Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(8):1227-1234. doi:10.7150/ijms.73080 This issue Cite
Research Paper
The influence of cerebral small vessel diseases on the efficacy of repositioning therapy and prognosis of benign paroxysmal positional vertigo
Department of Otolaryngology, the First Affiliated Hospital of China Medical University, Shenyang 110001, China.
Received 2022-3-21; Accepted 2022-6-24; Published 2022-7-4
Abstract
Background: Although vascular risk factors have been found to be closely related to the development of benign paroxysmal positional vertigo (BPPV), the relationship between BPPV and cerebral small vessels diseases (CSVDs) has rarely been discussed in literature. This study set out to investigate the efficacy of repositioning therapy and prognosis among BPPV patients with CSVDs.
Methods: We enrolled 553 BPPV patients who had undergone brain MRI, and categorized them into two groups based on the presence or absence of CSVDs. After controlling for other confounders using a propensity score matching (PSM) approach, we compared the incidence of recurrence and residual dizziness (RD). Then, we analyzed the recurrence rate and RD incidence in 176 BPPV patients with CSVDs, and assessed potential risk factors.
Results: White matter hyperintensity (WMH, 72.2%) and lacunar infarction (LI, 65.9%) were the two CSVDs that were present in the highest proportion among the BPPV patients. The incidence of RD in patients with CSVDs was significantly higher compared to subjects without CSVDs. Patients with RD (n=100, 56.8%) were older, had more severe WMH, and had a higher incidence of brain atrophy; age and higher Fazekas score were independent risk factors. Among the recurrent patients (n=61, 34.7%), the ages were older, the Fazekas score of WMH was higher, and number of LIs was increased; age was the sole independent risk factor.
Conclusion: BPPV patients with a combination of CSVD comorbidities, especially elderly patients with WMHs, are more likely to develop RD, which needs to be paid more attention.
Keywords: Benign paroxysmal positional vertigo, BPPV, Cerebral small vessels diseases, CSVD, Recurrence, Residual dizziness, White matter hyperintensity
Introduction
Benign paroxysmal positional vertigo (BPPV), a peripheral vestibular disease, refers to repeated episodes of vertigo that are triggered by a change in head position [1]. It is the most common peripheral vertigo disease in clinic, with a lifetime prevalence of 2.4%, a 1-year prevalence of 1.6%, and a 1-year incidence of 0.6% [2]. BPPV accounts for 17% to 42% of vertigo patients. The high incidence of BPPV seriously endangers people's health [3]. Most BPPV patients do not think that it is a malignant due to its name, but it can have a serious impact on patients' daily activities. BPPV can have a huge impact on patient's quality of life, and is closely related to patients' anxiety and depression, due to an increased risk of falls [2, 4, 5].
In recent years, some studies have shown that vascular risk factors are closely related to the occurrence and prognosis of BPPV [6, 7]. The labyrinthine artery of the inner ear is a tiny terminal artery. Small vessel lesions that directly damage the tiny arteries of the inner ear lead to microcirculation disorders, which can cause damage to the blood supply vessels of the inner ear, leading to the shedding of otoliths, which may be involved in BPPV pathogenesis [7-9]. Abnormal cerebral small vessels, including arterioles, branch arterioles, capillaries and venules, can directly damage blood supply, leading to microcirculatory disorders [10]. However, there have been almost no reports regarding the relationship between BPPV and cerebral small vessels diseases (CSVDs).
Brain MRI is not been routinely conducted for patients with BPPV. However, the pathogenesis of acute vertigo can sometimes be very complicated, especially in middle-aged and elderly patients with more basic diseases and a complicated past medical history. Therefore, sometimes we may need an MRI to check whether such patients have other brain diseases [11, 12]. In clinical practice, we find that many BPPV patients have lesions of cerebral small vessels, including white matter lesions. As we know, MRI manifestations of CSVDs include white matter hyperintensity (WMH, T2WI and flair R), lacunar infarction (LI), enlarged perivascular space (EPVS), cerebral microbleed (CMB) and brain atrophy [13]. Therefore, we sought to determine the distribution of these CSVDs in BPPV patients. We also set out to investigate the influence of CSVDs on efficacy of repositioning therapy and prognosis of BPPV patients. To our knowledge, there are no studies on this topic yet.
Although great progress has been made in the diagnosis and treatment of BPPV in recent years, there are different prognoses for the same treatment method [14-16]. This is particularly true for the recurrence and residual dizziness (RD) of BPPV, which seriously affect quality of life. Therefore, what remains to be asked is whether the presence of CSVDs and what types of CSVDs have an effect on the prognosis of patients with BPPV? This study investigated the relationship between BPPV and CSVDs, and the influence of CSVDs on the efficacy of repositioning of BPPV which can help us find effective treatment and improving prognosis.
Materials and methods
Patients
This is a retrospective study that was granted approval by the Ethics Committee of the First Affiliated Hospital of China Medical University, and was granted a waiver of written informed consent based on the study's retrospective design and anonymous data collection. The study was performed in accordance with the declaration of Helsinki.
Patients who were diagnosed with BPPV and treated at the Department of Otorhinolaryngology, the First Affiliated Hospital of China Medical University from December 2016 to May 2020 were enrolled retrospectively. All patients had undergone brain MRI examination for complaining of acute-onset dizziness at emergency department or neurology department before been transferred to the vertigo clinic of ENT department. The inclusion criteria are as follows: 1. recurrent episodes of transient vestibular vertigo induced by gravity-related head position changes; 2. Dix-Hallpike test or Roll test can induce vertigo and characteristic nystagmus; 3. the patients were clearly conscious and able to cooperate in the canalith repositioning procedure (CRP) treatment, with effective results; 4. MRI was performed within 1 week after onset; 5. denied a previous history of dizziness.
The exclusion criteria were as follows: 1. insufficient medical records; 2. patients who failed complete follow-up; 3. history of head trauma or head surgery; 4. intracranial tumor; 5. inner and middle ear diseases including Ménière's disease, vestibular migraine, sudden deafness, vestibular neuritis, otitis media; 6. use of ototoxic drugs; 7. patients with bilateral or multiple semicircular involvements; 8. cerebrovascular diseases due to cardiac and large vessel obstruction; 9. progressive multifocal leukoencephalopathy; 10. history of atrial fibrillation, myocardial infarction, valvular heart disease, infective endocarditis, myocarditis, mitral stenosis, or aneurysm; 11. severe extracranial and intracranial large blood vessels diseases; 12. evidence of known vasculitis; 13. multiple sclerosis, or other metabolic or toxic leukoencephalopathy; 14. osteoporosis.
Intervention and follow-up
The eligible subjects were separated into two groups based on presence of CSVDs. Age, gender, brain MRI results, features of BPPV (including involved side and canals), duration of BPPV, times of CRP, as well as the recurrence and RD of BPPV after successful treatment were recorded. We used a propensity score analysis to match CSVDs patients with non-CSVDs patients at a ratio of 1:1.
Patients with posterior canal (PSC) BPPV were treated using the Epley maneuver. The Barbecue maneuver was performed for treatment of geotropic horizontal canal (HC) BPPV. The Gufoni maneuver was used for apogeotropic HC BPPV. Details of the Epley, Gufoni, and Barbecue maneuvers were described in clinical practice guidelines of BPPV established by the AAO-HNSF (American Academy of Otolaryngology-Head and Neck Surgery Foundation) [3]. In order to treat anterior canal (AC) BPPV, we used Yacovino maneuver [17].
RD was defined as a feeling of unsteadiness and/or light-headedness and/or dizziness in absence of true vertigo and nystagmus, despite successful repositioning. The recurrence was defined as further BPPV episodes similar as the primary attack and the positive result of positional nystagmus test during follow-up. Three days after CRP treatment, BPPV patients returned to clinic for evaluation of efficacy. A successful CRP was defined as an absence of both positional vertigo and positional nystagmus [3]. The patients who failed to the repositioning procedure were reevaluated and underwent CRP every 3 days until the positioning nystagmus disappeared. We recorded the number of times CRP was performed. Follow-up was done at one year after treatment. Then, we investigated the recurrence and RD by checking the patient's outpatient records and telephone.
MRI protocol
A 3-T system scanner (MAGNETOM Verio, SIEMENS, Erlangen, Germany) was utilized for MRI. Parameters of spin-echo T1-weighted imaging (T1WI) included repetition time (TR) of 2000 ms, echo time (TE) of 17 ms, slice thickness of 5 mm, and slice distance of 1.5 mm. Parameters of spin-echo T2-weighted imaging (T2WI) included TR of 3500 ms, TE of 95 ms, slice thickness of 5 mm, and slice distance 1.5 mm. Parameters of fluid attenuated inversion recovery (FLAIR) sequences included TR of 8000 ms, TE of 104 ms, slice thickness of 5 mm, and slice distance of 1.5 mm. Susceptibility weighted imaging(SWI) included TR of 27 ms, TE of 20 ms, slice thickness 5 mm, and slice distance of 1.5 mm. The field of view was 230 mm × 230 mm, while the construction matrix was 192×256, and standard T2 and FLAIR for T2WI and FLAIR.
Manifestations of CSVDs on MRI
The MRI features of vasogenic WMH are high-intensity signal on T2 weighted (T2WI) images and Flair sequences, which showed punctate, patchy or confluent high intensity signal in dots, patches or fusion, and iso-signal or low signal on T1 weighted (T1WI) images. We further graded WMH according to the Fazekas [18] rating scales criteria. WMH was then divided into either periventricular white matter hyperintense (PWMH) or deep white matter hyperintense (DWMH), with regards to the location. PWMH showed hyperintense signals surrounding the ventricles in T2WI and isointense or hypointense signals in T1WI. PWMH was graded as 0=absence, 1=“caps” or pencil-thin lining (≤5 mm), 2=smooth “halo” (6-10 mm) and 3=large conglomerate lesions (>10 mm). DWMH showed balanced and irregular hyperintense signals in subcortical white matter, as well as beside the watershed area in T2WI and isointense or hypointense signals in T1WI. DWMH was graded as 0=absence, 1=punctate foci, 2=beginning confluence of foci, and 3=large confluent areas, which has affected most white matter areas. The total score of WMH obtained was the sum of both locations scores (0-9 points).
LI is manifested as a well-defined lesion with a diameter of 3-15 mm, T1WI with low intensity signal and high intensity signal on T2WI, and needs to be distinguished from EPVS (the diameter of EPVS is usually less than 3 mm). We determined and counted the total number of LIs in each patient.
The imaging definition of CMB is on a SWI sequence, which shows a uniform, round, well-defined boundary, low-signal focus/lesion with a diameter of 2-5 mm and no peripheral edema. It is necessary to exclude the vascular flow void and calcification without hemorrhage.
The EPVS manifests as a gap consistent with the course of the blood vessel. In the vertical plane of the blood vessel, there is often a round or oval cerebrospinal fluid signal with a diameter of less than 3 mm. The signal intensity is low on T1WI images, high-signal on T2WI and low-signal on Flair images.
Brain atrophy is characterized by a reduction in brain volume, which is reflected by an enlargement of ventricles and sulcus.
All brain MRI were reviewed and evaluated by an experienced radiologist.
Statistical analyses
Software SPSS 25.0 was utilized for statistical data analyses. We used a propensity score analysis to match CSVDs patients with non-CSVDs patients at a ratio of 1:1 with a match tolerance 0.02 and based on age, gender, laterality, previous BPPV history, duration of BPPV, number of CRPs and involved canal. Categorical variables were represented as either counts or percentages. For comparison among groups, the Chi-square test, Fisher exact test and McNemar test were used. Independent-samples t-test and paired t-test were used for continuous variables which were normal distribution and described by mean ± standard deviation (SD). The Mann-Whitney U and Wilcoxon test were used for continuous variables with an abnormal distribution and described by median (quartile range, QR). A binary logistic regression model helped determine risk factors for recurrence and RD of BPPV. All variables with P<0.05 at univariate test statistics were regarded as covariates and included in multifactorial logistic regression model as potential predictors. The odds ratio (OR) and 95% confidence interval (CI) were calculated. A difference was considered statistically significant if P <0.05.
Results
Basic characteristics of patients
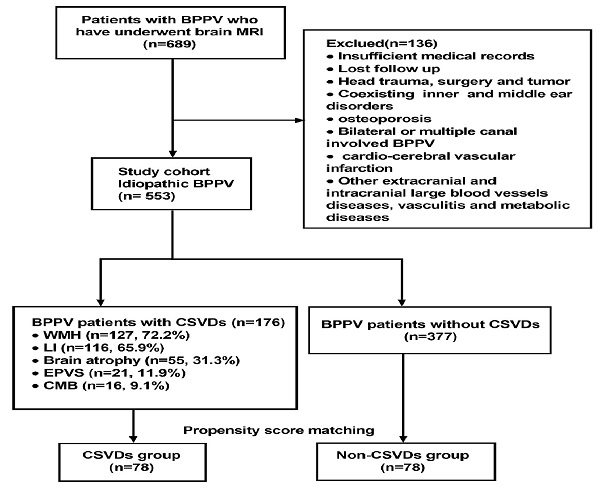
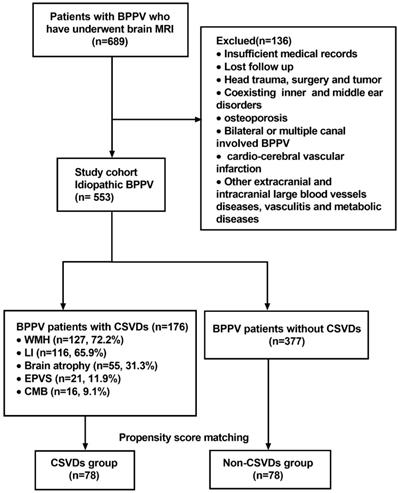
Overall, 689 patients who met the inclusion criteria were initially enrolled in the study. According to the exclusion criteria, 136 patients were then excluded at screening. Finally, a total of 553 patients were found to be eligible. Among them, 176 BPPV patients whose brain MRI showed the presence of CSVDs, including 74 males and 102 females with an age range of 31-91 years (median: 68). The distribution of CSVDs among 176 BPPV patients with CSVDs is shown in Figure 1. After matching on the propensity score, 78 patients in the CSVDs group and 78 patients in the non-CSVDs group were included in the study. A flow chart describing the study design is depicted in Figure 1.
Flow chart of the study design. Abbreviations: benign paroxysmal positional vertigo (BPPV); cerebral microbleed (CMB); cerebral small vessel disease (CVSD); enlarged perivascular space (EPVS); lacunar infarction (LI); white matter hyperintensities (WMH).
Comparison of clinical parameters between BPPV patients with and without CSVDs
After matching the two groups using propensity score analysis based on age, gender, laterality, previous history of BPPV, duration of BPPV, CRP times and involved semicircular canal types, we found a statistically significant difference in RD. Although there were more cases of 1-year recurrence in the CSVDs group, the difference was insignificant (Table 1).
Baseline clinical characteristics and the incidence of recurrence and RD in BPPV patients with and without CVSDs after matching on the propensity score
| CSVDs group n=78 (%) | Non-CSVDs group n=78 (%) | P value | |
|---|---|---|---|
| Age (years) | 68.15±9.23 | 68.73±10.15 | 0.598 |
| Gender (male) | 31(39.7%) | 30(38.5%) | 0.870 |
| Laterality (right) | 41(52.6%) | 43(55.1%) | 0.748 |
| Previous BPPV (yes) | 33(42.3%) | 30(38.5%) | 0.624 |
| Duration of BPPV (days) | 5.91±3.63 | 5.89±3.51 | 0.207 |
| Number of CRPs | 1.36±0.44 | 1.35±0.46 | 0.434 |
| Canal involved | |||
| PSC-BPPV | 55(70.5%) | 58(74.4%) | 0.771 |
| HSC-BPPV | 21(26.9%) | 19(24.4%) | |
| AC-BPPV | 2(2.6%) | 1(1.3%) | |
| 1- year recurrence | 27(34.6%) | 17(21.8%) | 0.075 |
| RD | 36(46.2%) | 21(26.9%) | 0.013 |
Abbreviations: anterior semicircular (ASC); benign paroxysmal positional vertigo (BPPV); canalith repositioning procedure/maneuvers (CRP); cerebral small vessel disease (CVSD); horizontal semicircular (HSC); posterior semicircular (PSC); residual dizziness (RD).
Analysis of recurrence rate and RD incidence in BPPV patients with CSVDs
As WMH (72.2%) and LI (65.9%) are the two CSVDs with the highest proportion in BPPV patients (Figure 1), we further scored WMH severity and counted the number of LIs in each patient. Among the 176 patients, 61 patients (34.7%) had a recurrence during the 1-year follow-up period. In the recurrent patients, the median age was older, the Fazekas score of WMH was higher, and number of LIs was more (Table 2). There were 100 patients (56.8%) who developed RD after the CRP treatment. The patients with RD had higher age, higher Fazekas score of WMH and higher incidence of brain atrophy (Table 2).
Multivariate logistic regression analysis of risk factors for recurrence and RD in BPPV patients with CSVDs
In multivariate logistic regression analysis for RD, when other factors remained unchanged, for every one point increase in Fazekas score of WMH, the probability for RD occurrence was 1.078 times higher compared to if the Fazekas score did not increase (OR=1.078, 95%CI: 1.041-1.116; p=0.010). Similarly, for every year increase in age, the probability for RD occurrence was 1.397 higher times compared to if the age did not increase (OR=1.397, 95%CI: 1.197-1.63; p=0.008). Hence, it can be concluded that increased severity of cerebral white matter lesions and age are risk factors for RD. Among the multivariate logistic regression analysis for recurrence, we found the increased age was an independent risk factor (OR=1.105, 95% CI: 1.060-1.152; p=0.012).
Analysis of recurrence rate and RD incidence in 176 BPPV patients with CSVDs
| Variables | Recurrence | RD | ||||
|---|---|---|---|---|---|---|
| Yes | No | P value | Yes | No | P value | |
| Subjects | 61(34.7%) | 115(65.3%) | 100(56.8%) | 76(43.2%) | ||
| Age b | 74(7.5) | 62(19) | <0.001 | 72(9.75) | 54(18.75) | <0.001 |
| Gender | ||||||
| Male | 22(29.7%) | 52(70.3%) | 0.242 | 39(52.7%) | 35(47.3%) | 0.348 |
| Female | 39(38.2%) | 63(61.8%) | 61(59.8%) | 41(40.2%) | ||
| Laterality | ||||||
| Right | 32(34.8%) | 60(65.2%) | 0.971 | 54(58.7%) | 38(41.3%) | 0.599 |
| Left | 29(34.5%) | 55(65.5%) | 46(54.8%) | 38(45.2%) | ||
| Previous BPPV | ||||||
| Yes | 34(41.5%) | 48(58.5%) | 0.076 | 46(56.1%) | 36(43.9%) | 0.857 |
| No | 27(28.7%) | 67(71.3%) | 54(57.4%) | 40(42.6%) | ||
| Duration of BPPV(days) a | 6.26±3.20 | 6.50±3.30 | 0.641 | 6.52±3.33 | 5.88±3.15 | 0.199 |
| Number of CRPs b | 2(1) | 1(1) | 0.138 | 2(1) | 1(1) | 0.500 |
| Canal involved | 0.113 | 0.730 | ||||
| PSC-BPPV | 35(57.4%) | 82(71.3%) | 64 (64%) | 53 (69.7%) | ||
| HSC-BPPV | 24(39.3%) | 28(24.3%) | 32 (32%) | 20 (26.3%) | ||
| AC-BPPV | 2(2.4%) | 5(4.3%) | 4 (4%) | 3 (3.9%) | ||
| CSVDs | ||||||
| WMH b | 5(3) | 3(6) | 0.030 | 5(3) | 0(4) | <0.001 |
| LI b | 4(3) | 2(5) | 0.025 | 3(5) | 2(5) | 0.119 |
| Brain atrophy (Yes) | 22(40.0%) | 33(60.0%) | 0.315 | 41(74.5%) | 14(25.5%) | 0.001 |
| (No) | 39(32.2%) | 82(67.8%) | 59(48.8%) | 62(51.2%) | ||
| CMB (Yes) | 7(43.8%) | 9(56.3%) | 0.423 | 12(75.0%) | 4(25.0%) | 0.124 |
| (No) | 54(33.8%) | 106(66.3%) | 88(55.0%) | 72(45.0%) | ||
| EPVS (Yes) | 8(38.1%) | 13(61.9%) | 0.724 | 14(66.7%) | 7(33.3%) | 0.332 |
| (No) | 53(34.2%) | 102(65.8%) | 86(55.5%) | 69(44.5%) | ||
Abbreviations: anterior semicircular (ASC); benign paroxysmal positional vertigo (BPPV); cerebral microbleed (CMB); canalith repositioning procedure/maneuvers (CRP); cerebral small vessel disease (CVSD); enlarged perivascular space (EPVS); horizontal semicircular (HSC); lacunar infarction (LI); posterior semicircular (PSC); residual dizziness (RD); white matter hyperintensities (WMH);
a Mean±standard deviation (SD), b Median (quartile range, QR).
Discussion
CSVDs are common among middle-aged and elderly people, and often are accompanied by no obvious clinical symptoms [19, 20]. In this study, we enrolled patients with BPPV who had undergone brain MRI as research subjects. We found that CSVDs was related to RD and recurrence of BPPV among BPPV patients.
The concomitant CSVDs among BPPV patients in this study include WMH, LI, EPVS, CMB and brain atrophy. Among them, WMH was the most common CSVD, which is consistent with the findings of Cha and others. Through investigation of the brain MRI of BPPV patients in the emergency department, Cha et al. found that about 70% of patients had WMH [12]. There were many factors causing WMH, among which hemodynamic changes, which are recognized by most researchers, leads to insufficient perfusion of deep brain tissue, decrease of blood flow, and finally ischemia and demyelinating changes in an area dominated by arterioles [21, 22]. WMH is an indicator of chronic microvascular ischemia [21]. Additionally, in this study, LI is also a common manifestation of CSVDs, which mostly located in the basal ganglia, frontal lobe, and parietal lobe. Reuck found that 25% of BPPV patients had small old cerebral infarcts on imaging, while none of them had a prior history of stroke [23]. Von Brevern et al. also noted that stroke can be an independent risk factor for BPPV [2]. WMH and LI are considered to be signs of small vessel disease in the brain, which can be detected by brain MRI [13].
Pathological changes, including cerebral small vessel stenosis or occlusion, are mostly chronic, and there are no obvious stroke events in clinic [10]. However, small vessel endothelium has been damaged, which causes a decrease in cerebral blood flow. In addition to causing brain tissue hypoperfusion and CSVDs, it can also cause ischemia of the inner ear [23, 24]. The inner ear is an organ with strong aerobic metabolism and is sensitive to ischemia [9, 23]. In particular, ischemia can cause local microcirculation disturbances to the vestibule, damage the elliptic sac, make otoliths more likely to fall off, and at the same time, affect the absorption of otoliths in the endolymph, which causes BPPV recurrence and occurrence of RD [7-9].
Frequent recurrence of BPPV seriously affects the quality of life and makes patients anxious. Hence, the early identification and prompt management of BPPV is very important. We excluded patients with a history of head trauma, Meniere's disease, migraine, osteoporosis, simultaneous bilateral or multiple canals involvement and other factors that could lead to the recurrence of BPPV [7, 14, 25-27]. In our study of 176 BPPV patients with CSVDs, the recurrent patients had higher age, higher Fazekas score of WMH, and more number of LIs. It has been found that the recurrence of BPPV is closely related to vascular risk factors, such as high hypertension, hyperlipidemia, diabetes, and smoking, among others [14, 27-31]. At the same time, they are many risk factors that are related to the onset of CSVDs [20, 32]. These vascular risk factors may cause insufficient perfusion of the anterior vestibular artery by changing blood vessels, leading to circulatory abnormalities of inner ear and causing damage to the macula and otoconia detachment [7-9]. Therefore, it is not difficult to understand that WMHs and LIs were more severe in the patients with recurrence. However, multivariate logic regression analysis of the patients with CSVDs indicated that only age was a risk factor for the recurrence of BPPV, after controlling the other factors. We considered that CSVDs may are likely more prevalent among the elderly and is not be an independent factor in the recurrence of BPPV. Some studies have indicated the recurrence of BPPV is related to gender, CRP times, previous BPPV, and is involved side and duration of disease [7, 14, 33]. However, some studies have the opposite view [12, 27, 31]. In this study, we did not find a correlation between these above factors and recurrence of BPPV. Only the previous history of BPPV was close to statistical significance in the difference in recurrence rate. Increasing age as a risk factor for BPPV recurrence has been consistently identified in many studies on the topic [3, 34, 35]. This may be due to change of otoconial morphology with age, which causes increases in detachment [36].
Several studies have identified that non-rotational dizziness and walking instability continues to exist after a successful repositioning of BPPV, with an incidence rate of 31.1% to 61%, and is termed as residual dizziness (RD) [37-40]. Generally, a positional nystagmus test is negative, without nausea and vomiting [16, 41, 42]. The duration of RD symptoms ranges from a few days to weeks, and even several months have been previously reported [12, 16, 37-39, 42]. The mechanism may be due to an additional vestibular dysfunction that coexists with BPPV and causes less-specific dizziness or incomplete central adaptation, which needs a longer time after repositioning [43-45]. Some researchers have even suggested that the remaining otoconial debris may not be completely repositioned, which can cause mild positional vertigo, but not enough to provoke any overt nystagmus [43, 46]. Some studies have also shown that BPPV is not only a disorder of semicircular canals, but also an otolith dysfunction that may account for transient mild dizziness [47, 48]. Residual vertigo has a great impact on quality of life, is a risk factor for falls and causes anxiety [42, 49].
In this study, we determined that the incidence of RD in the CSVDs group is higher compared to the non-CSVDs group, after matching on the propensity score; the differences are statistically significant. This suggests that BPPV patients with CSVDs are more likely to have RD. Chen et al. found a higher percentage of RD in BPPV patients with central neurologic disorders, a result which needs to be verified in a larger case-control study [50]. In addition, some studies have found that CSVD is closely related to vertigo [22, 51-53]. This study found that occurrence of RD is closely related to the severity of WMH. Logistic multiple regression analysis of 176 BPPV patients with CSVDs shows that severity of WMH and age are risk factors for RD. This is further supported by a study of Adamec et al., who found that the presence of WMH is a predictor for chronic RD among patients with previous vestibular neuritis [54]. In addition, Ahmad et al. found that the severity of WHM is higher among patients with unexplained dizziness compared to those with specific causes (BPPV, vestibular neuritis, vestibular migraine, Meniere's disease, and other diseases) [52]. All of these results suggest that WMH likely contributes to the development of RD. The reason for this analysis may be that WMH might affect vestibular cortex and related neural networks [21]. Some scholars have also suggested that WMH patients may have a cortical-subcortical disconnection syndrome [10, 52]. We speculate that a damage of the neural pathway may affect central vestibular adaptation and increase risk of RD.
In addition, some studies have shown that RD may be related to the duration of vertigo prior to CRP [37, 38, 45], but there are still some controversies [39, 42]. This study did not find any significant correlation between duration of BPPV and RD. The reason for the analysis may be selection bias, and that the analyzed population is patients with CSVDs. However, the recognition that age is a risk factor for RD is relatively consistent across studies [16, 35, 41, 42]. Our findings indicated that there is a significant correlation between age and occurrence of RD. The vestibular function of elderly patients suffers from age-related decline [36, 42]. In addition, elderly patients are more likely to develop anxiety, which has also been shown to promote occurrence of RD [5, 42]. Cha et al. found that brain atrophy was related to long-lasting dizziness after BPPV treatment and speculated that there may be a relationship between brain atrophy and otoneurologic diseases [12]. This study indicates that BPPV patients with CSVDs who develop RD have a higher incidence of brain atrophy, but the logic multivariate analysis does not find that brain atrophy is a risk factor for RD.
Therefore, it is of great clinical significance that we identify BPPV patients who are prone to RD and recurrence, as well as to intervene with treatment. In addition to CRPs, vestibular rehabilitation exercises have been shown to be effective at controlling RD symptoms and preventing BPPV recurrence [55, 56]. In addition, for BPPV patients with CSVDs, whether active treatment of CSVDs is helpful to the prognosis of BPPV, requires further research to prove it. This study had some limitations. First, a larger number of patients and longer follow-up time may be needed to provide more precise values in the future. Second, we excluded patients who did not complete brain MRI, which may cause selection bias. In addition, due to outpatient medical records, the patients' information was not fully complete for some potential risk factors, including psychological factors and otoneurologic examinations. Finally, we did not quantitatively evaluate the degree of brain atrophy, CMB and EPVS, but simply classified it as presence or absence.
Conclusion
We investigated the type and proportion of CSVD in BPPV patients with CSVDs and focused on determining the prognosis of BPPV patients with CSVDs, including recurrence rate and RD after successful CRP. WMH and LI were found to be the most prevalent types of CSVD in among BPPV patients. After controlling for other confounders using a propensity score matching approach, we found that patients with CSVDs were more likely to have RD. In addition, logistic regression analysis also indicated that increased age and WMH level were independent risk factors for RD. Accordingly, we conclude that older BPPV patients with WMHs are more likely to develop RD, and therefore need to be paid more attention. Perhaps, for BPPV patients with CSVDs, we should actively treat CSVDs at the same time as CRP treatment in order to reduce the risk of RD. We will expand the sample size in future studies and design controlled trials to further validate our findings.
Abbreviations
ASC: anterior semicircular; BPPV: benign paroxysmal positional vertigo; CMB: cerebral microbleed; CRP: canalith repositioning procedure/maneuvers; CVSD: cerebral small vessel disease; EPVS: enlarged perivascular space; HSC: horizontal semicircular; LI: lacunar infarction; PSC: posterior semicircular; PSM: propensity score matching; RD: residual dizziness; WMH: white matter hyperintensities.
Acknowledgements
Funding
This research was supported by Liaoning Province Natural Science Foundation (Doctoral Research Start-up Fund Project, 2021-BS-124).
Author Contributions
Jian Zang and Shuai Feng designed and wrote the manuscript. Jian Zang and Hongyang Zhang contributed to the data collection and prepared figures and tables.
Xuejun Jiang edited the manuscript text. All authors reviewed and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370:1138-47
2. von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T. et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78:710-5
3. Bhattacharyya N, Gubbels SP, Schwartz SR, Edlow JA, El-Kashlan H, Fife T. et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol Head Neck Surg. 2017;156:S1-s47
4. Özdilek A, Yalınay Dikmen P, Acar E, Ayanoğlu Aksoy E, Korkut N. Determination of Anxiety, Health Anxiety and Somatosensory Amplification Levels in Individuals with Benign Paroxysmal Positional Vertigo. J Int Adv Otol. 2019;15:436-41
5. Maas B, Bruintjes TD, van der Zaag-Loonen HJ, Winters SM, Masius-Olthof S, Colijn C. et al. Physical and Emotional Burden of the Epley Maneuver in the Elderly. Otol Neurotol. 2019;40:1082-7
6. Kao CL, Cheng YY, Leu HB, Chen TJ, Ma HI, Chen JW. et al. Increased risk of ischemic stroke in patients with benign paroxysmal positional vertigo: a 9-year follow-up nationwide population study in taiwan. Front Aging Neurosci. 2014;6:108
7. De Stefano A, Dispenza F, Suarez H, Perez-Fernandez N, Manrique-Huarte R, Ban JH. et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo. Auris Nasus Larynx. 2014;41:31-6
8. Cao Z, Zhao X, Ju Y, Chen M, Wang Y. Seasonality and Cardio-Cerebrovascular Risk Factors for Benign Paroxysmal Positional Vertigo. Front Neurol. 2020;11:259
9. Wada M, Naganuma H, Tokumasu K, Hashimoto S, Ito A, Okamoto M. Arteriosclerotic changes as background factors in patients with peripheral vestibular disorders. Int Tinnitus J. 2008;14:131-4
10. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689-701
11. Yang YJ, Choi JE, Kim MT, Kim SH, Lee MY, Yoo DS. et al. Measurement of horizontal ocular deviation on magnetic resonance imaging in various disease with acute vertigo. PLoS One. 2019;14:e0224605
12. Cha WW, Song K, Yu IK, Choi MS, Chang DS, Cho CS. et al. Magnetic resonance imaging predicts chronic dizziness after benign paroxysmal positional vertigo. Am J Otolaryngol. 2017;38:428-32
13. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822-38
14. Zhu CT, Zhao XQ, Ju Y, Wang Y, Chen MM, Cui Y. Clinical Characteristics and Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo. Front Neurol. 2019;10:1190
15. Yeo SC, Ahn SK, Lee HJ, Cho HJ, Kim SW, Woo SH. et al. Idiopathic benign paroxysmal positional vertigo in the elderly: a long-term follow-up study. Aging Clin Exp Res. 2018;30:153-9
16. Dispenza F, Mazzucco W, Mazzola S, Martines F. Observational study on risk factors determining residual dizziness after successful benign paroxysmal positional vertigo treatment: the role of subclinical BPPV. Acta Otorhinolaryngol Ital. 2019;39:347-52
17. Yacovino DA, Hain TC, Gualtieri F. New therapeutic maneuver for anterior canal benign paroxysmal positional vertigo. J Neurol. 2009;256:1851-5
18. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351-6
19. Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. 2018;14:387-98
20. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: A clinical review. Neurology. 2019;92:1146-56
21. Gamba P, Pavia M. White matter lesions and vascular vertigo: clinical correlation and findings on cranial magnetic resonance imaging. Eur Rev Med Pharmacol Sci. 2016;20:2786-91
22. Dimitriadis PA, Saad M, Igra MS, Mandavia R, Bowes C, Hoggard N. et al. White matter lesions in magnetic resonance imaging of the brain in 56 patients with visual vertigo. J Laryngol Otol. 2018;132:550-3
23. De Reuck J. Vascular risk factors in patients with peripheral vestibular disorders. Acta Neurol Belg. 2010;110:303-5
24. Cerchiai N, Mancuso M, Navari E, Giannini N, Casani AP. Aging with Cerebral Small Vessel Disease and Dizziness: The Importance of Undiagnosed Peripheral Vestibular Disorders. Front Neurol. 2017;8:241
25. Ogun OA, Janky KL, Cohn ES, Buki B, Lundberg YW. Gender-based comorbidity in benign paroxysmal positional vertigo. PLoS One. 2014;9:e105546
26. Yamanaka T, Shirota S, Sawai Y, Murai T, Fujita N, Hosoi H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope. 2013;123:2813-6
27. Babac S, Djeric D, Petrovic-Lazic M, Arsovic N, Mikic A. Why do treatment failure and recurrences of benign paroxysmal positional vertigo occur? Otol Neurotol. 2014;35:1105-10
28. Webster G, Sens PM, Salmito MC, Cavalcante JD, Santos PR, Silva AL. et al. Hyperinsulinemia and hyperglycemia: risk factors for recurrence of benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2015;81:347-51
29. D'Silva LJ, Staecker H, Lin J, Sykes KJ, Phadnis MA, McMahon TM. et al. Retrospective data suggests that the higher prevalence of benign paroxysmal positional vertigo in individuals with type 2 diabetes is mediated by hypertension. J Vestib Res. 2016;25:233-9
30. Gioacchini FM, Albera R, Re M, Scarpa A, Cassandro C, Cassandro E. Hyperglycemia and diabetes mellitus are related to vestibular organs dysfunction: truth or suggestion? A literature review. Acta Diabetol. 2018;55:1201-7
31. Tan J, Deng Y, Zhang T, Wang M. Clinical characteristics and treatment outcomes for benign paroxysmal positional vertigo comorbid with hypertension. Acta Otolaryngol. 2017;137:482-4
32. Han F, Zhai FF, Wang Q, Zhou LX, Ni J, Yao M. et al. Prevalence and Risk Factors of Cerebral Small Vessel Disease in a Chinese Population-Based Sample. J Stroke. 2018;20:239-46
33. Pérez P, Franco V, Cuesta P, Aldama P, Alvarez MJ, Méndez JC. Recurrence of benign paroxysmal positional vertigo. Otol Neurotol. 2012;33:437-43
34. Balatsouras DG, Koukoutsis G, Fassolis A, Moukos A, Apris A. Benign paroxysmal positional vertigo in the elderly: current insights. Clin Interv Aging. 2018;13:2251-66
35. Nahm H, Han K, Shin JE, Kim CH. Benign Paroxysmal Positional Vertigo in the Elderly: A Single-center Experience. Otol Neurotol. 2019;40:1359-62
36. Iwasaki S, Yamasoba T. Dizziness and Imbalance in the Elderly: Age-related Decline in the Vestibular System. Aging Dis. 2015;6:38-47
37. Seok JI, Lee HM, Yoo JH, Lee DK. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J Clin Neurol. 2008;4:107-10
38. Teggi R, Quaglieri S, Gatti O, Benazzo M, Bussi M. Residual dizziness after successful repositioning maneuvers for idiopathic benign paroxysmal positional vertigo. ORL J Otorhinolaryngol Relat Spec. 2013;75:74-81
39. Kim HA, Lee H. Autonomic dysfunction as a possible cause of residual dizziness after successful treatment in benign paroxysmal positional vertigo. Clin Neurophysiol. 2014;125:608-14
40. Kim MB, Lee HS, Ban JH. Vestibular suppressants after canalith repositioning in benign paroxysmal positional vertigo. Laryngoscope. 2014;124:2400-3
41. Giommetti G, Lapenna R, Panichi R, Mobaraki PD, Longari F, Ricci G. et al. Residual Dizziness after Successful Repositioning Maneuver for Idiopathic Benign Paroxysmal Positional Vertigo: A Review. Audiol Res. 2017;7:178
42. Martellucci S, Pagliuca G, de Vincentiis M, Greco A, De Virgilio A, Nobili Benedetti FM. et al. Features of Residual Dizziness after Canalith Repositioning Procedures for Benign Paroxysmal Positional Vertigo. Otolaryngol Head Neck Surg. 2016;154:693-701
43. Di Girolamo S, Ottaviani F, Scarano E, Picciotti P, Di Nardo W. Postural control in horizontal benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol. 2000;257:372-5
44. Pollak L, Davies RA, Luxon LL. Effectiveness of the particle repositioning maneuver in benign paroxysmal positional vertigo with and without additional vestibular pathology. Otol Neurotol. 2002;23:79-83
45. Stambolieva K, Angov G. Postural stability in patients with different durations of benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol. 2006;263:118-22
46. Di Girolamo S, Paludetti G, Briglia G, Cosenza A, Santarelli R, Di Nardo W. Postural control in benign paroxysmal positional vertigo before and after recovery. Acta Otolaryngol. 1998;118:289-93
47. von Brevern M, Schmidt T, Schönfeld U, Lempert T, Clarke AH. Utricular dysfunction in patients with benign paroxysmal positional vertigo. Otol Neurotol. 2006;27:92-6
48. Gall RM, Ireland DJ, Robertson DD. Subjective visual vertical in patients with benign paroxysmal positional vertigo. J Otolaryngol. 1999;28:162-5
49. Uz U, Uz D, Akdal G, Celik O. Efficacy of Epley Maneuver on Quality of Life of Elderly Patients with Subjective BPPV. J Int Adv Otol. 2019;15:420-4
50. Chen CC, Cho HS, Lee HH, Hu CJ. Efficacy of Repositioning Therapy in Patients With Benign Paroxysmal Positional Vertigo and Preexisting Central Neurologic Disorders. Front Neurol. 2018;9:486
51. Okroglic S, Widmann CN, Urbach H, Scheltens P, Heneka MT. Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS One. 2013;8:e53455
52. Ahmad H, Cerchiai N, Mancuso M, Casani AP, Bronstein AM. Are white matter abnormalities associated with "unexplained dizziness"? J Neurol Sci. 2015;358:428-31
53. Kaski D, Rust HM, Ibitoye R, Arshad Q, Allum JHJ, Bronstein AM. Theoretical framework for "unexplained" dizziness in the elderly: The role of small vessel disease. Prog Brain Res. 2019;248:225-40
54. Adamec I, Krbot Skorić M, Ozretić D, Habek M. Predictors of development of chronic vestibular insufficiency after vestibular neuritis. J Neurol Sci. 2014;347:224-8
55. Wu P, Cao W, Hu Y, Li H. Effects of vestibular rehabilitation, with or without betahistine, on managing residual dizziness after successful repositioning manoeuvres in patients with benign paroxysmal positional vertigo: a protocol for a randomised controlled trial. BMJ Open. 2019;9:e026711
56. Rodrigues DL, Ledesma ALL, Pires de Oliveira CA, Bahmad F Jr. Effect of Vestibular Exercises Associated With Repositioning Maneuvers in Patients With Benign Paroxysmal Positional Vertigo: A Randomized Controlled Clinical Trial. Otol Neurotol. 2019;40:e824-e9
Author contact
![]() Corresponding author: Jian Zang, Department of Otolaryngology, the First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning, China. E-mail: jzangedu.cn.
Corresponding author: Jian Zang, Department of Otolaryngology, the First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning, China. E-mail: jzangedu.cn.

 Global reach, higher impact
Global reach, higher impact