3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(6):1065-1071. doi:10.7150/ijms.71380 This issue Cite
Research Paper
Median Effective Dose of Dexmedetomidine Inducing Bradycardia in Elderly Patients Determined by Up-and-Down Sequential Allocation Method
1. Department of Anesthesiology, Shidong Hospital Affiliated to University of Shanghai for Science and Technology, Shanghai, China.
2. Department of Anesthesiology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai, China.
*These authors contributed equally to this work.
Abstract
Purpose: When dexmedetomidine is used in elderly patients, high incidence of bradycardia is reported. Given age-related physiological changes in this population, it is necessary to know the safety margin between the loading dose of dexmedetomidine and bradycardia. Therefore, we conducted this study to investigate the median effective dose (ED50) of dexmedetomidine causing bradycardia in elderly patients.
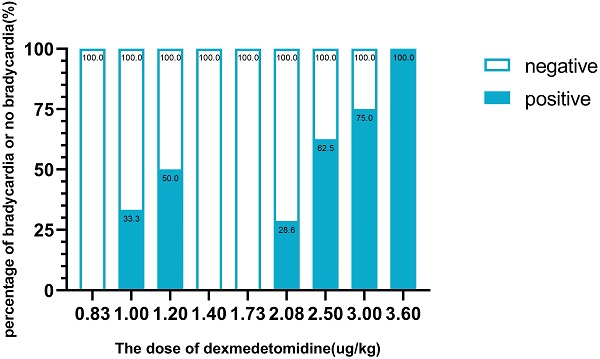
Methods: Thirty patients with ages over 65 years undergoing elective general surgery were enrolled. The Dixon and Massay sequential method were applied to determine the loading dose of dexmedetomidine, starting from 1.0 µg/kg. The dose for the follow-up subjects increased or decreased according to the geometric sequence with the common ratio 1.2, based on the 'negative' or 'positive' response of the previous subject. Positive mean that the subject developed bradycardia during the test. Hemodynamic data including heart rate and systolic blood pressure were recorded. The level of sedation was assessed with the Observer Assessment of Alertness and Sedation Scale (OAA/S).
Results: Bradycardia occurred in 13 patients (43.3%). The ED50 of dexmedetomidine causing bradycardia was 1.97 µg/kg (95% CI, 1.53-2.53 µg/kg). OAA/S scores at 10 min after the beginning of the dexmedetomidine infusion and 10 min after the termination of dexmedetomidine administration showed no significant differences between the positive and negative groups (P > 0.05).
Conclusion: The ED50 of dexmedetomidine causing bradycardia in our cohort was higher than clinical recommended dose. A higher loading dose appears acceptable for a faster onset of sedation under careful hemodynamic monitoring.
Trial registration: ChiCTR 15006368.
Keywords: dexmedetomidine, bradycardia, elderly patient, intravenous anaesthesia, adverse effects

 Global reach, higher impact
Global reach, higher impact