Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(11):1695-1705. doi:10.7150/ijms.75285 This issue Cite
Review
Insights into Exosome in the Intervertebral Disc: Emerging Role for Disc Homeostasis and Normal Function
Department of Orthopedic, Xijing Hospital, Fourth Military Medical University. Western Changle Road, Xi'an, 710032, Shannxi Provence, P. R. China.
#Equal contributors to this work.
Received 2022-5-19; Accepted 2022-9-16; Published 2022-9-25
Abstract
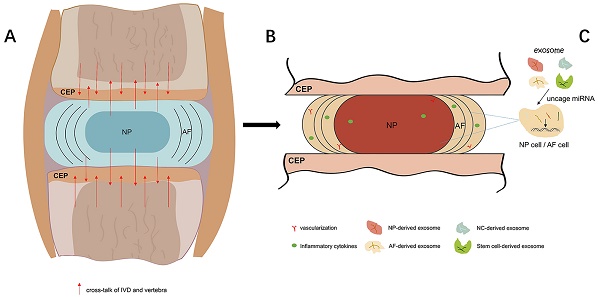
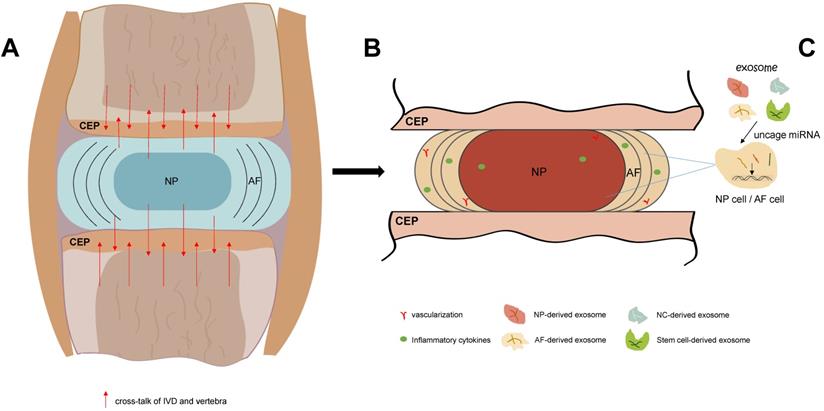
Low back pain (LBP) is a chronic condition that causes great individual suffering and economic burden. The major contributor of LBP is intervertebral disc degeneration (IDD), which is caused by a spectrum of homeostasis alteration, including the apoptosis of nucleus pulposus (NP) and annulus fibrosus (AF) cells, degradation of extracellular matrix (ECM), calcification of cartilaginous endplates (CEP) and so on. Currently, the therapeutic strategy for IDD includes conservative and surgery treatment. Nevertheless, none of them could reverse the progressive destruction of the intervertebral disc. Hence, it is pivotal to pursue a new therapeutic approach. Exosomes, nano-sized substances with diameters of 30-150 nm, can be synthesized and secreted by various types of cells. They play an important role in intercellular communication. Increasing evidence implicates that exosomes could impact the intracellular transcription activities, thereby inhibiting or accelerating the proliferation and apoptosis of cells. Thus, it is a new therapeutic source for IDD. This review chiefly focuses on generalizing and clarifying the roles of exosomes in the onset and deterioration of IDD, and their therapeutic potential.
Keywords: exosome, intervertebral disc, low back pain, stem cells, intervertebral disc degeneration
Introduction
Low back pain (LBP) is a very common symptom which not only leads to individual suffering, but social and economic burden [1]. The direct economic expenditure of LBP and health care is estimated to be around $87 billion in the US a year [2]. In Korean, LBP is the second disease in socioeconomic burden and expenditure for management [3]. The highest incidence of LBP happens to the people over 44 years old [2]. With the average life expectancy longer than ever, it is urgent to discover the safest and most efficacious treatment for LBP.
The etiology of LBP is multifactorial, and the major contributor is considered to be intervertebral disc degeneration (IDD) [4-8]. The risk factors of IDD mainly include age [9], genetic predisposition [10-12], mechanical damage [13], high body mass index, obesity [14] and so on [15]. Current therapeutic strategies for IDD contain conservative and surgical options, but the outcomes are not invariable pleased [16]. Besides, patients might not rehabilitate after surgery. Therefore, it is urgent to find a new therapeutic means which could reverse the degeneration of intervertebral disc (IVD) and free the patients from soreness.
Exosome was first visualized and named in the 1980s, which was considered to be the dumpster of cell and a way to dispose of unwanted molecules [17-20]. However, recent studies gradually find that the function of exosome is far more than just delivering unwanted molecules [21, 22]. It, as a tiny membrane vesicle which carries miRNA, mRNA and protein, is the mediator of intercellular communication and plays a pivotal role in the onset and deterioration of IDD [23, 24]. This review tries to summarize the observations about various cells-derived exosome and their function in the outset and development of IDD. In addition, we also discussed the exosome-based biological therapy of IDD.
Intervertebral Disc Degeneration
Structure of IVD
IVD, derived as the tissue that bears load of spine, constitute one-third of the height of spine [25, 26]. As the largest avascular structure of the body, IVD is composed of nucleus pulposus (NP), annulus fibrosus (AF) and cartilaginous endplates (CEP) [27]. NP, a high-pressured and hydrophilic structure, is composed of water, cell and extracellular matrix (ECM). The ratio of water in NP is around 70-90%, which indicates that NP is the highest water content part of IVD [28]; chondrocyte-like cells and notochordal cells are the main part of NP cells (NPc), which could secrete cartilage-like ECM components; ECM, made up of collagen, elastin, proteoglycans, and glycoproteins, participates in the metabolism and mechanical function of NP. AF, a ring-shaped disc of fibrosus connective tissue, is the fundamental load-bearing complex of IVD. It is composed of outer and inner annulus. The outer annulus principally comprises fibroblasts and the collagenated lamella; the inner annulus mainly consists of chondrocyte-like cells. CEP, a cartilaginous structure, forms the interface between adjacent vertebral segments [29, 30]. Due to the avascular feature of IVD, the essential nutrients and oxygen all supplied via the concentration gradients exchange in CEP.
In IVD, the volume of cells is only 1%, with the average cells density is nearly 6000/mm3. NP cells density is 5-103/mm3, AF cells density is 9-103/mm3 [31, 32]. The rare density of IVD cells signifies that when the IVD is impaired, the restoration rate is slow.
Pathogenesis of IDD
A spectrum of pathogenic factors and intercellular effects participate in the IDD progression [33]. For instance: superfluous mechanical stress; excessive oxidative stress; adverse inflammatory cytokines [34-40]. Besides, diabesity and high body mass index which might change the micro-vasculature of CEP and adjacent vertebral body also influence the nutrient supply into IVD, thus accelerating the development of IDD [39-41].
The metabolic dysregulation of NPc leads to the suppression of synthesis of ECM; the lamellae in the AF become irregular; and the calcification of the tiny pores within CEP decreases the supply of nutrition and oxygen [42-45]. All of which further aggravate the degeneration of IVD, unbalance the homeostasis of IVD, thus inducing the senescence and apoptosis of NPc and strengthening the permeation of inflammatory cytokines [46-50]. Significantly, AF cells are sensitive to mechanical pressure. The alteration of ECM composition and senescence of NPc leading to exceeding load onto AF, thus inducing the apoptosis of AF cells and rupture of AF [51, 52]. In addition, vascellums and nerves grow into IVD, causing pain [53-57]. Taken together, these multifactorial changes eventually cause the collapse of IVD structure.
Current Therapy of IDD
Currently, the therapeutic strategy for IDD mainly includes conservative and operative options [16]. The conservative therapeutic strategy includes non-pharmacological and pharmacological therapy. The operative therapeutic strategy includes discectomy with/without fusion and total disc replacement (TDR) [58, 59]. However, in conservative therapeutic strategy, the effect of non-pharmacological therapy is ambiguous; pharmacological therapy might cause drug addiction. In operative therapeutic strategy, complications after discectomy surgery might inevitable; the area of application of TDR is harsh [60].
The conservative and operative therapeutic strategies mainly aimed at relieving symptoms rather than reversing pathogenic progression of IDD. Therefore, it is essential to explore a novel therapeutic strategy which could delay and/or reverse the pathogenic process of IDD. Significantly, the stem cells and stem cell-derived exosome (SC-exo) based therapy is a newly developing non-invasive therapeutic approach. It has potent ability to suppress the IDD progression [61, 62]. Hence, summarizing the function of exosome in IDD and property of exosome-based biotherapy is necessary.
Exosome Derived from IVD Cells
Structure and Function of Exosome
Exosome is a nano-sized substance which has spheroid membranes of a uniform lipid bilayer. It is a type of extracellular vesicle [63], which also includes microvesicles and apoptotic bodies [22]. The diameter range of exosome are in the 30-150 nm; microvesicles are in the 200-1000 nm; apoptotic bodies are in the 800-5000 nm [64, 65]. Average diameter of exosome is 100 nm, with the density ranging from 1.13 g/ml to 1.19 g/ml [66-69]. It was first described by Stahl and Johnstone in the 1980s, as nano-sized vesicles discovered during reticulocyte maturation [17,70]. At the earliest, it was hypothesized that the function of exosome was to eliminate unwanted proteins of cellular from cytoplasm [19]. With further exploration in exosome, studies suggest that the function of exosome is potent and remarkable. It is not only the dumpster of cell, but could conduct the intercellular communication and response induction [21, 71].
Numerous types of cells could secrete exosome. It is detected in various body fluids, such as breast milk, cerebral spinal fluid, amniotic fluid, bile, saliva, semen, blood, lymph and amniotic fluid [72-77]. Exosome is derived from endocytic pathway, in which the early secretory endosome was formed during the inward budding of the intracellular endsomal membrane, intracellular multivesicular bodies containing intraluminal vesicles are formed. With the maturation of the endosome, intraluminal vesicles were secreted as exosome by fusion with the plasma membrane [78, 79]. Hitherto, studies reveal that exosome could secrete various bioactive molecules, such as mRNA, miRNA and proteins, and affect receptor cells by transmitting bioactive molecules [24]. However, more studies are needed to explore the underlying mechanism of their roles.
Exosome Derived from NP
Increasing evidence implicates that NPc and NP stem cell-derived exosome participate in the process of IDD. Chen et al. took the IDD structure of rat caudal vertebra, re-cultured it in medium, thus utilizing IL-1β inducing senescence of NPc and isolating the senescence NPc-derived-exosome (SNPC-Exo) [80]. They uncovered that incubated with SNPC-Exo made normal NPc evinced a degradation-related manifestation, particularly in the declined capacity of colony and proliferation. Besides, they revealed that SNPC-Exo mainly regulates and promotes the senescence of NPc by targeting the P53/P21 signaling pathway. All these results supported that SNPC-Exo might possess bioactive substances that derived from SNPC and play a key role in accumulating the senescence of normal NPc.
Various inflammatory and pre-inflammatory cytokines, such as IL-1β, IL-6, MMP-13 and TNF-α, participate in the process of IDD [81-86]. Zhang et al. [87] discovered that degenerative NPc could secrete exosome which carried miR-16 and directly inhibited the anti-apoptotic IGF-1 / IGF-1R signaling pathway, thereby accumulating the apoptosis of NPc. This research demonstrated that degenerative NPc could affect the normal NPc by secreting exosome to withhold the anti-apoptotic pathway, leading to the senescence and degeneration of NPc.
Accumulating evidence implicated that NPc-derived-exosome (NPc-exo) not only could influence NPc, but exerting effect to CEP. Feng et al. [88] revealed that degenerative NPc-exo (dNPc-exo) could be taken up by CEP cells (CEPc), thereby decreasing the expression of Bcl-2, increasing the expression of Bax and Caspase-3, which are cell apoptosis makers. Thus, they elucidated that dNPc-exo could induce the apoptosis of CEPc. Also, they suggested that dNPc-exo could promote the degradation of ECM and the IDD process. Collectively, this study demonstrated the intercommunication between NPc-exo and CEPc, showing that all parts of IVD cells have intimate correlation.
Autophagy, as a catabolic self-digestion progression, could sustain the cellular homeostasis via dislodging dysfunctional organelle debris or hazardous macromolecules [89]. In human chondrocytes, rapamycin could activate autophagy of articular chondrocytes, thereby promoting the secretion of extracellular vesicles [90]. Zhang et al. [91] utilized rapamycin to accumulate the autophagy of NPc and found that the deliverance of NPc-exo was promoted. They further investigated that rapamycin-induced NPc-exo could carry miR-27a to target and inhibit MMP-13, thereby suppressing the degradation of ECM and delaying IDD progression. Also, they elucidated that autophagy could promote the secretion of NPc-exo via targeting the RhoC/ROCK2 pathway [92]. This research provides a potential strategy for fabricating a vast amount of bio-synthesizing exosome.
Notochordal cells (NC) is the precursor cell of NPc. It appears in the embryonic stage and is gradually replaced by NPc during the development period in human. The remaining NC in the NP progressively disappeared after adolescence, and the degenerative process of IVD commenced [93]. Our group [94] revealed that notochordal cell-derived exosome (NC-exo) could mitigate the vascularization process of IDD. We, for the first time, unearthed the NC-exo and demonstrated that NC-exo could alleviate angiogenesis via carrying high expressed miR-140-5p to endothelial cells, thus regulating the downstream Wnt/β-catenin pathway. Additionally, we suggested that, for NC, 0.5 MPa is a suitable mechanical condition for luring the secretion of NC exosomes and its capacity of anti-vascularization. Consequently, with a spectrum of experiments, we elucidated that NC-exo plays a pivotal role in the anti-angiogenesis effect of IVD and IDD progression.
Nevertheless, the specific biological mechanism of NPc-exo/NC-exo, its role in the process of IDD and its intercommunication with other IVD cells remains vague, needing further exploration and illustration.
Exosome Derived from AF
Recently, we [95] isolated AF-derived exosome (AF-exo) and stimulated the depravity of AF cell (AFc). With a spectrum of experimentations, we unveiled that degenerative AFc (dAFc) could secrete exosome, thus excreting pro-vascularization effect by promoting cell migration and inflammatory factor expression. Interestingly, the exosome secreted by non-degenerative AFc (ndAFc) could prevent blood vessels from growing in and retain the homeostasis of IVD. So, we manifested that ndAFc and dAFc derives different types of exosome and acts opposite roles in the degradation process of IVD. Nevertheless, there is still no study investigates about the underlying molecular mechanism of AF-exo function.
Exosome Derived from CEP
While investigation in CEP chondrocyte-derived exosome is scarce, studies have shown the existence of CEP stem cell-derived exosome (CESCs-exo).
Luo et al. [96] isolated and extracted the cartilage endplate stem cells (CESCs) and CESCs-exo. They revealed that CESCs-exo could promote the autophagy and withhold the apoptosis of NPc by activating PI3K/AKT/autophagy signaling pathway. Notably, they found that non-degenerative CESCs-exo is more effective than degenerative CESCs-exo. Besides, they figured out that non-degenerative CESCs-exo could impetus CESCs changing into NPc. CEP inflammation, however, influences this progression and aggravates IDD procession. Therefore, this study showed that CESCs-exo plays a striking role in the NPc degeneration. In the follow-up research, Luo et al. [97] found that CESCs-exo could activate HIF-1α/Wnt signaling pathway via autocrine mechanisms, thus promoting the secretion of TGF-β1 and GATA4. All of which suggested that CESCs-exo could accumulate the CESCs transforming into NPCs and delay the development of IDD. Chen et al. [98] detected that miR-125-5p, secreted by CESCs-exo, could target histone methyltransferase (SUV39H1), thereby promoting NPc autophagy, suppressing NPc apoptosis and delaying ECM degradation.
Collectively, all of these studies confirmed that CESCs-exo participates in the process of IDD. Thus, it is feasible to hypothesize that exosome derived from CEP cells (CEP-exo) also involves in IDD procession. Like AF-exo, non-degenerative CEP-exo and degenerative CEP-exo might play different roles: non-degenerative CEP-exo delays and reverses the process of IDD; degenerative CEP-exo induces and accelerates the process of IDD. However, this hypothesis needs further research to prove.
Exosome Derived from Stem Cells
Stem cells, the most primitive cells at the top of the origin of cell lines, have multi-directional differentiation potential and self-renewal potency. They are abundant and easy to obtain, can proliferate in the low oxygen circumstances. Notably, mesenchymal stem cells (MSC), as a type of stem cells, has the ability of self-renewal and multi-directional differentiation [99-101]. It was first isolated from bone marrow, and then unearthed in many tissues. For example: periosteum; muscle; placenta; fat; umbilical cord; umbilical cord blood; and other tissues [102-104]. It has great therapeutic potential. In the field of IDD, the studies mainly focus on bone marrow mesenchymal stem cells (BMMSC), adipose-derived mesenchymal stem cells (ADSC), human placental mesenchymal stem cells (HPMSCs) and urine-derived stem cells (USCs).
Exosome Derived from Bone Marrow Mesenchymal Stem Cells
BMMSC is derived from mesoderm and has multi-differentiation potential. Increasing lines of evidence have shown that BMMSC could be applied in curing diseases. For instance: spinal cord injury; bone regeneration; IDD; and so on. Nevertheless, it still has some unsolved problems, such as immunological reaction and potential tumorigenesis. Besides, severe environment of IDD also arrest BMMSC proliferation. Interestingly, BMMSC derived-exosome (BMMSC-exo) can resist the influence of harsh environment of IDD. Li et al. [105] cultured the NPc in different PH circumstances, and incubated it with BMMSC-exo. They revealed that, with the decreased of PH value, NPc and ECM showed a series of degradation: The proliferation of NPc was descended; the degradation of ECM was strengthened. Significantly, they discovered that BMMSC-exo could decelerate the apoptosis of NPC, promote the synthesis of chondrocyte ECM, and downregulate the matrix-degrading enzymes. All of which revealed that not only does MSC-derived-exosome (MSC-exo) have approximate function with MSC, it could also survive in abnormal biological ambience of IDD. This study elucidated that MSC-exo might be a better choice for biologic therapy.
Numerous of studies have explored the BMMSC-exo function and its communication with NPc and APc. Lu et al. [106] first illustrated the intercommunication between BMMSC-exo and NPc-exo. They unearthed that NPc-exo could promote the BMMSC migration and induce BMMSC differentiation to the NP-like phenotype. Additionally, they revealed that BMMSC-exo could promote NPc proliferation and ECM production in degenerative NP. This research proved the potent function of BMMSC-exo and its intercommunication with NPc. It also elucidated that NP could impact MSC via secreting NPc-exo. Hu et al. [107] isolated the BMMSC-exo and co-cultured it with NPc. They found that BMMSC-exo plays a prominent part in NPc apoptosis process which was induced by compression. BMMSC-exo could inhibit compression-induced NPc apoptosis by suppressing oxidative stress. Therefore, when develops the BMMSC-based injectable biological drug, considering its interrelate with IVD cells is pivotal.
BMMSC-exo and its target pathways have been explored extensively. Advanced glycation end products (AGEs) [108-110], formed by non-enzymatic reaction of reducing sugars with free amino groups of macromolecules, leads to ER stress, thus activating the unfolded protein response (UPR) [111, 112]. Furthermore, UPR could initiate the C/EBP homologous protein, which is a responsible protein that modulates and induces cell apoptosis. Liao et al. [113] suggested that the endoplasmic reticulum (ER) stress markers and NPc apoptosis promoted in the process of IDD. They revealed that, under the AGEs stimulation, BMMSC-exo could alleviate ER stess-induced NPc apoptosis through AKT/ERK signaling pathway.
PI3K/AKT/mTOR signaling pathway is a pivotal regulator of autophagy [114, 115]. Li et al. [116] revealed that BMMSC-exo could ameliorate the inflammation and apoptosis of AFc by inhibiting the expression of PI3K/AKT/mTOR signaling pathway.
Wen et al. [117] uncovered that BMMSC-exo which carried miR-199a could inhibit and reverse the process of IDD by targeting GREM1 and downregulating the TGF-β pathway. Zhu et al. [118] found that BMMSC-exo could alleviate the NPc degeneration and IDD progression by delivering miR-142-3p to target MLK3, thereby suppressing MAPK signaling pathway. Wang et al. [119] unveiled that BMMSC-exo could deliver miR-129-5p, target SOX4, inhibit the activation of Wnt/β-catenin pathway, thus promoting the proliferation of degenerative NPc and the synthesis of ECM. Zhu et al. [120] explored that BMMSC-exo could attenuate the apoptosis of NPc and degradation of ECM by carrying miR-532-5p to inhibit target RASSF5 pathway, which is a considerable apoptotic and/or senescence pathway.
However, most of studies about BMMSC-exo places emphasis on exploring its communication with NPc. More research is needed to explore BMMSC-exo correlation with AF and CEP cells.
Exosome Derived from Adipose-Derived Mesenchymal Stem Cells
Despite accumulating evidence suggesting the benefits of BMMSC, it still has some limitation. For instance: expensive cost; collection difficulty; and the trauma to patient [121]. Thus, it is essential to explore other types of MSC which could be collected and isolated easily. Notably, ADSC has received interest because of its easy access [122]. ADSC and ADSC-derived exosome (ADSC-exo) could be applied in treating diseases and tissue damage, such as peripheral nerve injury, ischemic stroke and ruptured tendon [123-127]. Xing et al. [128] supported that ADSC-exo could inhibit the release of NLRP3, thus affecting the pyroptosis of NPc. Also, it could alleviate the expression of MMPs, thereby blocking the catabolism of ECM. They constructed a thermosensitive acellular ECM hydrogel coupled with ADSC exosomes (dECM-exo), which will be discussed later.
The studies about the function of ADSC-exo in IDD procession still scarce. But the application of ASDC in treating other diseases could provide inspiration. Hepatic ischemia-reperfusion (I/R) injury is a complex procession which includes hypoxia, apoptosis, inflammatory mediator and lipid peroxidation [129, 130]. In addition, GSK-3β could accumulate the expression of anti-apoptotic protein (Bcl-2 and survivin) in cells; ERK1/2 could induce the anti-apoptotic function by alleviating Bax protein and increasing of Bcl-2. Zhang et al. [131] revealed that ADSC-exo could carry PGE2, induce the inactivation of GSK-3β, upregulate ERK1/2, thus alleviating the secretion of inflammatory mediators and inhibiting the apoptosis of cells. Therefore, ADSC-exo might also carry PGE2, thereby alleviating the apoptosis of NPc. However, further research is needed to testify this hypothesis.
Exosome Derived from Human Placental Mesenchymal Stem Cells
As known to all, BMMSC is gold standard when choosing MSC, whereas the hardship of obtaining BMMSC is a difficult problem [99, 121]. Increasing evidence implicates that HPMSCs is easily obtained and ethically favored. Thus, it could be an alternative choice [132-134].
Pyroptosis, as an inflammatory cell death, is a pivotal mediator of inflammatory response [135]. Yuan et al. [136] suggested that HPMSCs-derived exosome (HPMSCs-exo) could carry miR-26a-5p to inhibit METTL14/NLRP3 signaling pathway, which is a noticeable pathway interrelates with the pyroptosis and pro-inflammatory cytokines [137-139]. Besides, HPMSCs-exo could alleviate the inflammatory conditions by suppressing cytokine release, thereby alleviating the pyroptosis of NPc.
ZNFs, the functional proteins related to the regulation of gene expression, are regulated by various types of miRNA [140]. Accumulating studies suggested that ZNFs could regulate the cell proliferation. Wu et al. [141] supported that miR-1247 could directly repress ZNF346 expression, and thus inhibiting the progression of childhood neuroblastoma. Interestingly, increasing evidence implicates that ZNF121 has the capacity of regulating cell proliferation and apoptosis [142]. Yuan et al. [143] unearthed that miR-4450 specifically targeted and inhibited the expression of ZNF121. Besides, they found that the knockdown of miR-4450 showed protective effect on NPc. Therefore, they elucidated that HPLMSC-exo could carry antagomiR-4450 to upregulate the expression of ZNF121, thereby alleviating the degradation of NPc.
Exosome Derived from Urine-derived Stem Cells
USCs could be obtained from non-invasive sources, and have lower cost of culture and faster proliferative rate [144, 145]. Interestingly, Qin et al. [145] suggested that USCs have longer telomere sequences and higher telomerase activity than other types of MSC, which is related to the proliferation ability. Thus, USCs is a promising source of exosome extraction and stem cell therapy.
MATN3 could promote IL-1ra expression and alleviate the IL-1β-induced catabolic matrix proteinases secretion [146]. Guo et al. [147] discovered that USCs-exo could carry MATN3, thereby reversing the degradation of ECM and the process of IDD.
ER stress, which is mentioned above, could induce the apoptosis of NPc [148]. Xiang et al. [149] identified that USCs-exo could inhibit the secretion of CHOP, GRP78, caspase-3 and caspase-12, thus inhibiting ER stress and the NPc apoptosis. They also revealed that USCs-exo could alleviate ER stress-induced apoptosis by activating the AKT and ERK signaling pathway.
Exosome-based Therapeutic Strategy
Accumulating studies have detected that exosome plays a striking role in containing the homeostasis of IVD. For instance: increasing autophagy; inhibiting inflammatory response pathway; promoting the synthesis of ECM; and alleviating pyroptosis [122, 150]. Hence, it is a better source for biological therapeutic strategy.
Xing et al. [128] developed an IVD biological hydrogel which is an ECM biological scaffolds loaded with exosome (dECM@exo) derived from ADSC. dECM, as an acellular scaffold, is a structure which loads the exosome. They uncovered that dECM@exo could slow the release of exosome while showing high load rate of exosomes. It is a regulator of inflammatory complexes and metalloproteinases. The combination of acellular scaffolds and exosome, both have low immunogenicity, makes exosome-based therapy be a better choice than cell-based therapy.
PI3K/AKT/mTOR signaling pathway is a vital regulator of autophagy [151]. Luo et al. [152] revealed that Sphk2 could activate PI3K/AKT signaling pathway, thus promoting the autophagy of NPc and reversing the process of IDD. They encapsulated the CESCs overexpressing Sphk2 in an ECM of costal cartilage (ECM-Gels) and injected it near the CEP of rat. And then, they found that ECM-Gels could produce Sphk2-engineered exosomes which penetrated the AF and transported Sphk2 into NPc, activated the PI3K/AKT signaling pathway, thereby accumulating the autophagy of NPc.
Taken together, compared with others, exosomes have many preponderances, such as lower immune response and higher transfer efficiency [153]. Thus, exosome-based therapeutic strategy has potent treatment potential and profound therapeutic implications, even further discovery is conducive to the development of it. Until now, some laboratories and companies have begun to investigate the engineered and mass-produced exosome. However, no exosome injection drugs for IDD have been approved for launch. The studies still stay in animal and preclinical experiment stage.
Discussion
LBP, as a chronic and prevalent condition, gives great burden to social economy and quality of life [154, 155], which is mainly caused by IDD. Nevertheless, the current therapeutic strategies for IDD could not achieve satisfactory results. Hence, it is essential to pursue a new therapeutic strategy. Exosome, which plays a striking role in IDD progression, has got more and more attention. Accumulating evidence implicates that exosome participates in the degradation process of IVD. Therefore, when investigates the pathological changes of IVD, taking exosome into consideration is pivotal (Table 1).
Exosome and its targeting ways
| Exosome derived from: | Carry | Target | Reference |
|---|---|---|---|
| SNPc | unknown | P53/P21 | [80] |
| DNPc | miR-16 | IGF-1/IGF-1R | [87] |
| RINPc | miR-27a | MMP-13 | [91] |
| NC | miR-140-5p | Wnt/β-catenin | [94] |
| CESCs | unknown | PI3K/AKT/autophagy | [96] |
| CESCs | unknown | HIF-1α/Wnt | [97] |
| CESCs | miR-125-5p | SUV39H1 | [98] |
| CESCs | Sphk2 | PI3K/AKT | [152] |
| BMMSC | miR-199a | GREM1/ TGF-β | [117] |
| BMMSC | miR-142-3p | MLK3/ MAPK | [118] |
| BMMSC | miR-129-5p | SOX4 Wnt/β-catenin | [119] |
| BMMSC | miR-532-5p | RASSF5 | [120] |
| BMMSC | unknown | AKT/ERK | [113] |
| BMMSC | unknown | PI3K/AKT/mTOR | [116] |
| ADSC | unknown | NLRP3 | [128] |
| HPMSCs | miR-26a-5p | METTL14/NLRP3 | [136] |
| HPMSCs | antagomiR-4450 | ZNF121 | [143] |
| USCs | MATN3 | unknown | [147] |
| USCs | unknown | AKT/ERK | [149] |
DNPc: degenerative NPc; RINPc: rapamycin-induced NPc.
The process of IDD and exosome-based therapeutic strategy. A. In IVD, with the redundant mechanical load and other elements, the homeostasis of IVD is blemished. IVD also produce cross-talk which could influence the adjacent vertebrae. B. As the gradually boosts of IDD, vascular and nerves grows into IDD, and inflammatory cytokines cluster in IVD and surround tissues. C. Exosome that derived from non-stem cell or stem cell could carry various types of miRNA, implicate the proliferation and apoptosis of IVD cells, and thus delaying and reversing the IDD progression.
As known to all, exosome-based drugs have great therapeutic potential. Nevertheless, it is essential to characterize the difficulties we faced. First, as a product secreted by various types of cells, exosome could be affected by multiple factors. For example: the source of cells; the state of cells; and the condition of culture. Secondly, IDD is an intricate process which includes manifold pathological changes. It is necessary to choose the exosome which aims at major pathological changes of IDD. Thirdly, as the largest avascular tissue in the body, the physiological condition of IVD is harsh. For instance: long-term internal high pressure; low pH; low nutrition; low oxygen; and complex inflammatory environment. All of which impact the activity and function of exosome [48]. Last but not least, the accurate dose and injection position of exosome-based drugs are still unclear. Collectively, it is pivotal to even further explore specific function of exosome and precise dose of exosome-based injectable drugs.
Conclusion
Exosome, as a substance which transmits information between cells, has attracted more and more attention. As a new direction for the therapeutic approach of IDD, exosome could influence IVD cells via various ways. For example: accumulating the autophagy; increasing the ECM synthesis; alleviating the apoptosis; and inhibiting the pyroptosis. Thus, it has remarkable potential to delay and reverse the onset and development of IDD. Further study is needed to explore the regulation mechanism of exosome, its intercommunication with IVD cells, and the safety/effectiveness of exosome-based therapeutic strategy (Figure 1).
Abbreviations
LBP: low back pain; IVD: intervertebral disc; IDD: intervertebral disc degeneration; NP: nucleus pulposus; NPc: nucleus pulposus cells; AF: annulus fibrosus; AFc: annulus fibrosus cells; ECM: extracellular matrix; CEP: cartilaginous endplates; CEPc: cartilaginous endplates cells; TDR: total disc replacement; SC-Exo: stem cell-derived exosome; SNPC-Exo: senescence nucleus pulposus cells-derived-exosome; NPc-exo: nucleus pulposus cells-derived-exosome; dNPc-exo: degenerative nucleus pulposus cells-derived-exosome; NC: Notochordal cells; NC-exo: notochordal cell-derived exosome; AF-exo: annulus fibrosus-derived exosome; dAFc: degenerative annulus fibrosus cells; ndAFc: non-degenerative annulus fibrosus cells; CESCs-exo: CEP stem cell-derived exosome; CESCs: cartilage endplate stem cells; CEP-exo: exosome derived from cartilaginous endplates cells; MSC: mesenchymal stem cells; BMMSC: bone marrow mesenchymal stem cells; ADSC: adipose-derived mesenchymal stem cells; HPMSCs: human placental mesenchymal stem cells; USCs: urine-derived stem cells; BMMSC-exo: bone marrow mesenchymal stem cells derived-exosome; MSC-exo: mesenchymal stem cells-derived-exosome; AGEs: advanced glycation end products; UPR: unfolded protein response; ER: endoplasmic reticulum; ADSC-exo: adipose-derived mesenchymal stem cells-derived exosome; HPMSCs-exo: HPMSCs-derived exosome; dECM@exo: ECM biological scaffolds loaded with exosome; ECM-Gels: extracellular matrix of costal cartilage.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82002348).
Availability of data and materials
All data analyzed during this study are included in this manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Global burden of 369 diseases, injuries in 204 countries, territories, 1990-2019. a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396:1204-22
2. Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A. et al. US Spending on Personal Health Care and Public Health, 1996-2013. Jama. 2016;316:2627-46
3. Kim TE, Lee RG, Park SY, Oh IH. Measuring Trends in the Socioeconomic Burden of Disease in Korea, 2007-2015. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2022;55:19-27
4. Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487-92
5. Kurunlahti M, Tervonen O, Vanharanta H, Ilkko E, Suramo I. Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine. 1999;24:2080-4
6. de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A. et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine. 2010;35:531-6
7. Scheele J, de Schepper EI, van Meurs JB, Hofman A, Koes BW, Luijsterburg PA. et al. Association between spinal morning stiffness and lumbar disc degeneration: the Rotterdam Study. Osteoarthritis and cartilage. 2012;20:982-7
8. Teraguchi M, Yoshimura N, Hashizume H, Muraki S, Yamada H, Minamide A. et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthritis and cartilage. 2014;22:104-10
9. Patil P, Niedernhofer LJ, Robbins PD, Lee J, Sowa G, Vo N. Cellular senescence in intervertebral disc aging and degeneration. Current molecular biology reports. 2018;4:180-90
10. Trefilova VV, Shnayder NA, Petrova MM, Kaskaeva DS, Tutynina OV, Petrov KV. et al. The Role of Polymorphisms in Collagen-Encoding Genes in Intervertebral Disc Degeneration. Biomolecules. 2021 11
11. Ikuno A, Akeda K, Takebayashi SI, Shimaoka M, Okumura K, Sudo A. Genome-wide analysis of DNA methylation profile identifies differentially methylated loci associated with human intervertebral disc degeneration. PloS one. 2019;14:e0222188
12. Kelempisioti A, Eskola PJ, Okuloff A, Karjalainen U, Takatalo J, Daavittila I. et al. Genetic susceptibility of intervertebral disc degeneration among young Finnish adults. BMC medical genetics. 2011;12:153
13. McMorran JG, Gregory DE. The effect of compressive loading rate on annulus fibrosus strength following endplate fracture. Medical engineering & physics. 2021;93:17-26
14. Özcan-Ekşi EE, Kara M, Berikol G, Orhun Ö, Turgut VU, Ekşi M. A new radiological index for the assessment of higher body fat status and lumbar spine degeneration. Skeletal radiology. 2022;51:1261-71
15. Wang Y, Videman T, Battié MC. ISSLS prize winner: Lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine. 2012;37:1490-6
16. Chen BL, Guo JB, Zhang HW, Zhang YJ, Zhu Y, Zhang J. et al. Surgical versus non-operative treatment for lumbar disc herniation: a systematic review and meta-analysis. Clinical rehabilitation. 2018;32:146-60
17. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-78
18. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. The Journal of cell biology. 1985;101:942-8
19. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). The Journal of biological chemistry. 1987;262:9412-20
20. Johnstone RM. Revisiting the road to the discovery of exosomes. Blood cells, molecules & diseases. 2005;34:214-9
21. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology. 2014;30:255-89
22. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200:373-83
23. Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015;4:27066
24. Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-32
25. Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone. 2012;50:771-6
26. Rider SM, Mizuno S, Kang JD. Molecular Mechanisms of Intervertebral Disc Degeneration. Spine surgery and related research. 2019;3:1-11
27. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. The Journal of bone and joint surgery American volume. 2006;88(Suppl 2):10-4
28. Meachim G, Cornah MS. Fine structure of juvenile human nucleus pulposus. Journal of anatomy. 1970;107:337-50
29. Roberts S, McCall IW, Menage J, Haddaway MJ, Eisenstein SM. Does the thickness of the vertebral subchondral bone reflect the composition of the intervertebral disc? European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 1997;6:385-9
30. Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine. 1989;14:166-74
31. Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. Journal of anatomy. 1975;120:113-30
32. Pereira DR, Silva-Correia J, Oliveira JM, Reis RL. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. Journal of tissue engineering and regenerative medicine. 2013;7:85-98
33. Colombier P, Clouet J, Hamel O, Lescaudron L, Guicheux J. The lumbar intervertebral disc: from embryonic development to degeneration. Joint bone spine. 2014;81:125-9
34. Farfan HF. The torsional injury of the lumbar spine. Spine. 1984;9:53
35. Adams MA, Hutton WC. The effect of fatigue on the lumbar intervertebral disc. The Journal of bone and joint surgery British volume. 1983;65:199-203
36. Rauck RL, Gargiulo CA, Ruoff GE, Schnitzer TJ, Trapp RG. Chronic low back pain: new perspectives and treatment guidelines for primary care: Part II. Managed care interface. 1998;11:71-5
37. Chan WC, Au TY, Tam V, Cheah KS, Chan D. Coming together is a beginning: the making of an intervertebral disc. Birth defects research Part C, Embryo today: reviews. 2014;102:83-100
38. Cao G, Yang S, Cao J, Tan Z, Wu L, Dong F. et al. The Role of Oxidative Stress in Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2022;2022:2166817
39. Francisco V, Pino J, González-Gay M, Lago F, Karppinen J, Tervonen O. et al. A new immunometabolic perspective of intervertebral disc degeneration. Nature reviews Rheumatology. 2022;18:47-60
40. García-Vega D, González-Juanatey JR, Eiras S. Diabesity in Elderly Cardiovascular Disease Patients: Mechanisms and Regulators. International journal of molecular sciences. 2022 23
41. Bonnheim NB, Wang L, Lazar AA, Zhou J, Chachad R, Sollmann N. et al. The contributions of cartilage endplate composition and vertebral bone marrow fat to intervertebral disc degeneration in patients with chronic low back pain. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2022;31:1866-72
42. Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006;15(Suppl 3):S312-6
43. Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis research & therapy. 2007;9:R45
44. Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochemical Society transactions. 2007;35:652-5
45. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford, England). 2008;47:809-14
46. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307-14
47. Urban JP, Roberts S. Development and degeneration of the intervertebral discs. Molecular medicine today. 1995;1:329-35
48. Gruber HE, Ingram JA, Norton HJ, Hanley EN Jr. Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine. 2007;32:321-7
49. Phillips KL, Chiverton N, Michael AL, Cole AA, Breakwell LM, Haddock G. et al. The cytokine and chemokine expression profile of nucleus pulposus cells: implications for degeneration and regeneration of the intervertebral disc. Arthritis research & therapy. 2013;15:R213
50. Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFα in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochemical and biophysical research communications. 2013;433:151-6
51. Ghelani RN, Zwambag DP, Gregory DE. Rapid increase in intradiscal pressure in porcine cervical spine units negatively impacts annulus fibrosus strength. Journal of biomechanics. 2020;108:109888
52. Chu G, Shi C, Lin J, Wang S, Wang H, Liu T. et al. Biomechanics in Annulus Fibrosus Degeneration and Regeneration. Advances in experimental medicine and biology. 2018;1078:409-20
53. Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Cross AK. et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis research & therapy. 2014;16:416
54. Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis research & therapy. 2008;10:R99
55. Lai A, Moon A, Purmessur D, Skovrlj B, Laudier DM, Winkelstein BA. et al. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. The spine journal: official journal of the North American Spine Society. 2016;16:420-31
56. Richardson SM, Purmessur D, Baird P, Probyn B, Freemont AJ, Hoyland JA. Degenerate human nucleus pulposus cells promote neurite outgrowth in neural cells. PloS one. 2012;7:e47735
57. Sun Z, Liu B, Luo ZJ. The Immune Privilege of the Intervertebral Disc: Implications for Intervertebral Disc Degeneration Treatment. International journal of medical sciences. 2020;17:685-92
58. McAfee PC, Cunningham B, Holsapple G, Adams K, Blumenthal S, Guyer RD. et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine. 2005;30:1576-83 discussion E388-90
59. Collis JS. Total disc replacement: a modified posterior lumbar interbody fusion. Report of 750 cases. Clinical orthopaedics and related research. 1985:64-7
60. Burgess-Hull AJ, Panlilio LV, Preston KL, Epstein DH. Trajectories of craving during medication-assisted treatment for opioid-use disorder: Subtyping for early identification of higher risk. Drug and alcohol dependence. 2022;233:109362
61. Krut Z, Pelled G, Gazit D, Gazit Z. Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration. Cells. 2021 10
62. Zhang W, Sun T, Li Y, Yang M, Zhao Y, Liu J. et al. Application of stem cells in the repair of intervertebral disc degeneration. Stem cell research & therapy. 2022;13:70
63. Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell cycle (Georgetown, Tex). 2009;8:2014-8
64. Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. American journal of cancer research. 2011;1:98-110
65. Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE. et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Scientific reports. 2015;5:17319
66. Sampey GC, Meyering SS, Zadeh MA, Saifuddin M, Hakami RM, Kashanchi F. Exosomes and their role in CNS viral infections. Journal of neurovirology. 2014;20:199-208
67. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics. 2010;73:1907-20
68. Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J. et al. Exosome-Induced Regulation in Inflammatory Bowel Disease. Frontiers in immunology. 2019;10:1464
69. Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. Journal of extracellular vesicles. 2018;7:1522236
70. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. The Journal of cell biology. 1983;97:329-39
71. Baixauli F, López-Otín C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Frontiers in immunology. 2014;5:403
72. Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. International immunology. 2005;17:879-87
73. Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S. et al. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PloS one. 2013;8:e78115
74. Dixon CL, Sheller-Miller S, Saade GR, Fortunato SJ, Lai A, Palma C. et al. Amniotic Fluid Exosome Proteomic Profile Exhibits Unique Pathways of Term and Preterm Labor. Endocrinology. 2018;159:2229-40
75. Yuan Z, Bedi B, Sadikot RT. Bronchoalveolar Lavage Exosomes in Lipopolysaccharide-induced Septic Lung Injury. Journal of visualized experiments: JoVE. 2018
76. Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Korvala J, Salo T, Sormunen R. et al. Human saliva-derived exosomes: comparing methods of isolation. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2015;63:181-9
77. Yamamoto H, Watanabe Y, Oikawa R, Morita R, Yoshida Y, Maehata T. et al. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA is a Useful Marker For Early Detection of Gastric Cancer in an H. pylori-Independent Manner. Clinical and translational gastroenterology. 2016;7:e184
78. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791-9
79. Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nature reviews Molecular cell biology. 2006;7:495-504
80. Chen CC, Chen J, Wang WL, Xie L, Shao CQ, Zhang YX. Inhibition of the P53/P21 Pathway Attenuates the Effects of Senescent Nucleus Pulposus Cell-Derived Exosomes on the Senescence of Nucleus Pulposus Cells. Orthopaedic surgery. 2021;13:583-91
81. Elfervig MK, Minchew JT, Francke E, Tsuzaki M, Banes AJ. IL-1beta sensitizes intervertebral disc annulus cells to fluid-induced shear stress. Journal of cellular biochemistry. 2001;82:290-8
82. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis research & therapy. 2005;7:R732-45
83. de Vries SA, van Doeselaar M, Meij BP, Tryfonidou MA, Ito K. The Stimulatory Effect of Notochordal Cell-Conditioned Medium in a Nucleus Pulposus Explant Culture. Tissue engineering Part A. 2016;22:103-10
84. Aulisa L, Papaleo P, Pola E, Angelini F, Aulisa AG, Tamburrelli FC. et al. Association between IL-6 and MMP-3 gene polymorphisms and adolescent idiopathic scoliosis: a case-control study. Spine. 2007;32:2700-2
85. Ye W, Ma RF, Ding Y, Huang DS, Chen WJ, Peng Y. et al. [Effect of interleukin-6 on the chondrocytes in the cartilage endplate of rabbits in vitro]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2007;27:1187-9
86. Séguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30:1940-8
87. Zhang QC, Zou YP, Hu SQ, Zhang TW, Zhou H, Liang B. et al. TNF-α-stimulated nucleus pulposus cells induce cell apoptosis through the release of exosomal miR-16 targeting IGF-1 and IGF-1R in rats. Annals of translational medicine. 2021;9:1376
88. Feng X, Li Y, Su Q, Tan J. Degenerative Nucleus Pulposus Cells Derived Exosomes Promoted Cartilage Endplate Cells Apoptosis and Aggravated Intervertebral Disc Degeneration. Frontiers in molecular biosciences. 2022;9:835976
89. Mizushima N. Physiological functions of autophagy. Current topics in microbiology and immunology. 2009;335:71-84
90. Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Grewal R, Ninomiya J, Coyne CB. et al. Autophagy modulates articular cartilage vesicle formation in primary articular chondrocytes. The Journal of biological chemistry. 2015;290:13028-38
91. Zhang QC, Hu SQ, Hu AN, Zhang TW, Jiang LB, Li XL. Autophagy-activated nucleus pulposus cells deliver exosomal miR-27a to prevent extracellular matrix degradation by targeting MMP-13. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2021;39:1921-32
92. Hu SQ, Zhang QC, Meng QB, Hu AN, Zou JP, Li XL. Autophagy regulates exosome secretion in rat nucleus pulposus cells via the RhoC/ROCK2 pathway. Experimental cell research. 2020;395:112239
93. Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue engineering. 2003;9:667-77
94. Sun Z, Liu B, Liu ZH, Song W, Wang D, Chen BY. et al. Notochordal-Cell-Derived Exosomes Induced by Compressive Load Inhibit Angiogenesis via the miR-140-5p/Wnt/β-Catenin Axis. Molecular Therapy - Nucleic Acids. 2020;22:1092-106
95. Sun Z, Zhao H, Liu B, Gao Y, Tang WH, Liu ZH. et al. AF cell derived exosomes regulate endothelial cell migration and inflammation: Implications for vascularization in intervertebral disc degeneration. Life Sciences. 2021 265
96. Luo L, Jian X, Sun H, Qin J, Wang Y, Zhang J. et al. Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem Cells. 2021;39:467-81
97. Luo L, Gong J, Zhang H, Qin J, Li C, Zhang J. et al. Cartilage Endplate Stem Cells Transdifferentiate Into Nucleus Pulposus Cells via Autocrine Exosomes. Frontiers in cell and developmental biology. 2021;9:648201
98. Chen D, Jiang X. Exosomes-derived miR-125-5p from cartilage endplate stem cells regulates autophagy and ECM metabolism in nucleus pulposus by targeting SUV38H1. Experimental cell research. 2022: 113066.
99. Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nature reviews Rheumatology. 2015;11:243-56
100. Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry Part A: the journal of the International Society for Analytical Cytology. 2018;93:19-31
101. Vizoso FJ, Eiro N, Costa L, Esparza P, Landin M, Diaz-Rodriguez P. et al. Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. International journal of molecular sciences. 2019 20
102. Maldonado-Lasunción I, Haggerty AE, Okuda A, Mihara T, de la Oliva N, Verhaagen J. et al. The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair. Cells. 2021 10
103. Zhou T, Yuan Z, Weng J, Pei D, Du X, He C. et al. Challenges and advances in clinical applications of mesenchymal stromal cells. Journal of hematology & oncology. 2021;14:24
104. Xu M, Shaw G, Murphy M, Barry F. Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Are Functionally and Genetically Different From Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells. 2019;37:754-65
105. Li M, Li R, Yang S, Yang D, Gao X, Sun J. et al. Exosomes derived from bone marrow mesenchymal stem cells prevent acidic pH-induced damage in human nucleus pulposus cells. Medical Science Monitor. 2020 26
106. Lu K, Li HY, Yang K, Wu JL, Cai XW, Zhou Y. et al. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Research and Therapy. 2017 8
107. Hu Y, Tao R, Wang L, Chen L, Lin Z, Panayi AC. et al. Exosomes Derived from Bone Mesenchymal Stem Cells Alleviate Compression-Induced Nucleus Pulposus Cell Apoptosis by Inhibiting Oxidative Stress. Oxidative Medicine and Cellular Longevity. 2021. 2021
108. Baker P, Cooper-Mullin CM, Jimenez AG. Differences in advanced glycation endproducts (AGEs) in plasma from birds and mammals of different body sizes and ages. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2022;267:111164
109. Liu L, Liu L, Xie J, Shen M. Formation mechanism of AGEs in Maillard reaction model systems containing ascorbic acid. Food chemistry. 2022;378:132108
110. Wu Y, Dong L, Song Y, Wu Y, Zhang Y, Wang S. Preventive effects of polysaccharides from Physalis alkekengi L. on dietary advanced glycation end product-induced insulin resistance in mice associated with the modulation of gut microbiota. International journal of biological macromolecules. 2022;204:204-14
111. Kohno K, Normington K, Sambrook J, Gething MJ, Mori K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Molecular and cellular biology. 1993;13:877-90
112. Qi M, Jiang Q, Yang S, Zhang C, Liu J, Liu W. et al. The endoplasmic reticulum stress-mediated unfolded protein response protects against infection of goat endometrial epithelial cells by Trueperella pyogenes via autophagy. Virulence. 2022;13:122-36
113. Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K. et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084-100
114. Barzegar Behrooz A, Talaie Z, Jusheghani F, Łos MJ, Klonisch T, Ghavami S. Wnt and PI3K/Akt/mTOR Survival Pathways as Therapeutic Targets in Glioblastoma. International journal of molecular sciences. 2022 23
115. Bhardwaj JK, Paliwal A, Saraf P, Sachdeva SN. Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary. Journal of cellular physiology. 2022;237:1157-70
116. Li ZQ, Kong L, Liu C, Xu HG. Human Bone Marrow Mesenchymal Stem Cell-derived Exosomes Attenuate IL-1β-induced Annulus Fibrosus Cell Damage. American Journal of the Medical Sciences. 2020;360:693-700
117. Wen T, Wang H, Li Y, Lin Y, Zhao S, Liu J. et al. Bone mesenchymal stem cell-derived extracellular vesicles promote the repair of intervertebral disc degeneration by transferring microRNA-199a. Cell cycle (Georgetown, Tex). 2021;20:256-70
118. Zhu L, Shi Y, Liu L, Wang H, Shen P, Yang H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: therapeutic potential for intervertebral disc degenerative diseases. Cell cycle (Georgetown, Tex). 2020;19:1727-39
119. Wang H, Li F, Ban W, Zhang J, Zhang G. Human Bone Marrow Mesenchymal Stromal Cell-Derived Extracellular Vesicles Promote Proliferation of Degenerated Nucleus Pulposus Cells and the Synthesis of Extracellular Matrix Through the SOX4/Wnt/β-Catenin Axis. Frontiers in Physiology. 2021 12
120. Zhu G, Yang X, Peng C, Yu L, Hao Y. Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Experimental cell research. 2020 393
121. Dou Y, Sun X, Ma X, Zhao X, Yang Q. Intervertebral Disk Degeneration: The Microenvironment and Tissue Engineering Strategies. Frontiers in bioengineering and biotechnology. 2021;9:592118
122. Xing H, Zhang Z, Mao Q, Wang C, Zhou Y, Zhou X. et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnology. 2021;19:264
123. Mathot F, Rbia N, Thaler R, Bishop AT, van Wijnen AJ, Shin AY. Introducing human adipose-derived mesenchymal stem cells to Avance(Ⓡ) nerve grafts and NeuraGen(Ⓡ) nerve guides. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2020;73:1473-81
124. Zhang J, Li C, Meng F, Guan Y, Zhang T, Yang B. et al. Functional tissue-engineered microtissue formed by self-aggregation of cells for peripheral nerve regeneration. Stem cell research & therapy. 2022;13:3
125. Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL. et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537-56
126. Li C, Fei K, Tian F, Gao C, Yang S. Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating miR-21-3p/MAT2B signaling transduction. Croatian medical journal. 2019;60:439-48
127. Ryu S, Lee JM, Bae CA, Moon CE, Cho KO. Therapeutic efficacy of neuregulin 1-expressing human adipose-derived mesenchymal stem cells for ischemic stroke. PloS one. 2019;14:e0222587
128. Xing H, Zhang Z, Mao Q, Wang C, Zhou Y, Zhou X. et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. Journal of Nanobiotechnology. 2021 19
129. Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. The Journal of clinical investigation. 1990;85:1936-43
130. Pan Y, Tan WF, Yang MQ, Li JY, Geller DA. The therapeutic potential of exosomes derived from different cell sources in liver diseases. American journal of physiology Gastrointestinal and liver physiology. 2022;322:G397-g404
131. Zhang Y, Li Y, Wang Q, Zheng D, Feng X, Zhao W. et al. Attenuation of hepatic ischemia-reperfusion injury by adipose stem cell-derived exosome treatment via ERK1/2 and GSK-3β signaling pathways. International journal of molecular medicine. 2022 49
132. Mathew SA, Naik C, Cahill PA, Bhonde RR. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cellular and molecular life sciences: CMLS. 2020;77:253-65
133. Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M. et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300-11
134. Gorodetsky R, Aicher WK. Allogenic Use of Human Placenta-Derived Stromal Cells as a Highly Active Subtype of Mesenchymal Stromal Cells for Cell-Based Therapies. International journal of molecular sciences. 2021 22
135. Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infection and immunity. 2005;73:1907-16
136. Yuan X, Li T, Shi L, Miao J, Guo Y, Chen Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Molecular Medicine. 2021 27
137. Coll RC, Robertson A, Butler M, Cooper M, O'Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PloS one. 2011;6:e29539
138. Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology. 2009;10:241-7
139. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821-32
140. Hawe JS, Wilson R, Schmid KT, Zhou L, Lakshmanan LN, Lehne BC. et al. Genetic variation influencing DNA methylation provides insights into molecular mechanisms regulating genomic function. Nature genetics. 2022;54:18-29
141. Wu T, Lin Y, Xie Z. MicroRNA-1247 inhibits cell proliferation by directly targeting ZNF346 in childhood neuroblastoma. Biological research. 2018;51:13
142. Luo A, Zhang X, Fu L, Zhu Z, Dong JT. Zinc finger factor ZNF121 is a MYC-interacting protein functionally affecting MYC and cell proliferation in epithelial cells. Journal of genetics and genomics = Yi chuan xue bao. 2016;43:677-85
143. Yuan Q, Wang X, Liu L, Cai Y, Zhao X, Ma H. et al. Exosomes Derived from Human Placental Mesenchymal Stromal Cells Carrying AntagomiR-4450 Alleviate Intervertebral Disc Degeneration Through Upregulation of ZNF121. Stem Cells Dev. 2020;29:1038-58
144. Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889-901
145. Qin D, Long T, Deng J, Zhang Y. Urine-derived stem cells for potential use in bladder repair. Stem cell research & therapy. 2014;5:69
146. Lu XD, Liu YR, Zhang ZY. Matrilin-3 alleviates extracellular matrix degradation of nucleus pulposus cells via induction of IL-1 receptor antagonist. European review for medical and pharmacological sciences. 2020;24:5231-41
147. Guo Z, Su W, Zhou R, Zhang G, Yang S, Wu X. et al. Exosomal MATN3 of Urine-Derived Stem Cells Ameliorates Intervertebral Disc Degeneration by Antisenescence Effects and Promotes NPC Proliferation and ECM Synthesis by Activating TGF- β. Oxidative Medicine and Cellular Longevity. 2021. 2021
148. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326-35
149. Xiang H, Su W, Wu X, Chen W, Cong W, Yang S. et al. Exosomes Derived from Human Urine-Derived Stem Cells Inhibit Intervertebral Disc Degeneration by Ameliorating Endoplasmic Reticulum Stress. Oxidative Medicine and Cellular Longevity. 2020. 2020
150. Li W, Zhang S, Wang D, Zhang H, Shi Q, Zhang Y. et al. Exosomes Immunity Strategy: A Novel Approach for Ameliorating Intervertebral Disc Degeneration. Frontiers in cell and developmental biology. 2021;9:822149
151. Li Y, Guo Y, Fan Y, Tian H, Li K, Mei X. Melatonin Enhances Autophagy and Reduces Apoptosis to Promote Locomotor Recovery in Spinal Cord Injury via the PI3K/AKT/mTOR Signaling Pathway. Neurochemical research. 2019;44:2007-19
152. Luo L, Gong J, Wang Z, Liu Y, Cao J, Qin J. et al. Injectable cartilage matrix hydrogel loaded with cartilage endplate stem cells engineered to release exosomes for non-invasive treatment of intervertebral disc degeneration. Bioactive Materials. 2021
153. Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X. et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22:261-76
154. Taylor VM, Deyo RA, Cherkin DC, Kreuter W. Low back pain hospitalization. Recent United States trends and regional variations. Spine. 1994;19:1207-12 discussion 13
155. Millecamps M, Czerminski JT, Mathieu AP, Stone LS. Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. The spine journal: official journal of the North American Spine Society. 2015;15:2524-37
Author contact
![]() Corresponding authors: Zhen Sun, E-mail: sunzhenedu.cn, Phone: +86 02984775288; Zhengxu Ye, E-mail: yzengxcom.
Corresponding authors: Zhen Sun, E-mail: sunzhenedu.cn, Phone: +86 02984775288; Zhengxu Ye, E-mail: yzengxcom.

 Global reach, higher impact
Global reach, higher impact