Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(12):1732-1742. doi:10.7150/ijms.77484 This issue Cite
Research Paper
Effect of No-ozone Cold Plasma on Regeneration after Crushed Mental Nerve Injury in rats
1. Department of Oral and Maxillofacial Surgery, Dental and Life Science Institute, Dental School, Pusan National University, Busan, South Korea.
2. Department of Research and Development, FEAGLE Corporations, 70‑6, Jeungsan‑ro, Mulgeum‑eup, Yangsan‑si, Gyeongsangnam‑do 50614, South Korea.
3. Department of Translational Dental Science, School of Dentistry, Pusan National University, Beomeo-ri, Mulgeum-eup, Yangsan-si, Gyeongsangnam-do, 50612, South Korea.
4. Department of Internal Medicine, School of Korean Medicine, Yangsan Campus of Pusan National University, Yangsan-si, Gyeongsangnam-do, 50612, South Korea.
5. Dental Research Institute, Pusan National University Dental Hospital, Yangsan, South Korea.
Received 2022-7-26; Accepted 2022-9-9; Published 2022-9-25
Abstract
Background: This experimental research aimed to determine whether No-ozone Cold Plasma (NCP) has regenerative effect on crushed injured sensory nerves in a rat model (Wistar A) and to evaluate whether NCP can be used as an alternative treatment method for sensory nerve injury in the oral-maxillofacial region.
Methods: A total of 10 Wistar A rats were used for this experiment. They were divided into three groups according to whether the mental nerve of the left mandible was injured and NCP was applied or not: group 1 (n=3) (non-mental nerve damage, non-MD) - the left mental nerve was exposed and non-damaged; group 2 (n=3) (mental nerve damage, MD) - the left mental nerve was exposed and damaged, NCP was not applied; and group 3 (n=4) (mental nerve damage and NCP, MD-NCP) - the left mental nerve was exposed and damaged, NCP was applied with regular intervals (three times a week).
Results: For the behavior analysis, von Frey test was used. Furthermore, the nerve tissues were examined with hematoxylin and eosin (H&E) staining, and the extent of neurorecovery was evaluated with the immunofluorescence staining of certain markers. The behavioral analysis showed that the function recovery sensory nerve was faster in group 3 (MD-NCP). In the histomorphologic and immunofluorescence analyses, the expression of the factors involved in neurorecovery was much higher in group 3 than in group 2 (MD).
Conclusions: The expeditious recovery of sensory nerve function as well as the higher expression of the factors indicating nerve function recovery in the NCP-treated group suggest that NCP has a positive effect on regeneration after sensory nerve crushing injury. Therefore, in the case of sensory impairment of the oral-maxillofacial region, no-ozone cold plasma can be applied for therapeutic effect.
Keywords: No-ozone Cold Plasma (NCP), Peripheral nerve injury, mental Nerve Crush Injury, Skeletal Muscle Healing, Macrophage, Myelin Sheath, Neuronal Axon.
Introduction
Dental implants have become commonplace for oral rehabilitation. As the use of dental implants increases, the frequency of side effects from them also increases. The most representative of these side effects is nerve damage due to implant placement [1, 2].
In the oral and maxillofacial region, the trigeminal nerve dominates the senses. The mental nerve is the most injured terminal branch of trigeminal nerves during oral and maxillofacial treatment [3, 4]. Nerve damage in the oral and maxillofacial area interferes with daily life, which adversely affects the patient's psychosocial state and causes his or her quality of life to deteriorate [5]. There have been reports of permanent neurological damage from 0 to 36% causing lip paresthesia after implant placement [6-8], but recent studies have reported that 2.95% of patients have transient neurological damage and 1.7% have permanent neurological damage [9, 10].
The over prepared implant hole allows the implant to enter the nerve canal or directly damage the nerve bundle [11]. This process usually results in nerve compression or transection damage. When nerve damage occurs due to implant placement, clinicians must decide whether to do nerve anastomosis, treat it with pharmacologic agents only, or remove the implant immediately.
Surgical methods like nerve anastomosis or nerve graft are the gold standard for the treatment of nerve injury, but because the micro surgeon can perform microsurgery only if the equipment is available, general clinics cannot easily perform it [12-15]. The postoperative repair and sensory nerve function recovery rates range from 50 to 60%, and the methods have been reported to be effective in some patients [16-20].Vitamin B, ATP, steroid, and alpha lipoic acid (ALA) are used as pharmacologic agents, but these have not yet been proven to be effective [21].
The use of plasma is one of the newly introduced alternative methods of treatment for nerve injury. Plasma is the fourth state of matter and has high energy. After its introduction in the 1850s, Plasma, a biomedicine, was introduced in the 21st century [22, 23].
Earlier, argon gas was used to reduce the amount of ozone and NO compounds generation from nozone (no-ozone) cold plasma device. However, lately Feagle Co. Ltd. (Yangsan, South Korea) has developed a NCP device, by which non-thermal plasma can be used with several applications. It has got distinctive features. It not only generate very limited amount of ozone but also maintain the optimum temperature of 35C or lower. This is the crucial feature in order to use the NCP in the study as the ozone and NO compounds can harm the respiratory system.
NCP contains reactive oxygen species, high-energy electrons, and ions. As such, it is known to modulate a number of biological responses, including inflammatory response [24, 25]. NCP is known to be highly applicable to cancer treatment and skin anti-aging, sterilization, teeth bleaching and wound healing promotion [26-32].
In previous studies, after inflicting crush or cut injury to the sciatic nerve of rats, NCP was applied to investigate the therapeutic effect [33]. These studies revealed that the function recovery and neuronal regeneration greatly improved after plasma application.
To quantify and measure the recovery of the function of the motor nerves, such as the sciatic nerve, the static sciatic index (SSI) can be used. It is difficult to quantify sensory nerve function recovery in animal experiments compared to motor nerve function recovery because animals cannot communicate their sensory experiences [34].
Thus, to test animals, sensory nerve function recovery, electrical, thermal, physical, or chemical stimuli can be used [35]. For mechanical testing, the von Frey test is a representative method that can be used for both rats and mice [36]. It makes use of the von Frey filament, a plastic filament with blunt ends that can apply 0.008-300 g pressure. Paw withdrawal, which occurs when pressure is applied to the experimental animals by the von Frey filament, indicates sensory nerve function recovery or pain. In the von Frey test in this study, however, pressure was applied to the facial part of the experimental animals, and head withdrawal was used to measure their sensory nerve function recovery.
The purpose of this study was to investigate the effects of NCP on nerve injury caused by crushing injury to the mental nerves of rats. This was the third study that evaluated NCP's effects on peripheral nerve regeneration, and the first using only a sensory nerve injury model.
Materials & Methods
Plasma generating device
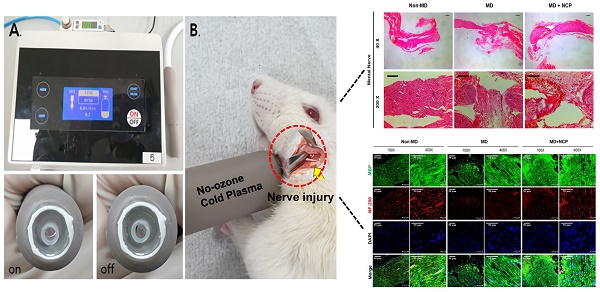
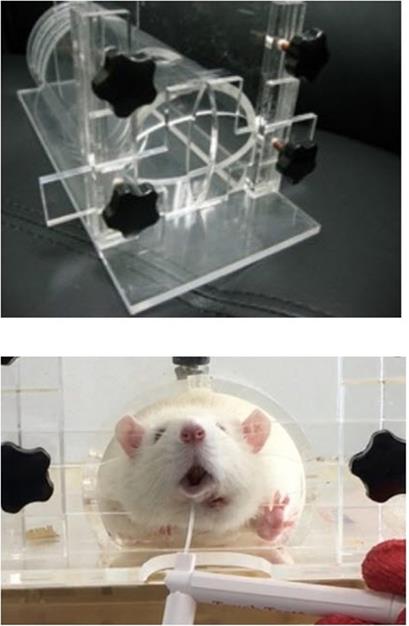
For this study, a dielectric-barrier-discharge-(DBD)-type NCP device developed by FEAGLE Corporation (Yangsan-si, South Korea) was adopted. Argon gas was used as a buffer gas, and 2.0 slm (standard liter per minute) of it was blown into the plasma source. NCP was formed by applying a high voltage (3 kV) to the plasma source. Plasma glow was formed within the electrodes, but it did not extend to the end of the electrodes. The temperature of the NCP flow at 1 cm from the electrode end was maintained under 35°C for 10 minutes, and no UVs were detected at this condition. Along with that, the value of ozone in this device was found to be 0.006ppm which seems to be much less than the level of ozone recommended by Food and Drug Administration (FDA) that is 0.05ppm.The distance of the skin from the electrodes was kept at 1 cm [Figure 1].[33, 37-39]
The NCP-generating device (A) and the NCP treating methods on rat (B).
Animal experiments
A total of 10 male Wistar A rats 6 weeks of age and weighing between 200 and 225 grams were used. The nerves of the rats were so close to their skins that they did not require re-incision to apply NCP. Rats' nerves are very similar to the human mental nerve in this regard, so the use of rats is very suitable for the experiment. All the experiments were conducted in accordance with the ethical guidelines of Pusan National University Institutional Animal Care and Use Committee (PNUIACUC, South Korea) and the regulations set out by International Association for the Study of Pain (IASP) in Animals (PNU-2019-2276). It is also complied with the ARRIVE guidelines. The experiment was conducted on three groups. Group 1 (n=3) was the control group, which underwent the same operation as that undergone by another group, but without any nerve injury. Groups 2 (n=3) and 3 (n=4) underwent the same surgical procedure, but only group 3 received NCP to the skin overlying the injured mental nerve 3 times a week for 8 weeks [Table 1] [Figure 2].
After the completion of the experiment, animal euthanasia was performed using CO2 gas chamber. The cage was kept as it was in CO2 gas chamber and the flow of the gas was maintained for about 1-3 minutes until the death was observed.
Surgical procedure
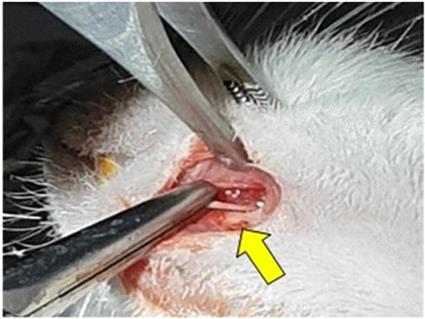
To anesthetize the animals, Intraperitoneal (IP) injection of a cocktail of 100 mg/kg Ketamine (Yuhan, Seoul, South Korea) and 10 mg/kg Xylazine (Rompun, BAYER KOREA Ltd., Seoul, South Korea) was used. A 0.3mL solution containing 1:100000 epinephrine and 2% lidocaine HCl was used for the local anesthesia, and cefazolin (50 mg/kg) was injected preoperatively subcutaneously, as a prophylactic antibiotic. After complete anesthesia was achieved, the rats were fixed with pins in a lateral position on the disinfected surgical plate. The surgical area of each rat's left mandible was shaved using an electric clipper, and was scrubbed with povidone iodine solution for disinfection. An about 1-cm-long skin incision was made at an angle from the left mandible, and the subcutaneous tissue was carefully dissected, with the mental nerve exposed. In group 1, the skin layer was sutured with 6-0 ETHILON synthetic non-absorbable monofilament suture in a continuous pattern, without any nerve injury. In groups 2 and 3, the mental nerve was crushed with a hemostat 5 mm proximal to the mental foramen for 30 seconds, and then skin suture was done with the same pattern as in group 1. Group 1 and Group 2 were not treated with NCP. In group 3, NCP was applied to the skin over the damaged mental nerve.
Time line: NCP application, nerve damage and behavior animation records.
Classification of Control and Experimental Groups.
| Exposure of Mental Nerve | Crush Injury of mental Nerve | NCP Applying | Number of Rats | |
|---|---|---|---|---|
| Group 1 Non-mental Nerve Damage (Non-MD) | O | X | X | 3 |
| Group 2 Mental Nerve Damage (MD) | O | O | X | 3 |
| Group 3 Mental Nerve Damage with NCP (MD-NCP) | O | O | O | 4 |
Non-MD: non-mental nerve damage; MD: mental nerve exposed and damaged without NCP; MD-NCP: mental nerve exposed and damaged with NCP.
After NCP treatment, rats were anesthetized, and the NCP was irradiated for 5 minutes at the distance of 0.5 cm from the skin. [Figure 3].
Behavior analysis
Behavioral analysis was conducted in the morning for 8 weeks, once every 2 weeks from 2 weeks after the surgery. Each rat's body was fixed in a cage made of a transparent acrylic plate (HEAD OUT RESTRAINER, Jeung Do Bio & Plant Co., Ltd., Seoul, South Korea), but even when the body was locked in the box, the rat's head was not fixed and could move freely. Behavior analysis was performed with a tablet PC (iPad Pro, Apple, California, USA) installed 20 cm away from the front of the box, and the behavioral experiment was recorded in a video.
The withdrawal test with von Frey filament (Stoelting Co., Wood Dale, IL, USA) was used for behavioral analysis. Withdrawal response was defined as the case where there is a movement opposite the direction of filament application. A 300g filament force was applied to both the left and right sides of each rat's lower lip. The withdrawal response rate difference between the experiment and opposite sites was recorded through video playback [Figure 4].
Histomorphologic analysis
One day after the final NCP treatment, all the experimental rats were sacrificed, and the nerve tissues of the surgical areas were isolated. All the tissue samples were fixed with 4% paraformaldehyde for 24 hr., and then embedded in paraffin. The tissue sections (5 μm thick) were subjected to hematoxylin and eosin (H&E) staining, and the morphology of the nerve tissues was then visualized by taking pictures of them using an iCM 9.0 digital camera system (IMT I-solution Inc., NY, USA) coupled with light microscopy (CX31, Olympus, Tokyo, Japan).
Immunofluorescence analysis
The 5-μm-thick tissue sections were treated with antibodies against CD68 and myelin basic protein (Santa-Cruz Biotechnology, Santa Cruz, CA, USA), neurofilament 200, S100, and tau (Abcam, Cambridge, MA, USA), and were incubated for 2 h at 37℃. After being washed 4 times with phosphate-buffered saline, the tissue sections were treated with anti-mouse Alexa Fluor-488 and anti-rabbit Alexa Fluor-594 (Thermo Fisher Scientific, Rockford, IL, USA) for 1 h at 37℃. After the washing, the nuclei of the cells within the tissues were counterstained with 4', 6-diamidino-2-phenylindole. The fluorescence from the tissues was observed, and images were captured using a Carl Zeiss LSM 780 confocal laser microscope.
Statistical analysis
Each experiment was repeated three times with standard deviations indicated as error bars. Experimental and control groups were compared using Excel's paired t-test (Microsoft, Redmond, WA, USA). Statistical significance was determined to be p < 0.05.
Results
Animal Behavioral Analysis showed that NCP treatment accelerated the recovery rate of mental nerve damage
Nerve injury: crushing with hemostat (yellow arrow).
Head out restrainer (Jeung Do Bio & Plant Co., LTD. Seoul, South Korea) and the Withdrawal test using von Frey filament.
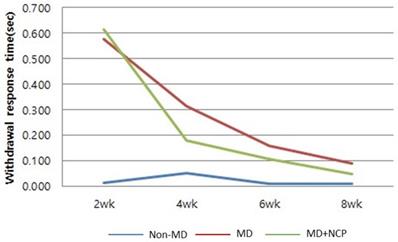
The behavioral recovery of the mental nerve was evaluated to address sensory nerve function recovery. In group 1 (non-mental-nerve damage, non-MD), within 2 weeks, the withdrawal response time was already almost the same as that on the unaffected right side. In groups 2 (mental nerve damage, MD) and 3 (mental nerve damage-NCP, MD-NCP), there was no significant difference in withdrawal response time at week 2. At week 4, the withdrawal response time rate was significantly decreased in group 3 than in group 2. At week 8, the withdrawal response time rate was already on the same level as that on the unaffected right side in both groups 2 and 3, but the recovery was slightly greater in group 3 [Figure 5].
NCP treatment accelerated the recovery of damaged areas of the mental nerve
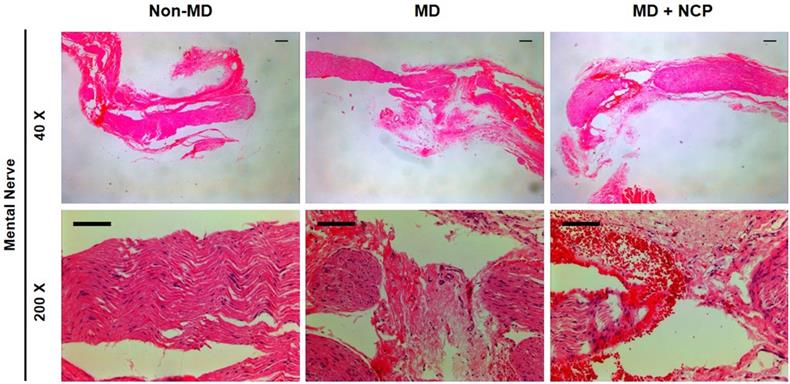
The differences in neural tissue recovery among the three groups were also observed, as can be seen in [Figure 6]. The part of the mental nerve where crushing damage was applied with a hemostat was observed under a microscope. In H&E staining, the nuclei of the cells were stained in violet to identify the cells, and the eosinophilic parts were the axon and myelin sheath of the nerve fibers [40]. There was a clear difference in the number of cell nuclei between groups 2 and 3. Obviously, there was a large number of cell nuclei in group 3, suggesting that the number of cells was higher.
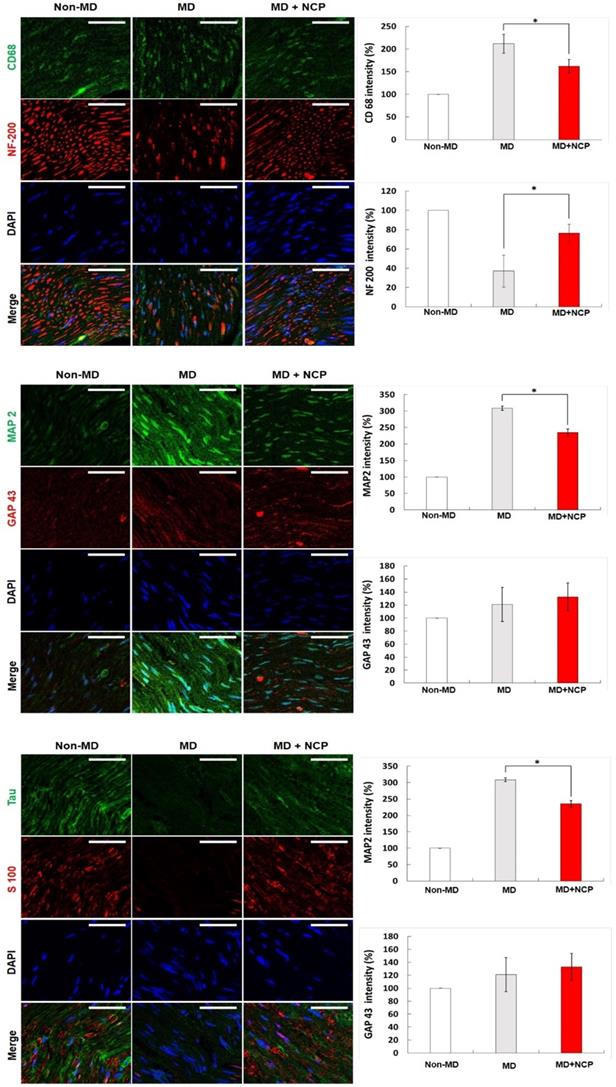
NCP restored the neuronal axon and myelin sheath and decreased the overexpression of CD68 positive macrophage
Immunofluorescence confirmed the markers for nerve regeneration, and compared the differences between the groups.
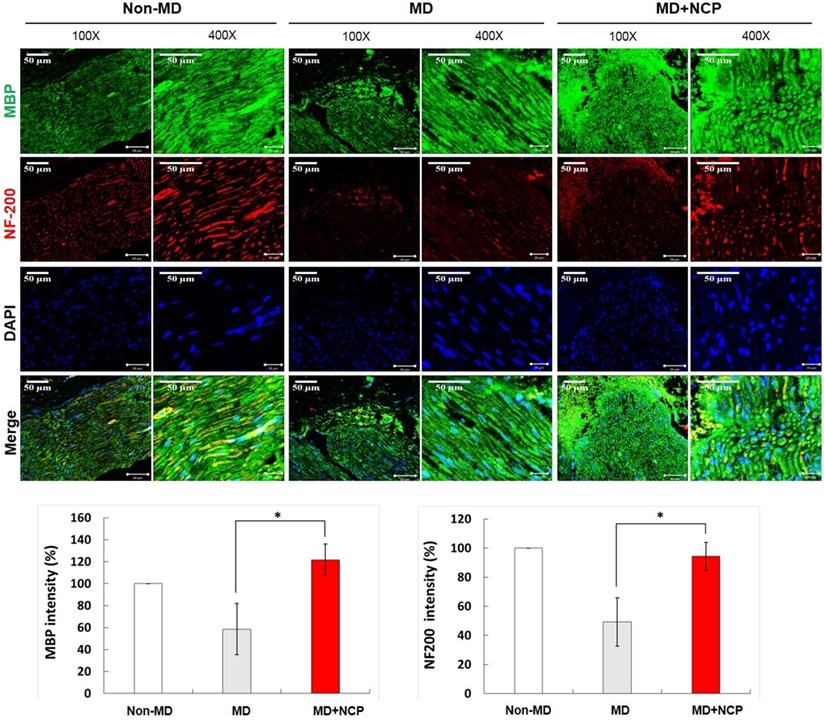
Compared with MBP, the crushed part of MD, the MD+NCP group was broken, but the MD group seemed to have maintained the damaged part as it was. The plasma-treated group, however, had more intense MBP expression, and it was observed that myelin was regenerated. [Figure 7].
CD68 and MAP2 were the most expressed in group 2, and their expression level in group 3 appeared to be similar to that in group 1.
NF-200 was highly expressed in group 3, as in group 1. In group 2, however, it was hardly expressed, and its continuity was lost, whereas in group 3, the continuity was recovered to the same level as in group 1. DAPI was more expressed in both groups 2 and 3 than in group 1, and was most expressed in group 3.
The effect of NCP on mental nerve crushing damage recovery: Withdrawal response time. At week 4, the withdrawal response time rate was significantly decreased in group 3 than in group 2.
As for GAP 43, it was more expressed in groups 2 and 3 than in group 1, and it was more expressed in group 3 than in group 2.
S100, and tau were significantly less expressed in group 2 and were expressed at similar levels in groups 3 and 1. Overall, group 3, which was treated with NCP, showed immunofluorescent staining similar to that in group 1 compared to group 2 (without NCP) [Figure 8].
Discussion
Plasma has been used biologically and medically for the last few decades. Low-pressure plasma, with the thermal effect of plasma, was initially mainly used, whereas No-ozone Cold Plasma (NCP) was developed and used in the early 1990s. The primary purpose of using plasma was material processing, such as rendering plastics or cloth more hydrophilic or hydrophobic. In the mid-1990s, however, the discovery of the bacterial inactivation or wound healing promotion effects of plasma led to the development of “plasma medicine” as the interest in the relationship between plasma and the biological cells increased [41, 42].
DBD, a type of NCP, is the source of reactive oxygen species (ROS) and reactive nitrogen species (RNS). OH, O, NO, H2O2, and O2- are known to have biological implications. Through these active molecules (both radical and non-radical), NCP was found to break the “equilibrium” of the redox process, to stimulate cell signaling, and to affect the cells [41].
The effect of plasma has been proven in various medical fields, including dentistry, and its utilization is increasing due to the convenience of the DBD plasma equipment. In addition, the ability to control the tissue activity of plasma can be considered in the treatment of tissue damage, such as nerve damage that can occur in dentistry [43, 44]
According to the results of a previous study of crush injury to the sciatic nerve, 3 weeks after the injury was incurred, the nerve function was fully recovered in the NCP-treated group whereas only 60% of the nerve function was recovered in the group whose injury was healed without NCP treatment [32]. As NCP was applied to the rats' skins and muscles during the experiment, histological examination was performed considering the possibility of promoting wound healing. In the NCP-treated group, the diameter and density of the cells near the muscle injury increased. This was due to the NCP satellite cell proliferation promotion effect [45], which is effective for the recovery of the muscles around the nerves as well as of the motor nerves.
The effect of NCP on mental nerve crushing damage recovery: The number of cells is the highest in the NCP treated group (H&E stain). *Scale bars 100µm
Immunofluorescence in neural tissue and MBP and NF-200 intensity (%) in the three groups. In the NCP treatment group, both MBP and DAPI stained more intensely. * MBP: Myelin Basic Protein, myelin sheath marker. * DAPI: 4', 6-diamidino-2-phenylindole. * Scale bars 50µm
To assess the recovery of the motor function of the sciatic nerve in the rat model, SSI, which was introduced by Bervar in 2000, can be used [46]. On the other hand, for analyzing sensory nerve function recovery, the von Frey test can be used. The von Frey filament is a plastic filament with blunt ends that can apply 0.008-300 g pressure. The filament is used to measure the amount of pressure needed to be applied to make an experimental rat withdraw its paw after its stimulation. To quantify, researchers can use the 50% threshold devised by Chaplan et al. [47].
In this study, the von Frey test was applied to the lower lip of the experimental rats. As the lower lips of rats, however, are covered by hair, unlike the paws, they are difficult to access, which limits the application of the von Frey filament thereto. In this experiment, the sensory nerve function recovery rate was determined by measuring the time to head evasion on the non-operated and surgical sides, with the gram of filament fixed.
Restrictions on the movement of rats into the restrainer can cause significant stress and can affect the experiment results. To prevent this, the restrainer was repeatedly adjusted for about 1 week before the experiment. In addition, the existing restrainer has a structure that suppresses the rat's neck in three directions (upper, left, and right), but the restrainer parts in the upper direction were removed in the experiment in this study. As such, it did not interfere with the breathing of the rats, but it inhibited the rats' movement and reduced the stress on them. In addition, the experiment was repeated to reduce the experiment errors and to improve the reproducibility.
Immunofluorescence in neural tissue in three groups (x400): NF-200, CD68, MAP2 were weakly expressed and GAP43, S100 and tau were strongly expressed in the NTP treatment group. * NF-200: Neurofilament-heavy chain, mature nerve cell marker. * CD68: Macrophage marker. * MAP2: Microtubule-associated protein 2. * GAP43: growth-associated protein 43. * Scale bars 50µm.
There have been no previous studies that conducted the von Frey test on the faces of rats, which limits the quantification of sensory nerve function recovery. In this regard, further research on sensory measurement is needed. The results of the behavioral analysis in this study showed that after 4 weeks, the withdrawal response rate of group 3 increased even more significantly. This suggests that NCP can make a positive contribution in promoting the rate of sensory nerve function recovery in crushing-injured nerves.
In the H&E staining, much more nuclei were observed in the MD-NCP group. This may confirm that NCP stimulated cell division to restore the injured area.
MBP is produced in the Schwann cells, which contain myelin, essential for maintaining the structure and function of the peripheral nerves [48]. Increased expression of MBP implies correction of the structural defects in damaged nerves. The expression of MBP increased in group 3 compared to group 2, which suggests that NCP promotes MBP expression and recovery of the function of injured mental nerves.
NF-200 is a marker found only in A-fiber, which is the myelinated nerve fiber distributed in the sensory nerves [49]. In the MD group in this study, there was continuity of NF-200, but in group 2, there was none, and in group 3, it was gradually recovered. In addition, the expression of NF-200 was increased significantly more in group 3 than in group 2, which also supports the conclusion that NCP promotes repair of damaged nerves.
DAPI is a marker that can confirm the total number of cells by binding to double-stranded DNA. In this experiment, DAPI was expressed more in groups 2 and 3 than in group 1. This suggests that several cells actively proliferated to repair the nerve damage, and especially that the DAPI expression in group 3 was higher than that in group 2 because NCP promotes cell proliferation.
CD68 expressed by macrophage was more expressed in group 2. Overexpressed CD68 may also slow inflammation by making it become chronic. This suggests that the inflammatory response persisted in group 2, and that inflammation was suppressed and nerve regeneration progressed in group 3.
MAP2 is responsible for the production of somatodendritic cytoskeleton, and GAP43 is a factor that initially regulates axon growth and plasticity. Nerve regeneration is mainly performed by axon, and increased dendrite production prevents axonal growth [46]. In group 3, which was treated with NCP, the dendrite production did not increase, suggesting that the environment for axon regeneration was well established.
The S100 and tau are essential for neuroskeleton and axon regeneration. Tau is one of the microtubule-associated proteins (MAPs) primarily present in the axons of normal neurons. It increases the stability of the microtubules in vitro. Although many studies on it have been reported, the actual role and importance of the tau cells have yet to be found and established. To date, research suggests that tau may be involved in axon development at the developmental stage of cells, and may regulate microtubule assembly and dynamics as well as the transport of axons in organelles like mitochondria. The significantly increased expression of tau in group 3 compared to group 2 in this study suggests that NCP promotes axon regeneration [50].
The aforementioned results are similar to those of previous studies that inflicted crushing and cut injury on the sciatic nerve. In the NCP-treated group, MBP, NF-200, GAP-43, S100B, and tau, which are involved in axonal regeneration, were increased, and CD68 and MAP2, which have negative effects on regeneration, were decreased [33].
The previous studies that analyzed the structural differences between the motor and sensory nerves showed that the motor neurons have more neurotrophic factors, such as insulin-like growth factor, basic fibroblast growth factor, and ciliary neurotrophic factor. It has also been reported that the motor neurons have larger Schwann cell basal lamina tubes than the sensory nerves. This resulted in more effective nerve regeneration when the motor nerve rather than the sensory nerve was grafted into the nerve gap. It can be inferred that structurally, the ability to regenerate damaged sensory nerves is slightly lower than the ability to regenerate damaged motor nerves. Therefore, the effect of NCP on sensory nerve function recovery after injury is more important. Such effect can be maximized by applying NCP to the injured sensory nerves that are slightly lacking in self-regeneration [51-57].
Both the histomorphologic and immunofluorescence analyses showed that NCP had a positive effect on promoting the recovery of the structure of the mental nerve with crushing injury. Also, in the behavioral analysis, NCP was found to have promoted the regeneration of the damaged sensory nerves. These results are in line with the results of the previous study. As this study was the first to have looked into the effect of NCP on the maxillofacial region, further study on the effect of NCP on the nerve regeneration of the maxillofacial region of other animal models is needed for the future application of NCP for nerve regeneration in the human maxillofacial region.
Conclusion
The results of this study, which examined the nerve regeneration after applying NCP to the crushed/injured mental nerve of rats shows that the use of NCP can have a positive effect in the progression of nerve functional recovery and neuronal regeneration. This suggests the possibility of non-invasive treatment of sensory impairment in the oral and maxillofacial region, such as sensory impairment due to dental implant placement, etc.
Abbreviations
NCP: No-Ozone Cold Plasma; non-MD: non-Mental nerve damage; MD: Mental nerve damage; MD-NCP: Mental nerve damage + No-Ozone Cold Plasma.
Acknowledgements
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by Korea government (MSIT) [Grant #: 2020R1A2C110051912].
Author contributions
Conceptualization: Dae- Seok Hwang; Methodology: Yoon- Seo Jang and Jeong- Hae Choi.; Formal analysis: Si- Yeon Park; Investigation: Si-Yeon Park and Yoon-Seo Jang; Data Curation: Si- Yeon Park and Yoon-Seo Jang; Writing—Original draft preparation: Si- Yeon Park; Writing—Review and Editing: Si- Yeon Park, M Shriya Jaiswal, Jin- Woo Hong and Dae- Seok Hwang; Supervision: M Shriya Jaiswal, Gyoo- Cheon Kim; Project administration and Funding acquisition: Dae- Seok Hwang. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ziccardi VB, Assael LA. Mechanisms of trigeminal nerve injuries. Atlas Oral Maxillofac Surg Clin North Am. 2001;9:1-11
2. Steinberg MJ, Kelly PD. Implant-related nerve injuries. Dent Clin North Am. 2015;59:357-73
3. Jerjes W, Upile T, Shah P, Nhembe F, Gudka D, Kafas P. et al. Risk factors associated with injury to the inferior alveolar and lingual nerves following third molar surgery-revisited. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:335-45
4. Pogrel MA, Kaban LB. Injuries to the inferior alveolar and lingual nerves. J Calif Dent Assoc. 1993;21:50-4
5. Abarca M, Van Steenberghe D, Malevez C, De Ridder J, Jacobs R. Neurosensory disturbances after immediate loading of implants in the anterior mandible: an initial questionnaire approach followed by a psychophysical assessment. Clinical Oral Investigations. 2006;10:269-77
6. Ellies LG, Hawker PB. The prevalence of altered sensation associated with implant surgery. Int J Oral Maxillofac Implants. 1993;8:674-9
7. Bartling R, Freeman K, Kraut RA. The incidence of altered sensation of the mental nerve after mandibular implant placement. Journal of Oral and Maxillofacial Surgery. 1999;57:1408-10
8. Wismeijer D, Van Waas MAJ, Vermeeren JIJF, Kalk W. Patients' perception of sensory disturbances of the mental nerve before and after implant surgery: a prospective study of 110 patients. British Journal of Oral and Maxillofacial Surgery. 1997;35:254-9
9. Dannan A, Alkattan F, Jackowski J. Altered Sensations of the Inferior Alveolar Nerve after Dental Implant Surgery: a Retrospective Study. Dentistry. 2013 13
10. Greenstein G, Carpentieri JR, Cavallaro J. Nerve damage related to implant dentistry: incidence, diagnosis, and management. Compend Contin Educ Dent. 2015;36:652-9 quiz 60
11. Worthington P. Injury to the inferior alveolar nerve during implant placement: a formula for protection of the patient and clinician. Int J Oral Maxillofac Implants. 2004;19:731-4
12. Zuniga JR, O'Connor B. Primary and secondary microneuroanastomotic repair of the mental nerve in the rat. International Journal of Oral and Maxillofacial Surgery. 1987;16:465-72
13. Ziccardi VB, Steinberg MJ. Timing of trigeminal nerve microsurgery: a review of the literature. J Oral Maxillofac Surg. 2007;65:1341-5
14. Renton T, Dawood A, Shah A, Searson L, Yilmaz Z. Post-implant neuropathy of the trigeminal nerve. A case series. British Dental Journal. 2012;212:E17-E
15. Bagheri SC, Meyer RA, Cho SH, Thoppay J, Khan HA, Steed MB. Microsurgical repair of the inferior alveolar nerve: success rate and factors that adversely affect outcome. J Oral Maxillofac Surg. 2012;70:1978-90
16. Susarla SM, Lam NP, Donoff RB, Kaban LB, Dodson TB. A Comparison of Patient Satisfaction and Objective Assessment of Neurosensory Function After Trigeminal Nerve Repair. Journal of Oral and Maxillofacial Surgery. 2005;63:1138-44
17. Pogrel MA. The results of microneurosurgery of the inferior alveolar and lingual nerve. Journal of Oral and Maxillofacial Surgery. 2002;60:485-9
18. Lam NP, Donoff RB, Kaban LB, Dodson TB. Patient satisfaction after trigeminal nerve repair. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2003;95:538-43
19. Strauss ER, Ziccardi VB, Janal MN. Outcome Assessment of Inferior Alveolar Nerve Microsurgery: A Retrospective Review. Journal of Oral and Maxillofacial Surgery. 2006;64:1767-70
20. Gregg JM. Studies of traumatic neuralgia in the maxillofacial region: Symptom complexes and response to microsurgery. Journal of Oral and Maxillofacial Surgery. 1990;48:135-40
21. Horasanli B, Hasturk AE, Arikan M, Togral G, Helvacioglu F, Dagdeviren A. et al. Comparative evaluation of the electrophysiological, functional and ultrastructural effects of alpha lipoic acid and cyanocobalamin administration in a rat model of sciatic nerve injury. J Back Musculoskelet Rehabil. 2017;30:967-74
22. Chirokov A, Gutsol A, Fridman A. Atmospheric pressure plasma of dielectric barrier discharges. Pure and Applied Chemistry. 2005;77:487-95
23. Kong MG, Kroesen G, Morfill G, Nosenko T, Shimizu T, van Dijk J. et al. Plasma medicine: an introductory review. New Journal of Physics. 2009;11:115012
24. Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied Plasma Medicine. Plasma Processes and Polymers. 2008;5:503-33
25. Heinlin J, Morfill G, Landthaler M, Stolz W, Isbary G, Zimmermann JL. et al. Plasma medicine: possible applications in dermatology. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2010;8:968-76
26. Arndt S, Wacker E, Li Y-F, Shimizu T, Thomas HM, Morfill GE. et al. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Experimental Dermatology. 2013;22:284-9
27. Partecke LI, Evert K, Haugk J, Doering F, Normann L, Diedrich S. et al. Tissue Tolerable Plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer. 2012;12:473
28. Choi JH, Lee HW, Lee JK, Hong JW, Kim GC. Low-temperature atmospheric plasma increases the expression of anti-aging genes of skin cells without causing cellular damages. Arch Dermatol Res. 2013;305:133-40
29. Klämpfl TG, Isbary G, Shimizu T, Li Y-F, Zimmermann JL, Stolz W. et al. Cold Atmospheric Air Plasma Sterilization against Spores and Other Microorganisms of Clinical Interest. Applied and Environmental Microbiology. 2012;78:5077-82
30. Park JK, Nam SH, Kwon HC, Mohamed AAH, Lee JK, Kim GC. Feasibility of nonthermal atmospheric pressure plasma for intracoronal bleaching. International Endodontic Journal. 2011;44:170-5
31. Antioxidants and Novel Therapeutics. Free Radical Biology and Medicine. 2009; 47: S136-S58.
32. Daeschlein G, Scholz S, von Woedtke T, Jünger M. Cold plasma antisepsis for skin and wounds: a new antimicrobial concept in dermatology; 2012
33. Lee ST, Jang YS, Kim UK, Kim HJ, Ryu MH, Kim GC. et al. Non-thermal plasma application enhances the recovery of transected sciatic nerves in rats. Exp Biol Med (Maywood). 2021;246:1287-96
34. Ahn DK, Park MK. Animal Models for Orofacial Neuropathic Pain. Hanyang Medical Reviews. 2011;31:107
35. Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597-652
36. Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39-50
37. Choi J-H, Lee H-W, Lee J-K, Hong J-W, Kim G-C. Low-temperature atmospheric plasma increases the expression of anti-aging genes of skin cells without causing cellular damages. Archives of Dermatological Research. 2013;305:133-40
38. Choi J-H, Song Y-S, Lee H-J, Hong J-W, Kim G-C. Inhibition of inflammatory reactions in 2,4-Dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Scientific Reports. 2016;6:27376
39. Park N-S, Yun S-E, Lee H-Y, Lee HJ, Choi J-H, Kim G-C. No-ozone cold plasma can kill oral pathogenic microbes in H2O2-dependent and independent manner. Scientific Reports. 2022;12:7597
40. Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of Axons after Nerve Transection Repair Is Enhanced by Degradation of Chondroitin Sulfate Proteoglycan. Experimental Neurology. 2002;176:221-8
41. Laroussi M, Lu X, Keidar M. Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. Journal of Applied Physics. 2017;122:020901
42. Laroussi M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Transactions on Plasma Science. 1996;24:1188-91
43. Keidar M, Robert E. Preface to Special Topic: Plasmas for Medical Applications. Physics of Plasmas. 2015;22:121901
44. Volotskova O, Dubrovsky L, Keidar M, Bukrinsky M. Cold Atmospheric Plasma Inhibits HIV-1 Replication in Macrophages by Targeting Both the Virus and the Cells. PLOS ONE. 2016;11:e0165322
45. Choi JW, Kang SU, Kim YE, Park JK, Yang SS, Kim YS. et al. Novel Therapeutic Effects of Non-thermal atmospheric pressure plasma for Muscle Regeneration and Differentiation. Scientific Reports. 2016;6:28829
46. Bervar M. Video analysis of standing-an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods. 2000;102:109-16
47. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55-63
48. Amici SA. Peripheral Myelin Protein 22 Is in Complex with 6beta4 Integrin, and Its Absence Alters the Schwann Cell Basal Lamina. Journal of Neuroscience. 2006;26:1179-89
49. Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkühler J. Modification of classical neurochemical markers in identified primary afferent neurons with Aβ-, Aδ-, and C-fibers after chronic constriction injury in mice. The Journal of Comparative Neurology. 2007;502:325-36
50. Mandelkow EM, Mandelkow E. Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006247-a
51. Shapiro LA, Whitaker-Azmitia PM. Expression levels of cytoskeletal proteins indicate pathological aging of S100B transgenic mice: an immunohistochemical study of MAP-2, drebrin and GAP-43. Brain Res. 2004;1019:39-46
52. Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. Journal of Cell Biology. 1990;110:1307-17
53. Rabinovsky ED. The multifunctional role of IGF-1 in peripheral nerve regeneration. Neurological Research. 2004;26:204-10
54. Grothe C, Haastert K, Jungnickel J. Physiological function and putative therapeutic impact of the FGF-2 system in peripheral nerve regeneration-lessons from in vivo studies in mice and rats. Brain Res Rev. 2006;51:293-9
55. Grothe C, Wewetzer K, Lagrange A, Unsicker K. Effects of basic fibroblast growth factor on survival and choline acetyltransferase development of spinal cord neurons. Developmental Brain Research. 1991;62:257-61
56. Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. The Journal of Neuroscience. 1990;10:3507-15
57. Moradzadeh A, Borschel GH, Luciano JP, Whitlock EL, Hayashi A, Hunter DA. et al. The impact of motor and sensory nerve architecture on nerve regeneration. Experimental Neurology. 2008;212:370-6
Author contact
![]() Corresponding author: Dae-Seok Hwang (DDS, PhD). Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Beomeo-ri, Mulgeum-eup, Yangsan-si, Gyeongsangnam-do, 50612, South Korea. Tel: +82-055-360-5110; Email: dshwangac.kr; ORCID: 0000-0001-6899-1769
Corresponding author: Dae-Seok Hwang (DDS, PhD). Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Beomeo-ri, Mulgeum-eup, Yangsan-si, Gyeongsangnam-do, 50612, South Korea. Tel: +82-055-360-5110; Email: dshwangac.kr; ORCID: 0000-0001-6899-1769

 Global reach, higher impact
Global reach, higher impact