced cancer cell proliferation by promoting cancer cell stemness. In addition, cancer stemness-related genes such as SOX2, ABCG2, NANOG, and OCT4 were also regulated by tumor-driven exosome-derived circRNAs, for example, exo-circSHKBP1 in NLCSC [87] (Figure 1C).
Tumor-driven exosomal circRNAs and metastasis
The distant metastases of tumor cells are the cause of 90% of human cancer deaths [128]. Many biological processes, such as tumor cell movement, adhesion and extracellular matrix degradation, are closely related to tumor metastasis [129]. Growing evidences indicate that tumor-driven exosomal circRNAs were involved in the regulation of the vital process. We found that tumor-driven exosomal circRNAs in many cancers, as oncogenes or tumor suppressor genes, can regulate the invasion and metastasis of tumor cells both in vivo and in vitro (as shown in Figure 1D). Mechanistically, most included studies indicated that most tumor-driven exosomal circRNAs mediate tumor cell invasion and metastasis by forming competing endogenous RNAs (ceRNAs) regulatory axis with miRNA and mRNA (as shown in Table 1). It is well known that circRNAs can act as miRNAs sponges to adsorb them, thereby weakening the inhibition of downstream mRNA transcription induced by miRNAs [10]. Changes in the physical coupling of cells to their microenvironment and activation of extracellular proteases were the two cores of tumor cell metastasis [16]. Several classes of proteins involved in the process including cell-cell adhesion molecules, integrins and extracellular proteases [130, 131]. The epithelial-to-mesenchymal transition (EMT) is a cell-biological program that induced normal epithelial cell to mesenchymal traits, thus finally enabling carcinoma cells to complete many of the steps of the invasion-metastasis cascade [132]. The deregulation of adhesion-related proteins plays an important role in EMT of cancers. In many cancers, tumor-driven exosomal circRNAs induced the tumor metastasis by regulating the expression of EMT-related protein makers. These tumor-driven exosomal circRNAs include circFNDC3B [38], circCOG2 [50], circ_100395 [79], circFBXW8 [82], circFARSA [89], circ_0001142 [103], circ-PDE8A [69], and circ_0020256 [64]. In addition to regulating the expression of EMT-related proteins, tumor-driven exosomal circRNAs also affected changes in other adhesins to promote tumor metastasis. In gastric cancer, circ_0000260 promoted the ability of migration and invasion in CDDP resistant GC cell by inducing an increase of the expression of fibronectin and vitronectin [61]. In HCC, circ-0004277 overexpression significantly promoted the metastasis of HCC cell by inhibiting the expression of ZO-1 (a member of the membrane-associated guanylate kinase (MAGUK) family of proteins, and regulates adherens junctions) [17].
ceRNA network mediated by tumor-driven exosomal circRNAs in cancers
| Exosomal circRNA | CircBase ID | miRNA | Target gene | Function | Ref. | ||
|---|---|---|---|---|---|---|---|
| Digestive system malignancy | |||||||
| Liver cancer | |||||||
| circTMEM45A | hsa_circ_0066659 | miR-665 | IGF2 | Proliferation, metastasis | 18 | ||
| circUHRF1 | hsa_circ_0048677 | miR-449c-5p | TIM-3 | Natural killer cell exhaustion, drug resistance | 19 | ||
| hsa_circ_0004658 | hsa_circ_0004658 | miR-499b-5p | JAM3 | Proliferation, metastasis | 20 | ||
| circ-DB | hsa_circ_0025129 | miR-34a | USP7 | Proliferation, deubiquitination | 21 | ||
| circRNA Cdr1 | hsa_circ_0001946 | miR-1270 | AFP | Proliferation, metastasis | 25 | ||
| circ-G004213 | hsa-circ-G004213 | miR-513b-5p | PRPF39 | Upregulating drug sensitivity | 26 | ||
| circTMEM181 | hsa_circ_0001663 | miR-488-3p | CD39 | Immunosuppression, drug resistance | 27 | ||
| circ-0072088 | hsa_circ_103809 | miR-375 | MMP-16 | Metastasis | 28 | ||
| circFBLIM1 | hsa_circ_0010090 | miR-338 | LRP6 | Glycolysis, metastasis, apoptosis | 29 | ||
| circANTXR1 | hsa_circ_0055033 | miR-532-5p | XRCC5 | Proliferation, metastasis | 31 | ||
| circRNA_100284 | hsa_circ_100284 | miR-217 | EZH2 | Proliferation, metastasis | 33 | ||
| Colon cancer | |||||||
| circ_0006174 | hsa_circ_104852 | miR-1205 | CCND2 | Proliferation, metastasis, drug resistance | 37 | ||
| circFNDC3B | hsa_circ_006156 | miR-937-5p | TIMP3 | Metastasis, angiogenesis | 38 | ||
| circ-133 | has_circ_0010522 | miR-133a | GEF-H1, RhoA | Proliferation, metastasis | 39 | ||
| ciRS-122 | hsa_circ_0005963 | miR-122 | PKM2 | Glycolysis, drug resistance | 41 | ||
| circPACRGL | has_circ_0069313 | miR-142-3p | TGF-β1 | Proliferation, metastasis, apoptosis | 42 | ||
| miR-506-3p | |||||||
| circFMN2 | hsa_circ_0005100 | miR-1182 | hTERT | Proliferation, metastasis | 44 | ||
| circ_IFT80 | hsa_circ_0067835 | miR-296-5p | MSI1 | Proliferation, metastasis, apoptosis, drug resistance | 45 | ||
| circ-RNF121 | hsa_circ_100876 | miR-1224-5p | FOXM1 | Glycolysis, proliferation, metastasis, apoptosis | 46 | ||
| circLONP2 | hsa_circ_0008558 | miR-17 | DGCR8 | Metastasis | 49 | ||
| circCOG2 | hsa_circ_101555 | miR-1305 | TGF-β1 | Proliferation, metastasis | 50 | ||
| circ_0007334 | hsa_circ_0007334 | miR-577 | KLF12 | Proliferation, metastasis, apoptosis | 53 | ||
| Gastric cancer | |||||||
| ciRS-133 | hsa_circ_0010522 | miR-133 | PRDM16 | Proliferation, metastasis, adipocyte metabolic | 54 | ||
| circNEK9 | hsa_circ_0032683 | miR-409-3p | MAP7 | Proliferation, metastasis | 55 | ||
| circ-PVT1 | circ-PVT1 | miR-30a-5p | YAP1 | Proliferation, metastasis, apoptosis, autophagy | 56 | ||
| circ-RanGAP1 | hsa_circ_0063526 | miR-877-3p | VEGFA | Proliferation, metastasis | 60 | ||
| circ_0000260 | hsa_circ_0000260 | miR-129-5p | MMP11 | Proliferation, metastasis, apoptosis, drug resistance | 61 | ||
| circ29 | hsa_circ_0044366 | miR-29a | VEGF | Proliferation, metastasis | 62 | ||
| circNHSL1 | hsa_circ_0006835 | miR-149-5p | YWHAZ | Metastasis, glutaminolysis | 63 | ||
| Bile duct/gallbladder cancer | |||||||
| circ_0020256 | hsa_circ_0020256 | miR-432-5p | E2F3 | Proliferation, metastasis | 64 | ||
| Esophagus cancer | |||||||
| circ0048117 | hsa-circ-0048117 | miR-140 | TLR4 | M2 macrophage polarization, metastasis | 66 | ||
| circ-SFMBT2 | hsa_circ_0000211 | miR-107 | SLC1A5 | Proliferation, metastasis, glutaminolysis | 67 | ||
| Pancreatic cancer | |||||||
| circZNF91 | hsa-circ-0109315 | miR-23b-3p | SIRT1 | Glycolysis, drug resistance | 68 | ||
| circ-PDE8A | hsa_circ_0036627 | miR-338 | MACC1 | Metastasis | 69 | ||
| Nervous system malignancy | |||||||
| Glioma | |||||||
| circMMP1 | hsa_circ_0024108 | miR-433 | HMGB3 | Proliferation, metastasis, apoptosis | 70 | ||
| circRNA 0001445 | hsa_circ_0001445 | miR-127-5p | SNX5 | Proliferation, metastasis, apoptosis | 75 | ||
| Respiratory neoplasms | |||||||
| Lung cancer | |||||||
| circSATB2 | hsa_circ_0008928 | miR-326 | FSCN1 | Proliferation, metastasis | 78 | ||
| circ_100395 | hsa_circ_100395 | miR-141-3p | LATS2 | Proliferation, metastasis, regulating signaling pathway | 79 | ||
| circ_102481 | hsa_circRNA_102481 | miR-30a-5p | ROR1 | Metastasis, apoptosis, drug resistance | 80 | ||
| circ_0076305 | hsa_circ_0076305 | miR-186-5p | ABCC1 | Drug resistance | 81 | ||
| circFBXW8 | circFBXW8 | miR-370-3p | TRIM44 | Proliferation, metastasis | 82 | ||
| circRNA-002178 | has_circ_002178 | miR-34 | PD1/PDL1 | Immunotherapy, diagnosis | 84 | ||
| circ_PIP5K1A | hsa_circ_0014130 | miR-101 | ABCC1 | Proliferation, metastasis, apoptosis, drug resistance | 85 | ||
| hsa_circ_0002130 | hsa_circ_0002130 | miR-498 | GLUT1/HK2/LDHA | Glycolysis, drug resistance | 86 | ||
| circSHKBP1 | hsa_circ_0000936 | miR-1294 | PKM2 | M2 macrophage polarization, proliferation, metastasis | 88 | ||
| circUSP7 | hsa_circ_0005152 | miR-934 | SHP2 | Immune infiltration, drug resistance | 90 | ||
| Malignant pleural mesothelioma | |||||||
| circPLK1 | hsa_circ_0038632 | miR-1294 | HMGA1 | Proliferation, metastasis | 92 | ||
| Female malignant tumor | |||||||
| Ovarian cancer | |||||||
| circWHSC1 | hsa_circ_0001387 | miR-145 | MUC1 | Proliferation, metastasis | 93 | ||
| miR-1182 | hTERT | ||||||
| circ-0001068 | hsa_circ_0001068 | miR-28-5p | PD1 | Immunotherapy | 95 | ||
| circFoxp1 | hsa_circ_0001320 | miR-22 | CEBPG | Proliferation, apoptosis, drug resistance | 97 | ||
| miR-150-3p | FMNL3 | ||||||
| circPUM1 | circPUM1 | miR-615-5p | NF-kB | Proliferation, metastasis, apoptosis | 98 | ||
| miR-6753-5p | MMP2 | ||||||
| Breast cancer | |||||||
| circHIF1A | hsa_circ_0032138 | miR-580-5p | CD44 | Proliferation | 99 | ||
| circPSMA1 | circPSMA1 | miR-637 | Akt1 | Immune infiltration, proliferation, metastasis | 101 | ||
| circ-MMP11 | hsa_circ_0062558 | miR-153-3p | ANLN | Proliferation, metastasis, apoptosis, drug resistance | 102 | ||
| circ_0001142 | hsa_circ_0001142 | miR-361-3p | PIK3CB | M2 macrophage polarization, proliferation, metastasis | 103 | ||
| circCARM1 | hsa_circ_0004552 | miR-1252-5p | PFKFB2 | Glycolysis | 104 | ||
| Head and neck malignancy | |||||||
| Oral squamous cell carcinoma | |||||||
| circGDI2 | hsa_circ_0005379 | miR-424-5p | SCAI | Glycolysis, proliferation, metastasis | 106 | ||
| circSPATA6 | hsa_circ_0008202 | miR-182 | TRAF6 | Proliferation, metastasis, apoptosis | 108 | ||
| Laryngeal squamous cell carcinoma | |||||||
| circRASSF2 | hsa_circ_0059354 | miR-302b-3p | IGF-1R | Proliferation, apoptosis | 111 | ||
| Urinary malignancy | |||||||
| Bladder carcinoma | |||||||
| circPRMT5 | hsa_circ_101320 | miR-30c | SNAIL1 | Metastasis | 119 | ||
| Prostate cancer | |||||||
| circXIAP | hsa_circ_0005276 | miR-1182 | TPD52 | Proliferation, metastasis, apoptosis, drug resistance | 120 | ||
| circRNA HIPK3 | hsa_circ_0000284 | miR-212 | BMI-1 | Proliferation, metastasis, apoptosis | 122 | ||
Extracellular proteases were very important for activation of matrix metalloproteinase (MMP). MMPs were significantly involved in metastasis. In HCC, exo-circ-0072088 could regulate the expression of MMP16 and promote the invasion and migration of HCC cells [28]. Similar mechanisms have been observed for GC [55] and esophageal cancer (EC) [67].
The oxygen and nutrients supplied by the vasculature are crucial for tumor cell function and survival. Lots of positive signals encourage angiogenesis in many cancers. Exo-circRNAs also promoted or inhibited the metastasis of tumor cells by regulating soluble mediating cell-matrix and cell-cell association-related factors and their receptors. In CRC, exosomal circFNDC3B, as a tumor suppressor, decreased the generation of the angiogenic activator VEGFA to inhibit the angiogenesis [36]. On the contrary, circ_0007334 promoted the migration and invasion of CRC cells by restraining the VEGFA expression of HUVECs in vivo and vitro [53].
Some special regulatory mechanisms still need to be emphasized. For example,in CRC, exo-circ-ABCC1 promotes the CRC cell migration by regulating CRC cell stemness[36]. circRHOBTB3 regulate intracellular reactive oxygen species (ROS) levels to inhibit tumor cell EMT by interacting with metabolic enzymes such as ENO1 and ENO2[52].
Tumor-driven exosomal circRNAs and apoptosis
Apoptosis is programmed cell death characterized by an elaborate sequence of morphological events such as nuclear condensation (pyknosis) and fragmentation (karyorrhexis), along with blebbing of the plasma membrane [133]. Apoptosis is needed to maintain the normal turnover of cells and embryonic development by facilitating cell death induced by a variety of stimuli. Any alteration to the normal apoptosis pathway (including the intrinsic and extrinsic pathways) can lead to various diseases including cancers [134]. Large studies indicated that apoptosis defects in cancers were closely related to the cause of chemoresistance [135], cancer metastasis [136], cancer angiogenesis [137], TME [138], tumor-promoting inflammation [139], tumor immune infiltration [140] and cancer stem cells [141]. In addition to the key molecular components of the intrinsic or extrinsic apoptotic pathway involved in the management of apoptosis, circRNAs also played an important role in this process. As presented in Table 1, we found by reviewing that many tumor-driven exosomal circRNAs indeed to be involved in cancer progression by promoting or inhibiting the apoptosis process.
Tumor-driven exosomal circRNAs production and these association with malignant tumor phenotypes: A: Tumors drive exosome circRNAs associated malignancies; B: Production process and release pathway of tumor derived exosomal circRNAs; C: Tumor-driven exosomal circRNAs and proliferation in pan-carcinoma; D: Tumor-driven exosomal circRNAs and metastasis in pan-carcinoma; E: Tumor-driven exosomal circRNAs and apoptosis in pan-carcinomas.
Autophagy, a special programmed cell death pathway, can be defined as a cellular process meant for the degradation and elimination of misfolded proteins and damaged organelles that function in adaptation to cellular stress events like starvation, development, cell death, and tumor suppression [142]. Studies had shown that the interference of autophagy in the process of apoptosis results in failure to achieve cell death [143]. Autophagy facilitates the cell survival mechanism through which the cells adapt to stress conditions and survive with stress, escaping cell death by apoptosis [143]. We also observed that tumor-driven exosomal circRNAs played an important role in autophagy process. For instance, circ-PVT1 knockdown repressed CDDP resistance via inhibiting autophagy in GC cells [56]. In breast cancer (BC), circ_0001142 could induce M2 polarization by regulating miR-361-3p and then affecting the level of autophagy in BC cells [103]. As another special programmed cell death pathway, ferroptosis also attracted more and more attentions from researchers. It is a newly reported iron-dependent PCD process that is characterized by the accumulation of an iron-dependent lethal lipid ROS response [144]. It is worth noting that some exosomal circRNAs could regulate programmed cell death in cancer via inducing ferroptosis. In lung adenocarcinoma (LUAD), exosomal circRNA_101093 from LAC cells upregulated expression of FABP3 protein and desensitize LUAD cells to ferroptosis via a FABP3-dependent reduction [77].
It can be concluded that tumor-driven exosomal circRNAs play vital roles in proliferation, metastasis, and apoptosis of cancers. This provides a general direction for the subsequent exploration of the mechanism in the genesis of various cancers (Figure 1E).
Roles and mechanisms of tumor-driven exosomal circRNAs in carcinogenesis
With the continuous development of next-generation sequencing approaches in the past two decades, the level of research on the molecular mechanisms of tumorigenesis has changed and became more systematic, which revealed that the presence of numerous ncRNAs enriches the molecular mechanisms of carcinogenesis [145]. As a new functional player in cancer biology, tumor-driven exosomal circRNAs have emerged as the important roles in regulating the development of cancers via different molecular mechanisms (including binding to proteins,sponging miRNAs, and interfering with the splicing of other RNAs) by means of the membrane structure of exosomes protecting circRNAs from degradation by enzymes and other chemicals.
ceRNA network with miRNA and mRNA
CeRNAs hypothesis was first put forward by Salmena et al. in 2011[146]. Among the many circRNAs regulatory mechanisms, circRNA/miRNA/mRNA network regulation was one of the most influential and deregulated mechanisms in cancers. Serving as miRNA sponge, circRNAs with miRNA binding sites bond directly to the corresponding one or more miRNAs to inhibit miRNA activity and thus regulated the expression of target genes [147]. Overwhelming evidence according to the results shown in Table 1 had demonstrated the profound impact of exosomal circRNA-mediated ceRNA interactions on multiple processes and events in the pathogenesis of diverse cancers such as cell proliferation, invasion, migration, apoptosis, chemoresistance or tumor microenvironment. Importantly, the roles of circRNAs in these processes were completed by exosome encapsulation and delivery. Thus, the regulatory mechanism of ceRNA network centered on exosomal circRNA played an important role in the management of biological behavior of malignant tumors.
Tumor-driven exosomal circRNAs regulate downstream gene expression
CircRNAs-protein interaction regulation pathway
In addition to the most well-accepted function of ceRNAs as miRNA sponges, tumor-driven exosomal circRNAs also directly interacted with proteins to perform physiological functions. Recent studies had shown that circRNA-protein interactions may play critical roles in a variety of diseases [20, 22, 35, 78, 92, 117]; Cytoplasm-retained circRNAs usually perform their functions by acting as scaffolds for RNA-binding proteins or other proteins [15, 148] (Figure 2A). For instance, circ-ABCC1 in CD133+cell extracted from CRC cells upregulated the expression of β-catenin in the nucleus and inhibited the expression of β-catenin in the cytoplasm by binding with β-catenin, which activating the wnt pathway, then mediated CRC cell stemness and metastasis [36]. In LUAD, exosomal cir93 increased the level of intracellular cir93 and interacted with FABP3, thus further desensitizing LUAD cells to ferroptosis [77]. In NSCLC, by help of the cyclization induced by eIF4A3, exosomal circFARSA promoted macrophage polarization to the M2 phenotype by downregulating PTEN expression and activating the PI3K/AKT pathway [89]. In HCC, circRNA-SORE from the cytoplasm binds YBX1 via the Y box sequence and prevent YBX1 from translocating into the nucleus to stabilize YBX1, which and then decreasing the ubiquitination of YBX1 induced by PRP19 in nucleus. Finally, the downstream target gene expressions of YBX1 including AKT, Raf1, ERK, c-Myc, and TGF-β1 would be affected and the patients treated with sorafenib developed resistance [22]. In glioma, circNEIL3 interacts with IGF2BP to inhibit the ubiquitin/proteasome-mediated degradation of IGF2BP3 to promote its protein expression and as well as that of its downstream targets, including CDK4/6, CD44 and c-MYC, thereby promoting malignant progression of glioma [73].
CircRNAs - miRNA interaction regulation pathway
In two articles, we also found a circRNA-miRNA interaction mode independent of the circRNA-centered ceRNA network regulation mechanism, which also played an important role in regulating cancer progression (Figure 2A). For example, Li et al. determined that exosomal circ_0044516 downregulate miR-29-3p expression by interacting with miR-29-3p to promote the proliferation and metastasis of prostate cancer cells [123]. In CRC, Jiang et al. found that exosomal circEPB41L2 sponged miR-21-5p and miR-942-5p to repress colorectal cancer progression by regulating the PTEN/AKT signalling pathway [47]. It was worth noting that the pattern in which this circRNA-miRNA interaction affects the activation of downstream PTEN/AKT signalling pathway identified by Jiang et al. does not be identified for specific mRNA targets. In essence, circRNA still plays the role of miRNA sponge in circRNA-miRNA interaction mechanism.
CircRNAs and epigenetic regulation
Epigenetics is defined as a heritable change in gene expression or chromosomal stability by utilizing DNA methylation, histone covalent modification or non-coding RNAs without a change in DNA sequence [149]. These effects of epigenetic changes had been well documented to contribute to tumor progression [150]. As a vital epigenetic mark, ncRNAs including lncRNA, circRNA, and miRNA mis-regulation eventually leaded to the activation of oncogenes or the deactivation of tumor suppressor genes governing the hallmarks of cancer such as sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis [151]. In this review, we also found that many tumor-driven exosomal circRNAs regulated oncogene or tumor suppressor gene expression by participating in or managing epigenetic alterations (Figure 2A). Firstly, these circRNAs could regulate protein ubiquitination and degradation at the post-transcriptional level. In gliomas, Pan et al. found that circNEIL3 interacts with IGF2BP in the cytoplasm, enhancing the stability of IGF2BP3 protein and inhibiting the E3 ubiquitin ligase HECTD4-mediated degradation of IGF2BP3 at the post-transcriptional level to promote the polarization of macrophage toward an immunosuppressive phenotype enabling gliomas to acquire angiogenic and immunosuppressive properties in turn promoting tumor progression. Meanwhile, exosomes wrapped circNEIL3 and secreted it to surrounding immune cells, making them lose their immune regulatory function and leading to carcinogenesis [73]. In HCC, exosome circ-DB and circRNA-SORE were also involved in the regulation of protein ubiquitination. Exosomal circ-DB absorbed miR-34a and enhanced the deubiquitination of cyclin A2 mediated by USP7, promoting HCC growth [21]. Exosomal circRNA-SORE stabilized YBX1 in the cytoplasm, thus preventing YBX1 from subsequently be degraded by the E3 ligase PRP19 in the nucleus and enhancing the sorafenib resistance of HCC [22]. In NSCLC, exosomal circFARSA regulated PTEN expression at a post-transcriptional level and activated the PI3K/AKT signaling pathway in macrophages by promoting PTEN ubiquitination and degradation, which induced M2 polarization [89]. Besides ubiquitination mediated by, circRNAs could induce protein phosphorylation in cancers. For example, exosomal circGLIS3 directly binds with p-Ezrin (T567) and elevates p-Ezrin (T567) level at the post-transcription, promoting the Ezrin T567 phosphorylation and contributing to the invasion and angiogenesis of glioma [74].
Upstream factors regulate tumor-driven exosomal circRNA expression
RNA-binding proteins (RBPs) were the most common factors regulating the expression of circRNAs in upstream. Large bioactivities such as tumor cell proliferation, differentiation, and apoptosis were closely related to the RBPs [152]. Studies suggested that various RBPs induced the upregulation of tumour-associated circRNAs[10, 105]. In glioma, an RNA-binding protein EWSR1 could bind to sequence 1 and sequence 3 of NEIL3 pre-mRNA and the binding ability was significantly upregulated under hypoxic conditions [73]. The study from Chen et al. indicated that eIF4A3 could directly bind to the sequences flanking circFARSA to mediate its circularization and expression [89].
In addition to RBPs that control circRNA expression in upstream, some special factors were also involved in regulating circRNA expression. In CRC, for example, Chen et al. found that antisense oligonucleotides artificially synthesized could negatively regulates circRHOBTB3 circularization and expression, inhibiting CRC growth and metastasis in vitro and in vivo [52]. In hypopharyngeal carcinoma, METTL3, a predominant methyltransferase for m6A modifification, could bind to circCUX1 and stabilize the expression of circCUX1 through m6A methylation modification regulating the progression of radiotherapy tolerance [109]. The above mechanisms were shown in Figure 2B.
Feedback-loop regulating mechanism
Numerous studies had associated feedback loop regulation with the development and therapeutic response of cancers [153]. The complex and variable signal networks led to the difficulties for cancers to develop precise targeted therapy drugs.
Roles and mechanisms of tumor-driven exosomal circRNAs in carcinogenesis: A: Tumor-driven exosomal circRNAs regulate downstream gene expression; B: Upstream factors regulate tumor-driven exosomal circRNA expression; C: Feedback-loop regulating mechanism by tumor-driven exosomal circRNAs.
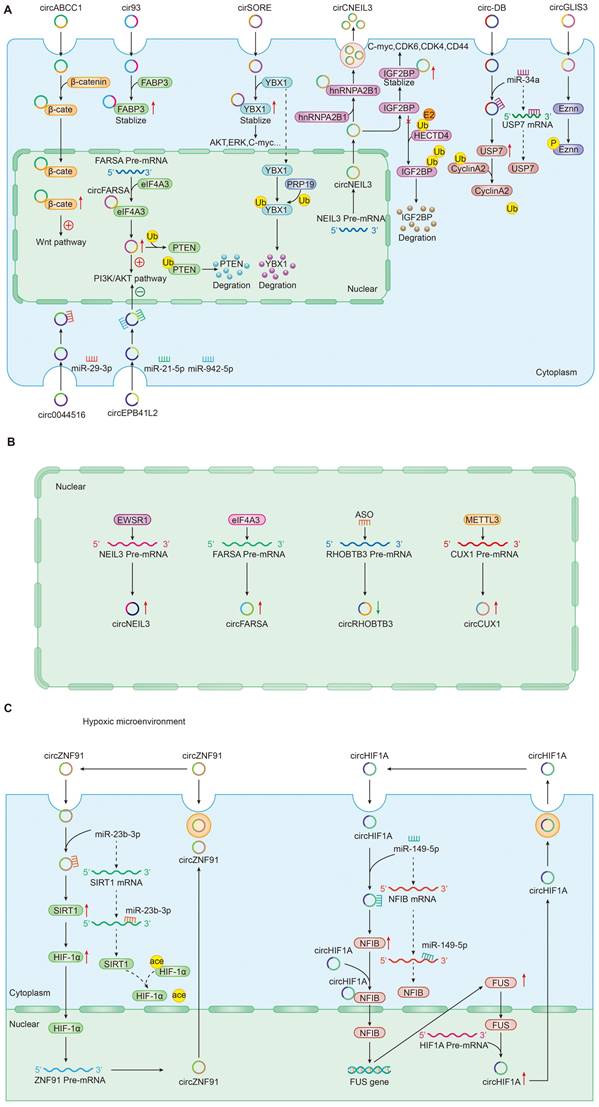
The feedback loop usually consists of a negative feedback loop and a positive feedback loop inhibiting or activating upstream and downstream signaling proteins [154]. In the process of exploring the mechanism of tumor-driven exosomal circRNAs mediated tumorigenesis, we also found the existence of feedback loop regulation mechanism (Figure 2C). In pancreatic cancer, hypoxic exosomal circZNF91/miR-23b-3p/SIRT1/HIF-1a formed a positive feedback loop that co-regulates gemcitabine resistance in pancreatic cancer cells under hypoxia. In this positive feedback loop, circZNF91 upregulated SIRT1 expression via competitively binding to miR-23b-3p and SIRT1 stabilizes HIF-1α protein via decreasing the acetylation of HIF-1α in pancreatic carcinoma (PC) cells,thus increasing the protein level of HIF-1α. As a transcription factor, HIF-1α could bind to the region at upstream of circZNF91 transcription start sites and promoted endogenous transcription of circZNF91[68]. Another a positive feedback loop was composed of circRNA 0001445/miRNA-127-5p/SNX5 involving in tumor progression including proliferation, migration and invasion. Similar feedback loops had been also found in triple-negative breast cancer. Exosomal circHIF1A could act as a miR-149-5p sponge and increase nuclear factor IB (NFIB) expression via post transcriptional regulation. In addition, circHIF1α could also bind to the NFIB protein directly in the cytoplasm and promote NFIB nuclear translocation. Interestingly, NFIB could act as a transcriptional activator of FUS (the FET family of ubiquitously expressed RNA-binding proteins) and upregulate the protein level of FUS. Meanwhile, in the 5' end flanking its intron, circHIF1A could bind to FUS as a modulator of circHIF1A formation. Finally, a positive feedback loop was formed by the circHIF1A/NFIB/FUS axis, thus increasing the TNBC growth and migration [105].
Tumor-driven exosomal circRNAs and metabolism
Cancer has long been considered a genetic disease characterized by a myriad of mutations that drive cancer progression [16]. But why do mutations in very different set of genes from most genetic disorders including cancers yield the same phenotypic outcome?[155] Recent accumulating evidence indicated that the dysregulated metabolism in cancer cells was more than a hallmark of cancer but might be the underlying cause of the tumor [155]. Since the notion of “Warburg effect” was proposed by Otto Warburg in 1927, cancers have been gradually identified as a metabolic disease [156]. Mechanically, these genetic alterations create an altered metabolic state allowing cancer cells to generate the large quantities of macromolecules (amino acids, nucleotides, and fatty acids) and metabolic intermediates which were required to fuel rapid cell growth and division resulting in carcinogenesis [157]. As an emerging category of regulatory molecules, circRNAs had been verified to control cancer metabolism [158]. In this review, we also found that tumor drives exosomal circRNAs participated in metabolic regulation (Figure 3).
Tumor-driven exosomal circRNAs and metabolism
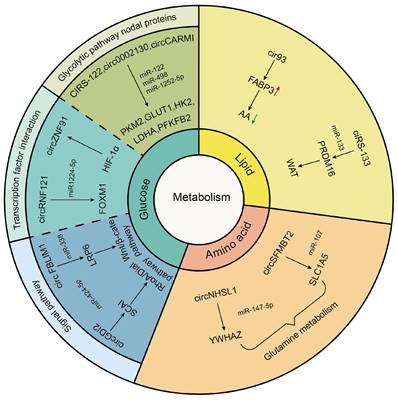
Tumor-driven exosomal circRNAs and glucose metabolism
In glucose metabolism, the Warburg effect is instrumental in malignancy. Normally, cells consume glucose to generate ATP through oxidative phosphorylation (OXPHOS) under the aerobic condition and channel into glycolysis only under the anoxic condition. However, cancer cells prefer glycolysis even in the normoxic [159]. On the one hand, this metabolism method relieved oxidative stress injury caused by mitochondria. One the other hand, excess lactic acid produced by the Warburg effect helped avoid immune surveillance environment [160, 161]. Tumor-driven exosomal circRNAs participated in glycolysis just by regulating expression of glucose transporters, metabolic enzymes, and oncogenes. The glucose transporters (GLUTs) and metabolic enzymes (including hexokinase (HK), 6-phosphfructa-1-kinase (PFK), lactate dehydrogenase, and pyruvate kinase) played a vital role in glycolysis progress. For instance, in CRC, exosomes derived from chemoresistant CRC cells could transfer ciRS-122 across cells and promote glycolysis to reduce drug susceptibility through the miR-122-PKM2 axis in CRC [41]. In NSCLC, exosomal hsa_circ_0002130 targeted miR-498 to regulate GLUT1, HK2 and LDHA and induce glycolysis, which enhanced osimertinib resistance [86]. A similar mechanism has been found in BC. BC stem cell exosome-derived circCARM1 played an important role in breast cancer cell glycolysis by sponging miR-1252-5p which regulated PFKFB2 expression [104].
Besides regulating expression of glucose transporters and metabolic enzymes, exosomal circRNAs also facilitated glycolysis by affecting functions of transcription factors (TFs). For example, Jiang et al. found that exosomal circ-RNF121 modulated CRC malignant progression via sponging miR-1224-5p to regulate the expression of Forkhead box M1 (FOXM1), an important transcription factor [46]. In addition, in pancreatic cancer (PCA), HIF-1α could bind to the region at upstream of circZNF91 transcription start sites and promoted endogenous transcription of circZNF91, thus regulating HIF-1α-associated glycolysis and increased GEM resistance in normoxic PCA cells [68].
Apart from the above influencing mechanisms, tumor-driven exosomal circRNAs also participated in glycolysis by affecting other signaling pathways. For instance, exosomal circGDI2 acted as a miRNA sponge through targeting the miR-424-5p/SCAI axis to regulate the RhoA-Dia1 signal transduction pathway weakening oral squamous cell carcinoma (OSCC) cell glycolysis [106]. In HCC, exosomal circFBLIM1 increased LRP6 expression by sponging miR-338 to regulate the activation of Wnt/b-catenin pathway and induce glycolysis and progression of patients with HCC [29].
No matter what mechanism circRNAs participated in regulating glycolysis, its core function was to act as a miRNA sponge to regulate the expression of downstream target genes. Thus, this ceRNA regulation played an important role in the management of glycolysis.
Tumor-driven exosomal circRNAs and lipid metabolism
Abundant lipid production is essential for cancer cell growth in tumor metabolism which needed adequate fatty acids (FAs) to replicate cellular membranes thus ensuring unlimited proliferation of cancer cells. Endogenous fatty acid synthesis (FAS) is triggered in various tumors [162]. In this review, we also found that tumor-driven exosomal circRNAs were involved in lipid metabolism in tumor cells. For example, arachidonic acid (AA) was a poly-unsaturated fatty acid and critical for ferroptosis associated increased peroxidation in the plasma membrane. Exosome released from LUAD cells increased intracellular cir93 to upregulate FABP3 and desensitize LUAD cells to ferroptosis via a FABP3-dependent reduction in global AA and prevention of AA incorporation into the plasma membrane [77].
In addition to participating in fatty acid metabolism, tumor-driven exosomal circRNAs played a vital role in adipose tissue transformation. It was well known that adipose tissue includes white adipose tissue (WAT) and brown adipose tissue (BAT). The former was used to store energy for the body and the latter was used for producing heat against cold and obesity conditions [163]. Although brown adipocytes can be induced within WAT depots, called as WAT browning, high percentage of WAT browning in adipose tissue was closely related to poor prognosis and death in patients [164, 165]. Thus, WAT browning in adipose tissue was undesired among tumor patients. The study of Zhang et al. indicated that exosomes derived from GC cells delivered ciRS-133 into pre-adipocytes, promoting the differentiation of pre-adipocytes into brown-like cells by activating PRDM16 and suppressing miR-133, which involved in WAT browning and played a key role in cancer-associated cachexia [54].
Tumor-driven exosomal circRNAs and amino acid metabolism
Glutamine, a non-essential amino acid with an amine functional group, is the most abundant amino acid circulating in the bloodstream. Glutamine was a major source of energy apart from glucose in proliferating cancer cells. The hydrolysis of glutamine also was a metabolic route essential for cancer cell survival and growth [166]. We found that some studies had investigated the impact of exosomal circRNAs on glutamine metabolism. For example, in gastric cancer, exosomal circNHSL1 could affect YWHAZ gene expression by sponging miR-149-5p and induce the glutaminolysis of GC cells [63]. In esophageal cancer, Chang et al. indicated that exosomal circ-SFMBT2 increased glutamine metabolism in EC cells by targeting miR-107 and upregulating SLC1A5 expression to promote the malignant development of EC [67].
Tumor-driven exosomal circRNAs and clinical applications
Tumor-driven exosomal circRNAs and diagnosis
The clinical application of tumor-driven exosomal circRNAs had rapidly attracted more and more attention. With the continuous development of high-throughput sequencing technology and the convenience of extracting exosomes from patient serums, a large number of studies had pointed out that many tumor-driven exosomal circRNAs could be used as molecular markers of some tumors to support diagnosis (Table 2). In CRC, for example, the combination of exosomal circLPAR1, CEA and CA19-9 increased the sensitivity and specifcity of diagnosis in CRC patients [35]. Circulating exosomal hsa-circ-0004771 could differentiate CRC patients from healthy controls [43]. In LUAD, serum exosomal circRNA-002178[84] and hsa_circ_0069313[83] could serve as a novel diagnosis biomarker for LUAD. Similarly, serum exosomal circKIAA1244 was also proved to serve as a novel circulating biomarker for detection of GC from healthy controls [57].
The detection and localization of tumors at their early stage is extremely crucial for treatment of cancer, and then to markedly prolong the life of the patients. Exosomal circRNAs were also being recognized as vital screening factors of early cancers. In CRC, a study suggested that the detection of early-stage CRC was confirmed by the detecting of serum exosomal circ-PNN [40]. In GC, Shao et al. found by bioinformatics analysis that hsa_circ_0065149 in plasma exosomes had higher sensitivity and specificity than traditional clinical biomarkers such as CEA, CA199, and CA125 and could be used as an indicator for early GC screening and prognosis prediction [58]. In addition, there had been many reports on the combination of multiple exosomal circRNAs to detect early cancer. For instance, the study from Xia et al. suggested that the exosome derived hsa_circ_0055202, hsa_circ_0074920 and hsa_circ_0043722 were firstly found to be used as the potential biomarker for early screening for glioblastoma multiforme (GBM) in healthy populations [71]. In astrocytoma, the features of exosomal circRNAs including hsa_circ_0003828, hsa_circ_0075828, and hsa_circ_0002976 from primary high-grade astrocytoma cells and cell-derived exosomes could be also used for screening in early high-grade astrocytoma [76].
Meanwhile, some tumor-driven exosomal circRNAs were proved to play a vital role in predicting cancer recurrence or metastasis and indicating poor prognosis. I) Predicting recurrence: The study from Wang et al. indicated that a risk score model consisted of eight circRNAs was used to predict the biochemical recurrence of prostate cancer (PCa) patients [121]. In OSCC, high exosomal circ_0000199 expression indicated the high tumor recurrence rate and mortality rate [107]. Likewise, high exosomal circMYC expression in the multiple myeloma patients' serums implied that the patients might have higher relapse rates and higher mortality rates [116]. In laryngeal squamous cell carcinoma, three serum exosomal circRNAs consisted of circ_0019201, circ_0011773 and circ_0122790 could be used as an early prediction model for occurrence of tumor [112]. II) Poor prognosis prediction: Through the detection of serum exosomes, the abnormal expression of some tumor-driven exosomal circRNAs also indicated the poor prognosis of cancer patients. For example, four exosomal circRNAs including hsa_circ_0005019, hsa_circ_0000880, hsa_circ_0051680, and hsa_circ_0006365 were used as markers for monitoring high-grade astrocytoma prognosis [76]. High exo_circRNA_0056616 expression represented a potential biomarker for screening and identifying lymph node metastasis risk in LUAD. III) Prediction treatment effect: In addition, serum exosomal circRNAs had also been used by researchers in the prediction of therapeutic efficacy. In nasopharyngeal carcinoma, the expression status of exosomal circMYC could be used as a biomarker for differentiating radioresistant patients from radiosensitive patients with nasopharyngeal carcinoma [110]. It can be seen that the detection of serum exosomal circRNAs has become an important means and method for doctors in cancer diagnosis, early screening, poor prognosis prediction and treatment expectation.
Tumor-driven exosomal circRNAs and clinical applications
| Clinical objective | Tumor-driven exosomal circRNAs | |
|---|---|---|
| Diagnosis | Cancer diagnosis and early cancer screening | circLPAR1, circ-PNN, circ_0055202, circ_0074920, circ_0043722, circRNA-002178, circKIAA1244, circ_0004001, circ_0004123, circ_0075792, circ_0065149, circ-0004771, circ_0003828, circ_0075828, circ_0002976, circ_0069313 |
| Predict cancer recurrence and metastasis | circ_30029, circ_117300, circ_176436, circ_112897, circ_112897, circ_178252, circ_115617, circ_14736, circ_17720, circ_0000199, circMYC, circ_0005019, circ_0000880, circ_0051680, circ_0006365, circ_0056616, circ_0019201, circ_0011773, circ_0122790, circ_0006602 | |
| Predicting treatment sensitivity | circMYC | |
| Treatment | Promote chemotherapy resistance | circ_0058493, circ_0006174, circUHRF1, circZNF91, circ_102481, circRNA-SORE, ciRS-122, circ_0042003, circ_PIP5K1A, circ_103801, circ_0002130, circFoxp1, circMYC, circTMEM181, circ_0000338, circUSP7 |
| Promote radiation resistance | circCUX1, circMYC, circ_IFT80 | |
| Increasing chemotherapy sensitivity | Cdr1, circ_G004213 | |
Tumor-driven exosomal circRNAs and drug-therapy or radiotherapy sensitivity and resistance
Drug-therapy and radiotherapy played an important role in the treatment of most solid cancers. Drug-therapy or radiotherapy resistance was a critical factor for tumor treatment failure and the development of locoregional relapse and distant metastases which seriously affected the prognosis of tumor patients. Different from the primary drug or radiotherapy resistance caused by genetic change, this acquired resistance were often induced by regularly exposing tumor cells to conventional drug-therapy or radiotherapy [167, 168]. It followed that the prediction and overcoming of drug-therapy or radiotherapy resistance were particularly important. As a novel kind of ncRNA, circRNAs had been shown to be involved in the induction of drug-radiotherapy resistance [169, 170]. Particularly, tumor-driven exosomal circRNAs were encapsulated and released by exosomes to change nearby cell characteristics in the tumor microenvironment (Table 2).
Tumor-driven exosomal circRNAs and drug-therapy
Tumor-driven exosomal circRNAs played an important role in cancer drug therapy. In terms of chemotherapy, in this review, we found that many tumor-driven exosomal circRNAs were involved in the mechanism of chemotherapy resistance of several tumors. In CRC, circ_0006174 in exosomes from doxorubicin-resistant CRC cells spread doxorubici resistance among CRC cells via the miR-1205-mediated overexpression of CCND2[37]. Exosomes derived from chemoresistant CRC cells could transfer ciRS-122 across cells and promote cell glycolysis to reduce drug susceptibility in chemosensitive cells through the miR-122-PKM2 axis [41]. Differentially upregulated hsa_circ_0000338 in exosomes could serve as a potential biomarker for early prediction of chemoresistance in CRC [48]. In LUAD, circ_0076305 promoted CDDP resistance of NLCSC cells through the miR-186-5p-ABCC1 axis [81]. Exosomal circ_PIP5K1A inhibited CDDP sensitivity by regulating miR-101/ABCC1 axis in NLCSC [85]. In PCA, exosomal circZNF91 was critical for the hypoxic exosome-promoted GEM resistance in normoxic cancer cells [68]. In glioma, hsa_circ_0042003 in exosomes derived from temozolomide (TMZ) resistance U251 cells conferred the resistance of U251 cells to TMZ [72]. In bone tumors, exosomal hsa_circ_103801 reduced the sensitivity of osteosarcoma cells to CDDP and strengthened the promotive effect of exosomes on the chemoresistance of osteosarcoma cells CDDP [124]. In addition, the expression of circMYC in circulating exosomes also played a vital role in bortezomib-resistant patients with multiple myeloma (MM) [116]. In ovarian cancer (OC), Luo et al. found that circFoxp1 conferred CDDP resistance to epithelial OC cells via sponges of miR-22 and miR-150-3p positively regulating the expressions of CEBPG and FMNL3 gene.
In addition to the regulatory role of chemotherapy resistance in cancers, tumor-driven exosomal circRNAs also played an important role in the resistance of targeted therapy and immunotherapy. For example, the study from Zhong et al. indicated that hsa_circ_0058493 was significantly correlated with imatinib-resistance in CML [117]. In HCC, exosomal circRNA-SORE was critical for maintaining sorafenib resistance [22]. In LUAD, tumor-derived exosomal circRNA_102481[80] and hsa_circ_0002130[86] both contributed to osimertinib resistance via ceRNA regulation pathway. Meanwhile, some exosomal circRNAs were also reported to be involved in immunotherapy resistance. In HCC, for example, exosomal circUHRF1 could enhance HCC resistance to anti-PD1 therapy by upregulating TIM-3 expression and suppressing miR-449c-5p activity [19]. Lu et al. found that high exosomal circTMEM181 favored the immunosuppressive microenvironment and endowed anti-PD1 resistance via sponging miR-488-3p and upregulating CD39 expression in macrophages [27]. In LUAD, exosomal circUSP7 was found to induce CD8+ T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC [90]. Surprisingly, we also found that some tumor-driven exosomal circRNAs, such as Cdr1as [94] and hsa-circRNA-G004213[26], could increase the sensitivity of drug therapy and improve the effect of tumor treatment in several cancers. These results demonstrated that exosomal circRNAs played multiple roles in regulating drug treatment mechanisms (Table 2).
Tumor-driven exosomal circRNAs and radiotherapy
Radiation therapy (RT) is the most effective method of cytotoxic treatment based on ionizing radiation, which was used in the treatment of most solid cancers [171]. However, the high frequency of relapses after RT was still found in different cancers. A critical factor for RT failure (the locoregional relapse or distant metastases) was that tumor-specific radioresistance[172]. Numerous studies demonstrated that tumor-driven exosomal circRNAs were closely associated with radioresistance. In hypopharyngeal squamous cell carcinoma, exosomal circCUX1 could induce tolerance to radiotherapy via regulating the expression of Caspase1 and decreasing the release of inflammatory factors [109]. As an oncogene, circMYC in exosomes could enhance the radiotherapy resistance in nasopharyngeal carcinoma cells [110]. A similar mechanism had been found in colon cancer. For instance, exosomal circ_IFT80 suppresses radiosensitivity in colorectal cancer by regulating miR-296-5p/MSI1 Axis [45] (Table 2).
Tumor-driven exosomal circRNAs and immune infiltration in TME
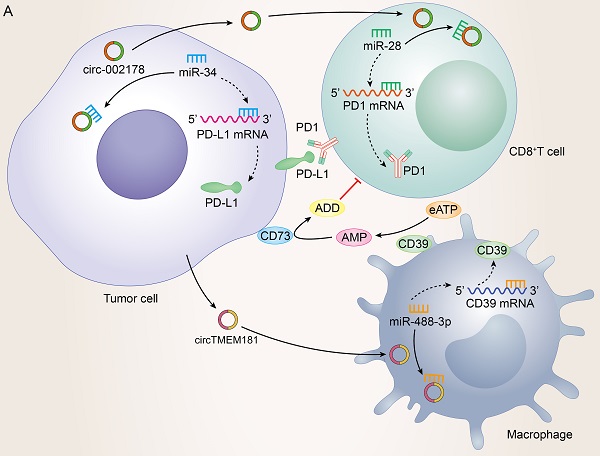
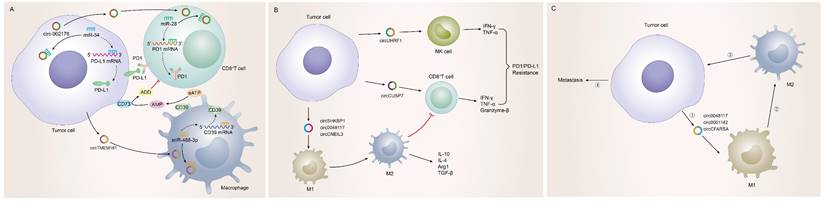
Possessing the capability of facilitating to interplay between cancer cells and surrounding cells, tumor-driven exosomal circRNAs had become a crucial role in intercellular communication [6, 9]. The TME was exactly a complicated ecosystem composed of stromal cells, extracellular matrix (ECM) components and exosomes which ensured to orchestrate cell-cell interactions. As an important component of stromal cells, immune cells could recognize antigens from tumor cells to kill them. However, most cancers could evade immune system-mediated destruction due to the mutations of immune and apoptotic pathway genes or the dysregulation of epigenetic regulation of immune escape genes [173-175]. circRNAs were closely related to the non-mutational epigenetic events in tumor immune escape. Numerous studies had identified many tumor-driven exosomal circRNAs epigenetically regulating immune infiltration, which mediated tumor immune escape and affected the efficacy of tumor immunotherapy. The specific mechanisms by which these circRNAs mediate immune cell infiltration and promote cancer development are summarized as follows: I) Immune escape: A research from HCC indicated that those HCC patients responding poorly to anti-PD1 therapy had high exosomal expression of circTMEM181. It was because of these high levels of exosomal circTMEM181 favored the immunosuppressive microenvironment. Exosomal circTMEM181 sponged miR-488-3p and upregulated CD39 expression in macrophages. Cell-specific CD39 expression in macrophages and CD73 expression in HCC cells synergistically activated the eATP-adenosine pathway which produces more adenosine, thereby impairing CD8+ T cell function and driving anti-PD1 resistance [27]. In LUAD, exosomal has-circRNA-002178 was reported to be related to immune escape and acted dual roles. Firstly, circRNA-002178 could act as a sponge for miR-34a in tumor cells to promote the PDL1 expression in tumor cells. Then tumor-derived exosomes could deliver circRNA-002178 into CD8+ T cells which enhanced PD1 expression by sponging the miR-28-5p. The cumulative expression of PDL1 and PD1 in tumor cells and CD8+ T cells finally inhibited the activation of immune cells and helped cancer cells escape T-cell-mediated death and resistanti-tumor immune responses [84] (Figure 4A). II) Regulate the secretion of cytokines by immune cells: Cytokines were major regulators of innate and adaptive immunity that enable cells of the immune system to communicate over short distances managing the function of immune cells [176]. For instance, Zhang et al. found that exosomal circUHRF1 promoted NK cell dysfunction in patients with HCC and inhibited NK cell-derived IFN-γ and TNF-α secretion thus driving resistance to anti-PD1 immunotherapy [19]. In LUAD, exosomal circUSP7 inhibits CD8+ T cell secretion of cytokines, including TNF-α, IFN-γ, Granzyme-B and perforin to promote resistance to anti-PD1 immunotherapy in NSCLC patients [90]. Similarly, exosomal circSHKBP1 could promote M2 polarization and increased the level of IL-10 and IL-4, simultaneously decreasing the secretion of TNF-a, IFN-g, granzyme-B, and perforin from CD8+ T cells [88]. In EC, hsa-circ-0048117 could facilitate the M2 macrophage polarization and increase the secretions of Arg1, IL-10 and TGF-β [66]. The changes in the levels of these cytokines ultimately affected the immune function of immune cells in the TME (Figure 4B). III) Immunosuppression and immune cell-mediated invasion and metastasis: Immunosuppression played an important role in TME to promote immune escape of tumor cells, which was the premise of promoting tumor development, metastasis and recurrence of nearby tumors, and resistance to therapy [177]. Some reports also indicated that exosomal circRNAs were closely related to the immunosuppression in TME: The study from Yang et al. indicated that exosomal circPSMA1 functioned as a tumor promoter through the circPSMA1/miR-637/Akt1-β-catenin (cyclin D1) regulatory axis, which can facilitate the immunosuppression of triple-negative breast cancer [101]. In glioma, exosomal circNEIL3 derived M2 macrophages infiltrated into the TME enabling them to acquire immunosuppressive properties by stabilizing IGF2BP3 and in turn promoting glioma progression [73]. Infiltrating immune cells in tumor immune microenvironment had been reported to participate in tumor invasion and metastasis [178, 179]. In this review, we also found that some tumor-derived exosomes were involved in the management of malignant tumor metastasis by affecting some immune cells. For instance, exosomal circ_0001142 in TME promoted macrophage polarization in BC in the presence of ERs, thus promoting the proliferation and metastasis of tumor cells [103]. In NSCLC, tumor-derived exosomal circFARSA polarized the macrophages to a M2 phenotype via PTEN ubiquitination and degradation, which further activated the PI3K/AKT signaling pathway and induced tumor cell metastasis [89]. In EC, exosomal hsa-circ-0048117 could act as sponge of miR-140 by competing with TLR4 to facilitate the M2 macrophage polarization eventually enhancing the ability of invasion and migration of tumor cells [66] (Figure 4C).
Tumor-driven exosomal circRNAs and immune infiltration in TME: A: Tumor cells regulate CD8+T cells and macrophages to participate in immune escape in TME by means of tumor-driven exosomal circRNAs; B: Tumor cells are involved in regulating PD1/PD-L1 drug resistance by regulating the secretion of cytokines by immune cells through exosome circRNAs; C: Tumor cells regulate immune cell-related tumor invasion and metastasis by means of tumor-driven exosomal circRNAs.
Of course, due to the complexity of the components in the TME, the 'cross-talk' interaction between tumor and stroma had a complex influence on the process of tumorigenesis and progression.
Conclusions and perspectives
With the in-depth exploration of the pathogenesis in the field of cancer treatment, circRNAs carried by tumor exosomes gradually showed an important role in cancer. These circRNAs in tumor-driven exosomes can deliver tumor-specific circRNAs to corresponding sites to promote or inhibit carcinogenesis through adjacent (such as paracrine or autocrine secretion of tumor cells) or distant secretion. Under the regulation of various mechanisms, tumor-driven exosomal circRNAs regulated the proliferation, invasion, migration and chemo-or-radiotherapy sensitivity of tumor cells. It was worth noting that the expression of many tumor-driven exosomal circRNAs were also regulated by upstream factors including some RNA binding proteins, oligonucleotides and other modification enzymes, which to some extent increased the complexity and uncertainty of exosomal circRNAs involvement in tumorigenesis and development. Encouragingly, some exosomal circRNAs had shown clinical application values in the future, such as early diagnosis of cancer or indicating poor prognosis.
Although it is still difficult to solve a series problems of tumor development by interfering with single exosomal circRNA, with the further study of the role of exosomal circRNAs in tumor, we also found that there were still several aspects worth looking forward to in the future. Firstly, more studies on the formation mechanism of exosomal circRNAs may provide more ideas and options for researchers in terms of inhibiting tumor formation by blocking the secretion of exosomes carrying oncogene circRNAs in the future. In addition, with the unique transport function of exosomes, some circRNAs acting as tumor suppressor genes can exert tumor suppressor effects on tumor cells. For instance, exosomes carrying therapeutic functions had been studied in clinical trials over the past few years and it was expected to become a new model of cancer treatment under the supervision of strict operational standards [8]. All the above mechanisms provide a theoretical basis for exosomal circRNAs as cancer therapeutic targets. Although the abundance of exosomal circRNAs is very low, exosomal circRNAs are widely present in human tissues and body fluids. Therefore, the research on exosomal circRNAs is a hot topic in the field of cancer diagnosis, efficacy or prognosis evaluation, and targeted therapy in future.
Supplementary Material
Supplementary tables.
Acknowledgements
Funding sources
This work was supported by the Natural Science Foundation of Liaoning Province (2020-MS-061), National Cancer Center Climbing Fund (NCC201906B02) and Shenyang Science and Technology Planning Project (22-321-33-53).
Author Contributions
Xiangyu Meng, Dong Yang and Bao Zhang: study concepts. Xiangyu Meng, Yan Zhao and Tao Zhang: study design. Xiangyu Meng, Dong Yang, Bao Zhang: data acquisition. Xiangyu Meng, Tao Zhang: quality control of data. Xiangyu Meng, Dong Yang, Bao Zhang, Tao Zhang: article preparation, writing. All authors: article editing. All authors: article review.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249
2. Xia C, Dong X, Li H. et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135(5):584-590
3. Vineis P, Wild CP, Global cancer patterns. causes and prevention. Lancet. 2014;383(9916):549-57
4. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-39
5. Ibrahim A, Marban E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol. 2016;78:67-83
6. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89
7. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367(6478)
8. Dwivedi M, Ghosh D, Saha A. et al. Biochemistry of exosomes and their theranostic potential in human diseases. Life Sci. 2023;315:121369
9. Zhang X, Yuan X, Shi H. et al. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83
10. Kristensen LS, Andersen MS, Stagsted LVW. et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675-691
11. Jeck WR, Sorrentino JA, Wang K. et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141-57
12. Cocquerelle C, Mascrez B, Hetuin D. et al. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7(1):155-60
13. Ivanov A, Memczak S, Wyler E. et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10(2):170-7
14. Salzman J, Chen RE, Olsen MN. et al. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9(9):e1003777
15. Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21(8):475-490
16. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70
17. Zhu C, Su Y, Liu L. et al. Circular RNA hsa_circ_0004277 Stimulates Malignant Phenotype of Hepatocellular Carcinoma and Epithelial-Mesenchymal Transition of Peripheral Cells. Front Cell Dev Biol. 2020;8:585565
18. Zhang T, Jing B, Bai Y. et al. Circular RNA circTMEM45A Acts as the Sponge of MicroRNA-665 to Promote Hepatocellular Carcinoma Progression. Mol Ther Nucleic Acids. 2020;22:285-297
19. Zhang PF, Gao C, Huang XY. et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19(1):110
20. Zhang L, Zhang J, Li P. et al. Exosomal hsa_circ_0004658 derived from RBPJ overexpressed-macrophages inhibits hepatocellular carcinoma progression via miR-499b-5p/JAM3. Cell Death Dis. 2022;13(1):32
21. Zhang H, Deng T, Ge S. et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2019;38(15):2844-2859
22. Xu J, Ji L, Liang Y. et al. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct Target Ther. 2020;5(1):298
23. Wang G, Liu W, Zou Y. et al. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine. 2019;40:432-445
24. Sun XH, Wang YT, Li GF. et al. Serum-derived three-circRNA signature as a diagnostic biomarker for hepatocellular carcinoma. Cancer Cell Int. 2020;20:226
25. Su Y, Lv X, Yin W. et al. CircRNA Cdr1as functions as a competitive endogenous RNA to promote hepatocellular carcinoma progression. Aging (Albany NY). 2019;11(19):8183-8203
26. Qin L, Zhan Z, Wei C. et al. Hsa-circRNA-G004213 promotes cisplatin sensitivity by regulating miR-513b-5p/PRPF39 in liver cancer. Mol Med Rep. 2021 23(6)
27. Lu JC, Zhang PF, Huang XY. et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J Hematol Oncol. 2021;14(1):200
28. Lin Y, Zheng ZH, Wang JX. et al. Tumor Cell-Derived Exosomal Circ-0072088 Suppresses Migration and Invasion of Hepatic Carcinoma Cells Through Regulating MMP-16. Front Cell Dev Biol. 2021;9:726323
29. Lai Z, Wei T, Li Q. et al. Exosomal circFBLIM1 Promotes Hepatocellular Carcinoma Progression and Glycolysis by Regulating the miR-338/LRP6 Axis. Cancer Biother Radiopharm. 2020
30. Huang XY, Huang ZL, Huang J. et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39(1):20
31. Huang C, Yu W, Wang Q. et al. CircANTXR1 Contributes to the Malignant Progression of Hepatocellular Carcinoma by Promoting Proliferation and Metastasis. J Hepatocell Carcinoma. 2021;8:1339-1353
32. Guo S, Hu C, Zhai X. et al. Circular RNA 0006602 in plasma exosomes: a new potential diagnostic biomarker for hepatocellular carcinoma. Am J Transl Res. 2021;13(6):6001-6015
33. Dai X, Chen C, Yang Q. et al. Exosomal circRNA_100284 from arsenite-transformed cells, via microRNA-217 regulation of EZH2, is involved in the malignant transformation of human hepatic cells by accelerating the cell cycle and promoting cell proliferation. Cell Death Dis. 2018;9(5):454
34. Chen W, Quan Y, Fan S. et al. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119-128
35. Zheng R, Zhang K, Tan S. et al. Exosomal circLPAR1 functions in colorectal cancer diagnosis and tumorigenesis through suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer. 2022;21(1):49
36. Zhao H, Chen S, Fu Q. Exosomes from CD133(+) cells carrying circ-ABCC1 mediate cell stemness and metastasis in colorectal cancer. J Cell Biochem. 2020;121(5-6):3286-3297
37. Zhang Y, Tan X, Lu Y. Exosomal transfer of circ_0006174 contributes to the chemoresistance of doxorubicin in colorectal cancer by depending on the miR-1205/CCND2 axis. J Physiol Biochem. 2022;78(1):39-50
38. Zeng W, Liu Y, Li WT. et al. CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol Oncol. 2020;14(11):2960-2984
39. Yang H, Zhang H, Yang Y. et al. Hypoxia induced exosomal circRNA promotes metastasis of Colorectal Cancer via targeting GEF-H1/RhoA axis. Theranostics. 2020;10(18):8211-8226
40. Xie Y, Li J, Li P. et al. RNA-Seq Profiling of Serum Exosomal Circular RNAs Reveals Circ-PNN as a Potential Biomarker for Human Colorectal Cancer. Front Oncol. 2020;10:982
41. Wang X, Zhang H, Yang H. et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 2020;14(3):539-555
42. Shang A, Gu C, Wang W. et al. Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-beta1 axis. Mol Cancer. 2020;19(1):117
43. Pan B, Qin J, Liu X. et al. Identification of Serum Exosomal hsa-circ-0004771 as a Novel Diagnostic Biomarker of Colorectal Cancer. Front Genet. 2019;10:1096
44. Li Y, Li C, Xu R. et al. A novel circFMN2 promotes tumor proliferation in CRC by regulating the miR-1182/hTERT signaling pathways. Clin Sci (Lond). 2019;133(24):2463-2479
45. Li L, Jiang Z, Zou X. et al. Exosomal circ_IFT80 Enhances Tumorigenesis and Suppresses Radiosensitivity in Colorectal Cancer by Regulating miR-296-5p/MSI1 Axis. Cancer Manag Res. 2021;13:1929-1941
46. Jiang Z, Hu H, Hu W. et al. Circ-RNF121 regulates tumor progression and glucose metabolism by miR-1224-5p/FOXM1 axis in colorectal cancer. Cancer Cell Int. 2021;21(1):596
47. Jiang Z, Hou Z, Li L. et al. Exosomal circEPB41L2 serves as a sponge for miR-21-5p and miR-942-5p to suppress colorectal cancer progression by regulating the PTEN/AKT signalling pathway. Eur J Clin Invest. 2021;51(9):e13581
48. Hon KW, Ab-Mutalib NS, Abdullah NMA. et al. Extracellular Vesicle-derived circular RNAs confers chemoresistance in Colorectal cancer. Sci Rep. 2019;9(1):16497
49. Han K, Wang FW, Cao CH. et al. CircLONP2 enhances colorectal carcinoma invasion and metastasis through modulating the maturation and exosomal dissemination of microRNA-17. Mol Cancer. 2020;19(1):60
50. Gao L, Tang X, He Q. et al. Exosome-transmitted circCOG2 promotes colorectal cancer progression via miR-1305/TGF-beta2/SMAD3 pathway. Cell Death Discov. 2021;7(1):281
51. Dou Y, Cha DJ, Franklin JL. et al. Circular RNAs are down-regulated in KRAS mutant colon cancer cells and can be transferred to exosomes. Sci Rep. 2016;6:37982
52. Chen C, Yu H, Han F. et al. Tumor-suppressive circRHOBTB3 is excreted out of cells via exosome to sustain colorectal cancer cell fitness. Mol Cancer. 2022;21(1):46
53. Bai L, Gao Z, Jiang A. et al. Circular noncoding RNA circ_0007334 sequestrates miR-577 to derepress KLF12 and accelerate colorectal cancer progression. Anticancer Drugs. 2022;33(1):e409-e422
54. Zhang H, Zhu L, Bai M. et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer. 2019;144(10):2501-2515
55. Yu L, Xie J, Liu X. et al. Plasma Exosomal CircNEK9 Accelerates the Progression of Gastric Cancer via miR-409-3p/MAP7 Axis. Dig Dis Sci. 2021;66(12):4274-4289
56. Yao W, Guo P, Mu Q. et al. Exosome-Derived Circ-PVT1 Contributes to Cisplatin Resistance by Regulating Autophagy, Invasion, and Apoptosis Via miR-30a-5p/YAP1 Axis in Gastric Cancer Cells. Cancer Biother Radiopharm. 2021;36(4):347-359
57. Tang W, Fu K, Sun H. et al. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer. 2018;17(1):137
58. Shao Y, Tao X, Lu R. et al. Hsa_circ_0065149 is an Indicator for Early Gastric Cancer Screening and Prognosis Prediction. Pathol Oncol Res. 2020;26(3):1475-1482
59. Rao M, Zhu Y, Qi L. et al. Circular RNA profiling in plasma exosomes from patients with gastric cancer. Oncol Lett. 2020;20(3):2199-2208
60. Lu J, Wang YH, Yoon C. et al. Circular RNA circ-RanGAP1 regulates VEGFA expression by targeting miR-877-3p to facilitate gastric cancer invasion and metastasis. Cancer Lett. 2020;471:38-48
61. Liu S, Wu M, Peng M. Circ_0000260 Regulates the Development and Deterioration of Gastric Adenocarcinoma with Cisplatin Resistance by Upregulating MMP11 via Targeting MiR-129-5p. Cancer Manag Res. 2020;12:10505-10519
62. Li S, Li J, Zhang H. et al. Gastric cancer derived exosomes mediate the delivery of circRNA to promote angiogenesis by targeting miR-29a/VEGF axis in endothelial cells. Biochem Biophys Res Commun. 2021;560:37-44
63. Hui C, Tian L, He X. Circular RNA circNHSL1 Contributes to Gastric Cancer Progression Through the miR-149-5p/YWHAZ Axis. Cancer Manag Res. 2020;12:7117-7130
64. Chen S, Chen Z, Li Z. et al. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis. 2022;13(1):94
65. Ren J, Chen S, Ye F. et al. Exploration of differentially-expressed exosomal mRNAs, lncRNAs and circRNAs from serum samples of gallbladder cancer and xantho-granulomatous cholecystitis patients. Bioengineered. 2021;12(1):6134-6143
66. Lu Q, Wang X, Zhu J. et al. Hypoxic Tumor-Derived Exosomal Circ0048117 Facilitates M2 Macrophage Polarization Acting as miR-140 Sponge in Esophageal Squamous Cell Carcinoma. Onco Targets Ther. 2020;13:11883-11897
67. Chang Z, Fu Y, Jia Y. et al. Circ-SFMBT2 drives the malignant phenotypes of esophageal cancer by the miR-107-dependent regulation of SLC1A5. Cancer Cell Int. 2021;21(1):495
68. Zeng Z, Zhao Y, Chen Q. et al. Hypoxic exosomal HIF-1alpha-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene. 2021;40(36):5505-5517
69. Li Z, Yanfang W, Li J. et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237-250
70. Yin K, Liu X. CircMMP1 promotes the progression of glioma through miR-433/HMGB3 axis in vitro and in vivo. IUBMB Life. 2020;72(11):2508-2524
71. Xia D, Gu X. Plasmatic exosome-derived circRNAs panel act as fingerprint for glioblastoma. Aging (Albany NY). 2021;13(15):19575-19586
72. Si J, Li W, Li X. et al. Heparanase confers temozolomide resistance by regulation of exosome secretion and circular RNA composition in glioma. Cancer Sci. 2021;112(9):3491-3506
73. Pan Z, Zhao R, Li B. et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer. 2022;21(1):16
74. Li Y, Chen J, Chen Z. et al. CircGLIS3 Promotes High-Grade Glioma Invasion via Modulating Ezrin Phosphorylation. Front Cell Dev Biol. 2021;9:663207
75. Han Y, Liu Y, Zhang B. et al. Exosomal circRNA 0001445 promotes glioma progression through miRNA-127-5p/SNX5 pathway. Aging (Albany NY). 2021;13(9):13287-13299
76. Li P, Xu Z, Liu T. et al. Circular RNA Sequencing Reveals Serum Exosome Circular RNA Panel for High-Grade Astrocytoma Diagnosis. Clin Chem. 2022;68(2):332-343
77. Zhang X, Xu Y, Ma L. et al. Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun (Lond). 2022;42(4):287-313
78. Zhang N, Nan A, Chen L. et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. 2020;19(1):101
79. Zhang C, Cao J, Lv W. et al. CircRNA_100395 Carried by Exosomes from Adipose-Derived Mesenchymal Stem Cells Inhibits the Malignant Transformation of Non-Small Cell Lung Carcinoma Through the miR-141-3p-LATS2 Axis. Front Cell Dev Biol. 2021;9:663147
80. Yang B, Teng F, Chang L. et al. Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging (Albany NY). 2021;13(9):13264-13286
81. Wang X, Wang H, Jiang H. et al. Circular RNAcirc_0076305 Promotes Cisplatin (DDP) Resistance of Non-Small Cell Lung Cancer Cells by Regulating ABCC1 Through miR-186-5p. Cancer Biother Radiopharm. 2021
82. Wang X, Lv J, He B. et al. CircFBXW8 Acts an Oncogenic Role in the Malignant Progression of Non-small Cell Lung Carcinoma by miR-370-3p-Dependent Regulation of TRIM44. Biochem Genet. 2022;60(4):1313-1332
83. Chen Y, Lou C, Ma X. et al. Serum exosomal hsa_circ_0069313 has a potential to diagnose more aggressive non-small cell lung cancer. Clin Biochem. 2022;102:56-64
84. Wang J, Zhao X, Wang Y. et al. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020;11(1):32
85. Shao N, Song L, Sun X. Exosomal circ_PIP5K1A regulates the progression of non-small cell lung cancer and cisplatin sensitivity by miR-101/ABCC1 axis. Mol Cell Biochem. 2021;476(6):2253-2267
86. Ma J, Qi Q, Li L. A Novel Serum Exosomes-Based Biomarker hsa_circ_0002130 Facilitates Osimertinib-Resistance in Non-Small Cell Lung Cancer by Sponging miR-498. Onco Targets Ther. 2020;13:5293-5307
87. He F, Zhong X, Lin Z. et al. Plasma exo-hsa_circRNA_0056616: A potential biomarker for lymph node metastasis in lung adenocarcinoma. J Cancer. 2020;11(14):4037-4046
88. Chen W, Tang D, Lin J. et al. Exosomal circSHKBP1 participates in non-small cell lung cancer progression through PKM2-mediated glycolysis. Mol Ther Oncolytics. 2022;24:470-485
89. Chen T, Liu Y, Li C. et al. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat Res Commun. 2021;28:100412
90. Chen SW, Zhu SQ, Pei X. et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer. 2021;20(1):144
91. Chen F, Huang C, Wu Q. et al. Circular RNAs expression profiles in plasma exosomes from early-stage lung adenocarcinoma and the potential biomarkers. J Cell Biochem. 2020;121(3):2525-2533
92. Zhang Q, Wang Z, Cai H. et al. CircPLK1 Acts as a Carcinogenic Driver to Promote the Development of Malignant Pleural Mesothelioma by Governing the miR-1294/HMGA1 Pathway. Biochem Genet. 2022;60(5):1527-1546
93. Zong ZH, Du YP, Guan X. et al. CircWHSC1 promotes ovarian cancer progression by regulating MUC1 and hTERT through sponging miR-145 and miR-1182. J Exp Clin Cancer Res. 2019;38(1):437
94. Zhao Z, Ji M, Wang Q. et al. Circular RNA Cdr1as Upregulates SCAI to Suppress Cisplatin Resistance in Ovarian Cancer via miR-1270 Suppression. Mol Ther Nucleic Acids. 2019;18:24-33
95. Wang X, Yao Y, Jin M. Circ-0001068 is a novel biomarker for ovarian cancer and inducer of PD1 expression in T cells. Aging (Albany NY). 2020;12(19):19095-19106
96. Wang J, Wu A, Yang B. et al. Profiling and bioinformatics analyses reveal differential circular RNA expression in ovarian cancer. Gene. 2020;724:144150
97. Luo Y, Gui R. Circulating exosomal circFoxp1 confers cisplatin resistance in epithelial ovarian cancer cells. J Gynecol Oncol. 2020;31(5):e75
98. Guan X, Zong ZH, Liu Y. et al. circPUM1 Promotes Tumorigenesis and Progression of Ovarian Cancer by Sponging miR-615-5p and miR-6753-5p. Mol Ther Nucleic Acids. 2019;18:882-892
99. Zhan Y, Du J, Min Z. et al. Carcinoma-associated fibroblasts derived exosomes modulate breast cancer cell stemness through exonic circHIF1A by miR-580-5p in hypoxic stress. Cell Death Discov. 2021;7(1):141
100. Yang SJ, Wang DD, Zhou SY. et al. Identification of circRNA-miRNA networks for exploring an underlying prognosis strategy for breast cancer. Epigenomics. 2020;12(2):101-125
101. Yang SJ, Wang DD, Zhou SL. et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/beta-catenin (cyclin D1) axis. Cell Death Dis. 2021;12(5):420
102. Wu X, Ren Y, Yao R. et al. Circular RNA circ-MMP11 Contributes to Lapatinib Resistance of Breast Cancer Cells by Regulating the miR-153-3p/ANLN Axis. Front Oncol. 2021;11:639961
103. Lu C, Shi W, Hu W. et al. Endoplasmic reticulum stress promotes breast cancer cells to release exosomes circ_0001142 and induces M2 polarization of macrophages to regulate tumor progression. Pharmacol Res. 2022;177:106098
104. Liu Y, Ma L, Hua F. et al. Exosomal circCARM1 from spheroids reprograms cell metabolism by regulating PFKFB2 in breast cancer. Oncogene. 2022;41(14):2012-2025
105. Chen T, Wang X, Li C. et al. CircHIF1A regulated by FUS accelerates triple-negative breast cancer progression by modulating NFIB expression and translocation. Oncogene. 2021;40(15):2756-2771
106. Zhang Y, Tang K, Chen L. et al. Exosomal CircGDI2 Suppresses Oral Squamous Cell Carcinoma Progression Through the Regulation of MiR-424-5p/SCAI Axis. Cancer Manag Res. 2020;12:7501-7514
107. Luo Y, Liu F, Guo J. et al. Upregulation of circ_0000199 in circulating exosomes is associated with survival outcome in OSCC. Sci Rep. 2020;10(1):13739
108. Fan X, Wang Y. Circular RNA circSPATA6 Inhibits the Progression of Oral Squamous Cell Carcinoma Cells by Regulating TRAF6 via miR-182. Cancer Manag Res. 2021;13:1817-1829
109. Wu P, Fang X, Liu Y. et al. N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021;12(4):298
110. Luo Y, Ma J, Liu F. et al. Diagnostic value of exosomal circMYC in radioresistant nasopharyngeal carcinoma. Head Neck. 2020;42(12):3702-3711
111. Tian L, Cao J, Jiao H. et al. CircRASSF2 promotes laryngeal squamous cell carcinoma progression by regulating the miR-302b-3p/IGF-1R axis. Clin Sci (Lond). 2019;133(9):1053-1066
112. Han J, Lin Q, Dong C. et al. Plasma cell-free circRNAs panel act as fingerprint predicts the occurrence of laryngeal squamous cell carcinoma. Aging (Albany NY). 2021;13(13):17328-17336
113. Yang C, Wei Y, Yu L. et al. Identification of Altered Circular RNA Expression in Serum Exosomes from Patients with Papillary Thyroid Carcinoma by High-Throughput Sequencing. Med Sci Monit. 2019;25:2785-2791
114. Zhang Y, Pisano M, Li N. et al. Exosomal circRNA as a novel potential therapeutic target for multiple myeloma-related peripheral neuropathy. Cell Signal. 2021;78:109872
115. Yu M, Yu J, Zhang Y. et al. A novel circRNA-miRNA-mRNA network revealed exosomal circ-ATP10A as a biomarker for multiple myeloma angiogenesis. Bioengineered. 2022;13(1):667-683
116. Luo Y, Gui R. Circulating Exosomal CircMYC Is Associated with Recurrence and Bortezomib Resistance in Patients with Multiple Myeloma. Turk J Haematol. 2020;37(4):248-262
117. Zhong AN, Yin Y, Tang BJ. et al. CircRNA Microarray Profiling Reveals hsa_circ_0058493 as a Novel Biomarker for Imatinib-Resistant CML. Front Pharmacol. 2021;12:728916
118. Wu Z, Sun H, Wang C. et al. Mitochondrial Genome-Derived circRNA mc-COX2 Functions as an Oncogene in Chronic Lymphocytic Leukemia. Mol Ther Nucleic Acids. 2020;20:801-811
119. Chen X, Chen RX, Wei WS. et al. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clin Cancer Res. 2018;24(24):6319-6330
120. Zhang H, Li M, Zhang J. et al. Exosomal Circ-XIAP Promotes Docetaxel Resistance in Prostate Cancer by Regulating miR-1182/TPD52 Axis. Drug Des Devel Ther. 2021;15:1835-1849
121. Wang S, Su W, Zhong C. et al. An Eight-CircRNA Assessment Model for Predicting Biochemical Recurrence in Prostate Cancer. Front Cell Dev Biol. 2020;8:599494
122. Tang Y, Liu J, Li X. et al. Exosomal circRNA HIPK3 knockdown inhibited cell proliferation and metastasis in prostate cancer by regulating miR-212/BMI-1 pathway. J Biosci. 2021 46
123. Li T, Sun X, Chen L. Exosome circ_0044516 promotes prostate cancer cell proliferation and metastasis as a potential biomarker. J Cell Biochem. 2020;121(3):2118-2126
124. Pan Y, Lin Y, Mi C. Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol Int. 2021;45(4):858-868
125. O'Leary VB, Smida J, Matjanovski M. et al. The circRNA interactome-innovative hallmarks of the intra- and extracellular radiation response. Oncotarget. 2017;8(45):78397-78409
126. Hadad SM, Jordan LB, Roy PG. et al. A prospective comparison of ER, PR, Ki67 and gene expression in paired sequential core biopsies of primary, untreated breast cancer. BMC Cancer. 2016;16(1):745
127. Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225-38
128. Sporn MB. The war on cancer. Lancet. 1996;347(9012):1377-81
129. Merchant N, Nagaraju GP, Rajitha B. et al. Matrix metalloproteinases: their functional role in lung cancer. Carcinogenesis. 2017;38(8):766-780
130. Aplin AE, Howe A, Alahari SK. et al. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50(2):197-263
131. Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3(11):895-904
132. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell. 2017;168(4):670-691
133. Feng S, Zha Z, Wang Z. et al. Anticancer activity of oleiferoside B involving autophagy and apoptosis through increasing ROS release in MCF-7 cells and SMMC-7721 cells. Nat Prod Res. 2021;35(22):4865-4869
134. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175-193
135. Kalal BS, Upadhya D, Pai VR. Chemotherapy Resistance Mechanisms in Advanced Skin Cancer. Oncol Rev. 2017;11(1):326
136. Kim YN, Koo KH, Sung JY. et al. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol. 2012;2012:306879
137. Watson EC, Grant ZL, Coultas L. Endothelial cell apoptosis in angiogenesis and vessel regression. Cell Mol Life Sci. 2017;74(24):4387-4403
138. Yaacoub K, Pedeux R, Tarte K. et al. Role of the tumor microenvironment in regulating apoptosis and cancer progression. Cancer Lett. 2016;378(2):150-9
139. Shin SA, Moon SY, Park D. et al. Apoptotic cell clearance in the tumor microenvironment: a potential cancer therapeutic target. Arch Pharm Res. 2019;42(8):658-671
140. Zhu J, Petit PF, Van den Eynde BJ. Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism. Cancer Immunol Immunother. 2019;68(5):835-847
141. Phi LTH, Sari IN, Yang YG. et al. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018;2018:5416923
142. Yun CW, Lee SH. The Roles of Autophagy in Cancer. Int J Mol Sci. 2018 19(11)
143. Das S, Shukla N, Singh SS. et al. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis. 2021;26(9-10):512-533
144. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-282
145. Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5-18
146. Salmena L, Poliseno L, Tay Y. et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353-8
147. Chen L, Shan G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021;505:49-57
148. Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. 2021;21(1):22-36
149. Baxter E, Windloch K, Gannon F. et al. Epigenetic regulation in cancer progression. Cell Biosci. 2014;4:45
150. Ilango S, Paital B, Jayachandran P. et al. Epigenetic alterations in cancer. Front Biosci (Landmark Ed). 2020;25(6):1058-1109
151. Audia JE, Campbell RM. Histone Modifications and Cancer. Cold Spring Harb Perspect Biol. 2016;8(4):a019521
152. Glisovic T, Bachorik JL, Yong J. et al. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582(14):1977-86
153. Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121(10):3804-9
154. Varusai TM, Kolch W, Kholodenko BN. et al. Protein-protein interactions generate hidden feedback and feed-forward loops to trigger bistable switches, oscillations and biphasic dose-responses. Mol Biosyst. 2015;11(10):2750-62
155. Gyamfi J, Kim J, Choi J. Cancer as a Metabolic Disorder. Int J Mol Sci. 2022 23(3)
156. Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211-218
157. Li C, Zhang G, Zhao L. et al. Metabolic reprogramming in cancer cells: glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer. World J Surg Oncol. 2016;14(1):15
158. Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32(5):453-61
159. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-14
160. DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148(6):1132-44
161. Stubbs M, Griffiths JR. The altered metabolism of tumors: HIF-1 and its role in the Warburg effect. Adv Enzyme Regul. 2010;50(1):44-55
162. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763-77
163. Enerback S. The origins of brown adipose tissue. N Engl J Med. 2009;360(19):2021-3
164. Petruzzelli M, Schweiger M, Schreiber R. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433-47
165. Kir S, White JP, Kleiner S. et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100-4
166. Chen JQ, Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim Biophys Acta. 2012;1826(2):370-84
167. Ayers D, Nasti A. Utilisation of nanoparticle technology in cancer chemoresistance. J Drug Deliv. 2012;2012:265691
168. Larionova I, Rakina M, Ivanyuk E. et al. Radiotherapy resistance: identifying universal biomarkers for various human cancers. J Cancer Res Clin Oncol. 2022;148(5):1015-1031
169. Hua X, Sun Y, Chen J. et al. Circular RNAs in drug resistant tumors. Biomed Pharmacother. 2019;118:109233
170. Ebahimzadeh K, Shoorei H, Mousavinejad SA. et al. Emerging role of non-coding RNAs in response of cancer cells to radiotherapy. Pathol Res Pract. 2021;218:153327
171. Baskar R, Itahana K. Radiation therapy and cancer control in developing countries: Can we save more lives? Int J Med Sci. 2017;14(1):13-17
172. Huang RX, Zhou PX. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5(1):60
173. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309-22
174. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014-22
175. Tomasi TB, Magner WJ, Khan AN. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55(10):1159-84
176. Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the Treatment of Cancer. J Interferon Cytokine Res. 2019;39(1):6-21
177. Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662-680
178. Charles N, Ozawa T, Squatrito M. et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6(2):141-52
179. Lathia JD, Heddleston JM, Venere M. et al. Deadly teamwork: neural cancer stem cells and the tumor microenvironment. Cell Stem Cell. 2011;8(5):482-5
Author contact
![]() Corresponding author: Tao Zhang, Department of Gastric Surgery, Cancer Hospital of China Medical University/Liaoning Cancer Hospital, No. 44 Xiaoheyan Road, Dadong District, Shenyang 110042, Liaoning, China. E-mail: 13940152108com; Tel: (024) 31916823.
Corresponding author: Tao Zhang, Department of Gastric Surgery, Cancer Hospital of China Medical University/Liaoning Cancer Hospital, No. 44 Xiaoheyan Road, Dadong District, Shenyang 110042, Liaoning, China. E-mail: 13940152108com; Tel: (024) 31916823.

 Global reach, higher impact
Global reach, higher impact