Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(2):186-193. doi:10.7150/ijms.79285 This issue Cite
Research Paper
Prognostic outcomes of cytomegalovirus reactivation after autologous stem cell transplantation
Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
Received 2022-9-26; Accepted 2022-12-2; Published 2023-1-9
Abstract
Background: Cytomegalovirus (CMV) reactivation is a common complication in patients undergoing allogeneic stem cell transplantation. However, the incidence of CMV reactivation is low after autologous stem cell transplantation (auto-SCT), and the prognostic value of CMV reactivation remains controversial. Moreover, reports on late CMV reactivation after auto-SCT are limited. We aimed to analyze the association between CMV reactivation and survival outcomes and develop a predictive model for late CMV reactivation in patients undergoing auto-SCT.
Methods: Data of 201 patients who underwent SCT at the Korea University Medical Center from 2007 to 2018 were collected. We analyzed prognostic factors for survival outcomes after auto-SCT and risk factors for late CMV reactivation using a receiver operating characteristic curve. Then, we developed a predictive risk model for late CMV reactivation based on results of the risk factor analysis.
Results: Early CMV reactivation was significantly associated with better overall survival (OS) (hazard ratio [HR], 0.329; P = 0.045) in patients with multiple myeloma; however, no significant differences were observed in patients with lymphoma. For late CMV reactivation, a serum lactate dehydrogenase level greater than the upper limit of normal (HR, 2.251; P = 0.027) and late CMV reactivation (HR, 2.964; P = 0.047) were independent risk factors for poor OS, while lymphoma diagnosis (vs. multiple myeloma; HR, 0.389; P = 0.016) was an independent risk factor for good OS. In risk factor analysis for late CMV reactivation, T-cell lymphoma diagnosis (odds ratio [OR], 8.499; P = 0.029), ≥ two prior chemotherapies (OR, 8.995; P = 0.027), failure to achieve complete response (CR) after transplantation (OR, 7.124; P = 0.031), and early CMV reactivation (OR, 12.853; P = 0.007) were significantly associated with late CMV reactivation. To develop the predictive risk model for late CMV reactivation, a score (1 to 1.5) was assigned for each of the above-mentioned variables. The optimal cutoff value (1.75 points) was calculated using the receiver operating characteristic curve. The predictive risk model showed good discrimination, with an area under the curve of 0.872 (standard error, 0.062; P < 0.001).
Conclusions: Late CMV reactivation was an independent risk factor for inferior OS, whereas early CMV reactivation was associated with better survival in patients with multiple myeloma. This risk prediction model could be helpful in identifying high-risk patients who require monitoring for late CMV reactivation and potentially benefit from prophylactic or preemptive therapy.
Keywords: cytomegalovirus, autologous stem cell transplantation, multiple myeloma, prognosis
Introduction
Cytomegalovirus (CMV) reactivation is a frequent complication in patients undergoing stem cell transplantation (SCT). It is commonly known to increase transplant-related mortality and lead to the development of specific disease after SCT, including hepatitis, pneumonia, gastroenteritis, and retinitis [1]. In contrast, some previous studies have reported that CMV reactivation after allogeneic SCT (allo-SCT) is associated with a reduced risk of early relapse in patients with acute myeloid leukemia (AML); however, it was not related to relapse in other diseases, such as chronic myeloid leukemia (CML), acute lymphoblastic leukemia, or lymphoma [2]. This finding is contradictory to previous findings on CMV reactivation after SCT [3]. Studies have reported that early CMV reactivation was associated with decreased relapse rate in patients with CML [4] and lymphoma [5]; however, another study showed that CMV reactivation was associated with increased non-relapse mortality and lower overall survival (OS) in patients with AML, CML, and myelodysplastic syndrome (MDS) [6]. In a study by Thomson et al., no association was found between CMV reactivation and relapse risk in patients with AML who received a novel immunosuppressive agent, alemtuzumab [7]. In contrast, asymptomatic CMV reactivation was found to be a prognostic factor for better OS in a small retrospective study conducted in allo-SCT recipients [8].
However, CMV reactivation is infrequently observed after autologous SCT (auto-SCT). The incidence of CMV reactivation after auto-SCT is considerably lower than that after allo-SCT (4-9% vs. 21-38%) [9]. Moreover, symptomatic CMV reactivation or infection is rare in the auto-SCT setting [10]. However, the incidence of CMV reactivation is high in patients with lymphoma and multiple myeloma based on treatment and disease-associated characteristics [10, 11]. Although CMV was found to cause life-threatening complications, such as pneumonia, in patients who underwent auto-SCT [12], most studies were conducted before the introduction of novel chemotherapeutic drugs [10]. Thus, currently available information is limited to the clinical progression and implications of CMV reactivation after auto-SCT. Although immune reconstitution after auto-SCT could affect the clinical outcomes of transplant recipients [13], the effect of CMV reactivation on prognosis after auto-SCT remains controversial.
This study aimed to evaluate the association between CMV reactivation and survival outcomes in patients undergoing auto-SCT. Based on the results of this study, we developed a predictive model for late CMV reactivation following auto-SCT. This model could help identify patients who may potentially benefit from optimized strategies for monitoring, preventing, and treating late CMV reactivation after auto-SCT.
Methods
Patients
The Korea University bone marrow transplantation registry is a longitudinal cohort containing data of patients who underwent SCT at the Korea University Medical Center (Korea University Anam, Guro, and Ansan Hospitals) from January 2007 to December 2018. According to this registry, 201 patients underwent auto-SCT: 107 with multiple myeloma; 50 with B-cell lymphoma; 35 with T-cell lymphoma; 8 with Hodgkin lymphoma, and 1 with amyloid light-chain (AL) amyloidosis. Patients with AL amyloidosis were excluded because critical medical data were missing. Of the 200 patients, 50 were not tested for CMV reactivation. Thus, we retrospectively analyzed data from 150 patients with multiple myeloma (n = 80), B-cell lymphoma (n = 39), T-cell lymphoma (n = 26), and Hodgkin lymphoma (n = 5). All methods were performed in accordance with relevant guidelines and regulations. This study was approved by the Institutional Review Board of the Korea University Medical Center, with a waiver of informed consent for the collection and analysis of retrospective data.
Definitions
CMV reactivation was defined as the detection of CMV viremia based on viral load greater than the lower limit of detection (LOD) using quantitative polymerase chain reaction (qPCR). Early and late CMV reactivations were defined as CMV reactivation 100 days before and after transplantation, respectively. The diagnosis of CMV disease requires identification of CMV in biopsy specimens. Treatment responses were assessed according to the International Myeloma Working Group response criteria [14] for multiple myeloma and the Lugano classification [15] for lymphoma. OS was measured from the start of transplantation until death from any cause. Progression-free survival (PFS) was defined as the time from the start of transplantation to disease progression or death from any cause. High-risk cytogenetics in multiple myeloma was defined as t(4;14), t(14;16), del(17/17p), TP53 deletion, or chromosome 1 abnormalities, including gain(1q) and del(1p).
CMV monitoring and management
CMV reactivation was monitored once or twice a week by qPCR in all patients until day 100 after transplantation and thereafter, only if clinically indicated. All patients received acyclovir for antiviral prophylaxis. CMV reactivation was preemptively treated with ganciclovir according to the physicians' decisions.
Prediction model
A predictive model for late CMV reactivation was constructed based on the results of risk factor analysis using a stepwise logistic regression method. The receiver operating characteristic (ROC) curves and area under the curve (AUC) were used to evaluate the performance and prediction accuracy of the model. The optimal cutoff point for estimating late CMV reactivation was identified as the point at which the AUC value was maximal.
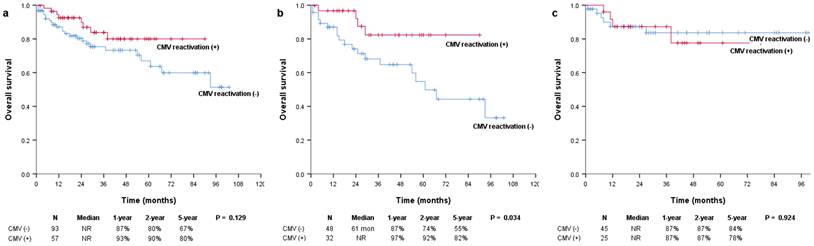
Kaplan-Meier survival curves for OS in (a) all patients and patients with (b) multiple myeloma and (c) lymphoma. OS: overall survival.
Statistical analysis
Categorical variables were evaluated using the chi-square test or Fisher's exact test, and continuous variables were evaluated using the Student's t-test. Backward stepwise logistic regression analysis was used to estimate the association between late CMV reactivation and clinical variables. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazards model with the backward stepwise elimination method was used to analyze the association between survival rates and other prognostic variables. All tests were two sided, and P-values < 0.05 were considered significant. Statistical analyses were performed using Statistical Package for the Social Sciences version 25.0 software (IBM Corporation, New York, NY, USA) and GraphPad Prism version 9.0.1 software (GraphPad Software Inc., San Diego, CA, USA).
Results
Patient characteristics
The baseline characteristics are summarized in Table 1. The median age of patients who underwent auto-SCT was 52 (17-69) years in all patients, 53 (27-69) years in patients with multiple myeloma, 51 (21-64) years in patients with B-cell lymphoma, 51 (17-65) years in patients with T-cell lymphoma, and 29 (19-54) years in patients with Hodgkin lymphoma. Of the 200 patients, 150 (75.0%) were tested for CMV reactivation. CMV reactivation was observed in 57 (38.0%) patients based on the LOD and in 28 (18.6%) patients based on the limit of quantification (LOQ). The median duration of CMV reactivation was 14 days (3-346). Preemptive therapy was provided to 24 patients, all of whom received ganciclovir. CMV reactivation was observed in 57 (38.0%) patients before 100 days and 7 (4.7%) patients after 100 days. Sixty-five (32.5%) patients received two or more chemotherapy lines before transplantation, and all patients underwent single transplantation.
Survival analyses in relation to CMV reactivation
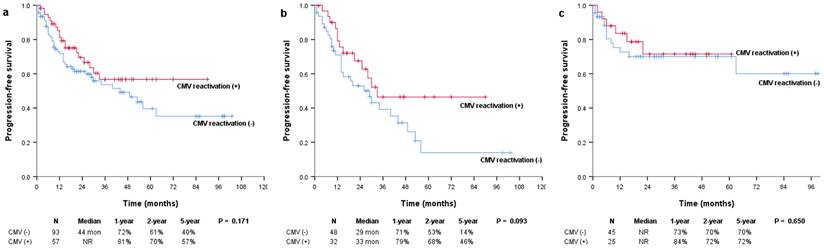
The median OS was not reached in either the positive or negative CMV reactivation group, and there were no significant differences between them (P = 0.129; Figure 1a). However, in the subgroup of patients with multiple myeloma, OS was significantly more prolonged in patients with CMV reactivation than in those without CMV reactivation (median, not reached vs. 61 months; P = 0.034) (Figure 1b). In patients with and without CMV reactivation, the 1-year OS rates were 97% and 87%, the 2-year OS rates were 92% and 74%, and the 5-year OS rates were 82% and 55%, respectively. There were no significant differences in the OS of patients with lymphoma (median, not reached vs. not reached; P = 0.924) (Figure 1c). The median PFS was not reached and was 44 months for patients with and without CMV reactivation, respectively (P = 0.171; Figure 2a). In patients with multiple myeloma, patients with CMV reactivation showed better PFS (median, 33 months vs. 29 months; P = 0.093) (Figure 2b) than patients without CMV reactivation; however, these results did not reach statistical significance. There were no significant differences in PFS between patients with lymphoma (median, not reached vs. not reached; P = 0.650) (Figure 2c).
Then, to confirm the association between CMV reactivation and survival prognosis of multiple myeloma, we conducted univariable and multivariable analyses including other relevant clinical factors. The characteristics of patients with multiple myeloma are summarized in Table 2, and the results are shown in Table 3. In the univariable analyses, CMV reactivation (hazard ratio [HR], 0.329; P = 0.045) was a significant favorable prognostic factor, whereas a serum lactate dehydrogenase (LDH) level higher than the upper limit of normal (HR, 2.894; P = 0.012) and high-risk cytogenetics (HR, 4.213; P = 0.002) were significant unfavorable prognostic factors. The high revised international staging system (R-ISS) stage was associated with poor survival, with borderline significance (HR, 2.459; P = 0.080). In the multivariable analysis using the backward stepwise elimination method, including variables of P < 0.2 in the univariable analyses, hemoglobin (Hb) level, platelet (PLT) count, type of M-protein, LDH level higher than the upper limit of normal, high-risk cytogenetics, R-ISS stage III, and CMV reactivation (HR, 0.213; 95% confidence interval [CI], 0.062-0.739; P = 0.015) were significant favorable prognostic factors, whereas a serum LDH level higher than the upper limit of normal (HR, 2.483; 95% CI, 1.046-5.893; P = 0.039) and non-IgG type of M-protein (HR, 2.614; 95% CI, 1.037-6.587; P = 0.042) were significant unfavorable prognostic factors. High-risk cytogenetics was associated with poor survival with borderline significance (HR, 2.404; 95% CI, 0.936-6.178; P = 0.068). Other factors did not show a significant association with OS in patients with multiple myeloma.
Baseline characteristics of the study cohort
| Total, n (%) (n = 200) | MM, n (%) (n = 107) | B-lymphoma, n (%) (n = 50) | T-lymphoma, n (%) (n = 35) | HL, n (%) (n = 8) | |
|---|---|---|---|---|---|
| Age, median years (range) | 52 (17-69) | 53 (27-69) | 51 (21-64) | 51 (17-65) | 29 (19-54) |
| Sex | |||||
| Male | 125 (62.5) | 66 (61.7) | 32 (64.0) | 21 (60.0) | 6 (75.0) |
| Female | 75 (37.5) | 41 (38.3) | 18 (36.0) | 14 (40.0) | 2 (25.0) |
| Patients tested for CMV reactivation | 150 (75.0) | 80 (74.8) | 39 (78.0) | 26 (74.3) | 5 (62.5) |
| CMV RT-PCR, copies | |||||
| < LOD | 93 (62.0) | 48 (60.0) | 28 (71.8) | 13 (50.0) | 4 (80.0) |
| LOD-LOQ | 29 (19.3) | 16 (20.0) | 6 (15.4) | 7 (26.9) | 0 |
| LOQ-10000 | 20 (13.3) | 12 (15.0) | 4 (10.3) | 4 (15.4) | 0 |
| ≥ 10000 | 8 (5.3) | 4 (5.0) | 1 (2.5) | 2 (7.7) | 1 (20.0) |
| CMV disease (pathology confirmed) | 1 (0.7) | 1 (1.3) | 0 | 0 | 0 |
| CMV treatment | |||||
| Ganciclovir | 24 (100) | 12 (100) | 6 (100) | 5 (100) | 1 (100) |
| Others | 0 | 0 | 0 | 0 | 0 |
| CMV duration, median days (range) | 14 (3-346) | 14 (3-57) | 11 (3-45) | 15 (7-346) | 37 (37) |
| Timing of CMV reactivation (≥ LOD) | |||||
| Early (before day 100) | 57 (38.0) | 32 (40.0) | 11 (28.2) | 13 (50.0) | 1 (20.0) |
| Late (after day 100) | 7 (4.7) | 1 (1.3) | 2 (5.1) | 3 (11.5) | 1 (20.0) |
| Both | 5 (3.3) | 0 | 2 (5.1) | 2 (7.7) | 1 (20.0) |
| Lines of therapy before transplantation | |||||
| 1 | 135 (67.5) | 81 (75.7) | 29 (58.0) | 25 (71.4) | 0 |
| ≥ 2 | 65 (32.5) | 26 (24.3) | 21 (42.0) | 10 (28.6) | 8 (100) |
| Number of transplantations | |||||
| 1 | 200 (100) | 107 (100) | 50 (100) | 35 (100) | 8 (100) |
| ≥ 2 | 0 | 0 | 0 | 0 | 0 |
Note: Data of one patient with amyloidosis are not shown.
CMV: cytomegalovirus, MM: multiple myeloma, HL: Hodgkin lymphoma, LOD: limit of detection, LOQ: limit of quantification.
Kaplan-Meier survival curves for PFS in (a) all patients and patients with (b) multiple myeloma and (c) lymphoma. PFS: progression-free survival.
Clinical characteristics according to early CMV reactivation in patients with multiple myeloma
| Parameter, n (%) / median (range) | Total (n = 80) | CMV reactivation + (≥ LOD), (n = 32) | CMV reactivation - (< LOD), (n = 48) | P |
|---|---|---|---|---|
| Age | 54 (27-69) | 57 (30-69) | 51 (27-65) | 0.317 |
| Sex | ||||
| Male | 48 (60.0) | 15 (46.9) | 33 (68.8) | 0.050 |
| Female | 32 (40.0) | 17 (53.1) | 15 (31.2) | |
| Hb level, g/dL | 9.7 (4.4-15.9) | 9.1 (4.4-13.7) | 10.0 (5.3-15.9) | 0.048 |
| ANC level, ×109/L | 2689 (646-13170) | 2637 (646-7830) | 2803 (1106-13170) | 0.149 |
| PLT count, ×109/L | 191.5 (53-487) | 178.5 (72-487) | 210.0 (53-358) | 0.961 |
| LDH level, IU/L | 351.5 (178-1820) | 377.5 (228-864) | 333.5 (178-1820) | 0.798 |
| < ULN | 55 (68.8) | 20 (62.5) | 35 (72.9) | 0.325 |
| ≥ ULN | 25 (31.2) | 12 (37.5) | 13 (27.1) | |
| β2-MG level, mg/L | 3.47 (0.56-24.8) | 3.60 (0.60-13.4) | 3.36 (0.56-24.8) | 0.321 |
| < 5.5 | 60 (75.0) | 25 (78.1) | 35 (72.9) | 0.598 |
| ≥ 5.5 | 20 (25.0) | 7 (21.9) | 13 (27.1) | |
| Albumin level, g/dL | 3.80 (1.90-4.90) | 3.35 (2.20-4.70) | 3.90 (1.90-4.90) | 0.016 |
| < 3.5 | 50 (62.5) | 14 (43.8) | 36 (75.0) | 0.005 |
| ≥ 3.5 | 30 (37.5) | 18 (56.2) | 12 (25.0) | |
| M-protein isotype | ||||
| IgG | 48 (60.0) | 17 (53.1) | 31 (64.6) | 0.305 |
| Non-IgG | 32 (40.0) | 15 (46.9) | 17 (35.4) | |
| BM plasma cell, % | ||||
| < 60 | 62 (77.5) | 24 (75.0) | 38 (79.2) | 0.662 |
| ≥ 60 | 18 (22.5) | 8 (25.0) | 10 (20.8) | |
| Cytogenetic risk* | ||||
| Others | 64 (80.0) | 26 (81.2) | 38 (79.2) | 0.819 |
| High | 16 (20.0) | 6 (18.8) | 10 (20.8) | |
| ISS risk group | ||||
| I or II | 60 (75.0) | 25 (78.1) | 35 (72.9) | 0.598 |
| III | 20 (25.0) | 7 (21.9) | 13 (27.1) | |
| R-ISS risk group | ||||
| I or II | 70 (87.5) | 29 (90.6) | 41 (85.4) | 0.732 |
| III | 10 (12.5) | 3 (9.4) | 7 (14.6) | |
| No. of previous CTx | ||||
| 1 | 59 (73.8) | 27 (84.4) | 32 (66.7) | 0.078 |
| ≥ 2 | 21 (26.2) | 5 (15.6) | 16 (33.3) | |
| Status at transplantation | ||||
| Non-CR | 36 (45.0) | 15 (46.9) | 21 (43.8) | 0.783 |
| CR | 44 (55.0) | 17 (53.1) | 27 (56.2) | |
| Conditioning regimen | ||||
| Bu/Cy | 10 (12.5) | 2 (6.2) | 8 (16.7) | 0.301 |
| Mel | 70 (87.5) | 30 (93.8) | 40 (83.3) | |
ANC: absolute neutrophil count, BM: bone marrow, BU: busulfan, CMV: cytomegalovirus, CR: complete response, CTx: chemotherapy, Cy: cyclophosphamide, Hb: hemoglobin, ISS: international staging system, LDH: lactate dehydrogenase, LOD: limit of detection, Mel: melphalan, MG: macroglobulin, PLT: platelet, R-ISS: revised international staging system, ULN: upper limit of normal.
* High-risk cytogenetics was defined as t(4;14), t(14;16), del(17/17p), TP53 deletion, or chromosome 1 abnormalities, including gain(1q) and del(1p).
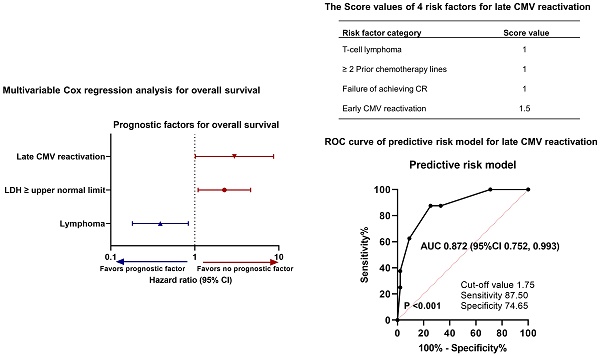
Furthermore, to analyze the effect of late CMV reactivation on OS, we also conducted Cox regression analyses including relevant prognostic factors (Table 4). In the univariable analyses, no factor was significantly associated with OS. In the multivariable analysis using the backward stepwise elimination method, including variables with P < 0.2 in the univariable analyses, Hb level, LDH level higher than the upper limit of normal, diagnosis of lymphoma, failure to achieve complete response (CR), two or more previous chemotherapy lines, and late CMV reactivation were significantly associated with OS. A poor prognosis was significantly associated with a serum LDH level higher than the upper limit of normal (HR, 2.251; 95% CI, 1.095-4.627; P = 0.027) and late CMV reactivation (HR, 2.964; 95% CI, 1.014-8.667; P = 0.047), whereas a good prognosis was significantly associated with the diagnosis of lymphoma (vs. diagnosis of multiple myeloma; HR, 0.389; 95% CI, 0.181-0.837; P = 0.016). In summary, in contrast to CMV reactivation, late CMV reactivation was a prognostic factor of poor survival in patients undergoing auto-SCT. Based on these results, we developed a new scale to predict late CMV reactivation.
Univariable and multivariable analyses of OS in multiple myeloma
| Prognostic factors | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | 0.991 | 0.948, 1.037 | 0.707 | |||
| Hb level, g/dL | 0.864 | 0.726, 1.028 | 0.100 | |||
| PLT count, ×109/L | 0.995 | 0.990, 1.001 | 0.112 | |||
| BM plasma cell, > 60% | 1.121 | 0.414, 3.030 | 0.822 | |||
| M-protein type, non-IgG | 1.931 | 0.822, 4.538 | 0.131 | 2.614 | 1.037, 6.587 | 0.042 |
| Albumin level, g/dL | 0.896 | 0.474, 1.692 | 0.735 | 0.550 | 0.249, 1.218 | 0.141 |
| ß2-microglobulin level, mg/L | 1.043 | 0.944, 1,153 | 0.404 | |||
| LDH, ≥ ULN | 2.894 | 1.264, 6.623 | 0.012 | 2.483 | 1.046, 5.893 | 0.039 |
| Cytogenetics, high risk* | 4.213 | 1.709, 10.388 | 0.002 | 2.404 | 0.936, 6.178 | 0.068 |
| R-ISS, stage III | 2.459 | 0.897, 6.737 | 0.080 | |||
| CMV reactivation, ≥ LOD | 0.329 | 0.111, 0.976 | 0.045 | 0.213 | 0.062, 0.739 | 0.015 |
| Failure to achieve CR | 1.176 | 0.519, 2.664 | 0.695 | |||
BM: bone marrow, CMV: cytomegalovirus, CR: complete response, Hb: hemoglobin, LDH: lactate dehydrogenase, LOD: limit of detection, OS: overall survival, PLT: platelet, R-ISS: revised international staging system, ULN: upper limit of normal.
* High-risk cytogenetics was defined as t(4;14), t(14;16), del(17/17p), TP53 deletion, or chromosome 1 abnormalities, including gain(1q) and del(1p).
Univariable and multivariable analyses for OS in relation to late CMV reactivation (overall patients)
| Prognostic factors | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | 1.021 | 0.987, 1.056 | 0.230 | |||
| Hb level, g/dL | 0.899 | 0.786, 1.027 | 0.117 | |||
| PLT count, ×109/L | 0.998 | 0.994, 1.002 | 0.293 | |||
| LDH level, ≥ ULN | 1.841 | 0.920, 3.684 | 0.085 | 2.251 | 1.095, 4.627 | 0.027 |
| Diagnosis of lymphoma | 0.532 | 0.253, 1.118 | 0.096 | 0.389 | 0.181, 0.837 | 0.016 |
| Failure to achieve CR | 1.633 | 0.822, 3.242 | 0.161 | |||
| Previous CTx lines, ≥ 2 | 1.365 | 0.677, 2.753 | 0.385 | |||
| Late CMV reactivation, ≥ LOD | 2.665 | 0.933, 7.615 | 0.067 | 2.964 | 1.014, 8.667 | 0.047 |
CMV: cytomegalovirus, CTx: chemotherapy, CR: complete response, Hb: hemoglobin, LDH: lactate dehydrogenase, LOD: limit of detection, OS: overall survival, PLT: platelet, ULN: upper limit of normal.
Prediction model for late CMV reactivation
To investigate the predictors of late CMV reactivation, we conducted a multivariable logistic regression analysis using the backward stepwise elimination method and included all clinically relevant variables. The results are shown in Table 5. Late CMV reactivation was independently predicted by a diagnosis of T-cell lymphoma (odds ratio [OR], 8.499; 95% CI, 1.242-58.151; P = 0.029), two or more previous chemotherapy lines (OR, 8.995; 95% CI, 1.280-63.216; P = 0.027), failure to achieve CR after SCT (OR, 7.124; 95% CI, 1.202-42.210; P = 0.031), and early CMV reactivation (OR, 12.853; 95% CI, 1.990-82.996; P = 0.007).
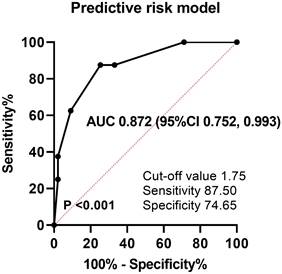
Then, to develop the predictive risk model for late CMV reactivation, a score from 1 to 1.5 was assigned for each of the four variables based on estimated coefficients in logistic analysis: diagnosis of T-cell lymphoma (1 point), two or more previous chemotherapy lines (1 point), failure to achieve CR (1 point), and early CMV reactivation (1.5 points). The total scores were calculated by adding these four values (0-4.5). The optimal cutoff value for the estimated late CMV reactivation was 1.75 points calculated using the ROC curve, with a sensitivity of 87.50% and specificity of 74.65% (Figure 3). Thus, 43 patients (28.7%) were assigned to the low-risk group (score < 1.75) and 107 patients (71.3%) to the high-risk group (score ≥ 1.75). The predictive risk model showed good discrimination ability with an AUC of 0.872 (standard error, 0.062; 95% CI, 0.752-0.993; P < 0.001).
ROC curve and AUC analysis for late CMV reactivation. AUC: area under the curve, ROC: receiver operating characteristic.
Multivariable logistic regression analysis of risk factors for late CMV reactivation
| Prognostic factors | Coefficient | OR | 95% CI | P | Points | |
|---|---|---|---|---|---|---|
| Estimate | SE | |||||
| T-cell lymphoma | 2.140 | 0.981 | 8.499 | 1.242, 58.151 | 0.029 | 1 |
| Previous CTx lines, ≥ 2 | 2.197 | 0.995 | 8.995 | 1.280, 63.216 | 0.027 | 1 |
| Failure to achieve CR | 1.963 | 0.908 | 7.124 | 1.202, 42.210 | 0.031 | 1 |
| Early CMV reactivation | 2.554 | 0.952 | 12.853 | 1.990, 82.996 | 0.007 | 1.5 |
CMV: cytomegalovirus, CR: complete response, CTx: chemotherapy, SE: standard error.
Discussion
In our retrospective cohort analysis, late CMV reactivation was associated with poor survival in auto-SCT recipients, whereas early CMV reactivation was associated with better survival in patients with multiple myeloma. Based on these results, we constructed a statistical model using relevant clinical parameters to predict late CMV reactivation in patients who underwent auto-SCT. Our model accurately predicted late CMV reactivation and identified patients who required monitoring for late CMV reactivation and who potentially benefit from prophylactic or preemptive therapy.
The prognostic value of CMV reactivation after SCT has been evaluated in several previous studies; however, these studies have shown contradictory results. A single-center study showed that CMV reactivation 100 days before allo-SCT was significantly associated with a 32% decrease in the risk of relapse in patients with AML [2]. In another study of patients with AML, CMV reactivation was an independent protective factor for the risk of relapse (HR, 0.77; P = 0.04) [16]. Furthermore, CMV reactivation has been associated with a lower risk of relapse in patients with CML and lymphoma [4, 5]. In contrast, CMV reactivation was an independent risk factor for non-relapse mortality after allo-SCT in another study, which reported a 31% overall increase in the risk of death without relapse [2]. A study using data from a large multicenter research database concluded that CMV is significantly associated with higher non-relapse mortality rate and lower OS in patients with AML, CML, and MDS [6]. In a study of patients with aplastic anemia who underwent allo-SCT, CMV reactivation was a significant independent prognostic factor for shorter OS in a multivariable analysis (HR, 1.65; P = 0.42) [17]. Thus, although it remains controversial, CMV reactivation seems to be an adverse prognostic factor in the allo-SCT setting.
CMV reactivation is a rare complication (2.9%) in patients who undergo auto-SCT [10], and previous reports investigating CMV reactivation or infection in relation to auto-SCT are limited. The major risk factors for CMV reactivation are well-known CMV serostatus, acute or chronic graft-versus-host disease (GVHD), donor type (unrelated or mismatched donor), and immunosuppressive treatment [18, 19]. However, these factors are not applicable in the auto-SCT setting. In a retrospective study that included 324 patients who underwent auto-SCT, the risk of CMV reactivation was positively associated with the diagnosis of non-Hodgkin lymphoma (OR, 4.9; P = 0.01) or multiple myeloma (OR, 4.6; P = 0.03), progressive disease at transplantation (OR, 4.9; P = 0.03), and age (OR, 1.04; P = 0.01) [11]. Tandem transplantation was also a significant risk factor for CMV reactivation (OR, 5.112; P = 0.02) in patients with multiple myeloma [20]. A retrospective study reported that early CMV reactivation, younger patient age, and acute GVHD were significant risk factors for late CMV reactivation after allo-SCT [21]. However, the risk factors associated with late CMV reactivation in patients who underwent auto-SCT have not been extensively studied.
The present study analyzed the risk factors for late CMV reactivation after auto-SCT because late CMV reactivation was an independent predictor of poor survival after auto-SCT in our setting. According to a previous report, OS was significantly lower in patients with lymphoma with CMV reactivation than in those without CMV reactivation; however, no difference in OS was observed in patients with multiple myeloma [11]. These findings were partially comparable to our results that CMV reactivation was associated with favorable OS in patients with multiple myeloma but not in patients with lymphoma. Nevertheless, the prognostic implications of CMV reactivation in relation to auto-SCT remain unclear. Among 7 patients with late CMV reactivation, 2 (28.6%) achieve CR at SCT and 5 (71.4%) received two or more line of therapy before SCT. Among 57 patients with early CMV reactivation, 35 (61.4%) achieve CR at SCT and 13 (22.8%) received two or more line of therapy before SCT. Based on our data, patients with late CMV reactivation have more advanced and refractory disease than those with early CMV reactivation and this could affect different survival outcomes between early and late CMV reactivation. We also found that a diagnosis of T-cell lymphoma, two or more chemotherapy lines before transplantation, failure to achieve CR, and early CMV reactivation were significant risk factors for late CMV reactivation. Early CMV reactivation was significantly associated with late CMV reactivation based on our data on auto-SCT, similar to that in a previous study [21]; however, patient age was not associated with late CMV reactivation in the current study. We could not consider all relevant factors for late CMV reactivation in this analysis, and further evaluation is required to confirm our results.
The present study has several limitations. First, it was conducted based on a retrospective analysis of a relatively small cohort. Thus, confirmatory conclusions could not be drawn from the results of this study. Second, we used data obtained from multicenter and longitudinal cohorts; thus, the testing methods, including sensitivity (LOD) and detection range (LOQ) for CMV DNA viral load by qPCR, were not consistent. Third, CMV reactivation and prognostic values could be affected by specific chemotherapeutic agents, including immunologic or cancer-specific treatment. Future studies should consider the effects of novel agents before and after transplantation. Fourth, we could not perform immunological or functional analyses. The absence of CMV-specific immunity by 3 months after SCT was significantly associated with late CMV reactivation and increased mortality [22]. These experimental analyses would help clarify the discrepancy between early and late CMV reactivation in relation to prognosis after auto-SCT. Finally, although our risk prediction model showed good performance based on the AUC value (0.872), we could not conduct a validation analysis due to the low incidence of late CMV reactivation (4.7%) and absence of available external datasets. Additional analyses are needed to validate our model and determine whether it can be used to effectively predict late CMV reactivation.
In conclusion, late CMV reactivation is a rare complication in patients undergoing auto-PBSCT; however, it is also associated with poor survival outcomes. In addition, we found that early CMV reactivation was an independent risk factor for favorable survival in patients with multiple myeloma receiving auto-SCT. Prediction of late CMV reactivation using our model could be helpful in identifying high-risk patients who require monitoring for late CMV reactivation and might provide personalized CMV treatment strategies to clinicians.
Acknowledgements
We are indebted to all patients and contributing physicians. We also thank Jin Wha Lee for her role in the data collection and management.
Funding
This work was supported by a Korea University Grant (No. K2225741).
Author Contributions
B-H.L. and Y.P. proposed the study concept and design. B-H.L., M.J.J., E.S.Y., K-W.K., D.S.K., S.R.L., C.W.C., and B.S.K. contributed to the patient data collection. B.H.L. analyzed the data. Y.P. and H.J.S. helped with data interpretation. B.H.L. wrote the draft of the manuscript. Y.P. and H.J.S. revised the manuscript. All authors reviewed and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ljungman P. CMV infections after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;42(Suppl 1):S70-S2
2. Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM. et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316-24
3. Elmaagacli AH. CMV and relapse: what has conditioning to do with it? Biol Blood Marrow Transplant. 2014;20:1-2
4. Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA. et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48:1313-6
5. Koldehoff M, Ross SR, Duhrsen U, Beelen DW, Elmaagacli AH. Early CMV-replication after allogeneic stem cell transplantation is associated with a reduced relapse risk in lymphoma. Leuk Lymphoma. 2017;58:822-33
6. Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M. et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127:2427-38
7. Thomson KJ, Mackinnon S, Peggs KS. CMV-specific cellular therapy for acute myeloid leukemia? Blood. 2012;119:1088-90 author reply 90-1
8. Kim DH, Won DI, Lee NY, Sohn SK, Baek JH, Kim JG. et al. Survival benefit of asymptomatic cytomegalovirus reactivation after HLA-identical allogeneic peripheral blood stem cell transplantation. Transplantation. 2006;81:101-8
9. Fassas AB, Bolaños-Meade J, Buddharaju LN, Rapoport A, Cottler-Fox M, Chen T. et al. Cytomegalovirus infection and non-neutropenic fever after autologous stem cell transplantation: high rates of reactivation in patients with multiple myeloma and lymphoma. Br J Haematol. 2001;112:237-41
10. Jain T, John J, Kotecha A, Deol A, Saliminia T, Revankar S. et al. Cytomegalovirus infection in autologous stem cell transplant recipients in the era of rituximab. Ann Hematol. 2016;95:1323-7
11. Massoud R, Assi R, Fares E, Haffar B, Charafeddine M, Kreidieh N. et al. Cytomegalovirus reactivation in lymphoma and myeloma patients undergoing autologous peripheral blood stem cell transplantation. J Clin Virol. 2017;95:36-41
12. Konoplev S, Champlin RE, Giralt S, Ueno NT, Khouri I, Raad I. et al. Cytomegalovirus pneumonia in adult autologous blood and marrow transplant recipients. Bone Marrow Transplantation. 2001;27:877-81
13. Reimer P, Kunzmann V, Wilhelm M, Weissbrich B, Kraemer D, Berghammer H. et al. Cellular and humoral immune reconstitution after autologous peripheral blood stem cell transplantation (PBSCT). Ann Hematol. 2003;82:263-70
14. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328-e46
15. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-68
16. Takenaka K, Nishida T, Asano-Mori Y, Oshima K, Ohashi K, Mori T. et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant. 2015;21:2008-16
17. Takenaka K, Onishi Y, Mori T, Hirakawa T, Tada Y, Uchida N. et al. Negative Impact of Cytomegalovirus Reactivation on Survival in Adult Patients with Aplastic Anemia after an Allogeneic Hematopoietic Stem Cell Transplantation: A Report from Transplantation-Related Complication and Adult Aplastic Anemia Working Groups of the Japan Society for Hematopoietic Cell Transplantation. Transplant Cell Ther. 2021;27:82 e1- e8
18. Dziedzic M, Sadowska-Krawczenko I, Styczynski J. Risk Factors for Cytomegalovirus Infection After Allogeneic Hematopoietic Cell Transplantation in Malignancies: Proposal for Classification. Anticancer Res. 2017;37:6551-6
19. Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J. et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122:3359-64
20. Kim JH, Goulston C, Sanders S, Lampas M, Zangari M, Tricot G. et al. Cytomegalovirus reactivation following autologous peripheral blood stem cell transplantation for multiple myeloma in the era of novel chemotherapeutics and tandem transplantation. Biol Blood Marrow Transplant. 2012;18:1753-8
21. Ozdemir E, Saliba RM, Champlin RE, Couriel DR, Giralt SA, de Lima M. et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40:125-36
22. Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P. et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060-7
Author contact
![]() Corresponding authors: Yong Park, Department of Internal Medicine, Korea University College of Medicine, 73, Goryeodae-ro, Seongbuk-gu, Seoul 02841, Korea. E-mail: paark76net; Hwa Jung Sung, Department of Internal Medicine, Korea University College of Medicine, 73, Goryeodae-ro, Seongbuk-gu, Seoul 02841, Korea. E-mail: doctorsungac.kr.
Corresponding authors: Yong Park, Department of Internal Medicine, Korea University College of Medicine, 73, Goryeodae-ro, Seongbuk-gu, Seoul 02841, Korea. E-mail: paark76net; Hwa Jung Sung, Department of Internal Medicine, Korea University College of Medicine, 73, Goryeodae-ro, Seongbuk-gu, Seoul 02841, Korea. E-mail: doctorsungac.kr.

 Global reach, higher impact
Global reach, higher impact