Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(11):1680-1694. doi:10.7150/ijms.73701 This issue Cite
Research Paper
Atranorin driven by nano materials SPION lead to ferroptosis of gastric cancer stem cells by weakening the mRNA 5-hydroxymethylcytidine modification of the Xc-/GPX4 axis and its expression
1. Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
2. Shanghai Geriatric Institute of Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200031, China.
3. Department of Imaging, Dahua Hospital, Xuhui District, Shanghai 200237, China.
4. Department of Acupuncture, Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai 200086, China.
5. School of Life Science and Technology, Tongji University, Shanghai 200092, China.
*These authors contributed equally to this work and shared the first authorship.
Abstract
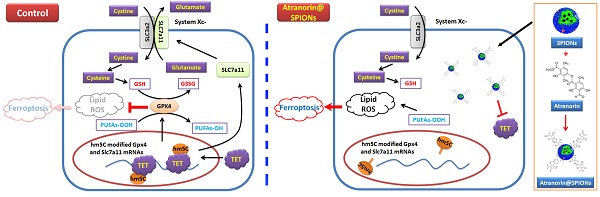
Gastric cancer is a highly malignant tumor. Gastric cancer stem cells (GCSCs) are the main causes of drug resistance, metastasis, recurrence, and poor prognosis. As a secondary metabolite of lichen, Atranorin has a variety of biological effects, such as antibacterial, anti-inflammatory, analgesic, and wound healing; however, its killing effect on GCSCs has not been reported. In this study, we constructed Atranorin complexes comprising superparamagnetic iron oxide nanoparticles (SPION) (Atranorin@SPION). In vitro and in vivo experiments confirmed that Atranorin@SPION could significantly inhibit the proliferation, invasion, angiogenesis, and tumorigenicity of CD44+/ CD24+ GCSCs, and induce oxidative stress injury, Fe2+ accumulation, and ferroptosis. Quantitative real-time reverse transcription PCR and western blotting results showed that Atranorin@SPION not only reduced the expression levels of GCSC stem cell markers and cell proliferation and division markers, but also significantly inhibited the expression levels of key molecules in the cystine/glutamate transporter (Xc-)/glutathione peroxidase 4 (GPX4) and Tet methylcytosine dioxygenase (TET) family proteins. The results of high performance liquid chromatography-mass spectrometry and Dot blotting showed that Atranorin@SPION significantly inhibited the mRNA 5‑hydroxymethylcytidine modification of GCSCs. Meanwhile, the results of RNA immunoprecipitation-PCR also indicated that Atranorin@SPIONs significantly reduced the 5-hydroxymethylcytidine modification level of GPX4 and SLC7A11 mRNA 3' untranslated region in GCSCs, resulting in a decrease in their stability, shortening their half-lives and reducing translation activity. Therefore, this study revealed that Atranorin@SPIONs induced ferroptosis of GCSCs by weakening the expression of the Xc-/GPX4 axis and the 5-hydroxymethylcytidine modification of mRNAs in the pathway, thereby achieving their therapeutic effect on gastric cancer.
Keywords: Atranorin, superparamagnetic iron oxide nanoparticle (SPION), Xc-/GPX4 axis, 5-hydroxymethylcytidine, gastric cancer stem cell (GCSCs), Ferroptosis

 Global reach, higher impact
Global reach, higher impact