Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(14):2087-2092. doi:10.7150/ijms.79162 This issue Cite
Review
Can CD147 work as a therapeutic target for tumors through COVID-19 infection?
1. Department of Radiology, the First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, P.R. China.
2. Department of Basic Nursing, College of Nursing, Jinzhou Medical University, Jinzhou, Liaoning, P.R. China.
3. Department of Pharmacy, Tianjin Union Medical Center, Tianjin, P.R. China.
4. Biological Anthropology Institute, College of Basic Medicine, Jinzhou Medical University, Jinzhou, Liaoning, P.R. China.
Received 2022-9-22; Accepted 2022-11-10; Published 2022-11-21
Abstract
In this review, we discussed an interesting case infected with “COVID-19” (Corona Virus Disease 2019). The patients with Hodgkin's lymphoma recovered after infection with COVID-19. It may be that COVID-19 activates the patient's immune system, or it may be a coincidence. COVID-19 spike protein can interact with CD147 and use it as an entry to invade host cells. CD147 is a partner of SLC3A2, which is the chaperone subunit of cystine/glutamate reverse transporter (system XC). The catalytic subunit of system XC is SLC7A11. SLC7A11 mediated cysteine uptake plays a key role in ferroptosis. Through literature review and data analysis, we suggest that CD147, as a new potential COVID-19 infection entry, may also lead to ferroptosis of host cells. Our hypothesis is that spike protein of COVID-19 induced ferroptosis in host cells via CD147/SLC3A2/SLC7A11 complex. This is another explanation for the cancer patient recovered after COVID-19 infection.
Keywords: ferroptosis, CD147, cancer, COVID-19, SLC7A11
Introduction
Recently, a novel coronavirus pneumonia case reported in the British Journal of Hematology was that a 61 year old man suffered from lymphatic cancer and was diagnosed with novel coronavirus pneumonia [1]. After four months, the virus disappeared and the man recovered from the cancer [1]. The authors gave two possible reasons, one is the reaction between pathogen specific T cells and tumor antigens, the other is the activation of natural killer cells by inflammatory cytokines [1]. In short, “COVID-19” (Corona Virus Disease 2019) activated the anti-tumor immune response, not only killed the new coronavirus, but also killed cancer cells. In fact, the specific mechanism is still unknown. Whether “COVID-19 kills tumor cells” is a coincidence is still under discussion. In this review, we will discuss a widely expressed marker of cancers, CD147, which is also a key protein of COVID-19 infection [2]. CD147 combines with SCL3A2 (CD98hc) to form CD147-CD98hc complex to regulate metabolism and proliferation in physiological and pathological conditions [3]. SLC3A2 is a single transmembrane protein, which has an intracellular N-terminal and highly glycosylated extracellular domain as the C-terminal [4]. SLC7A11 is a 12 times transmembrane protein, and its N-terminal and C-terminal are located in the cytoplasm [5]. These two subunits are linked by covalent disulfide bonds to form cystine/glutamate reverse transporter (system XC) [6]. SLC7A11 is responsible for the main transport activity and is highly specific for cystine and glutamate [6]. SLC3A2 mainly acts as chaperone to maintain the stability of SLC7A11 protein and regulate the transport of SLC7A11 to the plasma membrane [6]. System XC is a sodium independent reverse transporter of cystine and glutamate [6]. We not only summarized the effect of CD147 on COVID-19, but also hope to explain the mechanism of tumor inhibition of COVID-19 through CD147 and its partners.
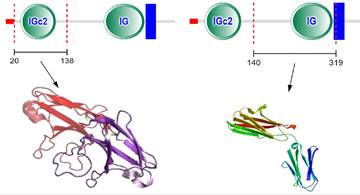
Schematic presentation of CD147 protein structure.
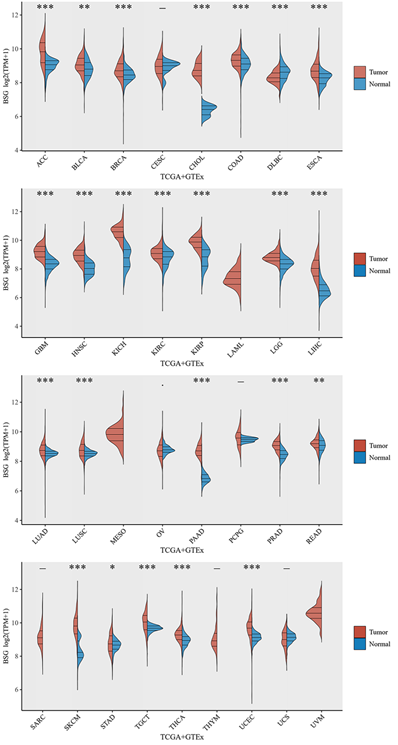
CD147 mRNA levels in different cancer types by TCGA and GTEx database. Abbreviations: ACC, Adrenocortical carcinoma; BLCA, Bladder Urothelial Carcinoma; BRCA, Breast invasive carcinoma; CESC, Cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, Cholangiocarcinoma; COAD, Colon adenocarcinoma; DLBC, Lymphoid Neoplasm Diffuse Large B-cell Lymphoma; ESCA, Esophageal carcinoma; GBM, Glioblastoma multiforme; HNSC, Head and Neck squamous cell carcinoma; KICH, Kidney Chromophobe; KIRC, Kidney renal clear cell carcinoma; KIRP, Kidney renal papillary cell carcinoma; LAML, Acute Myeloid Leukemia; LGG, Brain Lower Grade Glioma; LIHC, Liver hepatocellular carcinoma; LUAD, Lung adenocarcinoma; LUSC, Lung squamous cell carcinoma; MESO, Mesothelioma; OV, Ovarian serous cystadenocarcinoma; PAAD, Pancreatic adenocarcinoma; PCPG, Pheochromocytoma and Paraganglioma; PRAD, Prostate adenocarcinoma; READ, Rectum adenocarcinoma; SARC, Sarcoma; SKCM, Skin Cutaneous Melanoma; STAD, Stomach adenocarcinoma; TGCT, Testicular Germ Cell Tumors; THCA, Thyroid carcinoma; THYM, Thymoma; UCEC, Uterine Corpus Endometrial Carcinoma; UCS, Uterine Carcinosarcoma; UVM, Uveal Melanoma.
CD147 gene and protein
CD147 was first discovered in lung cancer cells by Biswas in 1982 [7]. It can induce fibroblasts to express collagenase, so it was named as tumor cell derived collagenase stimulating factor (TCSF) [7]. It also can induce the production of multiple matrix metalloproteinases (MMPs), which was renamed EMMPRIN [8-11]. CD147 has been given different names in different genera and tissues, such as EMMPRIN, TCSF, and basigin [9-11]. CD147 gene is located on chromosome 19p13.3, containing 1797 bp [12]. CD147 contains binding sites of specific protein 1 (SP1), activator protein 1 (AP1), transcription factor II (TFII) and early growth response factor 2 at its 5' end [12]. There are two binding sites of hypoxia inducible factor (HIF) in the 3' flanking sequence [13]. CD147 protein has 269 amino acid residues, which the relative molecular weight is 30-40kDa [14]. The protein structure of CD147 includes N-terminal signal sequence (21 amino acid residues), extracellular immunoglobulin like domain (185 amino acid residues), single transmembrane domain (24 amino acid residues) and C-terminal intracellular domain (39 amino acid residues) [14] (Figure 1). Each domain of CD147 interacts with different proteins and plays different functions [14]. The transmembrane region contains conserved hydrophobic amino acids, which can be used as the signal peptide of CD147 and the anchor site of cell membrane [14]. The intracellular domain of CD147 is highly conserved and plays a key role in the interaction with the arginine residues of monocarboxylate transporter (MCT)-1 and MCT-4 [15]. Moreover, the transmembrane region contains a typical leucine zipper domain, which is involved in membrane protein interaction and various intracellular signaling pathways [14, 15].
The role and the mechanism(s) of CD147 in tumor growth, metastasis and angiogenesis
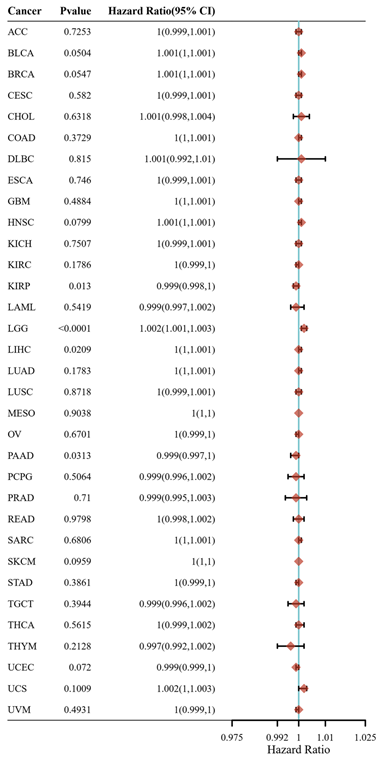
CD147 is upregulated in most tumor types (Figure 2) and a hazard factor for Bladder Urothelial Carcinoma (BLCA), Brain Lower Grade Glioma (LGG), and Liver hepatocellular carcinoma (LIHC) (Figure 3). The main function of CD147 is to induce adjacent fibroblasts and endothelial cells to produce MMPs [10]. CD147 rich vesicles released by tumor cells can promote the synthesis of MMPs through tumor matrix interaction [11]. The secreted MMPs can degrade extracellular matrix (ECM) and promote tumor growth, invasion and metastasis [16]. MMPs are secreted by CD147 stimulation, and then CD147 is removed from the cell membrane, thus forming a positive feedback loop [17]. The interaction between CD147 and integrin can affect the cytoskeleton rearrangement by activating focal adhesion kinase (FAK) signaling pathway [18]. The interaction between CD147 and Annexin II can inhibit the movement of hepatocellular cancer (HCC) cells, and promote the activation of SRC-dependent Rac1 signal through STAT3 [19]. CD147 mediates MMP dependent and independent angiogenesis [20]. CD147 is up-regulated in activated human umbilical vein endothelial cells and regulates angiogenesis through secretion of MMPs [20]. CD147 can induce the production of vascular endothelial growth factor (VEGF) by activating PI3K/Akt and MAPK signaling pathway [21]. CD147 also can directly bind to vascular endothelial growth factor receptor 2 (VEGFR-2) and regulate VEGF mediated VEGFR-2 activation [22].
CD147 is a potential entrance for COVID-19
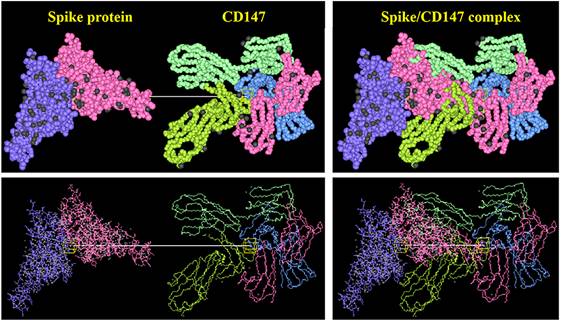
Novel coronavirus pneumonia is a new acute respiratory infectious disease [23]. Its new pathogen coronavirus (2019-nCoV or SARS-CoV-2) has the characteristics of fast propagation, wide coverage and strong infection [23]. According to the data of Johns Hopkins University in the United States, as of September, 2022, more than 600 million people have been diagnosed globally [24]. COVID-19 belongs to the beta coronavirus genus, and is an enveloped virus containing large RNA genome encapsulated by nucleocapsid protein (N) [25]. Three transmembrane proteins were integrated into the viral lipid envelope: spike protein (S) and two smaller proteins, membrane protein (M) and envelope protein (E) [26]. The spike protein of COVID-19 binds to ACE2 receptor on the surface of target cells and mediates subsequent viral uptake and fusion [27]. In the process of infection, coronavirus extensively reshapes the internal membrane structure of cells and produces virus replication organelles in which virus replication takes place [27]. As the first entry of COVID-19 infection, ACE2 is widely distributed in various tissues, such as heart, lung, kidney and testis, and plays an important role in controlling blood pressure, lung injury, preventing heart failure and kidney injury [28]. Therefore, ACE2 as a therapeutic target may cause serious side effects. Some studies have shown that because ACE2 is widely distributed in testis, COVID-19 can affect male reproductive function [28]. CD147 is a new potential way for COVID-19 to invade host cells besides ACE2 [29]. The combination of S protein and CD147 enhanced the invasion ability of virus to host cells (Figure 4). Plasmodium yoelii erythrocyte-binding-like protein (PyEBL) specifically interacts with CD147, which as the putative PyEBL receptor [30]. A recent interesting study shows that in malaria endemic countries, COVID-19 and malaria parasites used CD147 as a common receptor to enter cells, resulting in a low incidence rate of COVID-19 [31]. All these evidences suggested CD147 is a potential entrance for COVID-19. However, it is still in debate and needs further experiments to prove.
Hazard ratio of CD147 in cancers that calculated by R programming. Please refer to Figure 2 for the full name of the tumor.
Interaction between CD147 of host cells and spike protein of COVID-19 by HEX 8.0 software.
CD147 and ferroptosis
CD147 can inhibit autophagy by inhibiting PI3K signal pathway and down-regulating autophagy-related gene 6 (ATG6, Beclin 1) expression in ovarian cancer cells [32]. Intracellular domain of CD147 (CD147-ICD) enhanced autophagy, increased mitochondria-light chain 3 (LC3) protein level, and accumulated of autophagic vesicles in hepatocellular carcinoma cells though NF κB-TRAIL-caspase8-ATG3 pathway [33]. In prostate cancer cells, CD147 significantly inhibits starvation-induced autophagy via PI3K/Akt/mTOR pathway [34]. Ferroptosis is a new type of programmed cell death which is iron dependent and different from apoptosis, necrosis and autophagy [35]. The main mechanism of ferroptosis is that under the action of divalent iron or lipoxygenase, it catalyzes the lipid peroxidation of unsaturated fatty acids which are highly expressed on cell membrane to induce cell death; in addition, it also shows the decrease of glutathione peroxidase (GPX4) [36]. The failure of GPX4 results in the accumulation of reactive oxygen species (ROS) on membrane lipids, which requires the participation of iron ions [36].
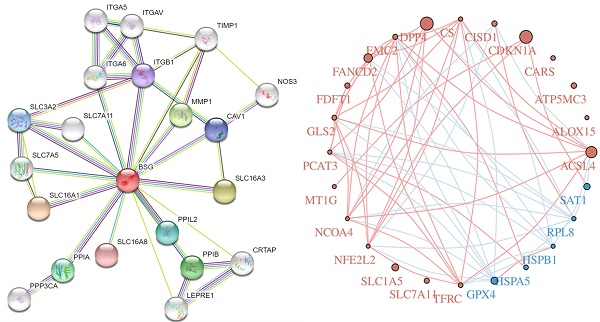
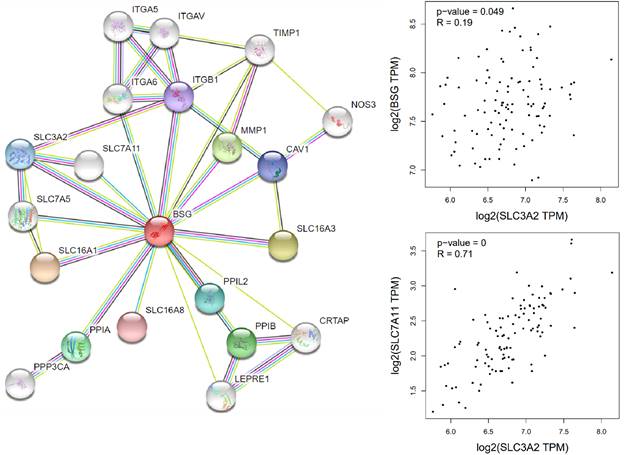
Cells mainly rely on system XC to obtain cystine from the extracellular environment, and then convert it into cysteine in the cytoplasm through the reduction reaction of consuming NADPH [37]. System XC can absorb cystine and excrete glutamate, which not only provides raw materials for intracellular glutathione synthesis, but also participates in the regulation of extracellular glutamate concentration [38]. Inhibition of SLC7A11 using pharmacological inhibitors will induce ferroptosis, such as erastin or sulfasalazine [39-41]. SLC7A11 gene silencing by siRNA interference also improves sensitive to erastin induced ferroptosis of cells [42]. SLC7A11 mediated cysteine uptake plays a key role in inhibiting oxidative response and maintaining cell survival under oxidative stress [42]. Based on the bioinformatic analysis, SLC7A11 is also a regulator for ferroptosis in EBV positive hodgkin lymphoma (HL) (Figure 5). To our knowledge, no previous study showed the interconnection of CD147 and SLC7A11. However, clear evidence demonstrated that the interaction of CD147 and SLC3A2, which is a subunit of system XC [43]. In this review, we analyzed the correlation of CD147, SLC7A11 and SLC3A2 in EBV-transformed lymphocytes by the Genotype-Tissue Expression (GTEx) database (Figure 6).
Ferroptosis related genes in Hodgkin's lymphoma by R programming.

Possible mechanism(s) of COIVD-19 inhibiting tumor cells
The current hypothesis is that after COIVD-19 enters the body, the patient's immune system is activated, killing the virus, but also killing the tumor cells [1]. Oncolytic virus therapy is close to this process, and currently the herpesvirus therapy has been approved for metastatic melanoma [44]. In 1891, Dr. William Coley, the father of tumor immunotherapy, injected Streptococcus into two patients with osteosarcoma [45]. Although the tumor also shrank, the patient died of infection [45]. It is very dangerous to activate the immune system by virus. Based on our bioinformatic analysis and literature review, our hypothesis is that COIVD-19 enters into host cells through CD147, leading to partial dysfunction of CD147. CD147 can affect the transport function of SLC3A2 through SLC3A2/CD147 complex, resulting in the dysfunction of SLC7A11. However, the truth needs to be confirmed in experiments.
Correlation of CD147, SLC3A2 and SLC7A11 in Hodgkin's lymphoma by GEPIA (http://gepia.cancer-pku.cn/index.html).
Conclusion
In this review, we used ferroptosis to explain the mechanism of COIVD-19 in the treatment of tumor. As an entry of COIVD-19, CD147 can also regulate SLC7A11 through SLC3A2/CD147 complex, which is an important factor of ferroptosis. Although the corresponding mechanism can be reasonably explained in theory, it needs to be proved by experimental evidence.
Acknowledgements
Funding
This study was supported by National Natural Scientific Foundation of China (No.81972784), and “Double First-Class” Disciplinary Construction Project of Liaoning Medical University.
Author contributions
P.X. designed the study and drafted the manuscript. H.L.R., G.M.W., and Z.Y.Z. designed figures. P.X., and D.H.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192(3):415
2. Xia P, Dubrovska A. Tumor markers as an entry for SARS-CoV-2 infection? FEBS J. 2020;287(17):3677-3680
3. Xu D, Hemler ME. Metabolic activation-related CD147-CD98 complex. Mol Cell Proteomics. 2005;4(8):1061-1071
4. Lee Y, Wiriyasermkul P, Jin C, Quan L, Ohgaki R, Okuda S. et al. Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat Struct Mol Biol. 2019;26(6):510-517
5. Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599-620
6. Liu L, Liu R, Liu Y, Li G, Chen Q, Liu X, Ma S. Cystine-glutamate antiporter xCT as a therapeutic target for cancer. Cell Biochem Funct. 2021;39(2):174-179
7. Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982;109:1026-1034
8. Saskia US, Alma Z, Peter S. Extracellular matrix metalloproteinase inducer EMMPRIN (CD147) in cardiovascular disease. Int J Mol Sci. 2018;19:507
9. Lai TM, Kuo PJ, Lin CY, Chin YT, Lin HL, Chiu HC, Fu MMJ, Fu E. CD147 self-regulates matrix metalloproteinase-2 release in gingival fibroblasts after coculturing with U937 monocytic cells. J Periodontol. 2020;91:651-660
10. Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193-3199
11. Liang L, Major T, Bocan T. Characterization of the Promoter of Human Extracellular Matrix Metalloproteinase Inducer (EMMPRIN). Gene. 2002;282:75-86
12. Huang C, Sun Z, Sun Y, Chen X, Zhu X, Fan C, Liu B, Zhao Y, Zhang W. Association of increased ligand cyclophilin A and receptor CD147 with hypoxia, angiogenesis, metastasis and prognosis of tongue squamous cell carcinoma. Histopathology. 2012;60:793-803
13. Yu XL, Hu T, Du JM, Ding JP, Yang XM, Zhang J. et al. Crystal structure of HAb18G/CD147: implications for immunoglobulin superfamily homophilic adhesion. J Biol Chem. 2008;283:18056-18065
14. Bai Y, Huang W, Ma LT, Jiang JL, Chen ZN. Importance of N-glycosylation on CD147 for its biological functions. Int J Mol Sci. 2014;15:6356-6377
15. Kim Y, Choi JW, Lee JH, Kim YS. Expression of lactate/H⁺ symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and The Cancer Genome Atlas data analyses. Hum Pathol. 2015;46:104-112
16. Yang J, Wang R, Li H, Lv Q, Meng W, Yang X. Lentivirus mediated RNA interference of EMMPRIN (CD147) gene inhibits the proliferation, matrigel invasion and tumor formation of breast cancer cells. Cancer Biomark. 2016;17:237-247
17. Redzic JS, Kendrick AA, Bahmed K, Dahl KD, Pearson CG, Robinson WA, Robinson SE, Graner MW, Eisenmesser EZ. Extracellular vesicles secreted from cancer cell lines stimulate secretion of MMP-9, IL-6, TGF-β1 and EMMPRIN. PLoS One. 2013;8:e71225
18. Qian AR, Zhang W, Cao JP, Yang PF, Gao X, Wang Z, Xu HY, Weng YY, Shang P. Downregulation of CD147 expression alters cytoskeleton architecture and inhibits gelatinase production and SAPK pathway in human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2008;27:50
19. Wang SJ, Cui HY, Liu YM, Zhao P, Zhang Y, Fu ZG, Chen ZN, Jiang JL. CD147 promotes Src-dependent activation of Rac1 signaling through STAT3/DOCK8 during the motility of hepatocellular carcinoma cells. Oncotarget. 2015;6:243-257
20. Yin J, Xu WQ, Ye MX, Zhang Y, Wang HY, Zhang J, Li Y, Wang YS. Up-regulated basigin-2 in microglia induced by hypoxia promotes retinal angiogenesis. J Cell Mol Med. 2017;21:3467-3480
21. Li F, Zhang J, Guo J, Jia Y, Han Y, Wang Z. RNA interference targeting CD147 inhibits metastasis and invasion of human breast cancer MCF-7 cells by downregulating MMP-9/VEGF expression. Acta Biochim Biophys Sin (Shanghai). 2018;50:676-684
22. Bougatef F, Menashi S, Khayati F, Naïmi B, Porcher R, Podgorniak MP, Millot G, Janin A, Calvo F, Lebbé C, Mourah S. EMMPRIN promotes melanoma cells malignant properties through a HIF-2alpha mediated up-regulation of VEGF-receptor-2. PLoS One. 2010;5:e12265
23. Jurek T, Teresiński G. Classification criteria for the deceased referred for forensic post-mortem examinations with regard to epidemiological risk posed by SARS CoV-2/COVID-19 during the pandemic. Arch Med Sadowej Kryminol. 2019;69:158-163
24. The Johns Hopkins Coronavirus Resource Center repository, [https://coronavirus.jhu.edu/]
25. Pal M, Berhanu G, Desalegn C, Kandi V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus. 2020;12:e7423
26. Boson B, Legros V, Zhou B, Siret E, Mathieu C, Cosset FL, Lavillette D, Denolly S. The SARS-CoV-2 Envelope and Membrane proteins modulate maturation and retention of the Spike protein, allowing assembly of virus-like particles. J Biol Chem. 2021;296:100111
27. Rodriguez JH, Gupta A. Contact residue contributions to interaction energies between SARS-CoV-1 spike proteins and human ACE2 receptors. Sci Rep. 2021;11:1156
28. Chaudhry F, Lavandero S, Xie X, Sabharwal B, Zheng YY, Correa A, Narula J, Levy P. Manipulation of ACE2 expression in COVID-19. Open Heart. 2020;7:e001424
29. Faghihi H. CD147 as an alternative binding site for the spike protein on the surface of SARS-CoV-2. Eur Rev Med Pharmacol Sci. 2020;24:11992-11994
30. Yuguchi T, Kanoi BN, Nagaoka H, Miura T, Ito D, Takeda H, Tsuboi T, Takashima E, Otsuki H. Plasmodium yoelii Erythrocyte Binding Like Protein Interacts with Basigin, an Erythrocyte Surface Protein. Front Cell Infect Microbiol. 2021;11:656620
31. Ulusan Bağcı Ö. Impact of the COVID-19 Duration on Neglected Parasitic Diseases. Turkiye Parazitol Derg. 2021;45(4):317-325
32. Hu Z, Cai M, Deng L, Zhu L, Gao J, Tan M, Liu J, Lin B. The fucosylated CD147 enhances the autophagy in epithelial ovarian cancer cells. Oncotarget. 2016;7(50):82921-82932
33. Wu B, Cui J, Yang XM, Liu ZY, Song F, Li L, Jiang JL, Chen ZN. Cytoplasmic fragment of CD147 generated by regulated intramembrane proteolysis contributes to HCC by promoting autophagy. Cell Death Dis. 2017;8(7):e2925
34. Fang F, Wang L, Zhang S, Fang Q, Hao F, Sun Y, Zhao L, Chen S, Liao H, Wang L. CD147 modulates autophagy through the PI3K/Akt/mTOR pathway in human prostate cancer PC-3 cells. Oncol Lett. 2015;9(3):1439-1443
35. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273-285
36. Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331
37. Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62
38. Bridges RJ, Natale NR, Patel SA. System xc- cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165:20-34
39. Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523
40. Xu X, Zhang X, Wei C, Zheng D, Lu X, Yang Y, Luo A, Zhang K, Duan X, Wang Y. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur J Pharm Sci. 2020;152:105450
41. Zheng Z, Hong X, Huang X, Jiang X, Jiang H, Huang Y, Wu W, Xue Y, Lin D. Comprehensive analysis of ferroptosis-related gene signatures as a potential therapeutic target for acute myeloid leukemia: A bioinformatics analysis and experimental verification. Front Oncol. 2022;12:930654
42. Yuan B, Liao F, Shi ZZ, Ren Y, Deng XL, Yang TT, Li DY, Li RF, Pu DD, Wang YJ, Tan Y, Yang Z, Zhang YH. Dihydroartemisinin Inhibits the Proliferation, Colony Formation and Induces Ferroptosis of Lung Cancer Cells by Inhibiting PRIM2/SLC7A11 Axis. Onco Targets Ther. 2020;13:10829-10840
43. Wu B, Wang Y, Yang XM, Xu BQ, Feng F, Wang B, Liang Q, Li Y, Zhou Y, Jiang JL, Chen ZN. Basigin-mediated redistribution of CD98 promotes cell spreading and tumorigenicity in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:110
44. Sussman TA, Funchain P, Singh A. Clinical Trials in Metastatic Uveal Melanoma: Current Status. Ocul Oncol Pathol. 2020;6:381-387
45. Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol. 2019;10:2965
Author contact
![]() Corresponding author: Pu Xia, PhD, Biological Anthropology Institute, College of Basic Medicine, Jinzhou Medical University, Jinzhou 121001, P.R. China. E-mail: xiapuEDU.CN.
Corresponding author: Pu Xia, PhD, Biological Anthropology Institute, College of Basic Medicine, Jinzhou Medical University, Jinzhou 121001, P.R. China. E-mail: xiapuEDU.CN.

 Global reach, higher impact
Global reach, higher impact