3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(12):1762-1769. doi:10.7150/ijms.76105 This issue Cite
Research Paper
Determinants for a low dose of alteplase and its relationship to a lower intracerebral bleeding risk in acute ischemic stroke
1. Department of Neurology, Fujian Provincial Hospital South Branch, Fuzhou, China.
2. Department of Neurology, Longyan First Hospital of Fujian Medical University, Longyan, Fujian, China.
3. Department of Neurology, Fujian Medical University Union Hospital, Fuzhou, China.
4. Institute of Clinical Neurology, Fujian Medical University, Fuzhou, China.
5. Department of Rehabilitation, Fujian Medical University Union Hospital, Fuzhou, China.
6. Department of Radiology, Fujian Medical University Union Hospital, Fuzhou, Fujian, China.
#These first two authors have contributed equally to this work.
Received 2022-6-13; Accepted 2022-9-24; Published 2022-10-3
Abstract
Background: Factors for the utilization of intravenous thrombolysis with a low-dose of alteplase (0.6mg/kg) and whether the low-dose of alteplase could reduce the risk of intracerebral bleeding in acute ischemic stroke (AIS) remains uncertain.
Aims: We aimed to investigate determinants for the utilization of intravenous thrombolysis with a low-dose of alteplase. We further assessed the association between the low-dose of alteplase and the intracerebral bleeding risk in AIS patients.
Method: We included AIS patients who received intravenous thrombolysis using alteplase in this multicenter retrospective observational study. We investigated the association between baseline characteristics and the utilization of a low-dose of alteplase to identify determinants. We assessed the association of the low-dose of alteplase with the risk of symptomatic intracranial hemorrhage (sICH) using a multivariable logistic regression model. We further compared the rate of sICH and any ICH in patients in the low-dose group to those in the standard-dose group, using propensity score-matching data.
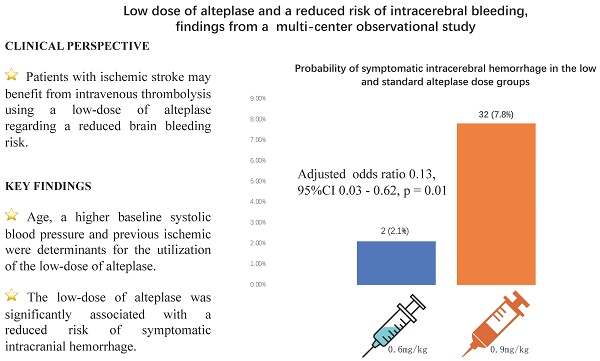
Results: A total of 506 AIS patients were included in this study. The mean age was 67 (interquartile range [IQR] 59-75), and 178 (35.2%) were women. A total of 96 patients were treated with the low-dose. Age (adjusted odds ratio [OR] 1.02, 95% confidence interval [CI] 1.00 -1.04, p = 0.042), having a previous ischemic stroke (adjusted OR 2.01, 95%CI 1.11 - 3.64 p = 0.021) and increasing baseline systolic blood pressure (adjusted OR 1.12, 95%CI 1.00 - 1.26, p = 0.049) were determinants for the utilization of the low-dose. Multivariable logistic regression analysis showed that the low-dose was significantly associated with a reduced risk of sICH (adjusted OR 0.13, 95%CI 0.03 - 0.62, p = 0.01). Propensity score analysis showed that the rate of sICH was significantly lower in the low-dose group compared to standard-dose group (2 [2.3%] vs 10 [11.4%], p = 0.032). There was no significant difference in the rate of any ICH between two groups (14 [15.9%] vs 18 [20.5%], p = 0.434).
Conclusions: Patients with increasing age, a higher baseline systolic blood pressure, and previous ischemic stroke were at a higher odd of receiving a low-dose of alteplase. The low-dose was associated with a lower risk of developing symptomatic intracranial hemorrhage.
Keywords: acute ischemic stroke, low-dose, alteplase, symptomatic intracranial hemorrhage
Introduction
Current stroke guidelines [1-3] recommend intravenous thrombolysis (IVT) using the recombinant tissue plasminogen activator (r-tPA) at a standard-dose (0.9mg/kg, alteplase) for patients with acute ischemic stroke (AIS). However, substantial concerns have been raised regarding the risk of adverse effects caused by r-tPA, of which the symptomatic intracranial hemorrhage (sICH) associates with poor clinical outcomes [4-6]. Whether a low-dose (0.6mg/kg, alteplase) of r-tPA could reduce the risk of intracerebral bleeding with similar efficacy as that of the standard-dose has long been debated. The Enhanced Control of Hypertension And Thrombolysis Stroke Study (ENCHANTED) showed that patients in the low-dose group experienced fewer sICH events than in the standard-dose group, although the low-dose was not non-inferior to standard-dose in reducing death and disability when administered within 4.5 hours of stroke onset [7]. The Japan stroke guideline recommends IVT using the low-dose of r-tPA [8]. Recently, several meta-analyses investigated the association between the low-dose and the risk of sICH, yielding inconsistent results [9,10]. The present study aimed to explore the potential determinants for the utilization of the low-dose of r-tPA, and whether the low-dose was associated with a lower intracerebral bleeding risk in this retrospective observational study.
Methods
Study design, participants, and setting
This is a retrospective, multi-center observational study conducted in stroke centers of three tertiary teaching hospitals in Southeast China. We included eligible adult (18 years or older) AIS patients who were treated with IVT using r-tPA within 4.5 hours after symptom onset. We applied the following exclusion criteria: 1. Patients with missing data regarding r-tPA dose; 2. Those who received arterial thrombolytic treatment; 3. Those who underwent interrupted IVT because of rapid neurological function improvement or severe side effects; 4. Those with missing data regarding sICH information. This study was approved by the local ethics committees at each center. Written informed consents were waived due to the nature of our retrospective study using routine anonymous data.
Data extraction
Two authors (J.C. and H.L.) reviewed the electronic medical records using a digital database, and extracted data regarding base demographics and clinical characteristics using standard digital collection sheets.
Intervention and Evaluation
The low-dose of alteplase (Boehringer Ingelheim Pharma GmbH) was defined as the dose of 0.6mg/kg body weight, and the standard-dose was defined as the dose of 0.9mg/kg body weight, based on current stroke guidelines [3,8]. A single dose of 0.9 mg/kg body weight (not exceeding 90 mg) or 0.6 mg/kg body weight (not exceeding 60 mg) was administered intravenously, with 10% (for 0.9mg/kg) or 15% (for 0.6 mg/kg) given as a bolus, followed by continuous infusion of the remainder within one hour. Stroke severity (presenting deficit severity) was assessed using the National Institutes of Health Stroke Scale (NIHSS) score by qualified clinicians before and after IVT treatment [11]. Functional outcome at discharge was assessed using a modified Rankin Scale (mRS, ranging from 0 to 6, with higher scores indicating greater disability) [12].
Outcome measures
Our primary outcome is symptomatic intracranial hemorrhage (sICH), defined as computed tomography (CT) evidence of new ICH with documented objective evidence of neurological decline or an increase of four points from the most recent NIHSS score, based on the European Cooperative Acute Stroke Study (ECASS-II) criteria [13]. Another safety outcome is any ICH, defined as CT-visible intracranial hemorrhage with and without evidence of neurological decline [13]. Additional outcomes include discharge NIHSS score, proportion of good functional outcome defined as a mRS score of 0 to 3 [12], and in-hospital mortality.
Statistics
Continuous variables were expressed as mean with standard deviation (SD) and median [interquartile range (IQR)] for those with normal and skewed distributions, respectively. Categorical variables were expressed as frequency with percentage. Between groups differences in demographic and clinical characteristics were calculated with t-test (for continuous variable if normally distributed) or Mann-Whitney test (if skewed distributions). Categorical variables were compared by Chi-square test or fisher's exact test, as appropriate. A multivariable logistic regression model was established to assess the association between baseline characteristics and the utilization of the low-dose. We calculated the unadjusted, and age- sex- adjusted odds ratio (OR) of sICH events with the low-dose of alteplase. The relationship between the low- dose and sICH risk was further assessed using multivariable logistic regression analysis by introducing those with a p < 0.1 in univariable analysis in addition to age and sex. To generate a comparable data set, we calculated a propensity score to estimate the individual probability of a patient receiving the low-dose of alteplase. Four variables were used as covariates (age, baseline NIHSS score, baseline systolic blood pressure (SBP), and history of a previous stroke). All statistical analyses were conducted using the SPSS 25.0 for windows (IBM). A p-value <0.05 was considered statistically significant.
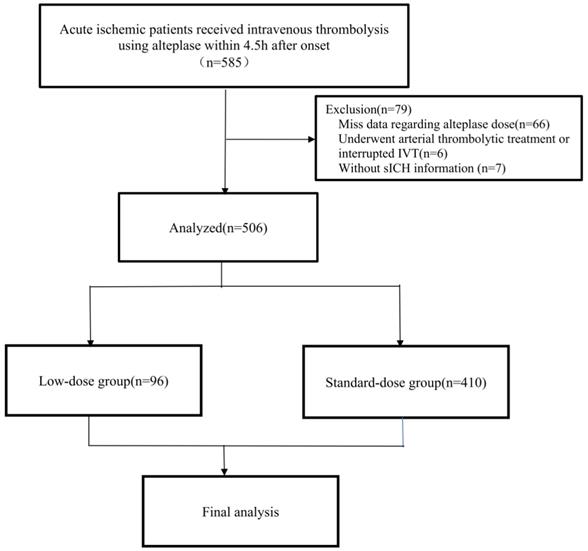
Flowchart of the study. Abbreviations: AIS, acute ischemic stroke; IVT, intravenous thrombolysis; sICH, symptomatic intracerebral hemorrhage.
Results
Between 2017 and 2021, a total of 585 AIS patients received IVT using r-tPA within 4.5 h after onset in this retrospective study. After excluding 79 patients (66 with missing data regarding r-tPA dose, 6 underwent arterial thrombolytic treatment or interrupted IVT, and 7 without sICH information), 506 patients were included in the final analysis (Figure 1). Ninety-six [19.0%] patients received a low dose of r-tPA. There were no significant differences in age (67 [IQR 59-75] vs 64 [58-74], p = 0.437), proportion of female sex (178 [35.2%] vs 29 [36.7%], p = 0.791), history of hypertension (342 [67.6%] vs 46 [58.2%], p = 0.102), diabetes (112 [22.1%] vs 14 [17.7%], p = 0.375), atrial fibrillation (162 [32.0%] vs 26 [32.9%], p = 0.874), previous ischemic stroke (67 [13.2%] vs 7 [8.9%], p = 0.276), and baseline NIHSS score (8 [IQR 4-15] vs 6 [IQR 3-11], p = 0.153) between those included and excluded in the final analysis.
Table 1 shows the differences in demographics and clinical characteristics among patients in the low-dose and standard-dose groups. Patients in the low-dose group were more likely to be older (median age, 69 [IQR 63-79] vs 67 [58-74], p = 0.011), had a previous ischemic stroke (20 [20.8%] vs 47 [11.5%], p = 0.015), a higher median baseline SBP (155mmHg [IQR 132-168] vs 147 [134 - 160], p = 0.017), and a higher baseline NIHSS score (8 [4-12] vs 6 [3-11], p=0.022). Regarding radiological findings, patients in the low-dose group were more likely to have hyperdense middle cerebral artery (MCA) sign on baseline non-contrast CT (12 [12.9%] vs 14 [3.5%], p < 0.001, data available in 490 patients).
Table 2 shows the association between baseline characteristics and the utilization of the low-dose, using a multivariable logistic regression analysis. The results showed that age (adjusted OR 1.02, 95%CI 1.00 - 1.04, p = 0.042), having a previous ischemic stroke (adjusted OR 2.01, 95%CI 1.11 - 3.64, p = 0.021) and having a higher baseline SBP (per-10mmHg increase, adjusted OR 1.12 95%CI 1.00 - 1.26, p = 0.049) were determinants for the utilization of the low-dose. There was a borderline association between baseline NIHSS score and the utilization of the low-dose (per-point increase, adjusted OR 1.04, 95%CI 1.00-1.08, p = 0.057).
Differences in baseline characteristics of patients in the standard-dose versus the low-dose alteplase
| Low-dose (n=96) | Standard-dose (n=410) | p-value | |
|---|---|---|---|
| Female (n, %) | 40 (41.7%) | 138 (33.7%) | 0.139 |
| Age (y, median, IQR) | 69 (63-79) | 67 (58-74) | 0.011 |
| Current smoker (n, %) | 29 (30.2%) | 111 (27.1%) | 0.537 |
| Hypertension (n, %) | 60 (62.5%) | 282 (68.8%) | 0.237 |
| Hyperlipidemia (n, %) | 37 (38.5%) | 146 (35.6%) | 0.590 |
| Diabetes (n, %) | 21 (21.9%) | 91 (22.2%) | 0.946 |
| Atrial fibrillation (n, %) | 33 (34.4%) | 129 (31.5%) | 0.582 |
| Previous ischemic stroke (n, %) | 20 (20.8%) | 47 (11.5%) | 0.015 |
| Previous ICH (n, %) | 0 | 0 | NA |
| Chronic heart failure (n, %) | 16 (16.7%) | 79 (19.3%) | 0.557 |
| Baseline SBP (mmHg, median, IQR) | 155 (132-168) | 147 (134-160) | 0.017 |
| Baseline blood glucose (mmol/L, median, IQR) | 6.8 (6.0-8.1) | 6.8 (5.9-8.5) | 0.788 |
| Baseline blood glucose ≥ 10mmol/L (n, %) | 14 (14.6%) | 63 (15.4%) | 0.848 |
| Baseline hypertense MCA sign (n, %)* | 12 (12.9%) | 14 (3.5%) | <0.001 |
| Type of occluded vessel (n, %) | 0.233 | ||
| Anterior circulation | 80 (84.2%) | 305 (74.6%) | |
| Posterior circulation | 6 (6.3%) | 39 (9.5%) | |
| Mixed | 6 (6.3%) | 36 (8.8%) | |
| Onset to thrombolysis time (min, median, IQR)† | 180 (130-237) | 170 (120-210) | 0.184 |
| Baseline NIHSS score (median, IQR) | 7.5 (4-12) | 6 (3-11) | 0.022 |
| sICH (n, %) | 2 (2.1%) | 32 (7.8%) | 0.074 |
| Fatal sICH (n, %) | 0 | 3 (0.73%) | >0.999 |
| Any ICH (n, %) | 15 (15.6%) | 74 (18.0%) | 0.574 |
| Discharge NIHSS | 2 (1-5) | 3 (1-6) | 0.931 |
| Discharge mRS 0-3 (n, %) | 74 (77.1%) | 296 (72.2%) | 0.331 |
| In-hospital mortality (n, %) | 0 | 12 (2.9%) | 0.135 |
Abbreviations: MCA, middle cerebral artery; NA, not applicable; NIHSS, national institute of health stroke scale; SBP, systolic blood pressure; sICH, symptomatic intracerebral hemorrhage;
*Data available in 490 patients.
†Data available in 470 patients.
A total of 34 (6.7%) sICH and 89 (17.6%) any ICH events were observed. Fatal sICH occurred in three patients who received the standard-dose alteplase, compared to none in the low-dose group. The rate of sICH was lower in the low-dose group compared to the standard-dose group, although only showing a trend towards the statistical significance (2[2.1%] vs 32 [7.8%], p = 0.074). The rate of any ICH was not significantly different between the low-dose and the standard-dose group (15 [15.6%] vs 74 [18.0%], p = 0.574). Patients in the low-dose and standard-dose groups did not differ in discharge NIHSS score (2 [1-5] vs 3 [1-6], p = 0.931), proportion of a discharge mRS score of 0-3 (74 [77.1%] vs 296 [72.2%], p = 0.331) and in-hospital mortality (0 vs 12 [2.9%], p = 0.135).
Table 3 summarizes the differences in baseline demographics and clinical characteristics between patients with and without sICH. Patients with sICH were older (median age, 73 [IQR 68-80] vs 67 [58-74], p < 0.001), more likely to have atrial fibrillation (25 [73.5%] vs 137 [29.0%], p < 0.001), diabetes (12 [35.3%] vs 100 [21.2%], p = 0.056), and a higher baseline NIHSS score (14 [IQR 9.5-17.5] vs 5 [3-11], p < 0.001). A lower proportion of hyperlipidemia were observed in patients with sICH compared to those without (7 [20.6%] vs 176 [37.3%], p = 0.05). Multivariable logistic regression analysis showed that the low-dose was significantly associated with a reduced risk of sICH (adjusted OR 0.13, 95%CI 0.03 - 0.62, p = 0.010, Table 4).
Table 5 shows that there were no significant differences in baseline demographics and clinical characteristics between patients who in the low-dose group and standard-dose group derived from a propensity score-matching cohort. The propensity score-matching analysis (88 in the low-dose group vs 88 in the standard-dose group) showed that the rate of sICH was significantly lower of the low-dose group compared to that of the standard-dose group (2 [2.3%] vs 10 [11.4%], p = 0.032). There was no significant difference in the rate of any ICH (14 (15.9%) vs 18 (20.5%), p = 0. 434), discharge NIHSS score (2 [1-5] vs 2 [1-7], p = 0.732), proportion of a mRS score of 0-3 (61 [69.3%] vs 56 [63.6%], p = 0.425), and in-hospital mortality (0 vs 3 [3.3%], p = 0.246) between two groups.
Discussion
The present study showed that AIS patients with increasing age, a higher baseline SBP and previous stroke were at a higher odd of receiving the low-dose of alteplase. Moreover, the low-dose of alteplase was associated with a lower risk of developing symptomatic intracranial hemorrhage.
Determinants for the utilization of the low-dose of alteplase
| Unadjusted (OR, 95%CI) | p-value | Age-sex-adjusted (OR, 95%CI) | p-value | Multivariable (OR, 95%CI) | p-value | |
|---|---|---|---|---|---|---|
| Age (continuous) | 1.03 (1.01-1.05) | 0.004 | / | / | 1.02 (1.00-1.04) | 0.042 |
| Female sex | 1.41 (0.89-2.22) | 0.140 | / | / | 1.28 (0.80-2.06) | 0.301 |
| Previous ischemic stroke | 2.03 (1.14-3.63) | 0.016 | 1.93 (1.07-3.48) | 0.029 | 2.01 (1.11-3.64) | 0.021 |
| Baseline NIHSS score (per-point increase) | 1.04 (1.00-1.08) | 0.031 | 1.03 (0.99-1.07) | 0.097 | 1.04 (1.00-1.08) | 0.057 |
| Baseline SBP (per-10 mmHg increase) | 1.13 (1.02 - 1.27) | 0.026 | 1.00 (1.00 - 1.24) | 0.071 | 1.12 (1.00 - 1.26) | 0.049 |
Abbreviations: SBP, systolic blood pressure; OR, Odds ratio; CI, confidence interval; NIHSS, national institute of health stroke scale.
Differences in baseline characteristics of patients who with and without sICH
| sICH (n = 34) | Non-sICH (n = 472) | p-value | |
|---|---|---|---|
| Female (n, %) | 15 (44.1%) | 163 (34.5%) | 0.258 |
| Age (median, IQR), y | 73 (68-80) | 67 (58-74) | <0.001 |
| Current smoker (n, %) | 7 (20.6%) | 133 (28.2%) | 0.339 |
| Hypertension (n, %) | 25 (73.5%) | 317 (67.2%) | 0.444 |
| Hyperlipidemia (n, %) | 7 (20.6%) | 176 (37.3%) | 0.05 |
| Diabetes (n, %) | 12 (35.3%) | 100 (21.2%) | 0.056 |
| Chronic heart failure (n, %) | 9 (26.5%) | 86 (18.2%) | 0.234 |
| Atrial fibrillation (n, %) | 25 (73.5%) | 137 (29.0%) | <0.001 |
| Previous ischemic stroke (n, %) | 4 (11.8%) | 63 (13.3%) | 0.999 |
| Baseline SBP (mmHg, median, IQR) | 142 (133-169) | 149 (134-163) | 0.573 |
| Baseline blood glucose (mmol/L, median, IQR) | 8.1 (6.5-11.6) | 6.8 (5.9-8.3) | 0.004 |
| Baseline blood glucose ≥ 10mmol/L (n, %) | 10 (29.4%) | 67 (14.2%) | 0.017 |
| Baseline MCA hypertense sign (n, %)* | 2 (6.3%) | 24 (5.2%) | 0.684 |
| Type of occluded vessel (n, %) | 0.203 | ||
| Anterior | 30 (88.2%) | 355 (75.5%) | |
| Posterior | 0 | 45 (9.6%) | |
| Mixed | 3 (8.8%) | 39 (8.3%) | |
| Onset to thrombolysis time (min)† | 169 (123-210) | 173 (120-210) | 0.951 |
| Baseline NIHSS score (median, IQR) | 14 (9.5-17.5) | 5 (3-11) | <0.001 |
| Low-dose (n, %) | 2 (5.9%) | 94 (19.9%) | 0.074 |
Abbreviations: MCA, middle cerebral artery; NIHSS, national institute of health stroke scale; SBP, systolic blood pressure; sICH, symptomatic intracerebral hemorrhage;
*Data available in 490 patients;
†Data available in 470 patients.
Previous studies showed that the utilization of the low-dose was mostly clinical relevant, including the prior use of antiplatelet treatments, antecedents of previous significant hemorrhage, or conditions that contraindicated the use of r-tPA [13]. Our data showed that patients who received a low dose of r-tPA were older (69 [63-79] vs 67 [58-74]), had a higher baseline SBP (155mmHg [132 - 168] vs 147 [134 - 160]), and a higher baseline NIHSS score (8 [4-12] vs 6 [3-11]). Our findings were supported by data from several multicenter, nationwide stroke registry-based observational studies [15-17]. The US stroke guideline does not limit advanced age [1]. However, the ECASS trials excluded those aged 80 years or older [13]. Observational data from the Safe Implementation of Treatments in Stroke (SITS) International Stroke Thrombolysis Register demonstrated the association between high baseline SBP and the risk of sICH [5]. Stroke severity assessed using the NIHSS score was the most relevant for an increased risk of sICH in AIS population. A meta-analysis of multiple randomized controlled trials showed that the risk of fatal sICH increased significantly with baseline NIHSS score in AIS patients who received intravenous alteplase [18]. However, IVT improved the overall likelihood of a good functional outcome at 3-6 months, irrespective of age or stroke severity [18]. Taken together, the low-dose of r-tPA would be favored when patients are thought to be at high bleeding risk, such as in older patients, or those with a higher baseline SBP and presenting a severe stroke.
In our cohort, the proportion of previous stroke was higher in the low-dose group compared to the standard-dose group (20 [20.8%] vs 47 [11.5%]). Patients with a history of ischemic stroke were excluded from most randomized controlled trials [12, 19, 20]. Consistent with our findings, an individual participant data of observational studies showed that the percentage of previous stroke was higher in patients who received a low-dose of alteplase compared to a standard-dose (1149 [20.6%] vs 657 [18.5%]), although the difference was not statistically significant [15]. There are several possible explanations. First, having a history of stroke within 90 days before the index stroke event is listed as a contradiction for IVT, based on the China stroke guideline [3]. However, some clinicians prefer to prescribe IVT to those with a low risk of intracerebral bleeding, based on their best clinical practice. A retrospective study [21] showed that having a history of previous stroke in less than three months did not translate to a worse outcome than those with first-ever stroke (OR: 1.62, 0.54 - 4.83). However, the wide 95% CI does not exclude the potential risk of intracerebral bleeding. Whether the low-dose of alteplase could reduce the risk of cerebral bleeding in patients with recurrent ischemic stroke remains unresolved and needs to be investigated in future large sample-sized studies.
Association between the low-dose of alteplase and the risk of sICH
| Unadjusted (OR, 95%CI) | p-value | Age- sex- adjusted (OR, 95%CI) | p-value | Multivariable | p-value | |
|---|---|---|---|---|---|---|
| Age (continuous) | 1.07 (1.03-1.10) | 0.001 | / | / | 1.06 (1.01-1.11) | 0.016 |
| Female sex | 1.50 (0.74-3.02) | 0.261 | / | / | 0.81 (0.35-1.85) | 0.614 |
| Hyperlipidemia | 0.44 (0.19-1.02) | 0.056 | 0.51 (0.22-1.22) | 0.129 | 0.55 (0.21-1.40) | 0.206 |
| Diabetes | 2.03 (0.97-4.24) | 0.060 | 1.80 (0.85-3.82) | 0.125 | 1.09 (0.40-2.96) | 0.878 |
| Atrial fibrillation | 6.79 (3.09-14.93) | <0.001 | 5.35 (2.40-11.94) | <0.001 | 3.20 (1.33-7. 70) | 0.009 |
| Baseline blood glucose (>10mmol/L) | 2.52 (1.15 - 5.50) | 0.021 | 2.70 (1.21 - 6.02) | 0.015 | 1.93 (0.64 - 5.83) | 0.245 |
| Baseline NIHSS score | 1.19 (1.12-1.26) | <0.001 | 1.18 (1.11-1.25) | <0.001 | 1.16 (1.08-1.24) | <0.001 |
| Low-dose | 0.251 (0.06-1.07) | 0.02 | 0.17 (0.04-0.76) | 0.02 | 0.13 (0.03-0.62) | 0.010 |
Abbreviations: OR, Odds ratio; CI, confidence interval; NIHSS, national institute of health stroke scale.
Differences in baseline characteristics of low dose and standard dose using the propensity score-matching sample
| Low-dose (n = 88) | Standard-dose (n = 88) | p-value | |
|---|---|---|---|
| Female, (n, %) | 36 (40.9%) | 26 (29.5%) | 0.115 |
| Age, (median, IQR),y | 68 (60-78) | 67 (61-72) | 0.151 |
| Hypertension, (n, %) | 54 (61.4%) | 67 (73.9%) | 0.076 |
| Hyperlipidemia, (n, %) | 34 (38.6%) | 42 (47.7%) | 0.223 |
| Diabetes, (n, %) | 19 (21.6%) | 23 (26.1%) | 0.479 |
| Chronic heart failure, (n, %) | 14 (15.9%) | 18 (20.5%) | 0.434 |
| Atrial fibrillation (n, %) | 29 (33.0%) | 33 (37.5%) | 0.528 |
| Previous ischemic stroke (n, %) | 14 (15.9%) | 8 (9.1%) | 0.171 |
| Baseline NIHSS score (median, IQR) | 7 (4-12) | 7 (4-12) | 0.732 |
| Hyperdense MCA sign (n, %)* | 10 (11.4%) | 6 (6.8%) | 0.294 |
| Type of occluded vessel (n, %) | 0.640 | ||
| Anterior | 78 (84.1%) | 71 (80.7%) | |
| Posterior | 5 (5.7%) | 4 (4.3%) | |
| Mixed | 6 (6.8%) | 11 (12.5%) | |
| Baseline SBP (mmHg) (median, IQR) | 155 (132-165) | 151 (136-167) | 0.696 |
| Baseline blood glucose (mmol/L) | 6.7 (6.0-8.1) | 7.0 (6.1-8.6) | 0.352 |
| Baseline blood glucose ≥ 10mmol/L (n, %) | 14 (15.9%) | 17 (54.8%) | 0.553 |
| Discharge NIHSS | 2 (1-5) | 2 (1-7) | 0.732 |
| Discharge mRS 0-3 (n, %) | 61 (69.3%) | 56 (63.6%) | 0.425 |
| sICH (n, %) | 2 (2.3%) | 10 (11.4%) | 0.032 |
| Any ICH (n, %) | 14 (15.9%) | 18 (20.5%) | 0.434 |
| Inhospital Mortality (n, %) | 0 (0.0%) | 3 (3.3) | 0.246 |
Abbreviations: MCA, middle cerebral artery; NIHSS, national institute of health stroke scale; SBP, systolic blood pressure; sICH, symptomatic intracerebral hemorrhage;
* Data available in 160 patients.
Our univariate analysis showed that the low-dose was non-statistically significantly different among patients with and without sICH (although there was a trend towards the statistical significance). However, our multivariate analysis showed that the low-dose was associated with a lower risk of sICH (adjusted OR 0.13, 95%CI 0.03 - 0.62, p = 0.01). One potential explanation is that patients in the low-dose group were older, had a higher baseline SBP, and presented a more severe stroke, all of which are risk factors for sICH [5,22]. Therefore, the between group differences may be underestimated. Our propensity score matching data confirmed the association between the low-dose alteplase and a reduced risk of sICH, after balancing these factors. Notably, the low-dose and standard-dose did not differ in discharge NIHSS, mRS 0-3 (good functional outcome), and in-hospital mortality. Our findings were supported by some previous studies [7,23]. The ENCHANTED trial [7] showed that major sICH occurred less frequently in the low-dose group compared to the standard-dose group (1.0% vs 2.1%, p=0.01). Moreover, the low-dose of alteplase was non-inferior to the standard dose in the ordinal analysis of mRS scores (unadjusted common OR 1.00; 95% CI 0.89 to 1.13; p=0.04 for non-inferiority). Data from a China national prospective stroke registry also revealed that in AIS patients with moderate severity of stroke, the lower-dose alteplase was associated with a lower risk of sICH and non-inferior performance in efficacy [23]. In contrast, data from a randomized controlled trial and an observational study [15,24] did not show significant differences in the occurrence of sICH events between the low-dose and the standard-dose group. Whether the low-dose of alteplase may have a safer profile in treating AIS needs to further investigated. For example, evidence on optimal alteplase dosage in bridging thrombectomy after IVT remains scarce. A Korean prospective observational study [25] showed that AIS patients who received different doses of alteplase in the context of bridging therapy had no difference in functional outcome, whereas the risk of ICH was lower in the low-dose group compared to the standard-dose group (8% vs. 3%, p = 0.056). The optimal amount of thrombolysis alteplase for bridging thrombectomy in AIS is worth further investigating.
There are several explanations for the heterogeneous results regarding the relationship between alteplase dose and sICH risk of the abovementioned studies. First, different definitions of sICH were used, which may affect the diagnostic rate of sICH. In the present study, the ECASS-II criteria was used, which is a well-validated method to assess the sICH occurrence. Second, ethnic differences should not be underestimated. It has been reported that Asian populations have a higher risk of developing intracerebral bleeding events caused by intravenous thrombolysis [26]. An observational study found that Japanese populations had a higher incidence of thrombolytic intracranial hemorrhage than Caucasians due to ethnic differences in clotting and fibrinolytic factor [27]. Moreover, Asians were more likely to benefit from the low-dose alteplase [28].
Our study has some limitations. First, this is a retrospective observational study with a moderate sample size, inevitably introducing selection bias. Second, we only included AIS patients within 4.5 hours after onset. Our results therefore could not be generalized to those with stroke on awakening from sleep or unknown onset, or treatment in an extended time-window. Second, we only included East-Asian stroke population; therefore our findings are not generalizable to other ethnic stroke populations. Third, the low number of sICH events does not permit subgroup analysis stratified by age or stroke severity. Data from previous observational studies of East-Asian stroke populations supported a trend that low-dose tPA seemed to be safer in elderly patients [29,30]. Fourth, we only assessed discharge functional outcomes; 90-day or longer-term follow-up of functional outcomes were not analyzed in the present study. Finally, our analysis was subject to casual post hoc grouping of alteplase doses, and thus, it is difficult to draw any conclusions based on the proper comparison.
Conclusion
The current study showed that patients with increasing age, a higher baseline blood pressure, and previous stroke were at a higher odd of receiving a low dose of alteplase for patients with acute ischemic stroke within 4.5 hours of onset. The low-dose was related to a reduced risk of developing symptomatic intracranial hemorrhage. Further studies are needed to determine a safer profile for a low dose of alteplase in a selected stroke patient population.
Abbreviations
AIS: acute ischemic stroke; MCA: middle cerebral artery; mRS: modified Rankin scale; NIHSS: National Institutes of Health Stroke Scale; IQR: Interquartile range; IVT: intravenous thrombolysis; OR: odds ratios; CI: confidence interval; r-tPA: recombinant tissue-plasminogen activator; SBP: systolic blood pressure; SD: Standard deviation.
Acknowledgements
Funding
This study was sponsored by Fujian provincial health technology project (2021CXA012).
Ethics approval and consent to participate
This study was approved by the local ethics committees. Written informed consents were waived due to the nature of our retrospective study using routine anonymous data.
Author contributions
Concept and design: J Chen and H Du. Acquisition, analysis, or interpretation of data: J Chen, H Li, and H Du. Drafting of the manuscript: J Chen, H Li, and H Du. Critical revision of the manuscript for important intellectual content: H Lei, S Fang, Q Yuan, Y Chen, D Chen, R Chen, Y Zhang, J Wei, G Chen, and N Liu. Statistical analysis: J Chen and H Du. H Du had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Availability of data and materials
Data are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K. et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019Dec;50(12):e344-e418 doi: 10.1161/STR.0000000000000211
2. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C. et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021Mar;6(1):I-LXII doi: 10.1177/2396987321989865
3. Liu L, Chen W, Zhou H, Duan W, Li S, Huo X. et al. Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. 2020Jun;5(2):159-176 doi: 10.1136/svn-2020-000378
4. Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis. 2012;34(2):106-114 doi: 10.1159/000339675
5. Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D. et al. SITS Investigators. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012Jun;43(6):1524-1531 doi: 10.1161/STROKEAHA.111.644815
6. Rao NM, Levine SR, Gornbein JA, Saver JL. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the National Institute of Neurological Disorders and Stroke tissue-type plasminogen activator trials. Stroke. 2014Sep;45(9):2728-2733 doi: 10.1161/STROKEAHA.114.005135
7. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, Levi C. et al. ENCHANTED Investigators and Coordinators. Low-Dose versus Standard-Dose Intravenous Alteplase in Acute Ischemic Stroke. N Engl J Med. 2016Jun16;374(24):2313-2323 doi: 10.1056/NEJMoa1515510
8. Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T. Japan Alteplase Clinical Trial II Group. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke. 2010Mar;41(3):461-465 doi: 10.1161/STROKEAHA.109.573477
9. Cheng JW, Zhang XJ, Cheng LS, Li GY, Zhang LJ, Ji KX. et al. Low-Dose Tissue Plasminogen Activator in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis. 2018Feb;27(2):381-390 doi: 10.1016/j.jstrokecerebrovasdis.2017.09.014
10. Liu H, Zheng H, Cao Y, Pan Y, Wang D, Zhang R. et al. Low- versus Standard-Dose Intravenous Tissue-Type Plasminogen Activator for Acute Ischemic Stroke: An Updated Meta-Analysis. J Stroke Cerebrovasc Dis. 2018Apr;27(4):988-997 doi: 10.1016/j.jstrokecerebrovasdis.2017.11.005
11. Criddle LM, Bonnono C, Fisher SK. Standardizing stroke assessment using the National Institutes of Health Stroke Scale. J Emerg Nurs. 2003Dec;29(6):541-546 doi: 10.1016/j.jen.2003.08.011
12. Meyer L, Bechstein M, Bester M. et al. Thrombectomy in Extensive Stroke May Not Be Beneficial and Is Associated With Increased Risk for Hemorrhage. Stroke. 2021Oct;52(10):3109-3117 doi:10.1161/STROKEAHA.120.033101
13. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D. et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998Oct17;352(9136):1245-1251 doi: 10.1016/s0140-6736(98)08020-9
14. Brunser AM, Mazzon E, Cavada G, Mansilla E, Rojo A, Almeida J. et al. Low dosis of alteplase, for ischemic stroke after Enchanted and its determinants, a single center experience. Arq Neuropsiquiatr. 2020Nov;78(11):681-686 doi: 10.1590/0004-282X20200048
15. Wang X, Li J, Moullaali TJ, Lee KJ, Kim BJ, Bae HJ. et al. Low-dose versus standard-dose alteplase in acute ischemic stroke in Asian stroke registries: an individual patient data pooling study. Int J Stroke. 2019Oct;14(7):670-677 doi: 10.1177/1747493019858777
16. Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC. et al. Low-Versus Standard-Dose Alteplase for Ischemic Strokes Within 4.5 Hours: A Comparative Effectiveness and Safety Study. Stroke. 2015Sep;46(9):2541-2548 doi: 10.1161/STROKEAHA.115.010180
17. Nakagawara J, Minematsu K, Okada Y, Tanahashi N, Nagahiro S, Mori E. et al. J-MARS Investigators. Thrombolysis with 0.6 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post-Marketing Alteplase Registration Study (J-MARS). Stroke. 2010Sep;41(9):1984-1989 doi: 10.1161/STROKEAHA.110.589606
18. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E. et al. Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014Nov29;384(9958):1929-1935 doi: 10.1016/S0140-6736(14)60584-5
19. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995Dec14;333(24):1581-1587 doi: 10.1056/NEJM199512143332401
20. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W. et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007Jan27;369(9558):275-282 doi: 10.1016/S0140-6736(07)60149-4
21. Karlinski M, Kobayashi A, Czlonkowska A, Mikulik R, Vaclavik D, Brozman M. et al. Safe Implementation of Treatments in Stroke-East Registry (SITS-EAST) Investigators. Intravenous Thrombolysis for Stroke Recurring within 3 Months from the Previous Event. Stroke. 2015Nov;46(11):3184-3189 doi: 10.1161/STROKEAHA.115.010420
22. Nisar T, Hanumanthu R, Khandelwal P. Symptomatic Intracerebral Hemorrhage after Intravenous Thrombolysis: Predictive Factors and Validation of Prediction Models. J Stroke Cerebrovasc Dis. 2019Nov;28(11):104360. doi: 10.1016/j.jstrokecerebrovasdis.2019.104360
23. Dong Y, Han Y, Shen H, Wang Y, Ma F, Li H. et al. Who may benefit from lower dosages of intravenous tissue plasminogen activator? Results from a cluster data analysis. Stroke Vasc Neurol. 2020Dec;5(4):348-352 doi: 10.1136/svn-2020-000388
24. Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I. et al. Japan Alteplase Clinical Trial (J-ACT) Group. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke. 2006Jul;37(7):1810-1815 doi: 10.1161/01.STR.0000227191.01792.e3
25. Kim JS, Kim YJ, Lee KB, Cha JK, Park JM, Hwang Y. et al. Low- versus Standard-Dose Intravenous Alteplase in the Context of Bridging Therapy for Acute Ischemic Stroke: A Korean ENCHANTED Study. J Stroke. 2018Jan;20(1):131-139 doi: 10.5853/jos.2017.01578
26. Mehta RH, Cox M, Smith EE, Xian Y, Bhatt DL, Fonarow GC. et al. Get With The Guidelines-Stroke Program. Race/Ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke. 2014Aug;45(8):2263-2269 doi: 10.1161/STROKEAHA.114.005019
27. Iso H, Folsom AR, Sato S, Wu KK, Shimamoto T, Koike K. et al. Plasma fibrinogen and its correlates in Japanese and US population samples. Arterioscler Thromb. 1993Jun;13(6):783-790 doi: 10.1161/01.atv.13.6.783
28. Ueshima S, Matsuo O. The differences in thrombolytic effects of administrated recombinant t-PA between Japanese and Caucasians. Thromb Haemost. 2002Mar;87(3):544-546
29. Huang Y, Sharma VK, Robinson T, Lindley RI, Chen X, Kim JS. et al. ENCHANTED investigators. Rationale, design, and progress of the ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy (ENCHANTED) trial: An international multicenter 2 × 2 quasi-factorial randomized controlled trial of low- vs. standard-dose rt-PA and early intensive vs. guideline-recommended blood pressure lowering in patients with acute ischaemic stroke eligible for thrombolysis treatment. Int J Stroke. 2015Jul;10(5):778-788 doi: 10.1111/ijs.12486
30. Chao AC, Hsu HY, Chung CP, Liu CH, Chen CH, Teng MM. et al. Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) Study Group. Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) study. Stroke. 2010May;41(5):885-890 doi: 10.1161/STROKEAHA.109.575605
Author contact
![]() Corresponding author: Asso Prof. Houwei Du, Department of Neurology, Fujian Medical University Union Hospital, 29 Xinquan Road, Gulou District, Fuzhou, China. 35000. E-mail: houweiduedu.cn, ORCID: https://orcid.org/0000-0002-5978-9734.
Corresponding author: Asso Prof. Houwei Du, Department of Neurology, Fujian Medical University Union Hospital, 29 Xinquan Road, Gulou District, Fuzhou, China. 35000. E-mail: houweiduedu.cn, ORCID: https://orcid.org/0000-0002-5978-9734.

 Global reach, higher impact
Global reach, higher impact