3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(8):1216-1226. doi:10.7150/ijms.74137 This issue Cite
Review
Role of extracellular vesicles in osteosarcoma
1. Department of Orthopaedics, the First Hospital of Jilin University, Changchun, Street Xinmin 71, China.
2. The first clinical medical college of Bin Zhou Medical College, Street Huanghe 661, China.
#These authors contributed equally to this work.
Received 2022-4-19; Accepted 2022-6-21; Published 2022-7-4
Abstract
Osteosarcoma is a malignant bone tumor characterized by the direct production of osteoid tissue from tumor cells. Extracellular vesicles are membranous vesicles released by cells into the extracellular matrix, which exist widely in various body fluids and cell supernatants, and stably carry some important signaling molecules. They are involved in cell communication, cell migration, angiogenesis and tumor cell growth. Increasing evidence has shown that extracellular vesicles play a significant role in osteosarcoma development, progression, and metastatic process, indicating that extracellular vesicles can be use as biomarker vehicles in the diagnosis and prognosis of osteosarcoma. This review discusses the basic biological characteristics of extracellular vesicles and focuses on their application in osteosarcoma.
Keywords: osteosarcoma, extracellular vesicles, immune escape, chemotherapy resistance, microenvironment
Introduction
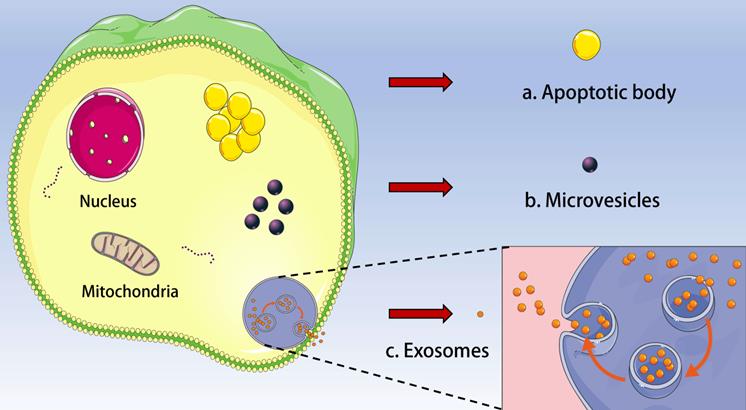
Extracellular vesicles (EVs) are a general term for all kinds of vesicle structures with membrane structure released by cells. Based on the biogenesis, diameter and biophysical properties, EVs can be divided into three subgroups: exosomes, microvesicles/ectosomes, and apoptotic body (Figure 1). EVs play a critical role in cell communication and body regulation through signal molecules such as proteins and lipids on the membrane and contents (neurotransmitters, enzymes, hormones and nucleic acids) wrapped inside the membrane [1-3]. Interestingly, EVs were initially regarded as “cell dust” and a mechanism for processing cellular components, and now EVs were considered as promising circulating biomarkers of diseases [4]. In addition, EVs are extensively involved in apoptosis, tumor development, angiogenesis and immune responses [5]. Almost all cells can secrete EVs under physiological or pathological conditions, and EVs can also be found in almost all body fluids such as blood, urine, and saliva [6]. EVs offer great advantages in cancer research.
Osteosarcoma (OS) is one of the most difficult diseases in the field of orthopedics and is also the focus of current medical research. OS, comprising around 1% of all human malignancies, is a heterogeneous malignant spindle cell tumor characterized by the formation of immature osteoid tissue or osteoid [7, 8]. In general, OS occurs mainly in adolescents, but there is a second incidence peak in older adults over 60 years of age [9]. The standard treatment for OS mainly includes surgery, post-operative chemotherapy and neoadjuvant chemotherapy. Although the 5-year survival rate of these treatment is 60-70% [10, 11], for patients with metastatic and/or recurrent OS, the original treatment method does not guarantee a favorable outcome [12, 13]. Therefore, the major translational objective in OS research is to find new therapeutic markers with great clinical potential. In this review, we mainly discuss the role of EVs in OS, including regulating OS metastasis, tumor microenvironment, immune escape, chemotherapy resistance, which make them to become promising biomarkers and therapeutic targets for OS.
Types of extracellular vesicle (EV). Based on the biogenesis, diameter and biophysical properties, EV can be divided into three subgroups: a. Apoptotic body; b. Microvesicles and c. Exosomes.
EVs biogenesis
Exosomes are produced by inward invagination through the endosomal membrane pathway [14]. Firstly, the inward budding of cellular plasma membrane promotes the formation of endosomes [15]. Vesicles are formed by further inward budding of limiting membranes inside endosomes, causing the formation of the multivesicular body (MVB), which is characterized by the intraluminal vesicles [16, 17]. Upon maturation, MVBs may fuse with the plasma membrane and secrete exosomes, or degrade the cargo by lysosomal fusion. During this process, transmembrane proteins, cytosolic contents, and peripheral proteins are intergrated into the invaginating membrane, and then MVBs fuse with the lysosome, resulting in the vesicular contents degradation [18, 19]. Similarly, MVBs can fuse with the plasma membrane and release vesicles to the extracellular space in an exocytotic manner [20]. The released small membrane-bound lipid vesicles with a diameter ranging from 30 to 200 nm are considered as exosomes.
In addition, EVs can directly bud in the plasma membrane. Microvesicles have extremely heterogeneous sizes ranging from 50 nm to 10 μm [21]. The mechanism of microvesicle biogenesis is correlated with non-apoptotic plasma membrane blebs which exist in the highly aggressive cancer cells [22]. These vesicles expand and contract on the cell surface to promote cell motility and can be released as microvesicles, which are rearranged by the actin cytoskeleton in the vesicle budding neck, leading to membrane scission [23-25]. Tumor cells during migration in vivo enable to adopt a phenotype called amoeba, which is related to the blebbing of extensive plasma membrane and microvesicle releasing, indicating that this type of EVs biogenesis plays an important role in tumor invasion and metastasis [25-28].
EVs cargo
In the tumor microenvironment, the functional properties of EVs depend on their cellular cargo and their metabolic dynamics [29]. One of the major challenges in this field is to identify the pro-tumorigenic components of cancer EVs and the pathways that lead to their binding into vesicles. EVs contain various contents as follow.
Protein cargo: Proteins are integrated into EVs by interrelating with components of the EV biogenesis machinery [30, 31]. Generally, EVs are very rich in the cytoskeleton protein, the cytoplasmic protein, the cell membrane protein, the heat shock protein, and proteins participating in vesicle transport, however, fewer organelle proteins exist in the cell [32].
DNA cargo: Several evidence has shown that the presence of the mitochondrial DNA, the fragmented genomic DNA and even the parasitic DNA in EVs [33-35]. Although the mechanism of DNA incorporation in EVs remains unknown, the fact that EV genomic DNA fragments are evenly distributed throughout the whole genome indicates a random process [36]. DNA fragments secreted by EVs prevent the activation of cytoplasmic DNA sensors, thereby promoting cellular homeostasis, which might be particularly critical in cancer because increased levels of DNA damage require efficient cytoplasmic DNA removal by EVs [37].
RNA cargo: EVs contain complete mRNA, fragmented mRNA, long non-coding RNA, ribosomal RNA, and miRNA [9]. EVs are loaded with various contents which reflect different states of the parent cell and affect the performance and functions of EVs.
EVs detection methods
Microscopy based methods
Microscopy based methods, such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), cryo-electron microscopy (Cryo-EM), and atomic force microscopy (AFM), are widely used in measuring the physical features (size, distribution, concentration, etc.) of EVs. SEM takes images of the EV sample through scanning the surface and provides information on the three-dimensional surface topography and the elemental composition of samples [38]. TEM has a high resolution and is capable of imaging objects <1 nm. Unlike SEM and TEM, which require extensive fixation and staining, Cryo-EM is able to analyze EVs in frozen samples because the interference of dehydration and chemical fixatives is excluded [39].
Nanoparticle tracking analysis
Nanoparticle Tracking Analysis (NTA) is a tracking method for determining particle concentration and size distribution. Nanoparticles in their suspensions undergo irregular Brownian motion due to the impact of surrounding solution molecules [40, 41]. According to the Stokes-Einstein equations, there is a quantitative relationship between the speed of movement of these particles per unit time and their own particle size, the viscosity of the solution and the temperature. Therefore, by observing the trajectory of the particles in solution, the particle size data associated with it are derived. At the same time, each observed particle is tracked and analyzed by the instrument's built-in high-speed camera and software, ultimately providing an analysis of the particle size number distribution and particle concentration that differs from that of conventional particle size meters [42].
Small particle flow cytometry
Conventional flow cytometry has limited resolution in detecting small particles less than 500 nm in diameter [43]. The high optical background due to the presence of small particles in sheath fluids is also a problem [44]. To address these challenges, highly sensitive flow cytometers, which can discriminate particles of 100 nm in diameter, are under development [45].
EVs in osteosarcoma
EVs are mediators in the tumor microenvironment and are thought to play a crucial role in communication between tumor cells and other cells. Recent evidence has shown that EVs also closely participated in the tumorigenesis, proliferation, metastasis, immune evasion and chemoresistance of OS, and are regarded as potential biomarkers and therapeutic targets for OS. Understanding the role of EVs in OS is crucial for future treatment and prolonged survival of OS patients. Roles of EVs in OS are thoroughly discussed as follow.
The role of EVs in metastasis of osteosarcoma
Despite recent advances in the diagnosis and treatment of OS, many patients still had the poor survival rate. It was estimated that 10% to 20% patients develop metastasis before diagnosis, with 5-year overall survival rates (OSR) of less than 20% [46]. Understanding the metastasis process is one of the current focuses of OS researches, which could help develop strategies for treating metastatic disease and improving OSR. Interestingly, EVs are considered to play an important role in tumor metastasis, and they participate in the metastasis of tumor cells mainly through three ways [47, 48]: tumor cells directly promote metastasis by secreting EVs; EVs influence the microenvironment of tumor cells; EVs indirectly mediate metastasis via transforming distant mesenchymal cells. MiRNA could be transported between tissues by EVs. Bioinformatics researches demonstrated that miRNAs could regulate metastasis through influencing mitogen activated protein kinase 1 (MAPK1), neuroblastoma Ras (NRAS), fibroblast growth factor receptor substrate 2 (FRS2), and Quaking (QKI) [49]. MiR-675 from serum EVs was found to be a potential new biomarker for OS metastasis, which also demonstrate that tumor cells could produce EVs to influence their own growth and metastasis [50]. MiR-143 could suppress the lung metastasis of OS, and the increasing of miR-143 could promote apoptosis, and inhibit OS cell growth [51, 52]. MiR-21 may remarkably influence the phenotype of OS cells, resulting in progression, metastasis, angiogenesis, and immune escape in OS [53]. MiR-1307 from OS cells derived EVs induced the proliferation, migration and invasion of OS cells via targeting AGAP1 [54]. Moreover, BMSCs-derived EVs miR-208a could promote the progression of OS via targeting PDCD4 [55]. EVs from adipose mesenchymal stem cells (AD-MSCs) could foster the metastasis of OS by increasing the level of COLGALT2 [56]. BMSCs-derived EVs encapsulated long non-coding PVT1 RNA and transported it to OS cells, promoting tumor growth and metastasis [57]. BMSCs-derived EVs LCP1 can induce metastasis via JAK2/STAT3 axis [58]. Macrophage-derived EVs lnc-LIFR-AS1 can induce the progression of OS cells via miR-29a/NFIA pathway [59]. The fusion protein Rab22a-NeoF1 can induce OS metastasis in lungs via the activation of RhoA [60]. In another study, TGF-β1 was found to enhance the level of the proteoglycan by suppressing miR-143, enhancing the metastasis of OS [61]. TGF-β expression has been detected in OS cells derived EVs, which participates in tumor invasion, angiogenesis, and metastasis. TGF-β could directly or indirectly influence the production of chemokine ligand 16 (CXCL16), regulating the metastasis of OS cells [62]. TGF-β from EVs could also induce the differentiation of monocytes and the accumulation of immature myeloid suppressor cells (MDSCs) [63, 64]. TGF-β was also confirmed to be present in the exosome surface, which could induce the production of IL-6 and IL-8 by MSCs, enhancing a pro-inflammatory OS microenvironment favorable for metastasis [65].
EVs have been identified as important factors in regulating tumor and mesenchymal cell induction of metastasis and in regulating oncogenic phenotypes [66, 67]. EVs could promote metastasis of OS to local or distant tissues and organs. OS cells could secret transforming EVs, which further confer tumor-like phenotypes on normal recipient cells, promoting metastasis [68]. EVs derived from OS cells could mediate fibroblast differentiation to cancer-related stromal fibroblasts (CAFs) phenotype via SMAD2 and TGF-β1 pathway, which show the possibility about OS cells influence distant cells to promote metastasis [69]. OS-derived EVs could also induce M2 type macrophages polarization to mediate invasion and distant metastasis of OS cells [70]. OS-derived EVs significantly induce mesenchymal stem cells (MSCs) metastasis via IL-6/STAT3 axis [71]. Compared with the non-metastatic OS-derived EVs, metastatic OS-derived EVs promoted the migration ability of osteoblasts [50]. In addition, the uptake of OS-derived EVs by endothelial cells mediated the production of pro-angiogenic factors, demonstrating EVs could modulate the invasiveness of cells through affecting OS microenvironment [72]. Another study found that injection of mesenchymal stem cells (MSCs) co-cultured with metastatic OS-derived EVs could promote tumor growth and metastatic dissemination to distant organs [65]. The capacity of stromal cells to influence OS cells has been confirmed in EVs secreted by CAFs. Compared with non-cancer fibroblast, transferring the exosomal cargo from CAFs to tumor cells significantly promoted migration and invasion, which may be attributed to the enrichment of miR-1228 in CAFs [73]. COL6A1 could be carried by OS-derived EVs and activate CAFs to promote OS metastasis [74]. Altogether, EVs and their contents could educate tumor cells, inducing a pro-metastatic and tumorigenic phenotype, and promote OS metastasis to local or distant tissues.
EVs and osteosarcoma microenvironments
In the microenvironment of OS, tumor cells, mesenchymal stem cells, immune cells, fibroblasts, osteoclasts, osteoblasts, and endothelial cells coexist and interact with each other, in which EVs play a vital role [5, 75, 76]. An important function of extracellular vesicles (EVs) is to communicate with target cells. The finding of extracellular vesicles in osteoblasts and osteoclasts provides a strong theoretical basis for studying the role of extracellular vesicles in the microenvironment of OS [77]. Interestingly, tumor cells can also secrete exosomes, which can promote tumor growth, metastasis and angiogenesis by regulating tumor microenvironment [78, 79].
OS is generally considered to be an osteoblastic cell line tumor, and osteoclasts have been shown to play a key role in OS invasiveness and adverse reactions to chemotherapy [80, 81]. Osteoclast formation and bone resorption are stimulated by the pro-osteoclastogenic cargo of OS-derived EVs [72, 82]. Raimondi et al. found the pro-osteoclastic miRNA cargo in OS-derived EVs, containing miR-148a-3p and miR-21-5p, which are involved in the establishment of tumor microenvironment [72]. These suggest that the pro-osteoclastogenic cargo of EVs have a specific role in modifying bone remodeling homeostasis in the OS bone microenvironment.
Potential effects of EVs from OS cells on bone marrow stroma have also been reported. Biomechanical stress in bone marrow stroma can increase intracellular calcium level, accelerate the formation of EVs, and elevate the expression of matrix metalloproteases (MMPs). The nuclear factor kappa-B (NF-κB) receptor activator ligand (RANKL) is regarded as an important factor regulating osteoclast differentiation because it plays an important role in activating MMPs and stimulating osteoclast formation [83]. Lim et al. found the nucleic acid transfer from the bone microenvironment to breast cancer cells via EVs [84]. Transmembrane 4 superfamily protein CD9 was found in OS tumor microenvironment, which is also a membrane fusion protein of osteoclast precursors and is associated with the regulation of osteoclast differentiation and maturation [62, 85]. CD-9 is abundant in EVs, which can regulate osteoclast differentiation. In cancers, overexpression of CD-9 induces osteoclast bone resorption in the bone micro environment [86]. Yi et al. [87] also found the regulatory function of CD-9 in MMPs induced cancer migration and invasion. Blocking of CD-9 by KMC8 could inhibit the formation of multinucleated osteoclasts and regulate the differentiation of osteoclasts [88]. The expression of transforming growth factor-β (TGF-β) in serum of OS patients was significantly increased, which can stimulate migration of OS cells [89]. TGF-β contained in extracellular vesicles can increase the accumulation of immature myeloid cells, which can accelerate osteoclastic bone resorption [64]. Thus, inhibiting EVs secretion may improve the bone microenvironment and suppress tumorigenesis.
Mesenchymal stem cells (MSCs) are believed to support tumor progression by vesicles secretion [90]. Therefore, there is increasing interest in studying the activity of MSCs derived EVs on tumor cells. EVs secreted by mesenchymal stem cells (MSCs) can change the phenotype of OS cells as regulators in the tumor microenvironment. Communication via EVs of stressed mesenchymal stem cells (SD-MSCs) significantly affects the metastasis potential of OS cells, which is closely related to the miRNA content of EVs [91]. EVs from human marrow mesenchymal stem cells (hBMSCs) can act as paracrine factors to activate Hedgehog signaling pathway in OS cells, regulating OS growth [92]. EVs from hBMSCs can promote the progression of OS by increasing the expression of HIF-1α and related gene through PI3K/AKT pathway [93]. Besides, EVs from hBMSCs can also promote tumorigenesis and metastasis of OS by accelerating oncogenic autophagy [94]. However, MSCs are not the only component of OS microenvironment. Further studies of how EVs act on OS cells will help to discover new mechanisms of the cell-to-cell communication in the microenvironment and to identify novel targets.
The EVs from OS cells can also regulate signaling pathways. EVs is widely involved in the regulation of multiple components of the Wnt pathway, which is closely related to OS progression [95]. It was discovered that the up-regulation of CD82 and CD9 mediated cells to secrete exosomes, which significantly inhibiting Wnt signaling [96]. However, the development of OS involves the interaction of various signaling pathways. Therefore, the in-depth study of their correlation can improve the therapy of OS.
EVs and immune escape
EVs derived from cancer could promote tumor immune escape through multiple mechanisms [97, 98]. Overexpression of PD-L1 is closely associated with metastasis in OS. In order to evade immune surveillance, cancer cells activate the programmed death ligand 1 (PD-L1) pathway [99]. On the other hand, malignant tumors released EVs carrying PD-L1, which can be used to predict the efficiency of anti-PD-1treatment [98]. EVs secreted by OS have a special cargo, which can mediate differentiation of CD4 + cells into T regulatory phenotype, and result in immune evasion [100]. EVs from the serum of dogs diagnosed with OS may help to discover the mechanism of immune evasion in OS. Increased expression of plasma protease C1 inhibitor and decreased expression of C1qa in exosomes of osteosarcoma dogs may prevent the activation of classic pathways as potential escape mechanisms [101]. In addition, EVs derived from metastatic OS cells can modulate TAMs signaling, promote M2 phenotype, and create an immunosuppressive, pro-tumor microenvironment [102].
EVs in chemotherapy resistance of osteosarcoma
Neoadjuvant chemotherapy combined with surgery is the main strategy for OS currently. However, some patients developed resistance to chemotherapy, posing a huge challenge to OS treatment. Recent studies have shown that EVs play a crucial role in multidrug resistance (MDR) of osteosarcoma [103-105], which is a major obstacle to successful therapy and good clinical outcome of OS [106]. OS-derived EVs can reduce the sensitivity of OS cells to doxorubicin and induce the MDR phenotype in doxorubicin-sensitive cells by transferring MDR-1 mRNA, suggesting a mechanism by which drug-resistant tumor cells spread drug unresponsiveness to sensitive cells, promoting chemotherapy resistance [105]. In addition, EVs induce MDR through the transfer of specific bioactive molecules, such as non-coding RNAs and proteins [107]. Moreover, miRNAs from EVs cargo have attracted much attention in this field because they can interfere with gene expression and participate in multiple drug resistance mechanisms [108, 109]. Xu et al. found that the level of microRNA and mRNA in exosomes of OS can be used to predict the sensitivity to chemotherapy. They isolated miR-135b and other RNAs that can make OS resistant to chemotherapy drugs from OS cell-derived exosomes [110]. Besides, EVs have be reported to induce chemoresistance of OS by transmitting circular RNAs (circRNAs), indicate exosomal circRNAs as new targets to be used for addressing OS chemoresistance [111]. Researchers also used OS animal models and patient samples to investigate the role of protein cargos, showing that exosomes exhibited unique protein signatures associated with drug resistance [112]. These studies explain part of the mechanism of drug resistance in OS cells and provide a new reference for the selection of drugs in OS patients. Therefore, isolating EVs and analyzing of their cargo may reveal new biomarkers that can be used to improve therapeutic efficacy. However, there are still some limitations to the clinical application of exosomes [113]. Subsequent study should seek to improve their production and storage processes to prevent loss of function and ensure the safety of EVs treatment.
EVs as biomarkers for diagnosis and prognosis in osteosarcoma
EVs have attracted extensive attention due to their possible roles in early diagnosis, prognosis prediction, and efficacy assessment of OS (Table 1). They can be easily obtained from body fluids, and their cargo is inside a membranous structure, which provides long-term storage stability prior analysis, making them viable for clinical application in the diagnosis and prognosis of OS [114, 115]. Nowadays, more and more researchers are using a variety of strategies to screen EVs markers, which can reflect the physiological and pathological state of cells. PD-L1 and exosomal N-cadherin detected from the serum of patients with OS can predict the progression of pulmonary metastasis [116]. SENP1 derived from EVs can be used as a novel prognostic biomarker in OS patients [117]. The excess of EVs related DNAs of repetitive elements suggests that they may serve as biomarkers for OS [118]. Moreover, CASC15 overproduction was discovered in EVs form the plasma of patients with OS, as well as the OS tissues and cell lines [119].
The RNAs in EVs cargo can also be used as effective biomarkers in the diagnosis and progression of OS. Elevated tumor mutation burden in RNA sequences from metastatic EVs plasma samples was observed in a pilot study [120]. The membrane of exosomes can make miRNA in exosomes relatively stable, thus increasing the feasibility of clinical application of miRNA in early diagnosis of OS [121-123]. Serum exosome miRNAs were regarded as a promising diagnostic biomarker that can distinguish differences in drug resistance in OS [110]. Another study found that lung metastasis resulted in significantly elevated expression of circulating EVs-derived miR-675, which may be used as a novel biomarker for OS metastasis in the future [50]. The level of exosomal miR-25-3p were also remarkably associated with poor prognosis of OS [109]. EVs derived miR-101 is considered as a possible circulating biomarker of OS metastasis [124]. Besides, several new miRNAs were found in OS cells and related EVs, indicating these miRNAs may possibly be applied as biomarkers for OS [125]. The upregulation of miR-21-5p and miR143-3p in the EVs of metastatic osteosarcoma cells also suggests their potential to become prognostic biomarkers for OS [49]. Through high-throughput sequencing, Ye et al. found exosomal miRNAs with different expression in OS and healthy controls, suggesting their possibility as new diagnostic biomarkers [126]. More recently, Zhang et al. demonstrated that exosome derived miR-101 could become a hopeful circulating biomarker of osteosarcoma metastasis [124].
EVs as biomarkers for diagnosis and prognosis in osteosarcoma
| Cargotype | Exosomal cargo | Source | Extraction method | Identification method | Clinical value | References |
|---|---|---|---|---|---|---|
| miRNA | miR-675 | Serum | Ultracentrifugation | Transmission electron microscope and western blot | Biomarker for predicting OS metastasis | [50] |
| miR-25-3p | Extracellular fluid | Ultracentrifugation | Scanning electron microscope and western blot | Diagnostic biomarkers to indicate poor prognosis of OS | [109] | |
| miR-101 | Plasma | Differential centrifugation | Scanning confocal microscope and western blot | Circulating biomarkers for OS detection | [124] | |
| miR-21-5p | Extracellular fluid | Ultracentrifugation | Nanoparticle tracking analysis | Diagnostic biomarkers for OS | [49] | |
| miR143-3p | Extracellular fluid | Ultracentrifugation | Nanoparticle tracking analysis | Diagnostic biomarkers for OS | [49] | |
| IncRNA | linc00852 | Extracellular fluid | Differential centrifugation | Transmission electron microscope and western blot | Biomarkers for OS | [133] |
| circRNA | hsa-circ-103801 | Serum | Ultracentrifugation | Transmission electron microscope and western blot | Prognostic biomarkers for OS | [111] |
| Protein | PD-L1 | Serum | Differential centrifugation | Transmission electron microscope, qRT-PCR, nanoparticle tracking analysis | Biomarkers to predict OS metastasis | [116] |
| N-cadherin | Serum | Differential centrifugation | Transmission electron microscope, qRT-PCR, nanoparticle tracking analysis | biomarkers to predict OS metastasis | [116] |
Additionally, EVs derived lncRNAs and circRNAs are also considered as promising biomarkers for OS, whose expression is correlated with diagnosis, prognosis and metastasis of osteosarcoma [127-132]. Li et al. found a positive feedback loop between EVs derived linc00852 and AXL, suggesting its potential to be a new OS biomarker [133]. Another study discovered that the levels of hsa-circ-103801 were elevated in serum of OS patients who had poor prognosis, demonstrating hsa-circ-103801 could be used as prognostic biomarker for OS [111].
EVs derived proteins are also regraded as promising biomarkers for predicting prognosis in OS patients. In terms of EVs-related proteins, circulating EVs-related TGF-β levels were significantly elevated in patients with OS [65]. EVs-associated proteins were also useful to distinguish serum of OS from serum of healthy animals [101]. Wang et al. obtained PD-L1 and N-cadherin from the serum of OS patients as biomarkers to predict OS metastasis [116]. Collectively, EVs derived cargoes have a promising prospect to be applied as new diagnostic and prognostic biomarkers for OS. However, there are still several challenges that need to be considered before clinical diagnostic applications of EVs [134]. Scholars need to focus on standardizing and improving EVs separation methods, as well as standardizing pre-analysis variables to ensure the reliably assess of EVs.
EVs in the treatment of osteosarcoma
The ability to exchange information and to deliver bioactive substances to target cells gives EVs great potential for treating human disease [135]. EVs can be transferred to target organs, and thus are widely considered as natural nanocarriers, which show great prospect for application as drug targeting vectors [136-142]. These researches opened a new area in OS research, linking emerging nanocarrier EVs to the progression, prognosis, and treatment of OS.
EVs derived RNAs may be used as therapeutic targets in OS. Several dysregulated EVs-derived miRNAs were discovered in patients with OS [126]. EVs derived from cisplatin-resistant OS cells could transfer the resistance to the recipient cells and inhibit apoptosis, which is closely associated with the expression of exosomal hsa_circ_103801 [111]. EVs-derived miR-206 could suppress OS progression through inhibiting the proliferation, migration and invasion of OS cells via targeting TRA2B [138]. EVs-derived miR-101 has been found to suppress metastasis in OS [124]. In another study, EVs-derived miR-1228 was found to facilitate migration and invasion of OS through inhibiting the level of SCAI in OS [73]. EVs-derived lncRNA LIFR-AS1 could mediate OS progression via miR-29a/NFIA axis [59]. However, upregulation of AGAP1 can suppress the function of EVs-derived miR-1307 in OS [54]. The application of EVs to encapsulate miRNAs has manifested advantages in the therapy of OS. Artificial miR-143 was introduced into BMSCs and encapsulated in EVs to reduce the metastasis capability of OS cells, and the exosome miRNA transport was better than other methods in intercellular transport [143].
In addition, EVs-derived proteins are also considered as potential targets for treating OS patients. OS-derived EVs were found to induce M2 type macrophages polarization by regulating Tim-3 level [70]. Knockdown of CASC15, which is elevated in OS derived EVs, could suppress OS progression through regulating miR-338-3p/RAB14 axis [119]. BMSCs-derived exosomal LCP1 mediates OS progression through the JAK2/STAT3 axis, while miR-135a-5p could inhibit tumorigenesis of OS induced by LCP1 [58]. Heterologous exosomes secreted by MSCs are regarded as reliable source of therapeutic exosomes [144, 145]. Combined use of TGF-β inhibitors and IL-6 blockers can reduce drug resistance and prevent the progression of osteosarcoma, which is based on the finding that EVs carry functional TGF-β molecules and elevate IL-6 production, promoting OS growth and metastasis [65].
Researchers have also explored the immune therapeutic effects of EVs in OS. EVs secreted from dendritic cells (DCs) had functional MHC, T-cell and costimulatory molecules, implying that DC vaccination secreted antibodies induce regulatory molecules could improve the immunotherapeutic efficacy in OS [146]. Kawano et al. discovered that the combination of DCs and anti-TGF-β antibody could inhibit the proliferation of OS cells, activate the systemic immune system, and improve the treatment efficacy of OS [147]. Another study discovered that doxorubicin-loaded exosomes derived from MSCs may be an excellent agent for OS in the future, considering the tumor-targeting function of BM-MSCs [148]. All of these studies demonstrate that these targets are promising as potential therapeutic approaches for OS, which may contribute to improve survival of OS patients.
Conclusions
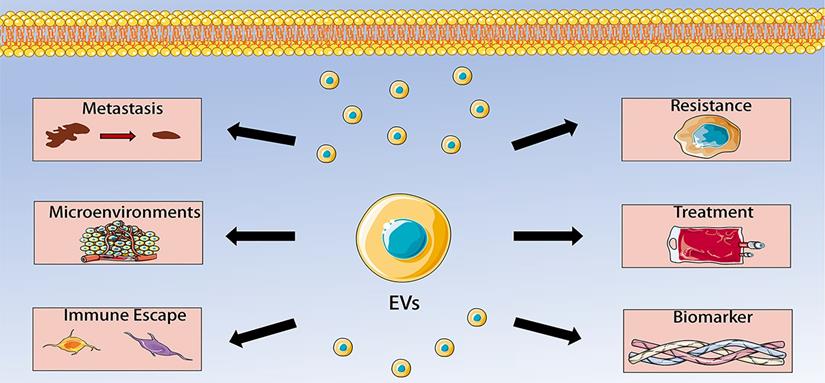
Extracellular vesicles have become the focus of research because of their intercellular communication functions. More and more studies have found that EVs can regulate tumor microenvironment, thereby influencing the occurrence, development, metastasis, immune escape and chemotherapy resistance of malignant tumors. OS has a complex tumor microenvironment in which tumor cells, stem cells, mesenchymal cells, immune cells, fibroblasts and endothelial cells communicate with each other, leading to OS progression. In this communication, extracellular vesicles play a key role. EVs are secreted by a variety of cells, such as osteosarcoma cells, BMSCs, ADSCs, CAFs, and macrophages. The EVs contain multiple kinds of cargoes, including miRNAs, lncRNAs, circRNAs, and proteins. EVs and their cargoes could regulate the activity of recipient cells, including angiogenesis, proliferation, invasion, migration, metastasis and chemoresistance. Moreover, the detection of serum EVs in patients with OS have great value of early diagnosis and prognosis of OS. EVs can also be applied as carriers to transfer therapy agents to targeted sites because they can avoid immune response. Collectively, data from recent studies reveal multiple functions of EVs in OS, including regulating OS metastasis, tumor microenvironment, immune escape, chemotherapy resistance, which make them to become promising biomarkers and therapeutic targets for OS (Figure 2). However, the specific mechanisms by which EVs participate in these processes have not been completely elucidated. Therefore, more studies are needed to determine the precise roles of EVs in the pathogenesis of OS. In addition, future studies should also advance EVs purification, characterization, storage and drug loading techniques, which could help to better detect EVs, understand their biological characteristics, and promote EVs into clinical practice. Another issue waiting to be addressed is identifying the exact content of EVs and their safety. There is no doubt that these problems have to be addressed before they can be used in the clinical practice of OS.
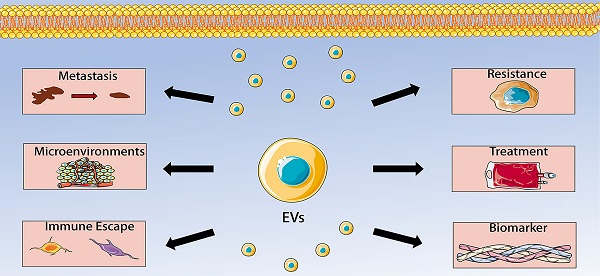
Extracellular vesicles (EVs) in osteosarcoma (OS). The role of EVs in OS includes regulating OS metastasis, tumor microenvironment, immune escape, chemotherapy resistance, which make them to become promising biomarkers and therapeutic targets for OS.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No12072129), Thanks for the financial support.
Author Contributions
Conceptualization YQ, LJ; investigation WB, WX, JY; original draft preparation YQ, LJ; review and editing ZD.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Song H, Zhao J, Cheng J, Feng Z, Wang J, Momtazi-Borojeni AA. et al. Extracellular Vesicles in chondrogenesis and Cartilage regeneration. Journal of Cellular and Molecular Medicine. 2021;25:4883-92
2. Li S, Wang X. The potential roles of exosomal noncoding RNAs in osteosarcoma. Journal of Cellular Physiology. 2021;236:3354-65
3. Abhange K, Makler A, Wen Y, Ramnauth N, Mao W, Asghar W. et al. Small extracellular vesicles in cancer. Bioactive Materials. 2021;6:3705-43
4. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New Technologies for Analysis of Extracellular Vesicles. Chemical Reviews. 2018;118:1917-50
5. Perut F, Roncuzzi L, Baldini N. The Emerging Roles of Extracellular Vesicles in Osteosarcoma. Front Oncol. 2019;9:1342
6. Liu Y, Xia Y, Smollar J, Mao W, Wan Y. The roles of small extracellular vesicles in lung cancer: Molecular pathology, mechanisms, diagnostics, and therapeutics. Biochimica Et Biophysica Acta-Reviews on Cancer. 2021 1876
7. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531-43
8. Aran V, Devalle S, Meohas W, Heringer M, Cunha Caruso A, Pinheiro Aguiar D. et al. Osteosarcoma, chondrosarcoma and Ewing sarcoma: Clinical aspects, biomarker discovery and liquid biopsy. Crit Rev Oncol Hematol. 2021;162:103340
9. Li S. The basic characteristics of extracellular vesicles and their potential application in bone sarcomas. J Nanobiotechnology. 2021;19:277
10. Lilienthal I, Herold N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int J Mol Sci. 2020 21
11. Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075-85
12. Gazouli I, Kyriazoglou A, Kotsantis I, Anastasiou M, Pantazopoulos A, Prevezanou M. et al. Systematic Review of Recurrent Osteosarcoma Systemic Therapy. Cancers (Basel). 2021 13
13. Ferrari S, Briccoli A, Mercuri M, Bertoni F, Picci P, Tienghi A. et al. Postrelapse survival in osteosarcoma of the extremities: Prognostic factors for long-term survival. Journal of Clinical Oncology. 2003;21:710-5
14. Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Current Opinion in Cell Biology. 2014;29:116-25
15. Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annual Review of Cell and Developmental Biology. 2007;23:519-47
16. Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H. et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nature Communications. 2017 8
17. Villarroya-Beltri C, Baixauli F, Mittelbrunn M, Fernandez-Delgado I, Torralba D, Moreno-Gonzalo O. et al. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nature Communications. 2016 7
18. Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nature Reviews Molecular Cell Biology. 2010;11:556-66
19. Luzio JP, Gray SR, Bright NA. Endosome-lysosome fusion. Biochemical Society Transactions. 2010;38:1413-6
20. Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 biology reports. 2011;3:15 -
21. Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31:4740-9
22. Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. Journal of Cell Biology. 2008;181:879-84
23. Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G. et al. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Current Biology. 2009;19:1875-85
24. Schlienger S, Campbell S, Claing A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Molecular Biology of the Cell. 2014;25:17-29
25. Sedgwick AE, Clancy JW, Balmert MO, D'Souza-Schorey C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Scientific Reports. 2015 5
26. Clancy JW, Sedgwick A, Rosse C, Muralidharan-Chari V, Raposo G, Method M. et al. Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nature Communications. 2015 6
27. Kim J, Morley S, Minh L, Bedoret D, Umetsu DT, Di Vizio D. et al. Enhanced shedding of extracellular vesicles from amoeboid prostate cancer cells Potential effects on the tumor microenvironment. Cancer Biology & Therapy. 2014;15:409-18
28. Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nature Reviews Cancer. 2017;17:131-40
29. Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1-11
30. Kim SB, Kim HR, Park MC, Cho S, Goughnour PC, Han D. et al. Caspase-8 controls the secretion of inflammatory lysyl-tRNA synthetase in exosomes from cancer cells. Journal of Cell Biology. 2017;216:2201-16
31. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature Cell Biology. 2012;14:677-85
32. Adamo G, Fierli D, Romancino DP, Picciotto S, Barone ME, Aranyos A. et al. Nanoalgosomes: Introducing extracellular vesicles produced by microalgae. Journal of Extracellular Vesicles. 2021 10
33. Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Research. 2014;24:766-9
34. Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A. et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E9066-E75
35. Sisquella X, Ofir-Birin Y, Pimentel MA, Cheng L, Abou Karam P, Sampaio NG. et al. Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nature Communications. 2017 8
36. Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M. et al. Identification of Double-stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. Journal of Biological Chemistry. 2014;289:3869-75
37. Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S. et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Communications. 2017 8
38. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology. 2006 Chapter 3: Unit 3.22-Unit 3
39. Tatischeff I, Larquet E, Falcon-Perez JM, Turpin P-Y, Kruglik SG. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. Journal of extracellular vesicles. 2012 1
40. Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P. et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine-Nanotechnology Biology and Medicine. 2011;7:780-8
41. Gardiner C, Ferreira YJ, Dragovic RA, Redman CWG, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. Journal of extracellular vesicles. 2013 2
42. Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS. et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nature Medicine. 2012;18:1835 -+
43. Orozco AF, Lewis DE. Flow Cytometric Analysis of Circulating Microparticles in Plasma. Cytometry Part A. 2010;77A:502-14
44. Van der Pol E, Van Gemert MJC, Sturk A, Nieuwland R, Van Leeuwen TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. Journal of Thrombosis and Haemostasis. 2012;10:919-30
45. Stoner SA, Duggan E, Condello D, Guerrero A, Turk JR, Narayanan PK. et al. High Sensitivity Flow Cytometry of Membrane Vesicles. Cytometry Part A. 2016;89A:196-206
46. Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722-35
47. Tauro BJ, Mathias RA, Greening DW, Gopal SK, Ji H, Kapp EA. et al. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics. 2013;12:2148-59
48. Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237-48
49. Jerez S, Araya H, Hevia D, Irarrázaval CE, Thaler R, van Wijnen AJ. et al. Extracellular vesicles from osteosarcoma cell lines contain miRNAs associated with cell adhesion and apoptosis. Gene. 2019;710:246-57
50. Gong L, Bao Q, Hu C, Wang J, Zhou Q, Wei L. et al. Exosomal miR-675 from metastatic osteosarcoma promotes cell migration and invasion by targeting CALN1. Biochem Biophys Res Commun. 2018;500:170-6
51. Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y. et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther. 2011;19:1123-30
52. Li WH, Wu HJ, Li YX, Pan HG, Meng T, Wang X. MicroRNA-143 promotes apoptosis of osteosarcoma cells by caspase-3 activation via targeting Bcl-2. Biomed Pharmacother. 2016;80:8-15
53. Wang S, Ma F, Feng Y, Liu T, He S. Role of exosomal miR-21 in the tumor microenvironment and osteosarcoma tumorigenesis and progression (Review). Int J Oncol. 2020;56:1055-63
54. Han F, Pu P, Wang C, Ding X, Zhu Z, Xiang W. et al. Osteosarcoma Cell-Derived Exosomal miR-1307 Promotes Tumorgenesis via Targeting AGAP1. Biomed Res Int. 2021;2021:7358153
55. Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235:4734-45
56. Wang Y, Chu Y, Li K, Zhang G, Guo Z, Wu X. et al. Exosomes Secreted by Adipose-Derived Mesenchymal Stem Cells Foster Metastasis and Osteosarcoma Proliferation by Increasing COLGALT2 Expression. Front Cell Dev Biol. 2020;8:353
57. Zhao W, Qin P, Zhang D, Cui X, Gao J, Yu Z. et al. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany N Y). 2019;11:9581-96
58. Ge X, Liu W, Zhao W, Feng S, Duan A, Ji C. et al. Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol Ther Nucleic Acids. 2020;21:900-15
59. Zhang H, Yu Y, Wang J, Han Y, Ren T, Huang Y. et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int. 2021;21:192
60. Zhong L, Liao D, Li J, Liu W, Wang J, Zeng C. et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. 2021;6:59
61. Li F, Li S, Cheng T. TGF-β1 promotes osteosarcoma cell migration and invasion through the miR-143-versican pathway. Cell Physiol Biochem. 2014;34:2169-79
62. Ota K, Quint P, Weivoda MM, Ruan M, Pederson L, Westendorf JJ. et al. Transforming growth factor beta 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone. 2013;57:68-75
63. Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J. et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621-33
64. Danilin S, Merkel AR, Johnson JR, Johnson RW, Edwards JR, Sterling JA. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology. 2012;1:1484-94
65. Baglio SR, Lagerweij T, Pérez-Lanzón M, Ho XD, Léveillé N, Melo SA. et al. Blocking Tumor-Educated MSC Paracrine Activity Halts Osteosarcoma Progression. Clin Cancer Res. 2017;23:3721-33
66. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019;49:347-60
67. Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C. et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18:39
68. Urciuoli E, Giorda E, Scarsella M, Petrini S, Peruzzi B. Osteosarcoma-derived extracellular vesicles induce a tumor-like phenotype in normal recipient cells. J Cell Physiol. 2018;233:6158-72
69. Mazumdar A, Urdinez J, Boro A, Migliavacca J, Arlt MJE, Muff R. et al. Osteosarcoma-Derived Extracellular Vesicles Induce Lung Fibroblast Reprogramming. Int J Mol Sci. 2020 21
70. Cheng Z, Wang L, Wu C, Huang L, Ruan Y, Xue W. Tumor-derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch Med Res. 2021;52:200-10
71. Lagerweij T, Pérez-Lanzón M, Baglio SR. A Preclinical Mouse Model of Osteosarcoma to Define the Extracellular Vesicle-mediated Communication Between Tumor and Mesenchymal Stem Cells. J Vis Exp. 2018
72. Raimondi L, De Luca A, Gallo A, Costa V, Russelli G, Cuscino N. et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis. 2020;41:666-77
73. Wang JW, Wu XF, Gu XJ, Jiang XH. Exosomal miR-1228 From Cancer-Associated Fibroblasts Promotes Cell Migration and Invasion of Osteosarcoma by Directly Targeting SCAI. Oncol Res. 2019;27:979-86
74. Zhang Y, Liu Z, Yang X, Lu W, Chen Y, Lin Y. et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. 2021;11:1473-92
75. Cappariello A, Rucci N. Tumour-Derived Extracellular Vesicles (EVs): A Dangerous "Message in A Bottle" for Bone. Int J Mol Sci. 2019 20
76. Lan M, Zhu XP, Cao ZY, Liu JM, Lin Q, Liu ZL. Extracellular vesicles-mediated signaling in the osteosarcoma microenvironment: Roles and potential therapeutic targets. J Bone Oncol. 2018;12:101-4
77. Garimella R, Eskew J, Bhamidi P, Vielhauer G, Hong Y, Anderson HC. et al. Biological characterization of preclinical Bioluminescent Osteosarcoma Orthotopic Mouse (BOOM) model: A multi-modality approach. J Bone Oncol. 2013;2:11-21
78. Xie F, Zhou X, Fang M, Li H, Su P, Tu Y. et al. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv Sci (Weinh). 2019;6:1901779
79. Perut F, Roncuzzi L, Zini N, Massa A, Baldini N. Extracellular Nanovesicles Secreted by Human Osteosarcoma Cells Promote Angiogenesis. Cancers (Basel). 2019 11
80. Avnet S, Longhi A, Salerno M, Halleen JM, Perut F, Granchi D. et al. Increased osteoclast activity is associated with aggressiveness of osteosarcoma. Int J Oncol. 2008;33:1231-8
81. Bago-Horvath Z, Schmid K, Rössler F, Nagy-Bojarszky K, Funovics P, Sulzbacher I. Impact of RANK signalling on survival and chemotherapy response in osteosarcoma. Pathology. 2014;46:411-5
82. Garimella R, Washington L, Isaacson J, Vallejo J, Spence M, Tawfik O. et al. Extracellular Membrane Vesicles Derived from 143B Osteosarcoma Cells Contain Pro-Osteoclastogenic Cargo: A Novel Communication Mechanism in Osteosarcoma Bone Microenvironment. Transl Oncol. 2014;7:331-40
83. Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX. et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40:834-44
84. Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA. et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550-60
85. Ishii M, Iwai K, Koike M, Ohshima S, Kudo-Tanaka E, Ishii T. et al. RANKL-induced expression of tetraspanin CD9 in lipid raft membrane microdomain is essential for cell fusion during osteoclastogenesis. J Bone Miner Res. 2006;21:965-76
86. Kischel P, Bellahcene A, Deux B, Lamour V, Dobson R, E DEP. et al. Overexpression of CD9 in human breast cancer cells promotes the development of bone metastases. Anticancer Res. 2012;32:5211-120
87. Yi T, Kim HJ, Cho JY, Woo KM, Ryoo HM, Kim GS. et al. Tetraspanin CD9 regulates osteoclastogenesis via regulation of p44/42 MAPK activity. Biochem Biophys Res Commun. 2006;347:178-84
88. Herr MJ, Kotha J, Hagedorn N, Smith B, Jennings LK. Tetraspanin CD9 promotes the invasive phenotype of human fibrosarcoma cells via upregulation of matrix metalloproteinase-9. PLoS One. 2013;8:e67766
89. Celotti F, Colciago A, Negri-Cesi P, Pravettoni A, Zaninetti R, Sacchi MC. Effect of platelet-rich plasma on migration and proliferation of SaOS-2 osteoblasts: role of platelet-derived growth factor and transforming growth factor-beta. Wound Repair Regen. 2006;14:195-202
90. Cortini M, Avnet S, Baldini N. Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett. 2017;405:90-9
91. Vallabhaneni KC, Hassler MY, Abraham A, Whitt J, Mo YY, Atfi A. et al. Mesenchymal Stem/Stromal Cells under Stress Increase Osteosarcoma Migration and Apoptosis Resistance via Extracellular Vesicle Mediated Communication. PLoS One. 2016;11:e0166027
92. Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y. et al. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell Physiol Biochem. 2017;42:2242-54
93. Lin S, Zhu B, Huang G, Zeng Q, Wang C. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: the role of PI3K/AKT and HIF-1α. Hum Cell. 2019;32:64-74
94. Huang Y, Liu W, He B, Wang L, Zhang F, Shu H. et al. Exosomes derived from bone marrow mesenchymal stem cells promote osteosarcoma development by activating oncogenic autophagy. J Bone Oncol. 2020;21:100280
95. Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M. et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837-51
96. Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079-91
97. Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458-66
98. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382-6
99. Huang X, Zhang W, Zhang Z, Shi D, Wu F, Zhong B. et al. Prognostic Value of Programmed Cell Death 1 Ligand-1 (PD-L1) or PD-1 Expression in Patients with Osteosarcoma: A Meta-Analysis. J Cancer. 2018;9:2525-31
100. Troyer RM, Ruby CE, Goodall CP, Yang L, Maier CS, Albarqi HA. et al. Exosomes from Osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp Cell Res. 2017;358:369-76
101. Brady JV, Troyer RM, Ramsey SA, Leeper H, Yang L, Maier CS. et al. A Preliminary Proteomic Investigation of Circulating Exosomes and Discovery of Biomarkers Associated with the Progression of Osteosarcoma in a Clinical Model of Spontaneous Disease. Transl Oncol. 2018;11:1137-46
102. Wolf-Dennen K, Gordon N, Kleinerman ES. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology. 2020;9:1747677
103. Namee NM, O'Driscoll L. Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev Cancer. 2018;1870:123-36
104. Zhao L, Liu W, Xiao J, Cao B. The role of exosomes and "exosomal shuttle microRNA" in tumorigenesis and drug resistance. Cancer Lett. 2015;356:339-46
105. Torreggiani E, Roncuzzi L, Perut F, Zini N, Baldini N. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int J Oncol. 2016;49:189-96
106. Li S, Sun W, Wang H, Zuo D, Hua Y, Cai Z. Research progress on the multidrug resistance mechanisms of osteosarcoma chemotherapy and reversal. Tumour Biol. 2015;36:1329-38
107. Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S. et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55
108. Chen R, Wang G, Zheng Y, Hua Y, Cai Z. Drug resistance-related microRNAs in osteosarcoma: Translating basic evidence into therapeutic strategies. J Cell Mol Med. 2019;23:2280-92
109. Yoshida A, Fujiwara T, Uotani K, Morita T, Kiyono M, Yokoo S. et al. Clinical and Functional Significance of Intracellular and Extracellular microRNA-25-3p in Osteosarcoma. Acta Med Okayama. 2018;72:165-74
110. Xu JF, Wang YP, Zhang SJ, Chen Y, Gu HF, Dou XF. et al. Exosomes containing differential expression of microRNA and mRNA in osteosarcoma that can predict response to chemotherapy. Oncotarget. 2017;8:75968-78
111. Pan Y, Lin Y, Mi C. Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol Int. 2021;45:858-68
112. Weinman MA, Ramsey SA, Leeper HJ, Brady JV, Schlueter A, Stanisheuski S. et al. Exosomal proteomic signatures correlate with drug resistance and carboplatin treatment outcome in a spontaneous model of canine osteosarcoma. Cancer Cell Int. 2021;21:245
113. Zhang XB, Zhang RH, Su X, Qi J, Hu YC, Shi JT. et al. Exosomes in osteosarcoma research and preclinical practice. Am J Transl Res. 2021;13:882-97
114. Jeyaram A, Jay SM. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. Aaps j. 2017;20:1
115. Grimaldi A, Zarone MR, Irace C, Zappavigna S, Lombardi A, Kawasaki H. et al. Non-coding RNAs as a new dawn in tumor diagnosis. Semin Cell Dev Biol. 2018;78:37-50
116. Wang J, Zhang H, Sun X, Wang X, Ren T, Huang Y. et al. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J Nanobiotechnology. 2020;18:151
117. Wang L, Wu J, Song S, Chen H, Hu Y, Xu B. et al. Plasma Exosome-Derived Sentrin SUMO-Specific Protease 1: A Prognostic Biomarker in Patients With Osteosarcoma. Front Oncol. 2021;11:625109
118. Cambier L, Stachelek K, Triska M, Jubran R, Huang M, Li W. et al. Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci Rep. 2021;11:94
119. Zhang H, Wang J, Ren T, Huang Y, Yu Y, Chen C. et al. LncRNA CASC15 is Upregulated in Osteosarcoma Plasma Exosomes and CASC15 Knockdown Inhibits Osteosarcoma Progression by Regulating miR-338-3p/RAB14 Axis. Onco Targets Ther. 2020;13:12055-66
120. Bao Q, Gong L, Wang J, Wen J, Shen Y, Zhang W. Extracellular Vesicle RNA Sequencing Reveals Dramatic Transcriptomic Alterations Between Metastatic and Primary Osteosarcoma in a Liquid Biopsy Approach. Ann Surg Oncol. 2018;25:2642-51
121. Li B, Song Y, Liu TJ, Cui YB, Jiang Y, Xie ZS. et al. miRNA-22 suppresses colon cancer cell migration and invasion by inhibiting the expression of T-cell lymphoma invasion and metastasis 1 and matrix metalloproteinases 2 and 9. Oncol Rep. 2013;29:1932-8
122. Gao Q, Lei F, Zeng Q, Gao Z, Niu P, Junnan. et al. Functional Passenger-Strand miRNAs in Exosomes Derived from Human Colon Cancer Cells and Their Heterogeneous Paracrine Effects. Int J Biol Sci. 2020;16:1044-58
123. Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL. et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90
124. Zhang K, Dong C, Chen M, Yang T, Wang X, Gao Y. et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10:411-25
125. Cuscino N, Raimondi L, De Luca A, Carcione C, Russelli G, Conti L. et al. Gathering Novel Circulating Exosomal microRNA in Osteosarcoma Cell Lines and Possible Implications for the Disease. Cancers (Basel). 2019 11
126. Ye Z, Zheng Z, Peng L. MicroRNA profiling of serum exosomes in patients with osteosarcoma by high-throughput sequencing. J Investig Med. 2020;68:893-901
127. Li JF, Song YZ. Circular RNA GLI2 promotes osteosarcoma cell proliferation, migration, and invasion by targeting miR-125b-5p. Tumour Biol. 2017;39:1010428317709991
128. Kun-Peng Z, Chun-Lin Z, Jian-Ping H, Lei Z. A novel circulating hsa_circ_0081001 act as a potential biomarker for diagnosis and prognosis of osteosarcoma. Int J Biol Sci. 2018;14:1513-20
129. Kun-Peng Z, Xiao-Long M, Chun-Lin Z. Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1. Int J Biol Sci. 2018;14:321-30
130. Yin WB, Yan MG, Fang X, Guo JJ, Xiong W, Zhang RP. Circulating circular RNA hsa_circ_0001785 acts as a diagnostic biomarker for breast cancer detection. Clin Chim Acta. 2018;487:363-8
131. Jin Y, Li L, Zhu T, Liu G. Circular RNA circ_0102049 promotes cell progression as ceRNA to target MDM2 via sponging miR-1304-5p in osteosarcoma. Pathol Res Pract. 2019;215:152688
132. Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X. Extracellular nanovesicles-transmitted circular RNA has_circ_0000190 suppresses osteosarcoma progression. J Cell Mol Med. 2020;24:2202-14
133. Li Q, Wang X, Jiang N, Xie X, Liu N, Liu J. et al. Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med. 2020;9:6354-66
134. Ayers L, Pink R, Carter DRF, Nieuwland R. Clinical requirements for extracellular vesicle assays. J Extracell Vesicles. 2019;8:1593755
135. Abhange K, Makler A, Wen Y, Ramnauth N, Mao W, Asghar W. et al. Small extracellular vesicles in cancer. Bioact Mater. 2021;6:3705-43
136. Ruan J, Miao X, Schlüter D, Lin L, Wang X. Extracellular vesicles in neuroinflammation: Pathogenesis, diagnosis, and therapy. Mol Ther. 2021;29:1946-57
137. Avni D, Avni O. Extracellular Vesicles: Schistosomal Long-Range Precise Weapon to Manipulate the Immune Response. Front Cell Infect Microbiol. 2021;11:649480
138. Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y. et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54-65
139. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
140. Masaoutis C, Korkolopoulou P, Theocharis S. Exosomes in sarcomas: Tiny messengers with broad implications in diagnosis, surveillance, prognosis and treatment. Cancer Lett. 2019;449:172-7
141. Brennan M, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020 30
142. Casadei L, Pollock RE. Extracellular vesicle cross-talk in the liposarcoma microenvironment. Cancer Lett. 2020;487:27-33
143. Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S. et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445:381-7
144. Liu H, Chen Y, Yin G, Xie Q. Therapeutic prospects of MicroRNAs carried by mesenchymal stem cells-derived extracellular vesicles in autoimmune diseases. Life Sci. 2021;277:119458
145. Ryan ST, Hosseini-Beheshti E, Afrose D, Ding X, Xia B, Grau GE. et al. Extracellular Vesicles from Mesenchymal Stromal Cells for the Treatment of Inflammation-Related Conditions. Int J Mol Sci. 2021 22
146. Shinder BM, Rhee K, Farrell D, Farber NJ, Stein MN, Jang TL. et al. Surgical Management of Advanced and Metastatic Renal Cell Carcinoma: A Multidisciplinary Approach. Front Oncol. 2017;7:107
147. Kawano M, Itonaga I, Iwasaki T, Tsuchiya H, Tsumura H. Anti-TGF-β antibody combined with dendritic cells produce antitumor effects in osteosarcoma. Clin Orthop Relat Res. 2012;470:2288-94
148. Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C. et al. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In vitro. Int J Nanomedicine. 2019;14:8603-10
Author contact
![]() Corresponding author: Dong Zhu, E-mail: zhu_dongjlu.edu.cn.
Corresponding author: Dong Zhu, E-mail: zhu_dongjlu.edu.cn.

 Global reach, higher impact
Global reach, higher impact