Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(13):1912-1919. doi:10.7150/ijms.76725 This issue Cite
Research Paper
Comparison of Clinical Characteristics and Outcomes of Hospitalized Patients Infected with the D614G Strain or Alpha Variant of COVID-19 in Taiwan: A Multi-Center Cohort Study
1. Department of Thoracic Medicine, Chang Gung Memorial Hospital, Chang Gung University, School of Medicine, Taoyuan, Taiwan.
2. Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Linkou, Taiwan.
3. Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Keelung, Taiwan.
4. School of Traditional Chinese Medicine, Chang Gung University, Taoyuan, Taiwan.
5. Graduate Institute of Health Industry Technology, Research Center for Chinese Herbal Medicine, Chang Gung University of Science and Technology, Taoyuan, Taiwan.
6. Department & Graduate Institute of Chemical Engineering & Graduate Institute of Biochemical Engineering, Ming Chi University of Technology, New Taipei, Taiwan.
7. School of Nursing, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan.
8. Department of Traditional Chinese Medicine, New Taipei Municipal Tucheng Hospital, New Taipei City, Taiwan.
9. Department of Pulmonary and Critical Care Medicine, New Taipei Municipal Tucheng Hospital, New Taipei City, Taiwan.
10. Division of Pediatric Infectious Diseases, Department of Pediatrics, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan.
#Contributed equally to this article as first authors.
Received 2022-7-1; Accepted 2022-10-8; Published 2022-10-24
Abstract
Objective: Direct comparison of the clinical traits of coronavirus disease 2019 (COVID-19) in strain D614G, which originated from Wuhan, China, and the Alpha variant, which contains 17 mutations, infected patients could help physicians distinguish between strains and make clinical decisions accordingly. This study sought to compare the clinical characteristics and outcomes of the D614G strain and Alpha variant of SARS-COV-2 and identify the predictors for viral RNA clearance and in-hospital mortality in patients with COVID-19.
Methods: This study recruited consecutive patients from four hospitals between March 1, 2020, and July 31, 2021. Demographic characteristics, laboratory results, and clinical outcomes were determined.
Results: Among the 239 enrolled patients, 11.2% (27/239) were infected with strain D614G and 88.7% (212/239) were infected with the Alpha variant. There were no significant differences in disease progression, rate of respiratory failure, subsequent development of acute respiratory distress syndrome (ARDS), acute kidney injury, cardiac injury, duration of stay in the intensive care unit or hospital, discharge rate, mortality rate, or viral RNA clearance time between the two groups. Multivariate Cox regression revealed that antibiotic therapy reduced the risk of delayed viral RNA clearance (hazard ratio [HR], 0.26; 95% confidence interval [CI], 0.13-0.55), while autoimmune disease increased the risk of delayed viral RNA clearance (HR, 3.98; 95% CI, 1.21-13.04). Elderly patients (age > 65 years) and patients with a history of cerebrovascular accident (CVA) were at increased risk of in-hospital mortality (HR, 5.14; 95% CI, 1.06-24.72 and HR, 3.62; 95% CI, 1.25-10.42, respectively).
Conclusions: There were no significant differences between the D614G strain and Alpha variant of COVID-19 in terms of clinical characteristics and outcomes. However, factors affecting viral RNA clearance and the risk of in-hospital mortality were identified. These results could help to inform the future prioritization of resource allocation and identify patients in need of intense monitoring.
Keywords: COVID-19, D614G, Alpha variant
Introduction
The emergence of coronavirus disease 2019 (COVID-19) has had an extensive impact on public health and economies globally. As of April 2022, there have been more than 494 million confirmed COVID-19 cases worldwide, with more than 6.1 million deaths attributed to the pandemic (https://covid19.who.int./). Taiwan was one of the few countries to achieve initial success in COVID-19 control without strict city lockdowns or school closures. Based on past experience of combating the severe acute respiratory syndrome coronavirus-1 (SARS-COV-1) outbreak in 2003, Taiwan responded to the COVID-19 pandemic with rapid measures as early as January 2020, resulting in only a few scattered household and workplace transmissions [1]. Most confirmed cases in Taiwan were imported from other countries, while further genetic sequencing confirmed that the D614G strain originated from Wuhan, China. The D614G strain, which emerged as the dominant strain globally in 2020, was associated with increased transmissibility but without increased incidence of severe illness [2]. However, when a country-wide outbreak started in Taiwan in May 2021, the Alpha variant was identified as the dominant strain of transmission rather than the D614G strain.
SARS-COV-2 is prone to genetic evolution, resulting in multiple variants that may exhibit different characteristics compared to ancestral strains. In late 2020, a variant of concern (VOC) known as lineage B.1.1.7, also referred to as the Alpha variant or GRY (GY/501Y.V1), was first reported based on whole-genome sequencing of samples from patients testing positive for SARS-COV-2 [3, 4]. The Alpha variant of SARS-CoV-2 exhibits 17 mutations, of which eight are found in the spike protein that mediates viral attachment and entry into human cells. At least three mutations potentially affect viral function. Mutation N501Y is a key contact residue in the receptor binding domain and enhances viral binding affinity to angiotensin-converting enzyme 2 (ACE2) [5, 6]. This VOC had been circulating in the United Kingdom (UK) since September 2020 and was reported to be 43% to 82% more transmissible, surpassing preexisting strains of SARS-COV-2 and emerging as the dominant strain [7]. The aforementioned outbreak of the COVID-19 Alpha variant in Taiwan in 2021 resulted in more than 500 deaths before coming to an end in August 2021. While strain D614G and the Alpha variant were each dominant in different parts of the world at one point, direct comparison of the two variants in terms of clinical characteristics and outcomes appeared somewhat lacking. Identifying differences in clinical traits in strains could help physicians to make clinical decisions based on the particular infecting strain. Therefore, we conducted a study to compare and contrast clinical characteristics and outcomes in COVID-19 patients infected with the D614G strain or the Alpha variant and also sought to identify the factors associated with SARS-COV-2 viral RNA clearance and in-hospital mortality.
Materials and methods
Study Population
This study reports on a multi-center, retrospective, observational study of subjects hospitalized due to COVID-19 and managed within the Chang Gung Health System. We recruited consecutive patients from four hospital branches, including two large tertiary care centers and two reginal hospitals. Subjects were included if they were > 18 years old and had had a laboratory-confirmed diagnosis of COVID-19 via polymerase chain reaction (PCR) testing. Data were collected for 239 consecutive patients admitted to the Chang Gung Health System between March 1, 2020, and July 31, 2021. In addition, 89 hospitalized patients during the same study period with the diagnosis of non-COVID-19 pneumonia were also collected for comparison. Due to the outbreak of COVID-19 pandemic, most of the medical wards were reassigned as quarantine wards. The hospitals only accepted referred non-COVID-19 pneumonia patients with at least one organ failure. Those non-COVID-19 pneumonia patients without organ failure were admitted to community hospitals. The study non-COVID pneumonia was approved by the Institutional Review Board of the Chang Gung Memorial Foundation (IRB No. 202100712B0). Informed consent was waived due to the retrospective nature of this study. All data were collected from electronic medical records.
Data Collection
Medical information, including baseline characteristics and comorbidities, as well as clinical, laboratory, treatment, and outcome data, was extracted using data collection forms, which were then checked independently by two trained physicians.
Definitions
All of the patients included in the study were diagnosed with COVID-19 in accordance with the guideline released by the Taiwan Centers for Disease Control (CDC): “Interim Guidelines for Clinical Management of SARS-CoV-2 Infection” [8]. There have been two waves of COVID-19 pandemic in Taiwan. The first wave occurred from January 2020 to January 2021, and the second wave occurred from May 2021 to July 2022. According to the report from whole genome sequencing by Taiwan CDC, the first wave of COVID-19 patients were infected with strain D614G while the second wave of COVID-19 patients were infected with the Alpha variant.
The patients were accordingly classify as (1) mild cases, involving mild clinical symptoms without manifestation of pneumonia in imaging; (2) moderate cases, involving fever and respiratory tract symptoms as well as manifestation of pneumonia in imaging; (3) severe cases, involving any of the following: (i) respiratory distress with respiratory rates ≥ 30 breaths/minute, (ii) oxygen saturation of ≤ 94% in resting state, (iii) an arterial oxygen tension (PaO2) over inspiratory oxygen fraction (FiO2) ratio of < 300 mmHg (1 mmHg = 0.133 kPa), (iv) multiple pulmonary lobes with images showing progression in more than 50% of lesions within 24-48 h; and (4) critically ill cases involving (i) respiratory failure requiring mechanical ventilation, (ii) shock, and (iii) multiple organ failure requiring monitoring and treatment in the intensive care unit (ICU). Progression was defined as an increase in severity, while unchanged severity throughout the observation period was not classified as progression. Acute kidney injury (AKI) events were defined as any of the following criteria occurring within 7 days after admission: an increase in serum creatinine level of ≥ 0.3 mg/ dL within a period of 48 h or an increase in serum creatinine level of ≥ 1.5 times from baseline within 7 days. Note that both of these criteria were suggested in “Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury” [9]. Cardiac injury was defined by at least one documented assay indicating elevated high sensitivity troponin-I [10].
COVID-19 Management Protocol
The strategies used in the management of COVID-19 were fairly homogenous across the four hospitals, all of which adopted protocols in line with the “Interim Guidelines for Clinical Management of SARS-CoV-2 Infection”. This involved regularly monitoring vital signs and oxygen saturation (severe cases were monitored continuously), strengthening supportive treatment, providing sufficient calories, and maintaining the stability of the internal environment (e.g., water, electrolytes, and acid-base balance). Supplemental oxygen therapy was immediately administered to patients upon hypoxemia presentation. The target oxygen saturation was a pulse oxygen saturation of ≥ 90%. In the event that standard oxygen therapy failed, high-flow nasal catheter oxygen or non-invasive ventilation was used, while invasive mechanical ventilation was initiated if non-invasive mechanical ventilation failed to provide benefits. Anti-viral therapy and anti-inflammatory agents were administered in accordance with standardized guidelines. Remdesivir was administered to patients with SPO2 ≤ 94 % under room air or supplied oxygen. Low-dose dexamethasone (6 mg per day for no more than 10 days) was administered to patients with SPO2 ≤ 94 % under room air or supplied oxygen, patients presenting respiratory failure, and patients requiring extracorporeal membrane oxygenation (ECMO). Tocilizumab was administered to patients with SPO2 < 94 % under room air or supplied oxygen, as well as those presenting with respiratory failure or requiring ECMO. Antimicrobial agents (oral or intravenous) were prescribed in accordance with the condition of the patient. Anti-viral and anti-inflammatory agents were provided by the Taiwan CDC in accordance with standard guidelines. It should be noted that all medical expenses are reimbursed by national health insurance in Taiwan.
Laboratory Data
All blood samples were obtained and analyzed using standardized laboratory methods. Routine hematological and biochemical testing included white blood cell count (WBC), lymphocyte count, prothrombin time (PT), activated partial prothrombin time (aPTT), C‐reactive protein (CRP), D-dimer, lactate dehydrogenase (LDH), ferritin, interleukin-6 (IL-6), and liver and kidney function tests. Repeat PCR testing was conducted at intervals decided by individual managing physicians. The PCR cycle threshold (Ct) values of nasopharyngeal samples were measured.
Statistical Analysis
All data were expressed as mean ± standard deviation or percentages unless otherwise indicated. The Student's t-test was used to compare the means of continuous variables and normally distributed data; otherwise, the Mann-Whitney test was used. Categorical variables were tested using the Chi-square test or Fisher's exact test. We implemented the Kaplan-Meier curve and log-rank test to compare in-hospital mortality and viral RNA clearance; the latter was defined as the time between the onset of symptoms to two consecutive PCR Cts > 30 since Ct values above this cut-off have been associated with a lack of viral culture [11, 12]. Univariate analysis was first performed to identify the predictors for viral RNA clearance and in-hospital mortality. All variables with a p-value of < 0.1 in univariate Cox regression analysis were entered into a multivariate Cox regression model to identify factors independently predictive of viral RNA clearance and in-hospital mortality A p-value of < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS software, version 24.0 (SPSS, Inc., Chicago, IL).
Results
Demographic Data
During the study period, 239 patients with COVID-19 met the inclusion criteria. Among them, 27 patients (11.2%) were infected with strain D614G and 212 patients (88.7%) were infected with the Alpha variant. Out of all the patients, 100 (41.8%) had severe disease, and 40 (16.7%) had critical disease. The mean age of the patients was 59.1 ± 14.5 years, and approximately half of them were male (50.6%). Eleven patients (4.6%) were active smokers. Hypertension was the most common comorbidity (37.2%) among all studied patients with COVID-19. Sixteen patients (6.7%) received high-flow nasal cannula therapy during the hospitalization period. The majority of the patients (70.7%) underwent antibiotic therapy, but only patients infected with the Alpha variant received anti-IL-6 therapy (31.1%). A dose of 6 mg dexamethasone per day was administered to patients who required any form of oxygenation therapy (165 patients; 69%). Patients infected with the Alpha variant presented with significantly lower lymphocyte counts (1058.2 ± 666.1 vs 1613.4 ± 1049.5, p = 0.014) and higher LDH values (371.1 ± 189.8 vs 270.7 ± 142.5, p = 0.041) than patients infected with D614G strain, as shown in Table 2. Detailed demographic and clinical characteristics of all included patients are presented in Tables 1 and 2.
Clinical Outcomes
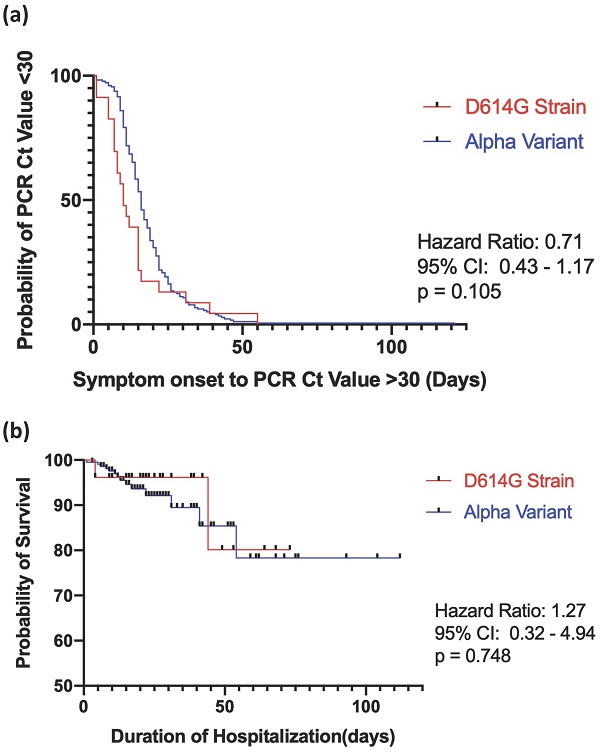
The results revealed that 94 hospitalized patients with COVID-19 (39.3%) experienced progression of the disease. A total of 65 patients (27.2%) experienced respiratory failure, and 44 (18.4%) subsequently developed ARDS. The mean durations of ICU and hospital stays were 23.0 ± 22.0 days and 23.0 ± 32.0 days, respectively. The in-hospital mortality rate was 7.1%. There were no significant differences between the D614G strain and Alpha variant groups with respect to respiratory failure rate or subsequent ARDS, AKI, cardiac injury, discharge rate, mortality rate, or duration of time spent in the ICU or hospitalized, as shown in Table 3. The Kaplan-Meier survival curve comparing viral RNA clearance for strain D614G and the Alpha variant is illustrated in Figure 1a, and in-hospital mortality is shown in Figure 1b. There were no statistical differences between the two groups in regard to viral RNA clearance time (hazard ratio [HR], 0.71, ref. Alpha variant; 95% confidence interval [CI], 0.43-1.17, log-rank test p = 0.105) or in-hospital mortality (HR, 1.27, ref. Alpha variant; 95% CI, 0.32-4.94, log-rank test p = 0.748).
Baseline characteristics of COVID-19 patients
| Total n = 239 | D614G Strain n = 27 | Alpha Variant n = 212 | p value | |
|---|---|---|---|---|
| Age, yr | 59.1 ±14.5 | 53.7 ± 19.5 | 59.8 ± 17.2 | 0.088 |
| Male gender | 121(50.6) | 9(33.3) | 112(52.8) | 0.505 |
| BMI | 25 ± 4.7 | 26.1 ± 5.5 | 24.8 ± 4.6 | 0.303 |
| Active smoker | 11(4.6) | 0(0) | 11(5.2) | 0.001 |
| Comorbidities | ||||
| Hypertension, n(%) | 89(37.2) | 8(29.6) | 81(38.2) | 0.086 |
| CAD | 35(14.6) | 1(3.7) | 34(16.0) | 0.008 |
| Heart failure | 10(4.2) | 1(3.7) | 9(4.2) | 0.895 |
| Atrial fibrillation | 10(4.2) | 0(0) | 10(4.7) | 0.001 |
| CVA | 12(5.0) | 3(11.1) | 9(4.2) | 0.286 |
| DM | 48(20.1) | 1(3.7) | 45(21.2) | 0.144 |
| CKD | 21(8.8) | 3(11.1) | 20(9.4) | 0.181 |
| COPD | 11(4.6) | 0(0) | 11(5.2) | 0.001 |
| Asthma | 7(2.9) | 1(3.7) | 6(2.8) | 0.801 |
| Autoimmune disease | 3(1.3) | 0(0) | 3(1.4) | 0.536 |
| Malignancy | 9(3.8) | 1(3.7) | 8(3.8) | 0.986 |
| HIV (+) | 6(2.5) | 0(0) | 6(2.8) | 0.990 |
| Therapies | ||||
| High flow nasal cannula | 16(6.7) | 1(3.7) | 15(7.1) | 0.511 |
| Antibiotic therapy, n(%) | 169(70.7) | 20(74.1) | 149(70.0) | 0.685 |
| Remdesivir | 135(56.5) | 3(11.1) | 132(62.3) | 0.000 |
| Dexamethasone | 165(69.0) | 1(3.7) | 164(77.4) | 0.000 |
| Tocilizumab | 66(27.6) | 0(0) | 66(31.1) | 0.000 |
| Disease severity on admission | ||||
| Mild | 56(23.4) | 6(22.2) | 50(23.6) | 0.233 |
| Moderate | 43(18.0) | 10(37.0) | 33(15.6) | |
| Severe | 100(41.8) | 8(29.6) | 92(43.4) | |
| Critical | 40(16.7) | 3(11.1) | 37(17.5) | |
Data are expressed as n (%) and mean ± SD. Abbreviations: COVID-19: coronavirus disease 2019; BMI: body mass index; CAD: coronary arterial disease; CVA: cerebrovascular accident; DM: diabetes mellitus; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus.
COVID-19 vs Non-COVID-19 Patients
Our study was conducted in tertiary referral centers and reginal hospitals. Due to the COVID-19 pandemic, we only admitted non-COVID-19 pneumonia patients with at least one organ failure. The hospital capacity for the non-COVID-19 patients was markedly reduced, and medical resources were re-allocated towards quarantine wards. The non-COVID-19 patients were predominantly male (69.7% vs 50.6%), with significantly higher rate of malignancy and immunosuppressive therapy (48.3% vs 3.8% and 36.0% vs 1.3%, respectively). Compared with COVID-19 patients, increased rate of respiratory failure (73.0% vs 27.2%), longer length of hospital stay (33.8 ± 31.1 vs 23 ± 32 days), and subsequently higher mortality rate (55.1% vs 7.1%) were observed in the non-COVID-19 group (Table 4).
Kaplan-Meier survival curve for D614G vs the alpha variant in (a) SARS-COV-2 RNA clearance and (b) in-hospital mortality. Viral RNA clearance is defined as the time between the onset of symptoms to two consecutive PCR Cycle threshold>30. Log rank test p<0.05 indicates a statistical significance.
Baseline Laboratory data of patients with COVID-19
| Total n = 239 | D614G Strain n = 27 | Alpha Variant n = 212 | p value | |
|---|---|---|---|---|
| WBC, 1,000/μL | 6.4 ± 5.3 | 6.6 ± 2.0 | 6.5 ± 5.6 | 0.864 |
| Lymphocyte count | 1120.7 ± 737.7 | 1613.4 ± 1049.5 | 1058.2 ± 666.1 | 0.014* |
| Platelet, 1,000/μL | 216.2 ± 97.0 | 229.6 ± 100.5 | 214 ± 96.7 | 0.467 |
| PT, seconds | 14.1 ± 29.3 | 11.9 ± 0.5 | 14.3 ± 30.6 | 0.749 |
| aPTT, seconds | 29.5 ± 4.3 | 29.8 ± 3.8 | 29.4 ± 4.44 | 0.730 |
| D-dimer, mg/L | 1383.0 ± 2442.3 | 1236.8 ± 2265.8 | 1394.8 ± 2461.0 | 0.750 |
| BUN, mg/dL | 17.2 ± 13.1 | 16.4 ± 22.9 | 17.3 ± 11.4 | 0.715 |
| Creatinine, mg/dL | 1.04 ± 1.36 | 1.11 ± 2.04 | 1.03 ± 1.2 | 0.773 |
| eGFR | 86.2 ± 38.0 | 101.3 ± 49.5 | 84.3 ± 36.0 | 0.032 |
| AST, U/L | 39.4 ± 29.2 | 34.0 ± 20.0 | 40.0 ± 30.1 | 0.350 |
| ALT, U/L | 37.0 ± 37.1 | 30.2 ± 21.9 | 37.8 ± 38.3 | 0.365 |
| Total bilirubin, mg/dL | 0.49 ± 0.27 | 0.52 ± 0.3 | 0.49 ± 0.27 | 0.778 |
| Na, mEq/L | 136.3 ± 4.5 | 137.9 ± 4.5 | 136.1 ± 4.4 | 0.054 |
| K, mEq/L | 3.9 ± 1.9 | 3.6 ± 0.4 | 3.9 ± 2.0 | 0.526 |
| LDH, U/L | 362.0 ± 187.9 | 270.7 ± 142.5 | 371.1 ± 189.8 | 0.041* |
| CRP, mg/L | 55.7 ± 69.1 | 42.5 ± 63.7 | 57.4 ± 69.7 | 0.348 |
| Ferritin, ng/mL | 916.5 ± 1396.0 | 751.0 ± 936.1 | 925.2 ± 1417.7 | 0.740 |
| IL-6, pg/mL | 72.48 ± 157.3 | 55.2 ± 101.4 | 74.6 ± 163.5 | 0.738 |
Data are expressed as mean ± SD. Abbreviations: WBC: white blood cell; PT: prothrombin time; aPTT: activated partial prothrombin time; BUN: blood urine nitrogen; AST: Aspartate Transaminase; ALT: alanine transaminase; LDH: lactate dehydrogenase; CRP: C‐reactive protein; IL-6: interleukine-6.
Univariate and Multivariate Cox Regression
Univariate analysis (Table 5) shows that for strain D614G, age > 65, CVA, autoimmune disease, dexamethasone, and antibiotics therapy were primarily selected. Furthermore, multivariate Cox regression analysis identified autoimmune disease history (adjusted HR, 3.98; 95% CI, 1.21-13.03, p = 0.022) as a positive predictor, and antibiotic therapy (adjusted HR, 0.26; 0.13-0.55, p < 0.001) as a negative predictor for delayed viral RNA clearance.
Treatment and outcomes of COVID-19 patients
| Total n = 239 | D614G Strain n = 27 | Alpha Variant n = 212 | Odds ratio 95% C.I. | p value | |
|---|---|---|---|---|---|
| Disease progression, n(%) | 94(39.3) | 8(29.6) | 86(40.6) | 0.617(0.258-1.473) | 0.261 |
| Respiratory failure, n(%) | 65(27.2) | 5(18.5) | 60(28.3) | 0.576(0.208-1.590) | 0.242 |
| ARDS, n(%) | 44(18.4) | 3(11.1) | 41(19.3) | 0.521(0.150-1.815) | 0.230 |
| Admission to ICU, n(%) | 64(26.8) | 5(18.5) | 59(278) | 0.589(0.213-1.629) | 0.187 |
| Acute kidney injury, n(%) | 47(19.7) | 4(14.8) | 43(20.3) | 0.684(0.225-2.081) | 0.503 |
| Cardiac injury, n(%) | 14(5.9) | 1(3.7) | 13(6.1) | 0.589(0.74-4.687) | 0.615 |
| Duration from symptom to 1st Ct No.>30, days | 17.8 ± 12.1 | 14.0 ± 12.5 | 18.3 ± 12.0 | - | 0.112 |
| Mortality, n(%) | 17(7.1) | 3(11.1) | 14(6.6) | 1.768(0.474-6.598) | 0.393 |
| Duration of hospitalization, days | 23.0 ± 32.0 | 41.9 ± 81.1 | 20.7 ± 17.1 | - | 0.187 |
| Duration of ICU stay, days | 23.0 ± 22.0 | 31.6 ± 28.8 | 21.9 ± 21.0 | - | 0.357 |
Data are expressed as n (%) and mean ± SD. Abbreviations: ARDS: acute respiratory distress syndrome; ICU: intensive care unit; Ct: cycle threshold.
Demographic characteristics and outcomes of patients with non-COVID-19 and COVID-19
| Non-COVID-19 n = 89 | COVID-19 n = 239 | p value | |
|---|---|---|---|
| Age, year | 62.7 ± 13.1 | 59.1 ±14.5 | 0.104 |
| Male gender, n(%) | 62 (69.7) | 121(50.6) | 0.001* |
| BMI | 23.3 ± 5.4 | 25 ± 4.7 | 0.013* |
| Active smoker, n(%) | 37 (41.6) | 11(4.6) | <0.001* |
| Co-morbidities | |||
| Hypertension, n(%) | 35 (39.3) | 89(37.2) | 0.790 |
| CAD, n(%) | 3 (3.4) | 35(14.6) | 0.759 |
| Heart failure, n(%) | 0 (0) | 10(4.2) | 0.001* |
| Atrial fibrillation, n(%) | 0 (0) | 10(4.2) | 0.001* |
| CVA, n(%) | 24 (27.0) | 12(5.0) | 0.845 |
| DM, n(%) | 24 (27.0) | 48(20.1) | 0.223 |
| CKD, n(%) | 10 (11.2) | 21(8.8) | 0.524 |
| COPD, n(%) | 4 (4.5) | 11(4.6) | 0.949 |
| Asthma, n(%) | 24 (27.0) | 7(2.9) | 0.185 |
| Immunosuppressive therapy, n(%) | 32 (36.0) | 3(1.3) | <0.001* |
| Malignancy, n(%) | 43 (48.3) | 9(3.8) | <0.001* |
| Clinical outcomes | |||
| Respiratory failure, n(%) | 65 (73.0) | 65(27.2) | <0.001* |
| Admission to ICU, n(%) | 65 (73.0) | 64(26.8) | <0.001* |
| Length of ICU stay, days | 26 ± 22 | 23 ± 22 | 0.553 |
| Length of hospital stay, days | 33.8 ± 31.1 | 23 ± 32 | 0.001* |
| Mortality, n(%) | 49 (55.1) | 17(7.1) | <0.001* |
Data are expressed as n (%) and mean ± SD. Abbreviations: COVID-19: coronavirus disease 2019; BMI: body mass index; CAD: coronary arterial disease; CVA: cerebrovascular accident; DM: diabetes mellitus; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit.
Risk factors associated with delayed viral RNA clearance
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| D614G Strain (ref. Alpha strain) | 0.27 | 0.11-0.68 | 0.005 | 0.20 | 0.49-1.21 | 0.097 |
| Age>65 years | 0.66 | 0.43-1.02 | 0.064 | 0.66 | 0.41-1.06 | 0.090 |
| Obesity (BMI>30) | 1.35 | 0.64-2.81 | 0.423 | |||
| Current/Ex-smoker | 0.82 | 0.42-1.64 | 0.599 | |||
| Hypertension | 0.69 | 0.45-1.07 | 0.105 | |||
| Coronary Artery Disease | 0.95 | 0.58-1.55 | 0.838 | |||
| Heart failure | 0.79 | 0.24-2.52 | 0.691 | |||
| Arrhythmia | 0.51 | 0.15-1.68 | 0.273 | |||
| Cerebrovascular Accident | 0.41 | 0.16-1.03 | 0.059 | 0.69 | 0.26-1.82 | 0.464 |
| Chronic Kidney Disease | 1.09 | 0.57-2.06 | 0.782 | |||
| Diabetes mellitus | 0.99 | 0.59-1.65 | 0.988 | |||
| COPD | 1.13 | 0.54-2.35 | 0.737 | |||
| Asthma | 2.11 | 0.77-5.81 | 0.145 | |||
| Autoimmune Disease | 4.16 | 1.30-13.3 | 0.004 | 3.98 | 1.21-13.04 | 0.022* |
| HIV | 1.47 | 0.20-10.89 | 0.702 | |||
| Pneumonia at admission | 0.74 | 0.45-1.21 | 0.234 | |||
| Dexamethasone therapy | 1.69 | 0.98-2.91 | 0.057 | 2.24 | 0.98-5.11 | 0.054 |
| Tociluzimab therapy | 0.86 | 0.56-1.31 | 0.493 | |||
| Remdesivir therapy | 1.37 | 0.88-2.13 | 0.160 | |||
| Antibiotics Therapy | 0.38 | 0.22-5.81 | 0.001 | 0.26 | 0.13-0.55 | <0.001* |
Abbreviations: COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; HR: hazard ratio.
Risk factors associated with hospital mortality
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| D614G Strain (ref. Alpha strain) | 1.20 | 0.342-4.25 | 0.770 | |||
| Age>65 | 8.55 | 1.93-37.72 | 0.005 | 5.14 | 1.06-24.72 | 0.041* |
| Obesity (BMI>30) | 2.12 | 0.59-7.57 | 0.244 | |||
| Current/ex-smoker | 0.48 | 0.60-3.73 | 0.490 | |||
| HTN | 1.48 | 0.56-3.90 | 0.421 | |||
| Coronary Artery Disease | 2.79 | 1.04-7.46 | 0.041 | 1.76 | 0.49-6.24 | 0.378 |
| Heart failure | 3.20 | 0.72-14.22 | 0.125 | |||
| Arrhythmia | 3.06 | 0.69-13.41 | 0.138 | |||
| Cerebrovascular accident | 6.81 | 2.41-19.18 | <0.001 | 3.62 | 1.25-10.42 | 0.017* |
| Diabetes mellitus | 2.14 | 0.79-5.82 | 0.132 | |||
| CKD | 3.65 | 1.26-10.56 | 0.016 | 1.31 | 0.32-5.30 | 0.697 |
| COPD | 1.75 | 0.39-7.78 | 0.456 | |||
| Pneumonia at admission | 2.33 | 0.52-10.31 | 0.263 | |||
| Dexamethasone therapy | 1.26 | 0.412-3.88 | 0.682 | |||
| Tociluzimab therapy | 1.23 | 0.45-3.29 | 0.681 | |||
| Remdesivir therapy | 1.22 | 0.45-3.30 | 0.695 | |||
Abbreviations: COPD: chronic obstructive pulmonary disease; CXR: Chest X-ray; HR: hazard ratio.
After performing univariate and multivariate Cox regression analysis, age > 65 years (adjusted HR, 5.14; 95% CI, 1.06-24.72; p = 0.041) and history of CVA (adjusted HR, 3.62; 95% CI, 1.25-10.42; p = 0.017) were the only two independent factors associated with increased in-hospital mortality in patients with COVID-19 (Table 6).
Discussion
The results of this study showed that for patients infected with strain D614G or the Alpha variant of COVID-19, viral RNA clearance times, discharge rates, mortality rates, and duration of ICU or hospital stays did not differ significantly. This is in contrast to other clinical studies; for example, a large matched cohort study reported that mortality hazard was 1.64 times higher in patients infected with the Alpha variant than in patients infected with the previously circulating strains in the UK [13]. Furthermore, two other studies reported HRs of 1.61 and 1.67 versus non-Alpha strains [14, 15]. Although these previous studies demonstrated a higher risk of mortality with the Alpha variant, the administration of proven clinical therapies such as anti-IL-6, remdesivir, and dexamethasone at the time of the Alpha outbreak in Taiwan in mid-2021 may have played a role in reducing the mortality rate. Our results also indicated that patients infected with the Alpha variant presented with significantly lower lymphocyte counts and higher LDH levels than patients infected with strain D614G. SARS-CoV-2 infection often results in multiple organ injury accompanied by elevated levels of serum inflammatory mediators, indicating that COVID-19 is a systemic inflammatory illness rather than simply a lung disease [16]. Another study reported that elevated LDH levels were associated with a six-fold increase in the odds of developing severe disease for patients with COVID-19 [17]. Since lymphocyte count and LDH levels are both markers of inflammation and correlate with the severity of the disease, the higher incidences of severely and critically ill individuals found in our Alpha variant group, compared with the D614G group (43.4% vs. 29.6% vs and 17.5% vs. 11.7%, respectively, see Table 1) may have contributed to our finding.
In order to effectively allocate resources and adjust quarantine policy accordingly, it is important to identify prognostic factors for SARS-COV-2 RNA clearance. In our current study, we found that the presence of autoimmune disease in COVID-19 patients was associated with a four-fold increase in the risk of delayed viral RNA clearance. Past studies have shown that inflammatory bowel disease and rheumatological diseases are associated not only with an increased risk of community-acquired and opportunistic infections but also with higher mortality, especially for patients undergoing some form of immunosuppressive therapy [18, 19]. Several reports in the earlier phase of the pandemic also demonstrated that immunocompromised individuals are prone to have longer period of shedding of SARS-CoV-2. During the prolonged viral RNA shedding, some within-host viral evolution was observed. Potential factors contributing to the delayed viral clearance are the compromised immune status of the host and viral genetic evolution [20, 21]. Therefore, this evidence may support our finding that patients with autoimmune diseases under immunosuppressants were predisposed to prolonged periods of SARS-CoV-2 viral clearance.
Although the routine use of antibiotics has been controversial in treating COVID-19 patients, use of adequate broad-spectrum antibiotic therapy helps to prevent and manage secondary bacterial infections and sepsis [22-24]. We found patients receiving antibiotics to have a decreased risk of delayed viral clearance. While it is difficult to examine the exact microbial and immunological response, as well as the clinical benefits of antibiotics, past studies have shown that early administration of macrolides (such as azithromycin), which possess immune-modulating properties, helps to prevent severe lower respiratory tract illnesses in viral infections [25]. A study by Du et al. reported azithromycin demonstrated in vitro anti-viral effect against SARS-COV-2 and blocks the entry of SARS-CoV-2 in HEK293T-ACE2 and Caco2 cells [26]. Another observational study also reported the clinical benefits of antibiotics: the combination of remdesivir-azithromycin treatment significantly decreased the ICU admission rate [27]. However, the exact mechanism of antibiotics in SARS-COV-2 viral clearance remains to be investigated.
The infectivity of COVID-19 patients is determined by the presence of virus in bodily fluids, secretions, and excreta [28]; therefore, all patients with positive viral RNA detection need to be isolated to prevent further transmission. However, this policy has a huge impact in terms of medical and economic resources. Previous studies showed that successful viral culture was associated with PCR Ct values < 30; therefore, a PCR Ct value > 30 may be considered as a surrogate indicator for the end of the infective period. In many countries, isolated COVID-19 patients can be de-isolated after the relief of symptoms and two successive RNA Ct values > 30 in respiratory specimens [8, 29]. Our study demonstrated that patients presenting autoimmune disease as a comorbidity and lacking treatment with antibiotics are strong candidates for delayed viral clearance and should therefore be given priority in the allocation of resources. The incorporation of these variables into clinical and laboratory algorithms could potentially be useful for future research.
We also found that elderly patients aged > 65 years and patients with a history of stroke were at increased risk of in-hospital mortality. A recent meta-analysis of 14 studies identified age > 65 years to be one of the risk factors associated with mortality in COVID-19, with a pooled odds ratio of 4.59 [30]. Age-related defects in B-cells and T-cells in elderly patients could result in prolonged inflammatory responses and deficient viral clearance, leading to eventual death [31]. In addition, elderly patients with COVID-19 may have more pre-existing comorbidities and risk factors, including cerebrovascular disease [32, 33]. The results of the current study could help clinicians to identify and be mindful of high-risk patients who require more intensive monitoring and intervention. Although our results demonstrated no difference in clinical outcomes in hospitalized patients in D614G and the Alpha variant, taking into consideration that the treatment and preventive measures for COVID-19 evolved over time, the identified risk factors for disease progression and in-hospital mortality may still remain and be applicable to the current Omicron pandemic and future variants of concern.
One major limitation of the current study is its retrospective nature, which may have led to bias in the selection of study subjects. Moreover, the sample size was rather small, especially for patients infected with D614G; therefore, the results should be interpreted with caution. Nonetheless, it should be noted that in Taiwan, all confirmed cases before the Omicron pandemic were admitted to hospital for treatment, based on guidelines declared by the Taiwan CDC. This made it possible for us to follow up patients longitudinally and maintain consistency among hospitals in terms of treatment regimens. Furthermore, the non-COVID-19 patients during the same study period were more critically ill than COVID-19 patients. The admission criteria of non-COVID-19 pneumonia patients with at least one organ failure may lead to some selection bias. However, our results demonstrated the “crowding-out effect” on other diseases that require in-patient treatment when a major pandemic outbreak occurs in the real-world setting.
In conclusion, there were no significant differences in clinical characteristics and outcomes for the D614G strain and Alpha variant of COVID-19. However, we successfully identified that antibiotic therapy could potentially reduce the risk of delayed SARS-COV-2 RNA clearance, while patients with autoimmune disease were shown to be at increased risk of delayed viral RNA clearance. Furthermore, elderly patients and patients with a history of CVA were at increased risk of in-hospital mortality. These findings could inform the future prioritization of resource allocation and identify patients in need of intense monitoring.
Acknowledgements
Funding
This research was supported by Chang Gung Medical Research Projects (CIRPG3K2011) and the Ministry of Science and Technology, Taiwan (MOST 109-2327-B-182-002).
Author Contributions
All authors participated in the design, interpretation of studies, analysis of data, and review of the manuscript; THC, KWC, THY, YHS, and ACCH conducted the data collection, CSL, FTC, and THH provided conceptualization, and ACCH and SML wrote the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen SC. Taiwan's experience in fighting COVID-19. Nat Immunol. 2021;22:393-4
2. Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W. et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182:812-27 e19
3. Galloway SE, Paul P, MacCannell DR, Johansson MA, Brooks JT, MacNeil A. et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage - United States, December 29, 2020-January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:95-9
4. Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L. et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593:266-9
5. Gu H, Chen Q, Yang G, He L, Fan H, Deng YQ. et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603-7
6. Starr TN, Greaney AJ, Hilton SK, Crawford KHD, Navarro MJ, Bowen JE. et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. bioRxiv. 2020
7. Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021; 372
8. Interim Guidelines for Clinical Management of SARS-CoV-2 Infection (14th edition) https://fightcovid.edu.tw/cdc-guidelines/clinical-management. Accessed 01 Nov 2021. Taiwan Center of Disease Control.
9. Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649-72
10. Hannawi S, Hannawi H, Naeem KB, Elemam NM, Hachim MY, Hachim IY. et al. Clinical and Laboratory Profile of Hospitalized Symptomatic COVID-19 Patients: Case Series Study From the First COVID-19 Center in the UAE. Front Cell Infect Microbiol. 2021;11:632965
11. Young BE, Ong SWX, Ng LFP, Anderson DE, Chia WN, Chia PY. et al. Viral Dynamics and Immune Correlates of Coronavirus Disease 2019 (COVID-19) Severity. Clin Infect Dis. 2021;73:e2932-e42
12. Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L. et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin Infect Dis. 2020;71:2663-6
13. Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579
14. Davies NG, Jarvis CI, Group CC-W, Edmunds WJ, Jewell NP, Diaz-Ordaz K. et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270-4
15. Grint DJ, Wing K, Williamson E, McDonald HI, Bhaskaran K, Evans D, et al. Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Euro Surveill. 2021; 26
16. Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y. et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52
17. Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M. et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38:1722-6
18. Figueroa-Parra G, Aguirre-Garcia GM, Gamboa-Alonso CM, Camacho-Ortiz A, Galarza-Delgado DA. Are my patients with rheumatic diseases at higher risk of COVID-19? Ann Rheum Dis. 2020;79:839-40
19. Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y. et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-68
20. Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL. et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020;183:1901-12 e9
21. Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X. et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383:2291-3
22. Rawson TM, Wilson RC, Holmes A. Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect. 2021;27:9-11
23. Fattorini L, Creti R, Palma C, Pantosti A, Unit of Antibiotic R, Special P. et al. Bacterial coinfections in COVID-19: an underestimated adversary. Ann Ist Super Sanita. 2020;56:359-64
24. Baskaran V, Lawrence H, Lansbury LE, Webb K, Safavi S, Zainuddin NI. et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol. 2021 70
25. Bacharier LB, Guilbert TW, Martinez FD. Early Azithromycin Treatment to Prevent Severe Lower Respiratory Tract Illnesses in Children-Reply. JAMA. 2016;315:2122-3
26. Du X, Zuo X, Meng F, Han C, Ouyang W, Han Y. et al. Direct inhibitory effect on viral entry of influenza A and SARS-CoV-2 viruses by azithromycin. Cell Prolif. 2021;54:e12953
27. Ticinesi A, Tuttolomondo D, Nouvenne A, Parise A, Cerundolo N, Prati B. et al. Co-Administration of Remdesivir and Azithromycin May Protect against Intensive Care Unit Admission in COVID-19 Pneumonia Requiring Hospitalization: A Real-Life Observational Study. Antibiotics (Basel). 2022 11
28. Qi L, Yang Y, Jiang D, Tu C, Wan L, Chen X. et al. Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020;96:531-7
29. Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh M, et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. 2021
30. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;23:1416-24
31. Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Suppl 7):S504-12
32. Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020;46:1472-4
33. Vogrig A, Gigli GL, Bna C, Morassi M. Stroke in patients with COVID-19: Clinical and neuroimaging characteristics. Neurosci Lett. 2021;743:135564
Author contact
![]() Corresponding author: Shu-Min Lin, MD, Department of Thoracic Medicine, Chang Gung Memorial Hospital, 199 Dun Hua N. Rd. Taipei, Taiwan Tel: (886) 3-328 1200 ext. 8467 Fax: (886) 3-3282474. E-mail: smlin100com.
Corresponding author: Shu-Min Lin, MD, Department of Thoracic Medicine, Chang Gung Memorial Hospital, 199 Dun Hua N. Rd. Taipei, Taiwan Tel: (886) 3-328 1200 ext. 8467 Fax: (886) 3-3282474. E-mail: smlin100com.

 Global reach, higher impact
Global reach, higher impact