3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(6):737-748. doi:10.7150/ijms.78766 This issue Cite
Research Paper
Immune responses to inactivated COVID-19 vaccine were decreased in Chinese patients with chronic respiratory diseases
1. Department of Respiratory Medicine, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, China.
2. Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, Department of Infectious Diseases, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, China.
3. Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China.
4. Department of General Medicine, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, China.
#Co-first authors: Lei Yang and LingFang Xu
Received 2022-9-9; Accepted 2023-3-30; Published 2023-4-23
Abstract
Purpose: The effectiveness of inactivated vaccines against acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the causative agent of coronavirus disease 2019 (COVID-19), has become a global concern. Hence, the aim of this study was to evaluate vaccine safety and to assess immune responses in individuals with chronic respiratory disease (CRD) following a two-dose vaccination.
Methods: The study cohort included 191 participants (112 adult CRD patients and 79 healthy controls [HCs]) at least 21 (range, 21-159) days after a second vaccination. Frequencies of memory B cells (MBCs) subsets and titers of SARS-CoV-2 neutralizing antibodies (NAbs) and anti-receptor binding domain (RBD) IgG antibodies (Abs) were analyzed.
Results: As compared to the HCs, CRD patients had lower seropositivity rates and titers of both anti-RBD IgG Abs and NAbs, in addition to lower frequencies of RBD-specific MBCs (all, p < 0.05). At 3 months, CRD patients had lower seropositivity rates and titers of anti-RBD IgG Abs than the HCs (p < 0.05). For CoronaVac, the seropositivity rates of both Abs were lower in patients with old pulmonary tuberculosis than HCs. For BBIBP-CorV, the seropositivity rates of CoV-2 NAbs were lower in patients with chronic obstructive pulmonary disease than HCs (all, p < 0.05). Meanwhile, there was no significant difference in overall adverse events between the CRD patients and HCs. Univariate and multivariate analyses identified the time interval following a second vaccination as a risk factor for the production of anti-RBD IgG Abs and CoV-2 NAbs, while the CoronaVac had a positive effect on the titers of both Abs. Female was identified as a protective factor for CoV-2 NAb levels.
Conclusion: Inactivated COVID-19 vaccines were safe and well tolerated by CRD patients but resulted in lower Ab responses and the frequencies of RBD-specific MBCs. Therefore, CRD patients should be prioritized for booster vaccinations.
Introduction
To date, there have been more than 600 million confirmed cases of infection with severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the causative agent of coronavirus disease 2019 (COVID-19). Vaccination against COVID-19 is essential to prevent COVID-19 and has altered the trajectory of the pandemic by averting an estimated 19.8 million deaths [1]. Risk factors for severe forms of COVID-19 include a history of chronic respiratory disease (CRD), such as chronic bronchitis (CB), chronic obstructive pulmonary disease (COPD), and old pulmonary tuberculosis (OPTB) [2-4].
Patients with COPD reportedly develop a sustained antibody (Ab) response after two doses of vaccination, but at lower levels than healthy controls (HCs) [5]. However, Ab levels do not significantly decrease in patients with a history of CRD [6]. Currently, changes to Ab levels in CRD patients following vaccination remain unclear, thus additional studies are required.
In addition to the generation of antigen-producing plasma cells, vaccination also induces the differentiation of memory B cells (MBCs), which help to protect against re-exposure to a pathogen [7]. MBCs rapidly multiply and terminally differentiate into Ab-secreting cells [8, 9]. However, there is a lack of data on clonal expansion of MBCs in CRD patients.
Therefore, the aim of the present study was to assess the titers of MBCs, neutralizing antibodies (NAbs) against SARS-COV-2, and Abs against the receptor-binding domain (RBD) of immunoglobulin G (IgG) in CRD patients after vaccination with an inactivated COVID-19 vaccine.
Methods
Study approval and patient consent
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University and conducted in accordance with the ethical principles for medical research involving human subjects described in the Declaration of Helsinki. Prior to inclusion in this study, written informed consent was obtained from all subjects. This study is registered with ClinicalTrials.gov (NCT05043246).
Study cohort
The study cohort included 112 patients with CRDs (43 with COPD, 35 with OPTB, and 34 with CB) and 79 healthy volunteers recruited from the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China). All patients with COPD received low-dose β-agonists/corticosteroids with no recent acute attack and had a ratio of forced expiratory volume in 1 second/slow vital capacity of < 0.7. All patients with CB had symptoms of cough and excess sputum production for at least 3 months and up to 2 years or more. All patients with a prior history of tuberculosis infection had no symptoms of hot flushes, night sweats, or fibrosis/calcification on the lungs, and sputum samples were free of tubercle bacilli. The inclusion criteria for all participants were (i) vaccination with two doses of the BBIBP COVID-19 vaccine (BBIBP-CorV; China National Pharmaceutical Group Corporation, Beijing, China) or CoronaVac vaccine (Sinovac Biotech, Beijing, China) (ii) age ≥ 18 years, (iii) no prior history of COVID-19, (iv) normal immune function, and (v) no current pregnancy.
To standardize the timing of vaccination, “1 month” was defined as 21-45 days (46 CRD patients and 42 HCs), “2 months” as 46-75 days (26 CRD patients and 18 HCs), and “3 months” as 76-105 days (29 CRD patients and 15 HCs).
Ab responses
Plasma levels of RBD IgG Abs and NAbs were detected with the MAGLUMI 2000 Chemiluminescence Immunoassay System (Snibe, Shenzhen, China). Cut-off values for seropositivity of RBD IgG Abs and NAbs were >1 AU/mL and >0.15 μg/mL, respectively.
Identification of MBCs
Fresh peripheral blood samples were collected and centrifuged through a Ficoll density gradient (Histopaque; Sigma-Aldrich Corporation, St Louis, MO, USA). Brilliant Violet 421™ Streptavidin-conjugated Abs (BioLegend, San Diego, CA, USA) and biotinylated Abs against the RBD of the SARS-CoV-2 spike protein (Sino Biological, Beijing, China) at a molar ratio of 4:1 were used for identification of the MBCs. For flow cytometry (Beckman Coulter, Inc., Brea, CA, USA), the cells were washed with phosphate-buffered saline, suspended staining buffer containing 2% fetal bovine serum, and probed with Abs against IgG, IgM, cluster of differentiation 3 (CD3), CD19, CD21, and CD27 (all, Biolegend). The data were examined using FlowJo software (version 10.0.7; FlowJo, LLC, Ashland, OR, USA). Abs against CD3, CD19, CD21, and CD27 were used to differentiate MBCs into subsets of RBD-specific MBCs (CD3-/CD19+/RBD+/CD27+), RBD+ atypical MBCs (CD3-/CD19+/RBD+/CD21-/CD27-), RBD+ intermediate MBCs (CD3-/CD19+/RBD+/CD21+/CD27-), RBD+ activated MBCs (CD3-/CD19+/RBD+/CD21-/CD27+), and RBD+ resting MBCs (CD3-/CD19+/RBD+/CD21+/CD27+).
Statistical analysis
The chi-square test or Fisher exact test was used to compare categorical variables. For continuous variables, the Student's t-test or the Mann-Whitney U test was used for comparisons of two groups, and the Kruskal-Wallis test for three or more groups. All results of multiple comparisons were corrected using Dunn's multiple comparisons test. Univariate and multivariate linear regression analyses were used to identify risk and protective factors associated with Ab titers. All outcomes were adjusted. Data analysis was conducted using IBM SPSS Statistics for Windows, version 26.0 (IBM Corporation, Armonk, NY, USA). Figures were generated with GraphPad Prism software (version 9.2.0; GraphPad Software, Inc., San Diego, CA, USA). A probability (p) value < 0.05 was considered statistically significant.
Results
Population Characteristics
The study cohort included a total of 191 participants. As shown in Table 1, comparisons between the CRD patients and HCs found no significant differences in median age, percentage of males, mean body mass index, vaccine type, median number of days after 2nd vaccination, results of routine blood tests (white blood cells, hemoglobin, lymphocytes, and platelets), and liver function markers (aspartate transaminase and alanine aminotransferase).
General characteristics of CRD patients and HCs.
| CRD patients (n =112) | HCs (n = 79) | p | |
|---|---|---|---|
| Age (years), | 64.00 (20-84) | 64.00 (19-89) | 0.895 |
| median (range) | |||
| Sex | |||
| Female | 44.6% (50/112) | 51.9% (41/79) | 0.323 |
| Male | 55.4% (62/112) | 48.1% (38/79) | |
| Body mass index, | 23.23 (16.60-33.98) | 23.92 (17.98-29.42) | 0.110 |
| mean (range) | |||
| Vaccine type | |||
| CoronaVac | 59.8% (67/112) | 72.2% (57/79) | 0.079 |
| BBIBP-CorV | 40.2% (45/112) | 27.8% (22/79) | |
| Days after 2nd vaccination | 54 (21-159) | 44 (21-135) | 0.071 |
| median (range) | |||
| Routine blood tests | |||
| median (range) | |||
| White blood cell | 6.02 (2.67-12.54) | 6.09 (2.05-19.08) | 0.634 |
| Hemoglobin | 137 (89-175) | 138 (73-208) | 0.952 |
| Lymphocyte | 1.70 (0.21-3.64) | 1.75 (0.64-3.40) | 0.598 |
| mean (range) | |||
| Platelet | 197 (94-638) | 189 (80-1182) | 0.648 |
| Liver function | |||
| median (range) | |||
| Aspartate transaminase | 21 (6-118) | 20 (3-82) | 0.278 |
| Alanine aminotransferase | 21 (10-76) | 21 (11-60) | 0.156 |
The chi-square test was used for comparisons of categorical variables and the Mann-Whitney U test was used for comparisons of continuous variables.
Safety
Following immunization, all adverse events (AEs) within the first week were categorized using the criteria of the China Medical and Drug Administration (2019 edition). There were no significant differences in overall, local, and systemic AEs between the CRD patients and HCs. Notably, there were no severe AEs (Table 2).
Humoral immune responses to inactivated SARS-CoV-2 vaccines in CRD patients
Overall, when compared to HCs, CRD patients had significantly reduced seropositivity rates for anti-RBD IgG Ab and CoV-2 NAb(Table 3).
AEs associated with vaccination in CRD patients and HCs.
| Variable | HTN patients | HCs | p |
|---|---|---|---|
| Overall AEs | 16(14.3%) | 6 (7.6%) | 0.154 |
| Local AEs | |||
| Pain | 4 (3.6%) | 3 (3.8%) | 0.935 |
| Swelling | 1 (0.9%) | / | 1.000 |
| Pruritus | 1 (0.9%) | 1 (1.3%) | 0.805 |
| Systemic AEs | |||
| Fever | 3 (2.7%) | / | 1.000 |
| Fatigue | 3 (2.7%) | 2 (2.5%) | 0.950 |
| Drowsiness | 2 (1.8%) | 1 (1.3%) | 0.773 |
| Cough | 2 (1.8%) | / | 1.000 |
| Dizziness | 2 (1.8%) | / | 1.000 |
| Gastro spasm | 1 (0.9%) | / | 1.000 |
| Grade 3 and 4 AEs | / | / |
The data are presented as n (%). The chi-square and Fisher's exact tests were used for comparisons.
Seropositivity rates of both Abs in all CRD patients.
| Ab | CRD patients (n = 112) | HCs (n = 79) | p |
|---|---|---|---|
| Anti-RBD IgG | 69.60% (78/112) | 88.80% (70/79) | 0.003 |
| CoV-2 NAb | 62.50% (70/112) | 79.70% (63/79) | 0.011 |
The chi-square test was used for comparisons.
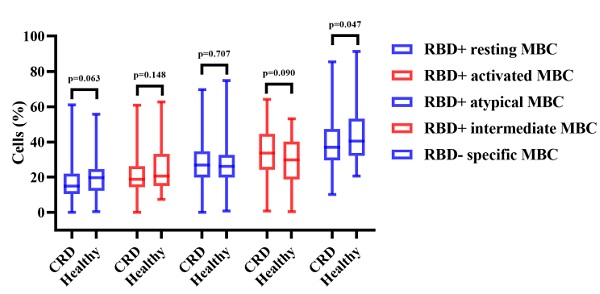
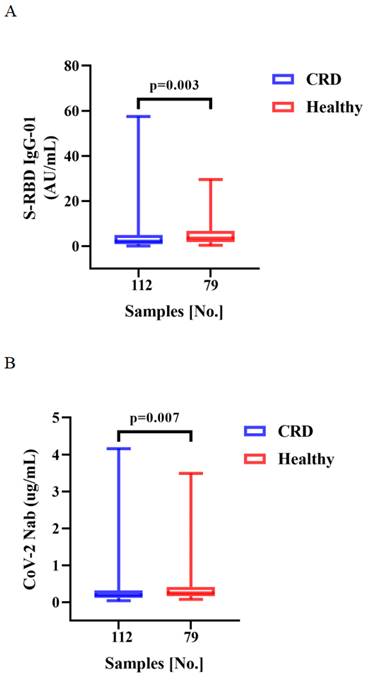
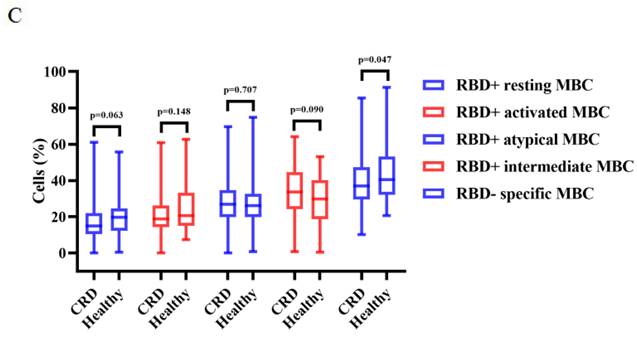
Vaccination induced production of anti-RBD IgG Abs and CoV-2 NAbs in all CRD patients and HCs. As compared to the HCs, CRD patients had significantly lower titers of anti-RBD IgG (median [IQR]: 2.10 [0.86-4.855] vs. 3.45 [1.84-6.79], respectively, p = 0.003) and SARS-CoV-2 NAbs (median [IQR]: 0.20 [0.12-0.32] vs. 0.25 [0.16-0.41], p = 0.007) (Figure 1A, B). However, the frequencies of RBD-specific MBCs were lower in CRD patients than the HCs (median [IQR]: 37.05 [29.43-47.30] vs. 40.4 [32.00-51.20], p = 0.047), while there was no significant difference in the frequencies of other MBC subsets (Figure 1C).
Between CRD patients and HCs, Figure 1A, B, the titers of anti-RBD IgG Abs and CoV-2 NAbs. Figure 1C show, the frequencies of RBD+ resting MBCs, RBD+ activated MBCs, RBD+ atypical MBCs, RBD+ intermediate MBCs, and RBD-specific memory B cells (MBCs). The Mann-Whitney U test was employed to compare the Ab titers and the frequencies of MBCs.
Humoral immune responses to inactivated SARS-CoV-2 vaccines in CRD patients over time
Between CRD patients and HCs, there was a significant difference in the seropositivity rates of anti-RBD IgG Ab after 3 months (Table 4).
A-C. Humoral immune responses to inactivated vaccines in CRD patients.
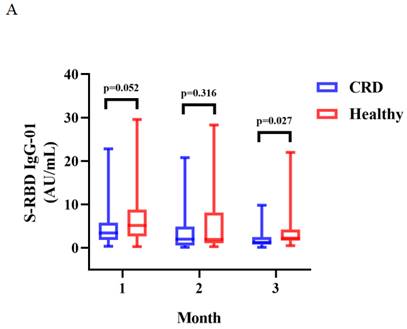
A-B. Antibody responses to inactivated vaccines in CRD at 1, 2, 3 month.
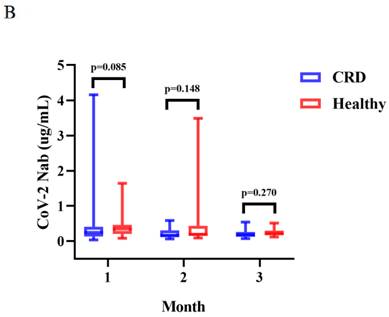
Seropositivity rates of both Abs in CRD patients at 1, 2, and 3 months.
| Ab/NAb | Time after second vaccination (months) | CRD patients | HCs | p |
|---|---|---|---|---|
| Anti-RBD IgG | 1 | 87.00% (40/46) | 93.00% (39/42) | 0.575 |
| 2 | 61.50% (16/26) | 83.30% (15/18) | 0.119 | |
| 3 | 62.10% (18/29) | 93.3% (14/15) | 0.027 | |
| CoV-2 NAb | 1 | 71.7% (33/46) | 83.7% (35/42) | 0.195 |
| 2 | 53.8% (14/26) | 77.80% (14/18) | 0.105 | |
| 3 | 62.10% (18/29) | 80.00% (12/15) | 0.226 |
The chi-square test was used for comparisons.
Between CRD patients and HCs, there was a significant difference in the titers of anti-RBD IgG at 3 months (median [IQR]: 1.29 [0.75-2.50] vs. 2.24 [1.68-4.22], p = 0.027) (Figure 2A). The titers of anti-RBD IgG Abs were slightly lower in CRD patients at 1 and 2 months as compared to HCs, as well as CoV-2 NAbs at 1, 2, and 3 months, although these differences were not significant (Figure 2A and B, respectively). In addition, the frequencies of RBD-specific MBCs was somewhat lower in CRD patients than the HCs at each time point, although this difference was not statistically significant (Supplementary Figure 1A-E).
The titers of anti-RBD IgG Abs and CoV-2 NAbs were measured in CRD patients and HCs at 1, 2, and 3 months were shown in Figure 2A-B. The Mann-Whitney U test was employed to compare the Ab titers.
Comparison of Humoral immune responses to CoronaVac and BBBIBP-CorV
As a result, the Corona Vac had higher seropositivity rates of both Abs than BBIBP-CorV in CRD patients (Table 5), and the CRD patients had higher seropositivity rates of anti-RBD IgG Ab than HCs (Corona Vac) (Table 6).
Titers of both Abs induced by CoronaVac were higher than those by BBIBP-CorV in CRD patients (median [IQR]: 2.67 [1.29-5.91] vs. 1.54 [0.57-3.37], p = 0.009, and 0.22 [0.13-0.38] vs. 0.15 [0.12-0.26], p = 0.023), while the titers of both Abs induced by BBIBP-CorV were higher in HCs than CRD patients (median [IQR]: 1.54 [0.57-3.37] vs. 2.64 [1.10-5.30], p = 0.035, and 0.15 [0.12-0.26] vs. 0.21 [0.14-0.36], p = 0.049) (Figure 3A, B). The frequencies of RBD+ resting MBCs was lower in the HCs than CRD patients after vaccination with CoronaVac (median [IQR]: 14.70 [10.20-23.00] vs. 19.70 [14.95-24.40], p = 0.017), while the frequencies RBD+ intermediate MBCs was higher after vaccination with BBIBP-CorV (median [IQR]: 32.40 [21.10-44.90] vs. 23.30 [2.09-40.03], p = 0.047). Meanwhile, the frequencies of RBD+ intermediate MBCs was higher with the CoronaVac than the BBIBP-CorV in the HCs (median [IQR]: 30.90 [23.40-40.70] vs. 23.30 [2.09-40.03], p = 0.037), while there was no significant difference in the frequencies of other MBC subsets (Figure 3C-G).
The titers of anti-RBD IgG Abs and CoV-2 NAbs in CRD patients and HCs (corona vac and BBIBP-corV) were shown in Figure 3A, B. The frequencies of RBD+ resting MBCs, RBD+ activated MBCs, RBD+ atypical MBCs, RBD+ intermediate MBCs and RBD-specific memory B cells (MBCs) in CRD patients and HCs (corona vac and BBIBP-corV) were shown in Figure 3C-G. The Mann-Whitney U test was used to compare the Ab titers and the frequencies of MBCs.
Humoral immune responses to inactivated SARS-CoV-2 vaccines in CRD subgroups
The seropositivity rates of both Abs (Corona Vac) were lower in OPTB patients than HCs, and the seropositivity rates of CoV-2 NAb(BBIBP-CorV) were lower in COPD patients than HCs, the seropositivity rates of CoV-2 NAb(Corona Vac) were significant different in CB, OPTB and COPD patients(Table 7).
There was no significant difference in the titers of both Abs after vaccination with Corona Vac or BBIBP-CorV in each CB, OPTB, COPD patients compared with HCs (all, p > 0.05). The titers of both Abs were similar in CB, OPTB, COPD patients after vaccination with Corona Vac(6.33[2.02-9.07] vs. 2.27[0.80-4.69] vs. 2.39[1.23-5.20], p = 0.103 and 0.32[0.20-0.49] vs. 0.22[0.11-0.43] vs. 0.19[0.13-0.31], p = 0.055, respectively), at the same time, the titers of both Abs were no significant difference in CB, OPTB, COPD patients after vaccination with BBIBP-CorV(1.54[0.67-2.25] vs. 1.99[0.57-3.87] vs. 0.89[0.51-4.91], p = 0.941 and 0.17[0.12-0.21] vs. 0.18[0.09-0.32] vs. 0.15[0.12-0.30], p = 0.947, respectively).
Seropositivity rates of both Abs by vaccine type in CRD patients and HCs.
| Ab | CoronaVac | BBIBP-CorV | p | |
|---|---|---|---|---|
| CRD patients | Anti-RBD IgG | 80.6% (54/67) | 55.6% (25/45) | 0.004 |
| CoV-2 NAb | 71.6% (48/67) | 44.9% (22/45) | 0.015 | |
| HCs | Anti-RBD IgG | 89.5% (51/57) | 86.4% (19/22) | 0.703 |
| CoV-2 NAb | 84.2% (48/57) | 68.2% (15/22) | 0.202 |
The chi-square test was used for comparisons.
Seropositivity rates for both Abs in CRD patients and HCs (CoronaVac and BBIBP-CorV).
| Ab | CRD patients | HCs | p | |
|---|---|---|---|---|
| CoronaVac | Anti-RBD IgG | 80.6% (54/67) | 89.5% (51/57) | 0.171 |
| CoV-2 NAb | 71.6% (48/67) | 84.2% (48/57) | 0.095 | |
| BBIBP-CorV | Anti-RBD IgG | 55.6% (25/45) | 86.4% (19/22) | 0.013 |
| CoV-2 NAb | 44.9% (22/45) | 68.2% (15/22) | 0.136 |
The chi-square test was used for comparisons.
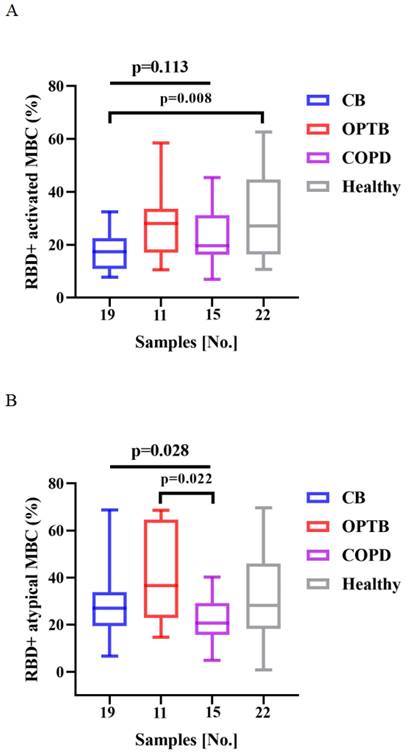
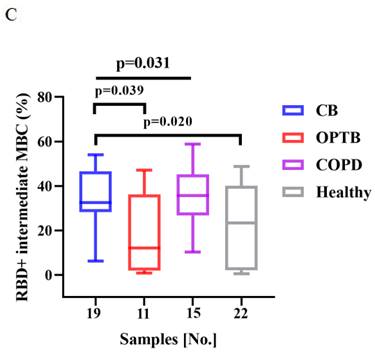
The frequencies of RBD+ activated MBCs was lower and that of RBD+ intermediate MBCs was higher in CB patients than HCs after vaccination with BBIBP-CorV (p = 0.008 and 0.020, respectively) (Figure 4A and C). There were significant differences in the frequencies of RBD+ atypical MBCs and RBD+ intermediate MBCs among the CB, OPTB, and COPD patients after vaccination with BBIBP-CorV, while the proportion of RBD+ atypical MBCs was lower in COPD patients than OPTB patients and the frequencies of RBD+ intermediate MBCs was higher in CB patients than OPTB patients (Figure 4B and C).
The frequencies of RBD+ activated MBCs and RBD+ intermediate MBCs of the CB, OPTB, and COPD patients and HCs after vaccination with BBIBP-CorV are shown in Figure 4A and C. The frequencies of RBD+ atypical MBCs and RBD+ intermediate MBCs in the CB, OPTB, and COPD patients after vaccination with BBIBP-CorV are shown in Figure 4B and C. The Kruskal-Wallis test and Dunn's multiple comparisons test were employed to compare the frequencies of MBCs.
Humoral immune responses to inactivated SARS-CoV-2 vaccines by age
As a result, there were no significant difference of the seropositivity rates of both Abs in CRD patients aged ≥ 60 and < 60 years, which is consistent with HCs (Table 8).
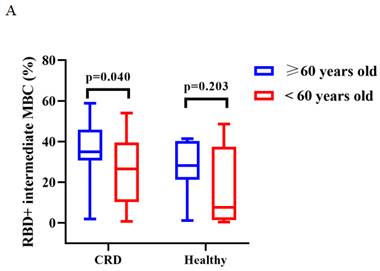
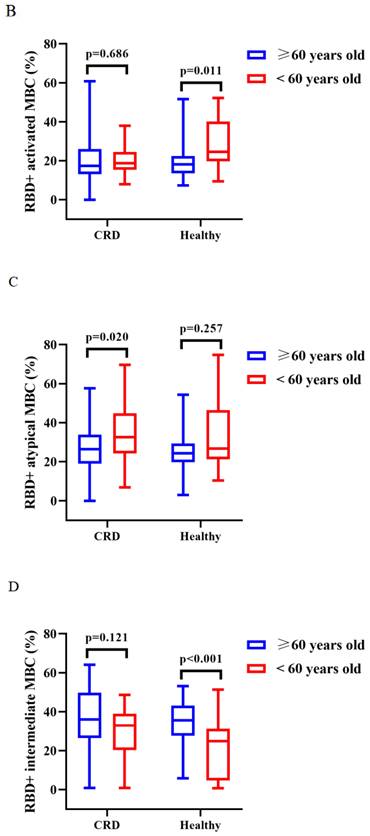
The titers of anti-RBD IgG Abs and CoV-2 NAbs were similar in CRD patients and HCs aged ≥ 60 and < 60 years after vaccination with CoronaVac (Supplementary Figure 3A, B) and BBIBP-CorV (Supplementary Figure 3C, D). After vaccination with CoronaVac, as compared to HCs aged ≥60 years, those aged <60 years had greater frequencies of RBD+ activated MBCs (median [IQR]: 18.05 [13.48-22.45] vs. 24.50 [19.65-40.20], p = 0.011) and lower frequencies of RBD+ intermediate MBCs (34.82 [95% CI = 31.29-38.34] vs. 21.04 [95% CI = 13.57-28.51], p < 0.001), while CRD patients aged ≥60 vs. <60 years had lower frequencies of RBD+ intermediate MBCs (35.09 [95% CI = 29.61-40.57] vs. 25.33 [95% CI = 16.98-33.68], p = 0.040) and RBD+ atypical MBCs (26.83 [95% CI = 23.28-30.38] vs. 35.33 [95% CI = 27.90-42.76], p = 0.020) (BBIBP-CorV: Figure 5A; CoronaVac Figure 5B-D), while there were no significant differences in the frequencies of the others MBC subsets (BBIBP-CorV: Supplementary Figure 3E-H; CoronaVac: Supplementary Figure 3I-J).
Seropositivity rates of both Abs in CRD subgroups.
| Ab vaccine | CB Patients | OPTB patients | COPD patients | HCs |
|---|---|---|---|---|
| Anti-RBD IgG (CoronaVac) | 100% (16/16) | 69.6% (16/23)* | 78.6% (22/28) | 89.5% (51/57) |
| #CoV-2 NAb (CoronaVac) | 100% (16/16) | 60.9% (14/23)* | 64.3% (18/28) | 84.2% (48/57) |
| Anti-RBD IgG (BBIBP-CorV) | 63.2% (12/19) | 54.5% (6/11) | 46.7% (7/15)* | 86.4% (19/22) |
| CoV-2 NAb (BBIBP-CorV) | 52.6% (10/19) | 54.5% (6/11) | 40.0% (6/15) | 68.2% (15/22) |
The chi-square, Fisher's exact, and Dunn's multiple comparisons tests were used for comparisons (*p < 0.05 vs. HCs, #p < 0.05 CB vs. OPTB vs. COPD).
Seropositivity rates of both Abs in CRD patients and HCs aged ≥60 and <60 years.
| Ab (vaccine type) | Age ≥ 60 years | Age < 60 years | p | |
|---|---|---|---|---|
| CRD patients | Anti-RBD IgG (BBIBP-CorV) | 50.0% (13/26) | 63.2% (12/19) | 0.380 |
| Cov-2 NAb (BBIBP-CorV) | 42.3% (11/26) | 57.9% (11/19) | 0.302 | |
| Anti-RBD IgG (CoronaVac) | 83.0% (39/47) | 75.0% (15/20) | 0.676 | |
| CoV-2 NAb (CoronaVac) | 72.3% (34/47) | 70.0% (14/20) | 0.846 | |
| HCs | Anti-RBD IgG (CoronaVac) | 85.0% (34/40) | 100.0% (17/17) | 1.000 |
| CoV-2 NAb (CoronaVac) | 80.0% (32/40) | 94.1% (16/17) | 0.347 | |
| Anti-RBD IgG (BBIBP-CorV) | 83.3% (10/12) | 90.0% (9/10) | 1.000 | |
| CoV-2 NAb (BBIBP-CorV) | 75.0% (9/12) | 60.0% (6/10) | 0.770 |
The chi-square test was used for comparisons.
A-G. Humoral immune responses to inactivated vaccines in CRD patients and HCs by vaccine type.
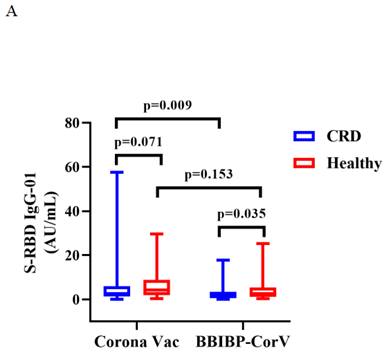
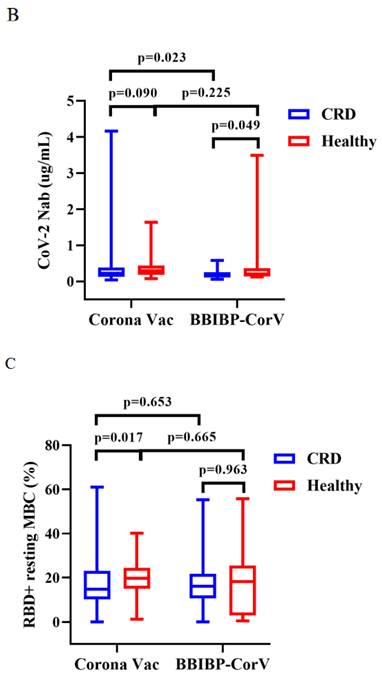
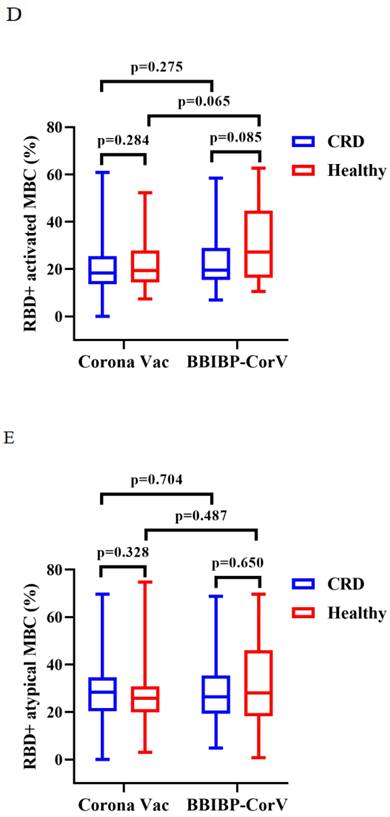
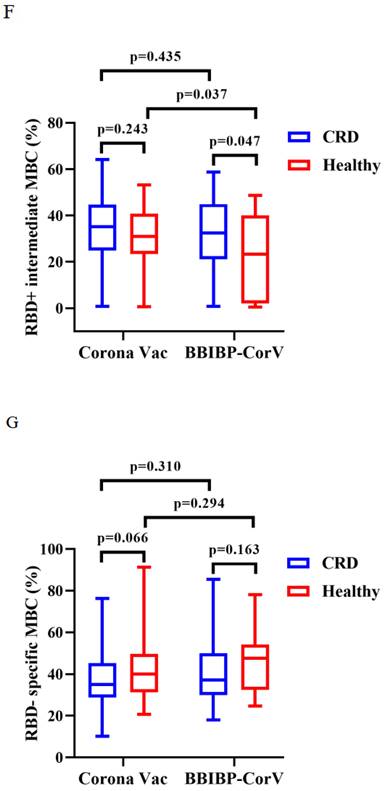
Figure 5A (BBIBP-CorV) and B-D (Corona Vac) shows, the frequencies of RBD+ activated MBCs, RBD+ atypical MBCs and RBD+ intermediate MBCs in CRD patients and HCs aged ≥60 and <60 years. The T test, Mann-Whitney U test were used to compare the Ab titers and the frequencies of MBCs.
Other confounding factors are listed in Tables 9 and 10. The duration of time following the second vaccination was identified as a risk factor for serum levels of anti-RBD IgG Abs and CoV-2 NAbs, while the type of vaccine (CoronaVac) had a protective impact on Ab response and female was a protective factor for CoV-2 NAb levels.
A-C. Frequencies of MBC subsets in response to inactivated vaccines in CRD subgroups.
A-D. Frequencies of MBC subsets in response to inactivated vaccines aged ≥60 and <60 years.
Discussion
In this prospective observational trial, Ab levels and responses of MBC subsets were measured to assess the efficacy and safety of inactivated SARS-CoV-2 vaccines.
The results clarified that inactivated vaccines were safe and well tolerated in CRD patients. The most prevalent local and systemic AEs were pain and exhaustion, respectively, while there were no severe AEs, such as myocardial infarction and thromboembolic events [10, 11]. As compared to earlier large-scale studies [12, 13], the likelihood of AEs following immunization in these patients was significantly reduced.
CRD patients had significantly lower serum Ab responses after the second vaccination, but were still detected after 6 months, consistent with previous studies [5, 15]. A previous study indicated that vaccine efficacy for non-immunocompromised patients with chronic diseases were similar between risk groups [14]. In addition, the seroconversion rate was lower for CRD patients as compared to the HCs, in agreement with a previously published study [16]. Overall, CRD patients had low immunogenicity to inactivated COVID-19 vaccines.
Anti-RBD IgG Ab and NAb levels were higher in the HCs as compared to the CRD patients for BBIBP-CorV, although not always significant for CoronaVac, possibly due to the relatively small study cohort. Interestingly, the response to BBIBP-CorV was lower in CRD patients than for CoronaVac. The results of linear regression analysis identified CoronaVac as a protective factor that promotes Ab responses, consistent with the findings of a previous study [17], but not for the HCs. These results suggest that BBIBP-CorV should not be administered to CRD patients. However, the lower titers of anti-RBD IgG Abs and NAbs of the CRD patients who received BBIBP-CorV may be due to the higher proportion of males, longer period between vaccinations, or the greater proportion of CB patients.
In this study, OPTB patients had lower seropositivity rates for both Abs following vaccination with CoronaVac, while COPD patients had lower seropositivity rates of CoV-2 NAbs following vaccination with BBIBP-CorV. However, this difference was not significant in other subgroups, possibly due to the higher proportion of males or the longer period between vaccinations and sample collection. The seropositivity rates of CoV-2 NAbs were significant difference after vaccination with CoronaVac among CB, OPTB, COPD patients, it might also be that OPTB patients have the higher proportion of males.
Univariate and multivariate analyses for anti-RBD IgG Ab in CRD.
| Univariate OR (95%CI) | P | Multivariate OR (95%CI) | P | |
|---|---|---|---|---|
| Gender (female) | 1.622(0.711~3.822) | 0.257 | 2.291(0.753~7.399) | 0.151 |
| Age (years) | 0.998(0.964~1.035) | 0.924 | 1.035(0.985~1.092) | 0.185 |
| BMI(Kg/m^2) | 1.122(0.982~1.292) | 0.096 | 1.161(0.974~1.408) | 0.108 |
| Days after 2nd dose | 1.023(1.010~1.037) | 0.001 | 1.030(1.014~1.050) | <0.001 |
| Vaccine type (BBIBP-corV) | 0.301(0.127~0.692) | 0.005 | 0.216(0.064~0.672) | 0.010 |
| HB (g/L) | 1.004(0.976~1.035) | 0.772 | ||
| WBC (10^9/L) | 1.326(1.037~1.730) | 0.029 | 1.252(0.915~1.771) | 0.178 |
| LC (10^9/L) | 1.596(0.798~3.276) | 0.190 | ||
| PLT (10^9/L) | 1.003 (0.997~1.008) | 0.314 | ||
| AST (IU/L) | 1.001(0.962~1.035) | 0.957 | ||
| ALT (IU/L) | 1.026(0.975~1.084) | 0.320 | ||
| Diseases | ||||
| OPTB | 0.411(0.126~1.229) | 0.121 | 0.426(0.091~1.862) | 0.260 |
| COPD | 1.027(0.402~2.654) | 0.956 | 0.495(0.123~1.916) | 0.310 |
| RBD+ resting MBCs (%) | 0.983(0.941~1.023) | 0.419 | 3.885(0.083~19.540) | 0.800 |
| RBD+ activated MBCs (%) | 1.020(0.984~1.056) | 0.270 | 3.854(0.052~19.518) | 0.801 |
| RBD+ atypical MBCs (%) | 1.022(0.995~1.051) | 0.116 | 6.213(0.033~39.413) | 0.737 |
| RBD+ intermediate MBCs (%) | 0.977(0.951~1.003) | 0.082 | 5.957(0.045~37.667) | 0.743 |
| RBD-specific memory B cells (MBCs) (%) | 1.006(0.977~1.035) | 0.694 | 1.556(0.747~4.893) | 0.366 |
Abbreviations: BMI, body mass index; WBC, white blood cell; HB, hemoglobin; LC, lymphocyte; PLT, platelet; AST, aspartate transaminase, ALT, alanine aminotransferase; RBD, receptor binding domain; MBC, memory B cell; CI, confidential interval; OR, odds ratio.
Univariate and multivariate analyses for CoV-2 NAb in CRD.
| Variable | Univariate OR (95%CI) | P | Multivariate OR (95%CI) | P |
|---|---|---|---|---|
| Gender (female) | 1.800(0.828~4.014) | 0.143 | 2.968(1.098~8.600) | 0.037 |
| Age (years) | 1.013(0.980~1.049) | 0.455 | 1.042(0.998~1.092) | 0.070 |
| BMI(Kg/m^2) | 1.054(0.930~1.198) | 0.411 | 1.056(0.905~1.240) | 0.492 |
| Days after 2nd dose | 1.011(1.000~1.024) | 0.063 | 1.016(1.002~1.032) | 0.032 |
| Vaccine type (BBIBP-corV) | 0.379(0.170~0.828) | 0.016 | 0.267(0.092~0.733) | 0.012 |
| HB (g/L) | 1.006(0.979~1.034) | 0.683 | ||
| WBC (10^9/L) | 1.238(0.982~1.585) | 0.078 | 1.105(0.834~1.490) | 0.496 |
| LC (10^9/L) | 1.173(0.610~2.273) | 0.630 | ||
| PLT (10^9/L) | 1.002(0.997~1.007) | 0.476 | ||
| AST (IU/L) | 0.994(0.955~1.027) | 0.736 | ||
| ALT (IU/L) | 1.016(0.967~1.070) | 0.518 | ||
| Diseases | ||||
| OPTB | 0.346(0.113~0.978) | 0.051 | 0.371(0.095~1.361) | 0.141 |
| COPD | 1.159(0.472~2.871) | 0.747 | 0.688(0.208~2.228) | 0.533 |
| RBD+ resting MBCs (%) | 0.977(0.937~1.015) | 0.244 | 0.182(0.099~21.374) | 0.720 |
| RBD+ activated MBCs (%) | 1.022(0.988~1.058) | 0.211 | 0.178(0.039~21.431) | 0.716 |
| RBD+ atypical MBCs (%) | 1.029(1.002~1.058) | 0.039 | 0.173(0.076~24.704) | 0.716 |
| RBD+ intermediate MBCs (%) | 0.974(0.949~0.998) | 0.038 | 0.163(0.031~23.010) | 0.708 |
| RBD-specific memory B cells (MBCs) (%) | 1.002(0.975~1.030) | 0.861 | 0.922(0.534~1.698) | 0.778 |
Abbreviations: BMI, body mass index; WBC, white blood cell; HB, hemoglobin; LC, lymphocyte; PLT, platelet; AST, aspartate transaminase, ALT, alanine aminotransferase; RBD, receptor binding domain; MBC, memory B cell; CI, confidential interval; OR, odds ratio.
The germinal center (GC) and an extra-follicular GC-independent mechanism can both produce MBCs [18, 19]. The GC is the primary source of class-switching and significant alterations in somatic Abs [18, 20]. Among the MBC subsets, the frequencies of IgG+ RBD-specific MBCs was the lowest [7]. MBCs, which differentiate into Ab-secreting cells upon secondary infection, are crucial to maintain long-term humoral immunity [21-23]. In a prior investigation, the frequencies of MBCs in peripheral blood were lower in COPD patients than HCs [24]. Additionally, counts of late and switched MBCs were decreased in COPD patients [25]. After therapy, classical MBCs remained at low levels in patients with tuberculous [26, 27]. Previous research has demonstrated that RBD-specific MBCs help to produce Abs [28]. In the present study, the frequencies of RBD-specific MBCs were lower in CRD patients than the HCs. Collectively, these data indicate that humoral immunity induced by inactivated COVID-19 vaccinations was compromised in CRD patients.
In this study, age was not associated with Ab responses in CRD patients, either by grouping or mixed-factor analysis. According to earlier studies [29, 30], the Ab response to the ChAdOx1nCoV-19 and mRNA-1273 COVID-19 vaccines in clinical trials was not influenced by age. However, in contrast to previous reports, age >70 years was related to decreased Ab responses in patients with COPD [31] and the immunological response was poor for those aged >55 years after vaccination with CoronaVac [32]. However, the sample size was relatively small, thus larger studies are needed.
The time interval after a second dose of the vaccine was linked to poor Ab responses by both univariate and multivariate analyses, which is consistent with known risk factors for suboptimal vaccination responses [33, 34]. This finding suggests the need for timely Ab detection and booster doses to maintain a stable Ab response. In a previous study, female was identified as a protective factor for CoV-2 NAb levels [35], consistent with the results of the present study. Higher body mass index was related to lower Ab titers [36], but this finding was not confirmed by univariate or multivariate analysis, possibly due to the relatively small sample size.
There were some limitations to this cross-sectional investigation that should be addressed. First, the study participants were recruited from a single center. Second, T cell levels were not analyzed. Third, longitudinal analysis was not conducted, as only cross-sectional comparisons were performed. Fourth, the small sample size prohibited assessments of potential effects. Fifth, the results might not be generalizable to other vaccines, settings, and ethnicities. Nonetheless, these findings will help to assess the safety and immunogenicity of the COVID-19 vaccination in CRD patients. Second, responses of two Abs were comprehensively analyzed to assess humoral and cellular immunity to the vaccine. Third, while the type of vaccine was protective for the two Abs, the amount of time since full immunization was confirmed as a risk factor for Ab levels.
In summary, the inactivated COVID-19 vaccines were safe and well tolerated, but the Ab response was modest and the frequencies of RBD-specific MBCs was reduced in CRD patients. Similar results were reported with the anti-RBD IgG response after 3 months. Hence, CRD patients should be prioritized for booster vaccinations.
Supplementary Material
Supplementary figures.
Acknowledgements
Funding
This work is supported by the National Science and Technology Major Project of China (2017ZX10202203-007, 2017ZX10202203-008, 2018ZX10302206-003) and a pilot project of clinical cooperation between traditional Chinese and western medicine for significant and complicated diseases of National Administration of Traditional Chinese Medicine: hepatic fibrosis. We also acknowledge the support of the National Natural Science Foundation of China (81772198), the Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0389), the Science and Technology Bureau of Chongqing, China (cstc2022ycjh-bgzxm00519), and supported by the kuanren talents program of the second affiliated hospital of Chongqing medical University.
Author contributions
Concept and design: Hong Ren, DePeng Jiang.
Funding acquisition: Hong Ren.
Participant recruitment and characterisation: Lei Yang, DePeng Jiang, Hong Ren.
Experiment execution: Lei Yang.
Acquisition, analysis or interpretation of data: Lei Yang, LingFang XU, DePeng Jiang, Hong Ren.
Supervision: DePeng Jiang, Hong Ren.
Administrative support: DePeng Jiang, Hong Ren.
Drafting and critical revision of manuscript: Lei Yang, Qiao Guo, Bing Deng, Yang Hong, LiangLiang Wang, YaLin Wang, DePeng Jiang, Hong Ren.
All authors contributed to the article and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-1302
2. Conway FM, Bloom CI, Shah PL. Susceptibility of Patients with Airway Disease to SARS-CoV-2 Infection. Am J Respir Crit Care Med. 2022;206:696-703
3. Aveyard P, Gao M, Lindson N. et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. 2021;9:909-23
4. Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond). 2020;52:902-7
5. Harboe ZB, Hamm SR, Pérez-Alós L. et al. Antibody responses and risk factors associated with impaired immunological outcomes following two doses of BNT162b2 COVID-19 vaccination in patients with chronic pulmonary diseases. BMJ Open Respir Res. 2022;9:e001268
6. Santotoribio JD, Franco-Garcia C, Mondejar R. et al. Clinical Evaluation of Serum Levels of SARS-CoV-2 Anti-Spike Protein IgG Antibodies in Infected Patients and Vaccinated Subjects. Clin Lab. 2022;68:10.7754 /Clin
7. Schaefer-Babajew D, Wang Z, Muecksch F. et al. Antibody feedback regulation of memory B cell development in SARS-CoV-2 mRNA vaccination. Preprint. medRxiv. 2022. 2022 08.05.22278483
8. Ogega CO, Skinner NE, Blair PW. et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. J Clin Invest. 2021;131:e145516
9. Sakharkar M, Rappazzo CG, Wieland-Alter WF. et al. Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol. 2021;6:eabg6916
10. Hippisley-Cox J, Patone M, Mei XW. et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931
11. Jabagi MJ, Botton J, Bertrand M. et al. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA. 2022;327:80-2
12. Xia S, Zhang Y, Wang Y. et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39-51
13. Tanriover MD, Doğanay HL, Akova M. et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey [published correction appears in Lancet. 2022 Jan 29;399(10323):436]. Lancet. 2021;398:213-22
14. Tartof SY, Slezak JM, Fischer H. et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407-16
15. Gandolfo C, Anichini G, Mugnaini M. et al. Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine. Vaccines (Basel). 2022;10:171
16. Hua Q, Zhang H, Yao P. et al. Immunogenicity and immune-persistence of the CoronaVac or Covilo inactivated COVID-19 Vaccine: a 6-month population-based cohort study. Front Immunol. 2022;13:939311
17. La Verde N, Riva A, Cona MS. et al. Immunogenicity of two doses of BNT162b2 and mRNA-1273 vaccines for solid cancer patients on treatment with or without a previous SARS-CoV-2 infection. Int J Cancer. 2023;152:661-71
18. Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15:149-59
19. Victora GD, Nussenzweig MC. Germinal Centers. Annu Rev Immunol. 2022;40:413-42
20. Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679-89
21. Brown SL, Bauer JJ, Lee J, Ntirandekura E, Stumhofer JS. IgM+ and IgM- memory B cells represent heterogeneous populations capable of producing class-switched antibodies and germinal center B cells upon rechallenge with P. yoelii. J Leukoc Biol. 2022;112:1115-35
22. Terreri S, Piano Mortari E, Vinci MR. et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe. 2022;30:400-8
23. Wang CY, Hwang KP, Kuo HK. et al. A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants. J Clin Invest. 2022;132:e157707
24. Lanzilli G, Traggiai E, Braido F. et al. Administration of a polyvalent mechanical bacterial lysate to elderly patients with COPD: Effects on circulating T, B and NK cells. Immunol Lett. 2013;149:62-7
25. Brandsma CA, Hylkema MN, Geerlings M. et al. Increased levels of (class switched) memory B cells in peripheral blood of current smokers. Respir Res. 2009;10:108
26. Jieyun Zhang, Ying Zhang, Qianting Yang. et al. Dynamic changes of T cell and B cell subsets in peripheral blood of tuberculosis patients during treatment. China Tropical Med. 2019 Vol.19,7
27. Sebina I, Biraro IA, Dockrell HM, Elliott AM, Cose S. Circulating B-lymphocytes as potential biomarkers of tuberculosis infection activity. PLoS One. 2014;9:e106796
28. Muecksch F, Wang Z, Cho A. et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022;607:128-34
29. Anderson EJ, Rouphael NG, Widge AT. et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383:2427-38
30. Lee SW, Moon JY, Lee SK. et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front Immunol. 2021;12:779212
31. Pelletier É, Desmeules P, Lacasse Y. et al. Antibody Response to Severe Acute Respiratory Syndrome Coronavirus-2 Vaccination in COPD: A Cohort Study. Chronic Obstr Pulm Dis. 2022;9:591-5
32. Medeiros GX, Sasahara GL, Magawa JY. et al. Reduced T Cell and Antibody Responses to Inactivated Coronavirus Vaccine Among Individuals Above 55 Years Old. Front Immunol. 2022;13:812126
33. Barin B, Kasap U, Selçuk F, Volkan E, Uluçkan Ö. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: a prospective, longitudinal population-based study. Lancet Microbe. 2022;3:e274-83
34. Ao L, Lu T, Cao Y. et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg Microbes Infect. 2022;11:1126-34
35. Pellini R, Venuti A, Pimpinelli F. et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928
36. Wei J, Stoesser N, Matthews PC. et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6:1140-49
Author contact
![]() Corresponding authors: DePeng Jiang, Department of Respiratory Medicine, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, email: gdp116cqmu.edu.cn. Hong Ren, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, Department of Infectious Diseases, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, email: renhong0531edu.cn
Corresponding authors: DePeng Jiang, Department of Respiratory Medicine, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, email: gdp116cqmu.edu.cn. Hong Ren, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, Department of Infectious Diseases, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, email: renhong0531edu.cn

 Global reach, higher impact
Global reach, higher impact