3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(2):219-224. doi:10.7150/ijms.80543 This issue Cite
Research Paper
Relationship between endoscopic gastric abnormalities and colorectal polyps: a cross-sectional study based on 33439 Chinese patients
Department of Gastroenterology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Received 2022-11-6; Accepted 2023-1-10; Published 2023-1-22
Abstract
Background: No study on the relationship between common abnormalities of the upper digestive tract and colorectal polyps (CPs) has been conducted.
Methods: 33439 patients were enrolled in this cross-sectional study, of which 7700 had available Helicobacter pylori (H.pylori) information. All participants underwent colonoscopy and esophagogastroduodenoscopy (EGD) simultaneously or within six months at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology from January 2015 to November 2021. The study assessed whether the risk of CPs was affected by the following gastroesophageal diseases: atrophic gastritis (AG), gastric polyps, Barrett's esophagus and reflux esophagitis, bile reflux, gastric ulcer, gastric mucosal erosion, superficial gastritis, and gastric H.pylori infection. The crude and adjusted odds ratios (ORs) of H.pylori on the occurrence of CPs were computed by logistic regression. Additionally, we also evaluated whether AG had an impact on the relationship between H.pylori infection and CPs.
Results: A total of 10600 cases (31.7%) were diagnosed as CPs. Multivariate logistic analysis showed that age, male (OR, 1.80; 95% confidence interval [CI], 1.61 to 2.02), gastric polyps (OR, 1.61; 95% CI, 1.05 to 2.46 for hyperplastic polyps; OR, 1.45; 95% CI, 1.09 to 1.94 for fundic gland polyps), H.pylori infection (OR, 1.21; 95% CI, 1.07 to 1.37) and atrophic gastritis (OR, 1.38; 95% CI, 1.21 to 1.56) were independent risk factors for colorectal polyps. Moreover, the combined effect of H.pylori infection and AG was slightly greater than the sum of their individual effects on the risk of CPs, but there was no additive interaction between them.
Conclusions: Gastric conditions including gastric polyps, H.pylori infection, and AG increased the risk of CPs. However, Barrett's esophagus and reflux esophagitis, bile reflux, erosive gastritis, gastric ulcer, and superficial gastritis might not have relationship with CPs occurrence.
Keywords: Colorectal polyp, Helicobacter pylori, Gastritis, Atrophy, Gastric polyp
Introduction
Colorectal carcinoma (CRC) is one of the most common malignancies with increasing incidence worldwide [1, 2]. The 5-year survival rate of patients with early-stage CRC exceeds 90% [3], while more than 85% of Chinese patients are in advanced stage at the time of diagnosis [4]. The untimely detection greatly affects the prognosis and survival of CRC patients. Colorectal polyps (CPs) are premalignant lesions associated with colorectal carcinogenesis [5]. It is reported that about 85%-90% of CRC originates from polyps [6]. Furthermore, one study reported that colonoscopic polypectomy reduced CRC-related long-term mortality by 53% [7]. Therefore, elucidating high-risk people with CPs is very important.
In recent years, the esophagogastroduodenoscopy (EGD) examination has been more and more widely performed and has become a standard examination in clinical work. However, due to the invasiveness, inconvenience, and required preparation, the colonoscopy examination is more onerous for patients than EGD. Although some researchers have explored the relationship between Helicobacter pylori (H.pylori) infection, atrophic gastritis (AG), and gastric polyps and the risk of colorectal tumors or polyps, the outcomes remain controversial, and most sample sizes are relatively small. In addition, no study has investigated the association between other upper gastrointestinal diseases and the occurrence of colorectal polyps.
Patient flow chart.
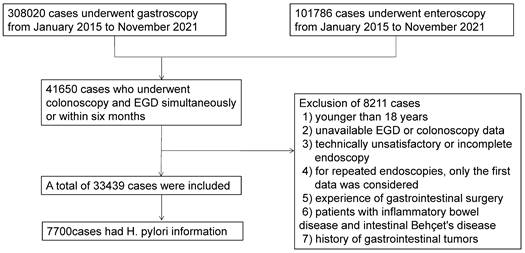
To solve this problem, we retrospectively analyzed the database of 33439 Chinese patients trying to find the possible association between various diseases of the upper digestive tract and CPs. We also evaluated whether AG impacted the relationship between H.pylori infection and CPs.
Materials and Methods
Study Population
We conducted a cross-sectional study of subjects who underwent colonoscopy and EGD at our institution from January 2015 to November 2021. During this period, 308020 cases underwent gastroscopy, and 101786 cases underwent colonoscopy. Forty-one thousand six hundred fifty subjects who had colonoscopy and EGD simultaneously or within six months were included. We excluded 8211 subjects for the following reasons: 1) younger than 18 years; 2) unavailable EGD or colonoscopy data; 3) technically unsatisfactory or incomplete endoscopy; 4) for repeated endoscopies, only the first data was considered; 5) experience of gastrointestinal surgery; 6) patients with inflammatory bowel disease and intestinal Behçet's disease; or 7) history of gastrointestinal tumors. Finally, 33439 subjects were included for analysis (Figure 1). The Institutional Ethics Board of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) approved this study (TJ-IRB20220437). The informed consent was waived, as we solely analyzed de-identified data.
Endoscopic impressions
At least one or two experienced gastroscopists performed gastrointestinal endoscopy examinations. Endoscopic findings were recorded as descriptive form in the endoscopy center database. EGD conditions considered relevant were the absence or presence of gastric mucosal atrophy, gastric polyps, Barrett's esophagus and reflux esophagitis, bile reflux, gastric ulcer, gastric mucosal erosion, and superficial gastritis. The diagnostic criteria of the above diseases were mainly based on endoscopic findings. In addition, 7700 subjects had available H.pylori information detected by urea breath test (UTB). The UTB value > 2.5% was diagnosed as H.pylori infection.
Statistical Analysis
Continuous variables are summarized as means ± standard deviation (SD), while categorical variables are reported as frequency (%). Chi-squared test and Mann-Whitney U-test were used for comparison between groups. Collinearity between gastric variables was checked with variance inflation factors, and none was collinear. Univariate logistic regression was performed to identify the association between gastric findings and CPs, and the variables with P < 0.1 were included for further multivariate analysis. Results are calculated as odds ratio (OR) and 95% confidence interval (CI). All two-tailed P values less than 0.05 were regarded as significant. Data analyses were carried out using SPSS statistics version 23 and R 4.1.2.
Additionally, we further analyzed whether the combined effect of H.pylori infection and AG was greater than the sum of their individual effects on CPs. Subjects were divided into four categories according to AG and H.pylori status: H.pylori (-) & AG (-), H.pylori (-) & AG (+), H.pylori (+) & AG (-), and H.pylori (+) & AG (+). Using logistic regression analysis, we estimated ORs of the other three groups with H.pylori (-) & AG (-) group as the reference category. We evaluated the existence of additive interaction by calculating the values of synergy index (S), the attributable proportion due to interaction (AP), and the relative excess risk due to interaction (RERI) [8], as proposed by Rothman [9]. The 95% CI of RERI and AP contains 0, and the 95% CI of S contains 1, indicating no additive interaction; the 95% CI of RERI and AP > 0, and the 95% CI of S > 1, indicating a positive interaction; the 95% CI of RERI and AP < 0, and the 95% CI of S < 1, indicating a negative interaction.
Results
General characteristics of subjects
Among 33439 enrolled cases, 10600 cases (31.7%) were diagnosed to have colorectal polyps and 22839 cases were confirmed as polyp-free controls. The mean age was 48.3±12.0 years and males comprised 58.3% of the population. The clinical characteristics of the subjects with CPs and polyp-free controls are listed in Table 1. The group of subjects with colorectal polyps was older and had a greater proportion of males, higher atrophic gastritis, higher Barrett's esophagus and reflux esophagitis, lower bile reflux, higher gastric polyps, higher gastric ulcer, higher erosive gastritis, lower superficial gastritis, and higher H.pylori infection as compared to the polyp-negative group.
Analysis of risk factors associated with colorectal polyps
Table 2 contains the logistic regression results of two study populations, one is the all 33439 study subjects, and the other is 7700 cases with available H.pylori information. The univariate analyses of the two populations were consistent, except for bile reflux in 7700 cases (P=0.052). They all revealed that age, sex, gastric polyps, Barrett's esophagus and reflux esophagitis, gastric ulcer, erosive gastritis, superficial gastritis, atrophic gastritis, and H.pylori were significantly associated with the risk of colorectal polyps.
General characteristics of patients with and controls without colorectal polyps
| Total (n = 33439) | Colorectal polyp (+) (n = 10600) | Colorectal polyp (-) (n = 22839) | P value | |
|---|---|---|---|---|
| Age, years | 48.3±12.0 | 52.2±11.0 | 46.6±12.1 | <0.001 |
| Male | 19499 (58.3) | 7046 (66.5) | 12453 (54.5) | <0.001 |
| Endoscopic manifestations | ||||
| Atrophic gastritis | 7129 (21.3) | 2932 (27.7) | 4197 (18.4) | <0.001 |
| Gastric polyps | 5859 (17.5) | 2521 (23.8) | 3338 (14.6) | <0.001 |
| BE+RE | 4130 (12.4) | 1579 (14.9) | 2551(11.2) | <0.001 |
| Bile reflux | 1758 (5.3) | 474 (4.5) | 1284 (5.6) | <0.001 |
| Gastric ulcer | 4122 (12.3) | 1470 (13.9) | 2652 (11.6) | <0.001 |
| Erosive gastritis | 15765 (47.1) | 5524 (52.1) | 10241(44.8) | <0.001 |
| Superficial gastritis | 11255 (33.7) | 2778 (26.2) | 8477 (37.1) | <0.001 |
| H.pylori (+) (7700) | 1843 (23.9) | 588 (26.9) | 1255 (22.8) | <0.001 |
Age is expressed as mean (SD) and all other data are expressed as number (proportion). BE, Barrett's esophagus; RE, reflux esophagitis; H.pylori, Helicobacter pylori.
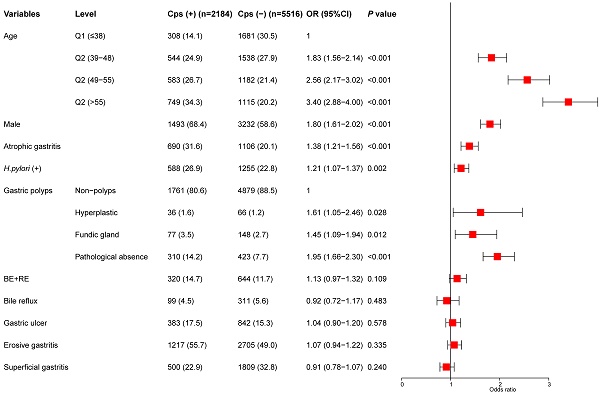
The indicators of univariate analysis in Table 2 (P<0.1) were all included in multivariate analysis. The results showed that age, sex, gastric polyps (including hyperplastic polyps and gastric fundus gland polyps), H.pylori infection, and AG were independent risk factors for colorectal polyps. In addition, we divided the age into four stages according to the quartile and conducted a trend test. Subjects with age in the highest quartile (> 55) had an OR of 3.40 (95% CI, 2.88-4.00) (P for trend < 0.001) (Figure 2).
Multivariate analysis for the risk of colorectal polyps.
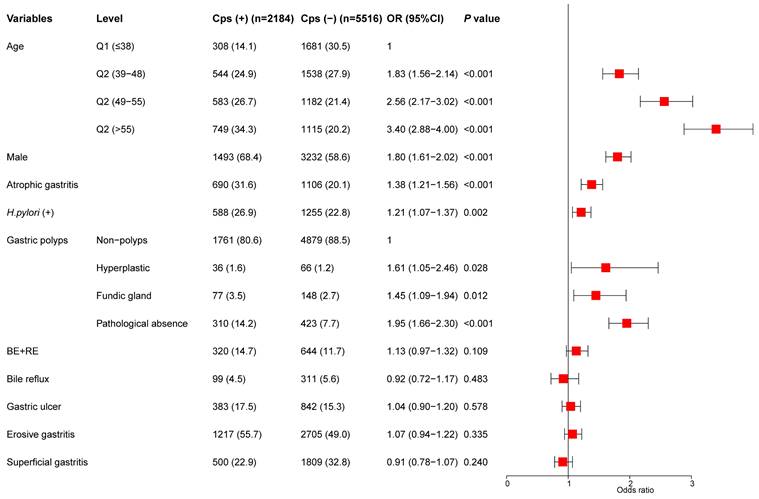
Univariate analysis for the risk of colorectal polyps
| Univariate analysis (n=33439) | Univariate analysis (n=7700) * | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, years | 1.04 (1.04-1.04) | <0.001 | 1.04 (1.04-1.05) | <0.001 |
| Male | 1.65 (1.58-1.74) | <0.001 | 1.53 (1.38-1.70) | <0.001 |
| Endoscopic manifestations | ||||
| Gastric polyps | 1.82 (1.72-1.93) | <0.001 | 1.84 (1.61-2.11) | <0.001 |
| BE+RE | 1.39 (1.30-1.49) | <0.001 | 1.30 (1.12-1.50) | <0.001 |
| Bile reflux | 0.79 (0.71-0.88) | <0.001 | 0.80 (0.63-1.00) | 0.052 |
| Gastric ulcer | 1.23 (1.15-1.31) | <0.001 | 1.18 (1.03-1.35) | 0.014 |
| Erosive gastritis | 1.34 (1.28-1.40) | <0.001 | 1.31 (1.18-1.45) | <0.001 |
| Superficial gastritis | 0.60 (0.57-0.63) | <0.001 | 0.61 (0.54-0.68) | <0.001 |
| Atrophic gastritis | 1.70 (1.61-1.79) | <0.001 | 1.84 (1.65-2.06) | <0.001 |
| H.pylori (+) | 1.25 (1.12-1.40) | <0.001 | 1.25 (1.12-1.40) | <0.001 |
BE, Barrett's esophagus; RE, reflux esophagitis; H.pylori, Helicobacter pylori. *H.pylori information available for 7700 patients.
Separate and combined effects of H.pylori infection and AG on colorectal polyps
| Exposure | N | n (%) with CPs | Crude OR (95% CI) | P value |
|---|---|---|---|---|
| AG (-) & H.pylori (-) | 4712 | 1169 (24.8) | 1 | |
| H.pylori (+) & AG (-) | 1192 | 325 (27.3) | 1.14 (0.98-1.31) | 0.082 |
| AG (+) & H.pylori (-) | 1145 | 427 (37.3) | 1.80 (1.57-2.07) | <0.001 |
| AG (+) & H.pylori (+) | 651 | 263 (40.4) | 2.05 (1.73-2.43) | <0.001 |
| Measure | Estimate (95% CI) | |||
| RERI | 0.12 (-0.28-0.53) | |||
| AP | 0.06 (-0.13-0.25) | |||
| S | 1.13 (0.75-1.71) |
AG, atrophic gastritis; CPs, Colorectal polyps; H.pylori, Helicobacter pylori; RERI, the relative excess risk due to interaction; AP , the attributable proportion due to interaction; S, the synergy index.
Separate and combined effects of H.pylori infection and AG on colorectal polyps
As presented in Table 3, the prevalence of CPs was 24.8% in AG (-) & H.pylori (-), 27.3% in H.pylori (+) & AG (-), 37.3% in AG (+) & H.pylori (-) and 40.4% in AG (+) & H.pylori (+). Compared with subjects without H.pylori infection and atrophy, subjects with AG (OR: 1.80, 95%CI: 1.57-2.07) had a higher incidence of polyps, while subjects with H.pylori-positive (OR: 1.14, 95%CI: 0.98-1.31) did not. Moreover, subjects with H.pylori infection and AG increased the risk of CPs, but the relative excess risk attributable to the interaction was not statistically significant (RERI: 0.12 (-0.28-0.53), AP: 0.06 (-0.13-0.25), S: 1.13 (0.75-1.71). Figure 3 shows the separate and combined ORs of H.pylori infection and AG on CPs.
Discussion
The current analysis analyzed the relationship between common gastroscopic manifestations and colorectal polyps in 33439 people. The results supported an elevated risk of CPs in individuals with gastric atrophy, gastric polyps, and H.pylori infection. It also showed that the risk of CPs might be further increased in subjects with concomitant AG and H.pylori infection.
Multiple publications have recently noticed the connection between H.pylori infection and CPs or CRC. However, these findings remain inconclusive. Although several research studies reported a positive association between H.pylori and CPs or CRC [10-13], other studies did not find any association [14-17]. We speculated that the contradictory results might be partly related to different H.pylori detection methods, regions and races, and small sample sizes. As for the relationship between AG and CPs, several studies have shown an association between them [13, 18]. Recently, Sonnenberg et al. [19] conducted a large retrospective study to assess the connection between colonic polyps and abnormal gastric histopathology, based on 302 061 patients from a national database. The data displayed that patients with gastric polyps, intestinal metaplasia, and H.pylori infection increased the risk of colonic polyps. Similarly, our current investigation found that H.pylori infection and AG were risk factors for CPs.
OR with contributions from different exposure categories.
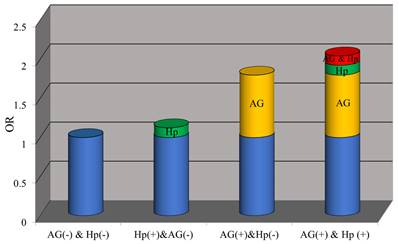
In addition, Lee et al. [20] found that patients with H.pylori infection (OR: 1.34, 95%CI: 1.04-1.72) had a higher risk of advanced CRC, especially when it coexists with AG (OR: 1.40, 95%CI: 1.03-1.91). We also found that the combined effects of H.pylori infection and AG were slightly greater than the sum of their individual effects on CPs. Therefore, we speculated that there might be additive interaction between H.pylori and AG and conducted additional analysis to verify our conjecture. Although our data showed that the additive interaction between the two on the risk of CPs was positive, it is not statistically significant. Nevertheless, we still recommend H.pylori eradication treatment, especially in patients with AG. Hu et al. [21] first demonstrated that H.pylori eradication could reduce the incidence of CPs, which needs to be confirmed in the future. Moreover, AG without H.pylori infection was independently associated with CPs, but H.pylori infection without AG was not. This result is similar to other findings [20, 22]. Therefore, prolonged H.pylori infection may be more critical to developing CPs because most AG is usually caused by longstanding H.pylori infection.
Several explanations have been proposed to clarify the mechanism of positive correlation between H.pylori infection and CPs. First, H.pylori infection and AG induce hypergastrinemia [23], which might promote the development of CRC by stimulating the proliferation of colorectal mucosa [24]. Several studies have shown a relationship between colorectal tumors and gastrin [25, 26], while others disagreed [27-29]. Second, chronic AG induced by long-term H.pylori infection suppresses acid secretion. Low gastric acid may change the gastrointestinal microflora and result in bacterial overgrowth [30, 31], which may play a role in colorectal carcinogenesis. Third, as early as 1992 and 1999, it was found that viable H.pylori existed in the feces of infected individuals [32, 33]. A few studies showed that CRC had a higher H.pylori detection rate compared with normal mucosa [34]. Therefore, H.pylori might locally activate the occurrence of CRC through direct contact with the mucosa.
Moreover, we also demonstrated that gastric polyps, whether hyperplastic polyps or gastric fundus gland polyps, increased the risk of CPs, whereas Genta et al. [35] found no relation between gastric fundic gland polyps and CPs in males. However, other previous reports showed an increased risk of CPs in individuals with hyperplastic polyps and fundic gland polyps [36, 37]. The existence of CPs also increased in subjects with gastric polyps in a meta-analysis (OR: 1.15, 95%CI: 1.04-1.26) [38]. The relevant mechanisms are still unclear. Gastric fundus gland polyps are the main component of gastric polyps, and most of them are signs of long-term proton pump inhibitors treatment [39]. Therefore, the etiology of the positive associations may relate to the reduction of gastric acid barrier. As for hyperplastic polyps, they are closely related to H.pylori infection and atrophic gastritis [40, 41], but whether this can explain the positive relationship is still unknown. Another suggestion is that lifestyle, environmental and genetic factors may have crucial roles in the mechanism of this correlation.
To our knowledge, male and elderly participants were at high risk of CPs and CRC. Likewise, our current research also determined an increased risk of CPs in men compared with women. Furthermore, the prevalence of CPs gradually increased with age, showing the highest OR (3.40; 95% CI, 2.88 to 4.00) in the older than 55 group.
The possible shortcomings of our study must be mentioned. Firstly, the current analysis relied almost entirely on endoscopic findings rather than histologic diagnoses, and there may be subjectivity and diagnostic variability among observers. However, our experienced endoscopists had received systematic training, and superior doctors further reviewed their uncertain examination reports. Therefore, the possibility of variability might be minimized. On the other hand, endoscopic diagnoses were also one of our advantages, increasing convenience and providing practical knowledge during daily clinical work. Secondly, we cannot obtain information about eating habits, lifestyle, disease history, medication history, and other confounding factors. However, in other studies, the associations stayed largely unaffected by these confounding factors. Thirdly, our data came from a single center in China. Thus, the generalizability of our findings needs to be confirmed by other populations. Despite these limitations, it is the first report with a relatively large sample size to investigate the relationship between gastroscopic abnormalities and CPs.
In conclusion, gastric conditions including gastric polyps, H.pylori infection, and AG were independent risk factors for CPs. Furthermore, H.pylori-infected subjects with AG might slightly enhance the risk of CPs. However, Barrett's esophagus and reflux esophagitis, bile reflux, gastric mucosal erosion, gastric ulcer, and superficial gastritis might not be related to the presence of CPs. Therefore, screening colonoscopy should be considered when patients are diagnosed as gastric polyps, AG, and H.pylori infection during EGD examination. For people undergoing gastroscopy, our study provides valuable evidence for physicians to recommend colonoscopy screening. In addition, further investigations are required to clarify the exact mechanism underlying the positive relationship between gastroscopic findings and CPs, and the effect of H.pylori eradication on susceptibility to CPs and cancer.
Acknowledgements
Funding
This work is supported by the Subproject of National Key Research and Development Program [No. 2021YFC2600263].
Ethical approval
All procedures followed were in accordance with the Helsinki Declaration. This study has been approved by the Ethical Committees of Tongji Hospital of Tongji Medical College.
Author contributions
LF, KZ, GW, RD and MZ conceived and designed this study. SX, YZ, WZ, WY and JL participated in data collection, interpretation and analysis. LF, KZ, GW and DT wrote the first draft of the manuscript. LF, KZ, RD, MZ and WZ prepared the figures and tables. JL, GW, SX, YZ, DT and WY revised the primary manuscript. All authors read and approved the final manuscript.
Data sharing statement
The data analyzed for this study can be accessible from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dekker E, Tanis PJ, Vleugels JLA. et al. Colorectal cancer. Lancet. 2019;394:1467-80
2. Fitzmaurice C, Akinyemiju TF, Al Lami FH. et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553-68
3. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-502
4. Allemani C, Matsuda T, Di Carlo V. et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-75
5. Li D, Liu L, Fevrier HB. et al. Increased Risk of Colorectal Cancer in Individuals with a History of Serrated Polyps. Gastroenterology. 2020;159:502-11.e2
6. Conteduca V, Sansonno D, Russi S. et al. Precancerous colorectal lesions (Review). Int J Oncol. 2013;43:973-84
7. Zauber AG, Winawer SJ, O'Brien MJ. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-96
8. Andersson T, Alfredsson L, Källberg H. et al. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575-9
9. Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506-11
10. Plummer M. Helicobacter pylori and colonic neoplasms. Am J Gastroenterol. 2013;108:216-7
11. Mizuno S, Morita Y, Inui T. et al. Helicobacter pylori infection is associated with colon adenomatous polyps detected by high-resolution colonoscopy. Int J Cancer. 2005;117:1058-9
12. Brim H, Zahaf M, Laiyemo AO. et al. Gastric Helicobacter pylori infection associates with an increased risk of colorectal polyps in African Americans. BMC Cancer. 2014;14:296
13. Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol. 2013;108:208-15
14. Machida-Montani A, Sasazuki S, Inoue M. et al. Atrophic gastritis, Helicobacter pylori, and colorectal cancer risk: a case-control study. Helicobacter. 2007;12:328-32
15. Liou JM, Lin JW, Huang SP. et al. Helicobacter pylori infection is not associated with increased risk of colorectal polyps in Taiwanese. Int J Cancer. 2006;119:1999-2000
16. Siddheshwar RK, Muhammad KB, Gray JC. et al. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol. 2001;96:84-8
17. Limburg PJ, Stolzenberg-Solomon RZ, Colbert LH. et al. Helicobacter pylori seropositivity and colorectal cancer risk: a prospective study of male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:1095-9
18. Kawahara Y, Kodama M, Mizukami K. et al. Endoscopic gastric mucosal atrophy as a predictor of colorectal polyps: a large scale case-control study. J Clin Biochem Nutr. 2019;65:153-9
19. Sonnenberg A, Turner KO, Genta RM. Associations between gastric histopathology and the occurrence of colonic polyps. Colorectal Dis. 2020;22:814-7
20. Lee JY, Park HW, Choi JY. et al. Helicobacter pylori Infection with Atrophic Gastritis Is an Independent Risk Factor for Advanced Colonic Neoplasm. Gut Liver. 2016;10:902-9
21. Hu KC, Wu MS, Chu CH. et al. Decreased Colorectal Adenoma Risk After Helicobacter pylori Eradication: A Retrospective Cohort Study. Clin Infect Dis. 2019;68:2105-13
22. Yan Y, Chen YN, Zhao Q. et al. Helicobacter pylori infection with intestinal metaplasia: An independent risk factor for colorectal adenomas. World J Gastroenterol. 2017;23:1443-9
23. Watson SA, Grabowska AM, El-Zaatari M. et al. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer. 2006;6:936-46
24. Thorburn CM, Friedman GD, Dickinson CJ. et al. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275-80
25. Georgopoulos SD, Polymeros D, Triantafyllou K. et al. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion. 2006;74:42-6
26. Ciccotosto GD, McLeish A, Hardy KJ. et al. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142-53
27. Fireman Z, Trost L, Kopelman Y. et al. Helicobacter pylori: seroprevalence and colorectal cancer. Isr Med Assoc J. 2000;2:6-9
28. Selgrad M, Bornschein J, Kandulski A. et al. Helicobacter pylori but not gastrin is associated with the development of colonic neoplasms. Int J Cancer. 2014;135:1127-31
29. Robertson DJ, Sandler RS, Ahnen DJ. et al. Gastrin, Helicobacter pylori, and colorectal adenomas. Clin Gastroenterol Hepatol. 2009;7:163-7
30. Kanno T, Matsuki T, Oka M. et al. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochem Biophys Res Commun. 2009;381:666-70
31. Kado S, Uchida K, Funabashi H. et al. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395-8
32. Thomas JE, Gibson GR, Darboe MK. et al. Isolation of Helicobacter pylori from human faeces. Lancet. 1992;340:1194-5
33. Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. Jama. 1999;282:2240-5
34. Soylu A, Ozkara S, Alis H. et al. Immunohistochemical testing for Helicobacter Pylori existence in neoplasms of the colon. BMC Gastroenterol. 2008;8:35
35. Genta RM, Schuler CM, Robiou CI. et al. No association between gastric fundic gland polyps and gastrointestinal neoplasia in a study of over 100,000 patients. Clin Gastroenterol Hepatol. 2009;7:849-54
36. Cao H, He N, Song S. et al. Is surveillance colonoscopy necessary for patients with sporadic gastric hyperplastic polyps? PLoS One. 2015;10:e0122996
37. Zhang S, Zheng D, Yang Z. et al. Patients with Gastric Polyps need Colonoscopy Screening at Younger Age: A Large Prospective Cross-Sectional Study in China. J Cancer. 2019;10:4623-32
38. Wu ZJ, Lin Y, Xiao J. et al. Clinical significance of colonoscopy in patients with upper gastrointestinal polyps and neoplasms: a meta-analysis. PLoS One. 2014;9:e91810
39. Carmack SW, Genta RM, Schuler CM. et al. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104:1524-32
40. Abraham SC, Singh VK, Yardley JH. et al. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. The American journal of surgical pathology. 2001;25:500-7
41. Di Giulio E, Lahner E, Micheletti A. et al. Occurrence and risk factors for benign epithelial gastric polyps in atrophic body gastritis on diagnosis and follow-up. Alimentary pharmacology & therapeutics. 2005;21:567-74
Author contact
![]() Corresponding author: Jiazhi Liao, No.1095 Jie Fang Avenue, Hankou, Wuhan, Hubei, 430030, China. E-mail address: liaojiazhitjmu.edu.cn.
Corresponding author: Jiazhi Liao, No.1095 Jie Fang Avenue, Hankou, Wuhan, Hubei, 430030, China. E-mail address: liaojiazhitjmu.edu.cn.

 Global reach, higher impact
Global reach, higher impact