3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(7):1198-1204. doi:10.7150/ijms.74751 This issue Cite
Research Paper
Cytoplasmic IGF2BP2 Protein Expression in Human Patients with Oral Squamous Cell Carcinoma: Prognostic and Clinical Implications
1. Department of Surgical Pathology, Changhua Christian Hospital, Changhua, Taiwan
2. Department of Medical Laboratory Science and Biotechnology, Central Taiwan University of Science and Technology, Taichung, Taiwan
3. Institute of Oral Sciences, Chung Shan Medical University, Taichung, Taiwan
4. Department of Dentistry, Chung Shan Medical University Hospital, Taichung, Taiwan
5. Antimicrobial Resistance Interdisciplinary Research Group, Singapore-MIT-Alliance for Research and Technology, Singapore, Singapore
6. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
7. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
8. School of Medicine, Chung Shan Medical University, Taichung, Taiwan
9. Division of Hematology and Oncology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
Received 2022-5-5; Accepted 2022-6-17; Published 2022-7-4
Abstract
Oral squamous cell carcinoma (OSCC) is particularly prevalent in Taiwan. The goal of this study was to determine the clinicopathological role of insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) proteins as an indicator of clinical outcomes in OSCC patients. In this study, immunohistochemical (IHC) analysis was used to examine IGF2BP2 protein expression in 244 OSCC patients. We investigated the relationships among IGF2BP2 expression, clinicopathological variables, and patient survival. Our results showed that IGF2BP2 cytoplasmic protein expression was significantly correlated with lymph node metastasis, cancer stage, and patient survival. Kaplan-Meier survival curves revealed that elevated cytoplasmic IGF2BP2 expression levels in OSCC patients were associated with poor overall survival. Moreover, multivariate cox proportional hazard models revealed that cytoplasmic IGF2BP2 expression, T status, and lymph node metastasis were independent prognostic factors for survival. In conclusion, IGF2BP2 protein was found to be a helpful predictive marker for OSCC patients, as well as a possible therapeutic target for OSCC treatment.
Keywords: IGF2BP2, tissue microarray, immunohistochemistry, oral squamous cell carcinoma, survival
Introduction
Oral squamous cell carcinoma (OSCC) is the most common malignant tumor in the head and neck region [1]. Tobacco addiction, excessive alcohol consumption, and human papillomavirus (HPV) infection have all been identified as major risk factors for carcinogenesis and the development of OSCC [2]. OSCC accounts for more than 90% of all oral cancers, with more than 300,000 new cases and 145,000 fatalities each year [3]. Despite advancements in therapy, the 5-year survival rate of OSCC patients remains low [4]. Advanced OSCC is characterized by unregulated growth with severe lymphatic metastases and a poor prognosis. As with other forms of cancer, OSCC is caused by a series of complex interactions involving a range of genes and proteins, resulting in a multifactor interaction [5-8]. Our lack of understanding as to the molecular processes underlying OSCC development underlines the need to develop new biomarkers as prognostic indicators and treatment targets [9].
Insulin-like growth factor (IGF) and IGF-binding protein play a vital role in the premalignant oral lesions and oral cancer [10-13]. Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) controls IGF2 translation by binding to the 5′ untranslated region (5′UTR) of IGF2 mRNA. In the realm of cancer research, IGF2BP2 is well-known for its regulation of differentiation potential in mouse neocortical neural precursor cells as well as myoblast proliferation, myogenesis, muscle cell motility, and energy consumption [14-16]. A number of studies have linked IGF2BP2 gene polymorphisms to the incidence of type 2 diabetes and cancer [17-20]. According to one recent study, IGF2BP2 knock-out mice fight obesity by regulating mRNA that encodes for mitochondrial proteins [21].
IGF2BP2 has been shown to promote tumor growth in cases of solid tumors and leukemia [22-27]. Recent research has identified IGF2BP2 as a potential oncogene, which, when overexpressed in liver cancer, causes excessive cell proliferation and invasion, resulting in a poor prognosis [28-31]. The overexpression of IGF2BP2 has also been shown to promote the development of glioblastoma multiforme by activating the IGF2/phosphoinositide 3-kinase (PI3K)/Akt pathway, thereby making glioblastoma resistant to temozolomide therapy [32]. In head and neck squamous cell carcinoma and OSCC tissues, the elevated mRNA or protein expression of IGF2BP2 is indicative of poor prognosis [33-35]. The upregulation of IGF2BP2 has also been shown to promote OSCC progression associated with cell proliferation, metastasis, and tumor-infiltrating immune cells [34].
Nonetheless, there is a pressing need to further elucidate the function of IGF2BP2 protein expression and other clinical variables in OSCC. In the current study, immunohistochemical (IHC) analysis was used to examine the expression of IGF2BP2 proteins tissue samples from 244 OSCC patients. We also examined the relationship between IGF2BP2 protein expression and OSCC clinicopathological variables and prognosis. Finally, we sought to identify potential prognostic markers to facilitate the early detection of OSCC.
Materials and Methods
Human patients and ethics statement
Patients (n = 244) were recruited from Changhua Christian Hospital in Taiwan. The most common forms of treatment included tumor removal and radical neck dissection followed by post-operative irradiation. A number of patients also received 5-fluorouracil (5-FU) and cisplatin chemotherapy. This study was also approved by the Changhua Christian Hospital's Ethics Committee in accordance with Institutional Review Board guidelines (IRB No. 150808, date of approval 03 July 2016). Prior to surgery, all OSCC patients provided written informed consent.
Tissue microarray preparation and evaluation
In accordance with the methods outlined in previous reports [36, 37], tissue microarrays (TMAs) were created using the OSCC samples, which included typical OSCC tissues and the surrounding epithelial tissue. The samples were fixed with paraffin to perforate tissue cylinders (2 mm in diameter) to produce OSCC and neighboring TMAs using a handmade, semiautomated tissue array. TMAs were created after the pathological evaluation of typical OSCC samples. Two senior pathologists validated the morphology of the malignancy based on representative lesions revealed by staining tissue slices using hematoxylin and eosin (H&E). The American Joint Committee on Cancer (AJCC, 7th Edition) Tumor, Node, Metastasis (TNM) staging system and the Edmondson-Steiner grading system were used to make pathological evaluations of tumor stages and histological differentiation.
IHC staining and scoring
IHC staining was performed in accordance with our previous studies [38, 39]. Following deparaffinization and hydration with various quantities of ethanol, the TMAs were antigen-retrieved using microwave radiation with 0.01 M citrate buffer (pH 6.0) and then incubated in 3 % H2O2 to block endogenous peroxidase activity, followed by incubation in 10% normal goat serum at 37°C for 1 h. The TMAs were combined with a solution containing monoclonal rabbit anti-human IGF2BP2 antibodies and held at 4°C overnight (Catalog number: ab124930; 1:50 dilution; Abcam, Cambridge, MA, USA). On the following day, the TMAs were tested for immune complex using a LASB 2 Kit (Dako, Carpinteria, CA, USA). The TMAs were stained using aminoethyl carbazole followed by hematoxylin to detect enzyme activity. The experiment involved a positive control (pancreatic cancer tissue as a known positive case) [27] as well as a negative control (samples not treated with the primary antibody) to assess the specificity of IGF2BP2 antibodies for IHC staining.
Statistical analysis
The clinicopathological variables of cytoplasmic IGF2BP2 protein expression and OSCC were assessed using Fisher's exact test or the Chi-Square test. The Kaplan-Meier method was used to create overall survival curves for OSCC patients with low and high cytoplasmic IGF2BP2 protein expression, and the log-rank test was used to estimate cumulative survival rates. The Cox proportional hazard regression model was used to confirm prognostic variables of OSCC using univariate and multivariate analyses after adjusting the stage, tumor size, lymph node metastasis and cell differentiation status. A p-value of <0.05 was used to identify statistically significant results [36, 37, 40]. Statistical Product and Service Solutions (SPSS, version 17) was used for all analysis (SPSS, Inc., Chicago, IL, USA).
Results
Demographics and characteristics of human patients with OSCC
Table 1 lists the demographics and data pertaining to patient characteristics. This study included 234 male OSCC patients (95.9%), ranging in age from 32 to 85. Patients were categorized according to disease stage according to criteria outlined by the American Joint Committee on Cancer (AJCC), as follows: stage I (n=43; 17.6%), stage II (n=54; 22.1%); stage III (n=29; 11.9%), and stage IV (n=118; 48.4%). The tumor size distribution was as follows: tumor size I (T1) (n=57; 23.4 %), tumor size II (T2) (n=78; 31.9 %), tumor size III (T3) (n=19; 7.80 %), and tumor size IV (T4) (n = 90; 36.9%). Patients were also categorized according to histological grade, as follows: well differentiated (Well; n=39; 16.0%), moderately differentiated (Moderate; n=198; 81.1%), and poorly differentiated (Poor; n=7; 2.9%).
Demographics and characteristics of human patients with oral squamous cell carcinoma
| Factors | (n = 244) | Percentage |
|---|---|---|
| Gender | ||
| Male | 234 | 95.9% |
| Female | 10 | 4.10% |
| Age (yrs) | ||
| Range | 32-85 | |
| Mean | 55.0 | |
| Medium | 53.0 | |
| AJCC cancer stage | ||
| I | 43 | 17.6% |
| II | 54 | 22.1% |
| III | 29 | 11.9% |
| IV | 118 | 48.4% |
| T (Tumor size) | ||
| T1 | 57 | 23.4% |
| T2 | 78 | 31.9% |
| T3 | 19 | 7.8% |
| T4 | 90 | 36.9% |
| N (Lymph node) | ||
| No | 151 | 61.9% |
| Yes | 93 | 38.1% |
| M (Metastasis) | ||
| No | 242 | 99.2% |
| Yes | 2 | 0.80% |
| Histological grade (differentiation) | ||
| Well | 39 | 16.0% |
| Moderate | 198 | 81.1% |
| poor | 7 | 2.9% |
IGF2BP2 protein expression in OSCC and clinicopathological variables
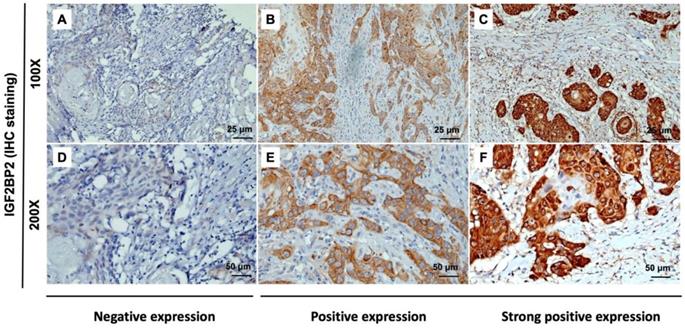
The expression of IGF2BP2 in OSCC cancer tissue was examined via IHC staining. As shown in Figure 1, samples were divided into two groups based on IGF2BP2 protein expression, as follows: (1) Low cytoplasmic staining of IGF2BP (negative expression); (2) high cytoplasmic staining of IGF2BP (positive and strong positive expression). The tissue samples were stratified as follows: Low IGF2BP2 expression (n=209; 85.7%) and high IGF2BP2 expression (n=35; 14.3%). The relationship between IGF2BP2 expression and clinicopathological variables in individuals with OSCC was used as the clinical basis in assessing the clinical relevance of IGF2BP2 protein expression using the Fisher exact test or the Chi-square test (Table 2). High IGF2BP2 expression was significantly linked to lymph node metastases, disease stage, and the survival of human patients with OSCC (p=0.004, p=0.027, p=0.038, and p=0.011, respectively). In OSCC patients, we did not observe a significant relationship between IGF2BP2 expression and age, histological grade, T status, distant metastasis, smoking, or betel quid chewing.
Clinicopathologic variables correlated with IGF2BP2 expression in human patients with oral squamous cell carcinoma
| Variables | Cytoplasmic Staining of IGF2BP2 | |||
|---|---|---|---|---|
| Low | High | (n=244) | p-valuea | |
| Age (yrs) | 55.1±10.9 | 54.3±10.0 | 0.331 | |
| Gender | ||||
| Male | 200 (95.7%) | 34 (97.1%) | 234 | 1.000a |
| Female | 9 (4.3%) | 1 (2.9%) | 10 | |
| Smoking | ||||
| No | 69 (39.7%) | 8 (30.8%) | 77 | 0.385 |
| Yes | 105 (60.3%) | 18 (69.2%) | 123 | |
| Betel quid chewing | ||||
| No | 56 (40.9%) | 7 (36.8%) | 63 | 0.737 |
| Yes | 81 (59.1%) | 12 (63.2%) | 93 | |
| AJCC cancer stage | ||||
| I, II | 89 (42.6%) | 8 (22.9%) | 97 | 0.027* |
| III, IV | 120 (57.4%) | 27 (77.1%) | 147 | |
| T (Tumor size) | ||||
| T1/T2 | 117 (56.0%) | 18 (51.4%) | 135 | 0.616 |
| T3/T4 | 92 (44.0%) | 17 (48.6%) | 109 | |
| Lymph node metastasis | ||||
| No | 137 (65.6%) | 14 (40.0%) | 151 | 0.004* |
| Yes | 72 (34.4%) | 21 (60.0%) | 93 | |
| Distant metastasis | ||||
| No | 207 (99.0%) | 35 (100%) | 242 | 1.000a |
| Yes | 2 (1.0%) | 0 (0%) | 2 | |
| Histological grade (differentiation) | ||||
| Well | 37 (17.7%) | 2 (5.7%) | 39 | 0.083 a |
| Moderate/Poor | 172 (82.3%) | 33 (94.3%) | 205 | |
| Survival | ||||
| ≤4 year | 86 (41.1%) | 21 (60.0%) | 107 | 0.038* |
| >4 year | 123 (58.9%) | 14 (40.0%) | 137 | |
| ≤5 year | 95 (45.5%) | 24 (68.6%) | 119 | 0.011* |
| >5 year | 114 (54.5%) | 11 (31.4%) | 125 | |
aThe p-value using Fisher's exact test or Chi-square test. *p<0.05
IHC analysis of cytoplasmic IGF2BP2 expression in human OSCC tissue showing negative (A and D), positive (B and E), and strong positive expression (C and F). Magnification: (top panel) 100x and lower panel (200x). Scale bars=25 and 50 µm.
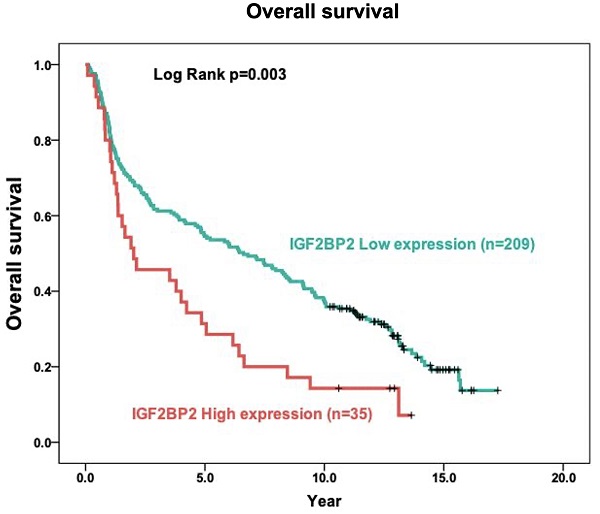
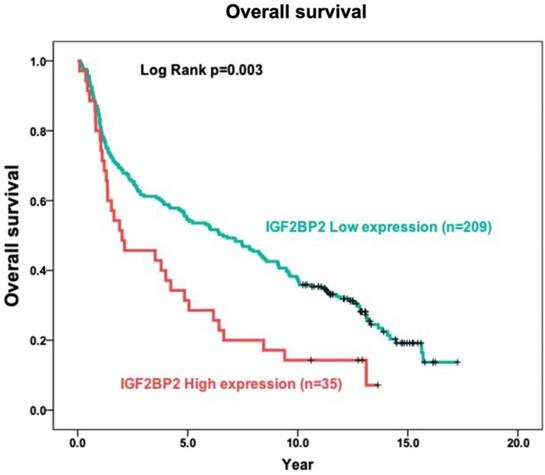
High IGF2BP2 protein expression levels are linked to shorter overall survival in OSCC patients
The role of IGF2BP2 in tumor prognosis was elucidated in terms of the relationship between IGF2BP2 expression and the overall survival of OSCC patients. In Kaplan-Meier analysis, the survival curves of OSCC patients with high IGF2BP2 expression were lower than those with low IGF2BP2 expression (p=0.003) using log-rank tests (Figure 2).
Prognostic indicators of clinicopathological variables and IGF2BP2 protein expression in OSCC patients identified using Cox proportional-hazards models
Univariate and multivariate analysis based on the Cox proportional-hazards model were used to determine the degree to which independent prognostic factors of IGF2BP2 expression affect overall survival of OSCC patients (Table 3). Univariate and multivariate analyses both revealed that the overall survival rate of OSCC patients was significantly linked to the expression of IGF2BP2 (p=0.003, 95% CI 1.213 to 2.644; p=0.039, 95% CI 1.530 to 2.289, respectively), histological grade (p=0.039, 95% CI 1.021 to 2.218), T status (p<0.001, 95% CI 1.239 to 2.139; p=0.013, 95% CI 1.132 to 2.887, respectively), lymph node metastasis (p<0.001, 95% CI 1.384 to 2.423; p=0.012, 95% CI 1.181 to 2.435, respectively) and stage (p<0.001, 95% CI 1.342 to 2.373).
Discussion
Cancer has become one of the most common causes of mortality among middle-aged and elderly individuals. OSCC is among the most common malignant tumors of the head and neck. The high recurrence and metastasis of the disease pose a severe threat to human health and welfare [41-43]. OSCC is generally detected in the middle or late stages, due to non-specific early clinical symptoms. Despite recent advancements in the treatment of OSCC, the 5-year survival rate remains low [44, 45]. OSCC is a malignant tumor affecting the head and neck region, which has also been shown to harm oral epithelial cells [46]. OSCC has been linked to genetic modifications, including mutations to chromosomes 3, 9, 11, and 13 [47, 48]. Increasing survival rates and improving the quality of life of OSCC patients will depend on the identification of molecular biomarkers and therapeutic techniques for the diagnosis and treatment of OSCC [49].
Overall survival and clinicopathologic variables of human patients with oral squamous cell carcinoma using univariate and multivariate analysis
| Variables (n = 244) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI)a | p-value | Hazard ratio (95% CI)a | p-value | |
| Expression of IGF2BP2 | ||||
| Low | 1.0 | 0.003* | 1.0 | 0.039* |
| High | 1.79 (1.213-2.644) | 1.53 (1.530-2.289) | ||
| AJCC cancer stage | ||||
| I, II | 1.0 | <0.001* | 1.0 | 0.642 |
| III, IV | 1.78 (1.342-2.373) | 0.88 (0.498-1.537) | ||
| T (Tumor size) | ||||
| T1/T2 | 1.0 | <0.001* | 1.0 | 0.013* |
| T3/T4 | 1.63 (1.239-2.139) | 1.81 (1.132-2.887) | ||
| Lymph node metastasis | ||||
| No | 1.0 | <0.001* | 1.0 | 0.012* |
| Yes | 1.83 (1.384-2.423) | 1.65 (1.181-2.435) | ||
| Histological grade (differentiation) | ||||
| Well | 1.0 | 0.039* | 1.0 | 0.136 |
| Moderate/Poor | 1.51 (1.021-2.218) | 1.38 (0.904-2.103) | ||
95% CI: 95% Confidence interval; aHazard ratio was adjusted for gender and age. *p<0.05
Relationship between cytoplasmic IGF2BP2 expression levels and overall survival in patients with OSCC based on the Kaplan-Meier method. Analysis was based on included 244 oral squamous cell carcinoma samples, using Kaplan-Meier analysis in conjunction with the log-rank test to establish survival curves.
Note that the specific mechanism underlying OSCC tumorigenesis has yet to be elucidated, and there are currently no reliable early indicators for the diagnosis or prognosis of OSCC [8, 50]. Identifying genes with distinct patterns of expression in OSCC tumors versus normal tissue could advance our understanding of OSCC etiology, while providing important diagnostic markers and therapeutic targets for OSCC therapy [51]. The aberrant expression of oncogenes and tumor suppressor genes has previously been demonstrated to have anti-tumor or tumor-promoting effects [52]. Several biomarkers have been linked to OSCC occurrence and disease progression, indicating that they play an important role in carcinogenesis. In the current study, we assessed IGF2BP2 protein expression levels within the context of the prognosis of OSCC patients.
Researchers have reported that IGF2BP2 is elevated in cases of malignancy. IGF2BP2 levels can also use as a prognostic indicator of acute myelocytic leukemia [25], breast cancer [53], endometrial adenocarcinoma [54], liposarcoma [55], pancreatic cancer [27], hepatocellular carcinoma [30] and OSCC [35]. Moreover, Lu et al. reported that IGF2BP2 may play an important role in the development of ESCC carcinogenesis [56]. In the current study, we discovered the overexpression of IGF2BP2 in OSCC patients (Figure 1), which is consistent with previous findings [35]. We also determined that IGF2BP2 overexpression is related to poor overall survival outcomes in OSCC patients (Figure 2), which suggests that it could perhaps be used as a prognostic indicator for use in OSCC risk classification. Elevated IGF2BP2 expression in OSCC cells has been linked to cell proliferation, metastasis, and tumor-infiltrating immune cells in in vitro experiments [34]. Those studies confirm our clinical results, which suggest that IGF2BP2 enhances OSCC epithelial cell proliferation and epithelial-mesenchymal transition (EMT), thereby promoting tumor growth and invasion in OSCC patients.
In the current study, we used clinical tissue samples from OSCC patients to characterize the connection between IGF2BP2 and clinicopathologic indicators. High cytoplasmic IGF2BP2 expression was strongly linked to disease stage and survival. The connections between positive IGF2BP2 protein expression and lymph node metastases, as well as between IGF2BP2 and AJCC cancer stage, suggest that IGF2BP2 may play a role in OSCC metastasis (Table 2). Our findings are consistent with previous studies in which IGF2BP2 mRNA expression levels were examined in the context of clinicopathological characteristics based on public clinical datasets [35]. Univariate and multivariate analyses both identified IGF2BP2 expression, histological grade, T status, lymph node metastases, and disease stage as key independent prognostic factors impacting the overall survival of OSCC patients (Table 3). Our findings suggest that IGF2BP2 may operate as an oncogene in OSCC cells, which is in line with earlier research [34, 35]. Note that this was the first study to examine the use of clinicopathological variables and IGF2BP2 protein expression within the context of OSCC prognosis. Further multistep research, including both in vitro and in vivo testing, will be required to corroborate our data and assess the efficacy of IGF2BP2 as a therapeutic target.
In conclusion, our findings demonstrate that IGF2BP2 protein expression is prevalent in OSCC tissues, and that protein expression levels were associated with histological grade, T status, lymph node metastasis, disease stage, and survival. Our results from 244 OSCC patients show a strong link between IGF2BP2 protein levels and survival rates. Our results also show that IGF2BP2 protein expression could potentially be used as an independent OSCC prognostic predictor and/or therapeutic target for OSCC treatment.
Acknowledgements
This study was supported by grants from Ministry of Science and Technology, Taiwan (MOST-108-2320-B-040-012-MY3).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
2. Antonsson A, de Souza M, Wood ZC, Carroll A, Van K, Paterson L. et al. Natural history of oral HPV infection: Longitudinal analyses in prospective cohorts from Australia. Int J Cancer. 2021;148:1964-72
3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
4. Chow LQM. Head and Neck Cancer. N Engl J Med. 2020;382:60-72
5. Su SC, Chang LC, Lin CW, Chen MK, Yu CP, Chung WH. et al. Mutational signatures and mutagenic impacts associated with betel quid chewing in oral squamous cell carcinoma. Hum Genet. 2019;138:1379-89
6. Chang YA, Weng SL, Yang SF, Chou CH, Huang WC, Tu SJ. et al. A Three-MicroRNA Signature as a Potential Biomarker for the Early Detection of Oral Cancer. Int J Mol Sci. 2018;19:758
7. Su CW, Lin CW, Yang WE, Yang SF. TIMP-3 as a therapeutic target for cancer. Ther Adv Med Oncol. 2019;11:1758835919864247
8. Chien MH, Lin CW, Cheng CW, Wen YC, Yang SF. Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin Ther Targets. 2013;17:203-16
9. Xu L, Li Q, Wang Y, Wang L, Guo Y, Yang R. et al. m(6)A methyltransferase METTL3 promotes oral squamous cell carcinoma progression through enhancement of IGF2BP2-mediated SLC7A11 mRNA stability. Am J Cancer Res. 2021;11:5282-98
10. Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001;37:477-92
11. Tsai CH, Yang SF, Chen YJ, Chou MY, Chang YC. The upregulation of insulin-like growth factor-1 in oral submucous fibrosis. Oral Oncol. 2005;41:940-6
12. Chen PN, Lin CW, Yang SF, Chang YC. Oral submucous fibrosis stimulates invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by activating MMP-2 and IGF-IR. J Cell Mol Med. 2021;25:9814-25
13. Yoon AJ, Zavras AI, Chen MK, Lin CW, Yang SF. Association between Gly1619ARG polymorphism of IGF2R domain 11 (rs629849) and advanced stage of oral cancer. Med Oncol. 2012;29:682-5
14. Fujii Y, Kishi Y, Gotoh Y. IMP2 regulates differentiation potentials of mouse neocortical neural precursor cells. Genes Cells. 2013;18:79-89
15. Li Z, Gilbert JA, Zhang Y, Zhang M, Qiu Q, Ramanujan K. et al. An HMGA2-IGF2BP2 axis regulates myoblast proliferation and myogenesis. Dev Cell. 2012;23:1176-88
16. Boudoukha S, Cuvellier S, Polesskaya A. Role of the RNA-binding protein IMP-2 in muscle cell motility. Mol Cell Biol. 2010;30:5710-25
17. Christiansen J, Kolte AM, Hansen T, Nielsen FC. IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J Mol Endocrinol. 2009;43:187-95
18. Liu X, Chen Z, Zhao X, Huang M, Wang C, Peng W. et al. Effects of IGF2BP2, KCNQ1 and GCKR polymorphisms on clinical outcome in metastatic gastric cancer treated with EOF regimen. Pharmacogenomics. 2015;16:959-70
19. Liu G, Zhu T, Cui Y, Liu J, Liu J, Zhao Q. et al. Correlation between IGF2BP2 gene polymorphism and the risk of breast cancer in Chinese Han women. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2015;69:297-300
20. Chou CH, Chang CY, Lu HJ, Hsin MC, Chen MK, Huang HC. et al. IGF2BP2 Polymorphisms Are Associated with Clinical Characteristics and Development of Oral Cancer. Int J Mol Sci. 2020;21:5662
21. Dai N, Zhao L, Wrighting D, Kramer D, Majithia A, Wang Y. et al. IGF2BP2/IMP2-Deficient mice resist obesity through enhanced translation of Ucp1 mRNA and Other mRNAs encoding mitochondrial proteins. Cell Metab. 2015;21:609-21
22. Huang RS, Zheng YL, Li C, Ding C, Xu C, Zhao J. MicroRNA-485-5p suppresses growth and metastasis in non-small cell lung cancer cells by targeting IGF2BP2. Life Sci. 2018;199:104-11
23. Ma YS, Shi BW, Guo JH, Liu JB, Yang XL, Xin R. et al. microRNA-320b suppresses HNF4G and IGF2BP2 expression to inhibit angiogenesis and tumor growth of lung cancer. Carcinogenesis. 2021;42:762-71
24. Gao T, Liu X, He B, Pan Y, Wang S. Long non-coding RNA 91H regulates IGF2 expression by interacting with IGF2BP2 and promotes tumorigenesis in colorectal cancer. Artif Cells Nanomed Biotechnol. 2020;48:664-71
25. He X, Li W, Liang X, Zhu X, Zhang L, Huang Y. et al. IGF2BP2 Overexpression Indicates Poor Survival in Patients with Acute Myelocytic Leukemia. Cell Physiol Biochem. 2018;51:1945-56
26. Ye S, Song W, Xu X, Zhao X, Yang L. IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF-1 degradation by miR-195. FEBS Lett. 2016;590:1641-50
27. Hu X, Peng WX, Zhou H, Jiang J, Zhou X, Huang D. et al. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 2020;27:1782-94
28. Jeng YM, Chang CC, Hu FC, Chou HY, Kao HL, Wang TH. et al. RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology. 2008;48:1118-27
29. Waly AA, El-Ekiaby N, Assal RA, Abdelrahman MM, Hosny KA, El Tayebi HM. et al. Methylation in MIRLET7A3 Gene Induces the Expression of IGF-II and Its mRNA Binding Proteins IGF2BP-2 and 3 in Hepatocellular Carcinoma. Front Physiol. 2018;9:1918
30. Wei Q. Bioinformatical identification of key genes regulated by IGF2BP2-mediated RNA N6-methyladenosine and prediction of prognosis in hepatocellular carcinoma. J Gastrointest Oncol. 2021;12:1773-85
31. Pu J, Wang J, Qin Z, Wang A, Zhang Y, Wu X. et al. IGF2BP2 Promotes Liver Cancer Growth Through an m6A-FEN1-Dependent Mechanism. Front Oncol. 2020;10:578816
32. Mu Q, Wang L, Yu F, Gao H, Lei T, Li P. et al. Imp2 regulates GBM progression by activating IGF2/PI3K/Akt pathway. Cancer Biol Ther. 2015;16:623-33
33. Deng X, Jiang Q, Liu Z, Chen W. Clinical Significance of an m6A Reader Gene, IGF2BP2, in Head and Neck Squamous Cell Carcinoma. Front Mol Biosci. 2020;7:68
34. Zhou L, Li H, Cai H, Liu W, Pan E, Yu D. et al. Upregulation of IGF2BP2 Promotes Oral Squamous Cell Carcinoma Progression That Is Related to Cell Proliferation, Metastasis and Tumor-Infiltrating Immune Cells. Front Oncol. 2022;12:809589
35. Wang X, Xu H, Zhou Z, Guo S, Chen R. IGF2BP2 maybe a novel prognostic biomarker in oral squamous cell carcinoma. Bioscience reports. 2022 42::BSR20212119
36. Lin YM, Lin CW, Lu JW, Yeh KT, Lin SH, Yang SF. Decreased Cytoplasmic Expression of ADAMTS14 Is Correlated with Reduced Survival Rates in Oral Squamous Cell Carcinoma Patients. Diagnostics (Basel). 2020;10:122
37. Lu JW, Tseng YS, Lo YS, Lin YM, Yeh CM, Lin SH. Prognostic Significance of Cytoplasmic SPNS2 Expression in Patients with Oral Squamous Cell Carcinoma. Medicina (Kaunas). 2021;57:164
38. Hsin CH, Chou YE, Yang SF, Su SC, Chuang YT, Lin SH. et al. MMP-11 promoted the oral cancer migration and Fak/Src activation. Oncotarget. 2017;8:32783-93
39. Lin CW, Yang WE, Lee WJ, Hua KT, Hsieh FK, Hsiao M. et al. Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase IX inhibition and is associated with favourable prognosis. Carcinogenesis. 2016;37:712-22
40. Ho YJ, Shih CP, Yeh KT, Shi B, Gong Z, Lin YM. et al. Correlation between high expression levels of jumonji domain-containing 4 and short survival in cases of colon adenocarcinoma. Biochem Biophys Res Commun. 2018;503:1442-9
41. Su SC, Chang LC, Huang HD, Peng CY, Chuang CY, Chen YT. et al. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis. 2021;42:127-35
42. Su SC, Yeh CM, Lin CW, Hsieh YH, Chuang CY, Tang CH. et al. A novel melatonin-regulated lncRNA suppresses TPA-induced oral cancer cell motility through replenishing PRUNE2 expression. Journal of pineal research. 2021;71:e12760
43. Yang SF, Huang HD, Fan WL, Jong YJ, Chen MK, Huang CN. et al. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral oncology. 2018;77:1-8
44. Yeh CM, Su SC, Lin CW, Yang WE, Chien MH, Reiter RJ. et al. Melatonin as a potential inhibitory agent in head and neck cancer. Oncotarget. 2017;8:90545-56
45. Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter RJ, Yang SF. Cancer metastasis: Mechanisms of inhibition by melatonin. Journal of pineal research. 2017;62:e12370
46. Gharat SA, Momin M, Bhavsar C. Oral Squamous Cell Carcinoma: Current Treatment Strategies and Nanotechnology-Based Approaches for Prevention and Therapy. Crit Rev Ther Drug Carrier Syst. 2016;33:363-400
47. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol. 2000;36:256-63
48. Su SC, Lin CW, Liu YF, Fan WL, Chen MK, Yu CP. et al. Exome Sequencing of Oral Squamous Cell Carcinoma Reveals Molecular Subgroups and Novel Therapeutic Opportunities. Theranostics. 2017;7:1088-99
49. Shi D, Li H, Zhang J, Li Y. CircGDI2 Regulates the Proliferation, Migration, Invasion and Apoptosis of OSCC via miR-454-3p/FOXF2 Axis. Cancer Manag Res. 2021;13:1371-82
50. Su CW, Chang YC, Chien MH, Hsieh YH, Chen MK, Lin CW. et al. Loss of TIMP3 by promoter methylation of Sp1 binding site promotes oral cancer metastasis. Cell death & disease. 2019;10:793
51. Zhong L, Liu Y, Wang K, He Z, Gong Z, Zhao Z. et al. Biomarkers: paving stones on the road towards the personalized precision medicine for oral squamous cell carcinoma. BMC Cancer. 2018;18:911
52. Lee EY, Muller WJ. Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol. 2010;2:a003236
53. Li Y, Francia G, Zhang JY. p62/IMP2 stimulates cell migration and reduces cell adhesion in breast cancer. Oncotarget. 2015;6:32656-68
54. Zhang L, Liu Y, Hao S, Woda BA, Lu D. IMP2 expression distinguishes endometrioid from serous endometrial adenocarcinomas. Am J Surg Pathol. 2011;35:868-72
55. Cleynen I, Brants JR, Peeters K, Deckers R, Debiec-Rychter M, Sciot R. et al. HMGA2 regulates transcription of the Imp2 gene via an intronic regulatory element in cooperation with nuclear factor-kappaB. Mol Cancer Res. 2007;5:363-72
56. Lu F, Chen W, Jiang T, Cheng C, Wang B, Lu Z. et al. Expression profile, clinical significance and biological functions of IGF2BP2 in esophageal squamous cell carcinoma. Exp Ther Med. 2022;23:252
Author contact
![]() Corresponding authors: Shun-Fa Yang, PhD. or Hsueh-Ju Lu, MD, PhD. Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo N. Road, Taichung, Taiwan, ROC. Fax: 886-4-24723229. E-mail: ysfedu.tw (Shun-Fa Yang); hsuehju0311com (Hsueh-Ju Lu).
Corresponding authors: Shun-Fa Yang, PhD. or Hsueh-Ju Lu, MD, PhD. Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo N. Road, Taichung, Taiwan, ROC. Fax: 886-4-24723229. E-mail: ysfedu.tw (Shun-Fa Yang); hsuehju0311com (Hsueh-Ju Lu).

 Global reach, higher impact
Global reach, higher impact