Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(6):1013-1022. doi:10.7150/ijms.72378 This issue Cite
Research Paper
Effect of tissue inhibitor of metalloproteinases-3 genetics polymorphism on clinicopathological characteristics of uterine cervical cancer patients in Taiwan
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
2. Department of Radiology, Taichung Veterans General Hospital, Taichung, Taiwan
3. Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital, Taichung, Taiwan
4. School of Medicine, Chung Shan Medical University, Taichung, Taiwan
5. Department of Surgery, Chung Shan Medical University Hospital, Taichung, Taiwan
6. Department of Medicine, National Taiwan University, Taipei, Taiwan
7. Neurological Institute, Taichung Veterans General Hospital, Taichung, Taiwan
8. Department of Obstetrics and Gynecology, Changhua Christian Hospital, Changhua, Taiwan
9. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
#Equal contribution as first authors
Received 2022-2-25; Accepted 2022-5-23; Published 2022-5-29
Abstract
Single nucleotide polymorphisms (SNPs) of tissue inhibitor of metalloproteinases-3 (TIMP-3) have been revealed to be related to various cancers. To date, no study explores the relationships between TIMP-3 polymorphisms and uterine cervical cancer. The purposes of this research were to investigate the associations among genetic variants of TIMP-3 and development and clinicopathological factors of uterine cervical cancer, and patient 5 years survival in Taiwanese women. The study included 123 patients with invasive cancer and 97 with precancerous lesions of uterine cervix, and 300 control women. TIMP-3 polymorphisms rs9619311, rs9862 and rs11547635 were checked and their genotypic distributions were determined by real-time polymerase chain reaction. It showed that women with genotypes CT/TT in rs9862 were found to display a higher risk of developing cervical cancer with moderate and poor cell differentiation. Moreover, it revealed that cervical cancer patients carrying genotypes CC in rs9619311 exhibited a poorer 5 years survival, as compared to those with TT/TC in Taiwanese women, using univariate analysis. In addition, pelvic lymph node metastasis was determined to independently predict 5 years survival in cervical cancer patients using multivariate analysis. Conclusively, TIMP-3 SNPs polymorphisms rs9619311 are related to cervical patient survival in Taiwanese women.
Keywords: tissue inhibitor of metalloproteinases-3, single nucleotide polymorphisms, uterine cervical cancer, lymph node metastasis, 5 years survival
Introduction
Matrix metalloproteinases (MMPs), capable of degrading extracellular matrix (ECM), are categorized to a zinc- as well as calcium-dependent endopeptidase family, and comprise 23 members [1]. Based on their substrate specificity, the MMPs are classified into membrane-type matrix metalloproteinases (MT-MMPs), collagenases, gelatinases, stromelysins and matrilysins [2, 3]. MMPs are involved in cell growth, invasion, survival, and adhesion in biological and pathological conditions, and then their activation can cause cell proliferation, invasion and metastasis of cancer cells [4-6]. Tissue inhibitors of MMPs (TIMPs) are endogenous inhibitors of MMPs, and imbalance between the activities of MMPs and TIMPs may have an impact on cancer progression [7-10]. The TIMPs are known as a family of at least four 20 to 29 kDa proteins (TIMPs 1-4) which reversibly inhibit the MMPs [11]. TIMP-3 can inhibit the action of pro-MMP-2 that pertains to gelatinases, and is demonstrated to control cell death and suppress cancer cell invasion and metastasis [12-15]. TIMP-3 overexpression was found to induce apoptosis in cancer cell lines of uterine cervix and breast [16].
Genetic polymorphisms are defined as allele base change in the shared DNA sequence of a gene where alternate alleles exceed 5% of some populations [17-19]. When the genetic polymorphisms display a variation on the promoter area, exon or 3'-untranslated region of a gene, they may influence the gene expression, and then facilitate the development of disease and cancer [20, 21]. Therefore, the genetic variations of TIMP-3 in promoter area such as rs9619311 (-1296 T/C) and in the exon regions such as rs9862 (exon 3; 249 C/T) and rs11547635 (exon 3; 261 C/T) can be inferred to be associated with the occurrence and progression of uterine cervical cancer [22].
Uterine cervical cancer is the fourth most common cancer in female individuals in the world [23]. It also ranked the eighth most common malignancy among women in 2014 according to the data of the Health Promotion Administration of the Ministry of Health and Welfare and Annual Cancer Registry Report in Taiwan. The age-standardized mortality rate of cervical cancer was calculated to 3.39 per 100000 women in this year, responsible for the seventh main cause of cancer mortality of females in the country. A continuous and multistep dysplasia progresses in cervical tumorigenesis [24, 25]. Cervical intraepithelial neoplasia 1 (CIN 1) is referred to low-grade squamous intraepithelial lesion in cytological term (LSIL; also regarded as low-grade CIN or mild dysplasia in histologic counterpart), in which mitotic and immature cells only present in the lower third of the epithelium; whereas CIN 2 and CIN 3 are summed as high-grade CIN in histologic term and defined as high-grade squamous intraepithelial lesion in cytological counterpart, (HSIL) where mitotic and immature cells separately occur in the middle (CIN 2 or moderate dysplasia in histologic counterpart) as well as upper thirds of the epithelium or whole epithelium [severe dysplasia or carcinoma in situ (CIS), respectively, and summed as CIN 3 in histologic term], based on the Bethesda system. Near 90 % of CIN 1 regresses to normal, whereas, high-grade CIN progresses into invasive cancer in considerable rate, and is considered as cervical precancerous lesions [26, 27]. Until now, no study investigates the involvement of TIMP-3 genetic polymorphisms in the development of cervical cancer. Therefore, we conduct the study to associate the single nucleotide polymorphisms (SNPs) of TIMP-3 with the carcinogenesis and progression of uterine cervical cancer, and further relate them to patient 5 years survival.
Materials and Methods
Female individual's enrollment
The research was conducted to consecutively enroll 123 women with cervical invasive cancer and 97 with precancerous lesions from the Department of Obstetrics and Gynecology in Chung Shan Medical University Hospital in Taichung, Taiwan, from February 1994 to February 2015. Meanwhile, 300 female subjects who received routine healthcare in the outpatient department of this hospital were recruited as the controls. They had no previous cervical cancer and precancerous lesions. The 123 cervical patients were staged according to the 2009 FIGO. The studied individuals were examined for the distribution of three TIMP-3 genetic polymorphisms rs9619311, rs9862 and rs11547635. For the patients who had cervical cancer or precancerous lesions, cervical punch biopsy was done under colposcopy guidance and pathological findings were reported with final diagnosis of invasive cervical cancer, moderate, severe dysplasia or CIS. These patients were referred to cervical neoplasia group. The control group was defined as including female subjects without cervical neoplasias based on the normal cytological report from cervical Papanicolaou smear and further determined by normal colposcopic report in the general examination. All subjects were Taiwanese female individuals who resided in central Taiwan. The marital status and education level of cervical neoplasia group were well compatible with those of control group. Recruited patients underwent the standard treatment protocols, which conformed to guidelines offered by National Comprehensive Cancer Network. The Institutional Review Board in the Chung Shan Medical University Hospital agreed with this research (CSMUH number: CS18208). Informed consents were completed by all participants.
Extraction of deoxyribonucleic acid (DNA) from the blood samples of all female individuals and determination of three TIMP-3 genetic polymorphisms
Laboratory technician performed venipuncture to obtain blood samples from all subjects. The samples were collected into Vacutainer tubes mixed with ethylenediaminetetraacetic acid, and then stored at 4°C. DNA was subsequently extracted from white blood cells in accordance with previous study [28, 29]. The extracted DNA was dissolved in pH 7.8 TE buffer. Thereafter, it was quantified by the determination of OD260. The OD260/OD280 ratio was defined and the range of 1.8-2.0 was in line with our criteria and was determined as pure for preventing its cross reactivity from the current homologous RNA in the samples. The final products were refrigerated at -20°C and used as templates for the polymerase chain reaction (PCR).
According to the data of the international HapMap project and the previous research of Su et al., three genetic polymorphisms of TIMP-3 rs9619311 (-1296 T/C), rs9862 (exon 3; 249 C/T), and rs11547635 (exon 3; 261 C/T) were determined [22]. Genotyping of these 3 TIMP-3 genetic variants was defined using the TaqMan SNP Genotyping Assay with an ABI StepOnePlusTM Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The results were further checked with SDS version 3.0 software (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
Welch test was done and post hoc analysis was further performed by Dunnett test to compare the age distribution among patients with cervical invasive cancer and precancerous lesions, and normal controls. Chi-square or Fisher's tests were done to associate genotypic distributions of TIMP-3 genetic polymorphisms with the incidence of cervical neoplasias. Age must be adjusted because the age of patients having precancerous lesions was inherently earlier than that of those with invasive cancer of uterine cervix. The p values were determined by chi-square or Fisher's exact tests and by logistic or multinomial logistic regression models for adjusting age. The adjusted odds ratios (AORs) with their 95% confidence intervals (CIs) were used to check the relationships among genotypic distributions of TIMP-3 genetic variants and the incidence of cervical neoplasias (including precancerous lesions and invasive cancer) by the logistic or multinomial logistic regression models for adjusting age. Chi-square or Fisher's exact tests were applied to associate TIMP-3 SNPs with clinicopathological characteristics including clinical stage, pathologic type, cell grading, stromal invasion depth and tumor diameter of cancer masses, as well as invasions of parametrium and vagina, and pelvic lymph node metastasis. In univariate analysis, the Kaplan-Meier curve model was performed to determine the significances of TMIP-3 genetic variants and clinicopathological parameters for patient survival relative to survival time, which were related to 5 years survival of cervical cancer patients. The log-rank test was applied to check the differences among them. In multivariate analysis, the influences of TIMP-3 SNPs and these clinicopathological variables on 5 years survival of the patients were assessed using the Cox proportional hazard model in correspondence to survival time. The hazard ratios (HRs) were then determined. The SPSS, version 18.0 and WinPepi Software, version 10.0 were performed for statistical analysis. P <0.05 was defined to have statistically significant difference.
Results
Patients with cervical neoplasm were significantly different from control women in the age distribution (50.8 ± 13.8 vs. 44.0 ± 10.0, p<0.001). Using the Welch test by Dunnett test as post hoc analysis, the age distributions were statistically different between patients with cervical cancer and those with precancerous lesions (56.2 ± 12.6 vs. 43.9 ± 12.3, p<0.001), and between patients with cervical cancer and control individuals (56.2 ± 12.6 vs. 44.0 ± 10.0, p<0.001). Whereas, no statistical difference was found in the age distribution between patients with precancerous lesions and control subjects (43.9 ± 12.3 vs. 44.0 ± 10.0, p= 1.000).
The genotypic frequencies of TIMP-3 polymorphisms rs9619311 accorded with Hardy-Weinberg equilibrium in the normal controls [χ2 value: 0.69, p=0.709, degree of freedom (d.f.)=2]. The genotypic distributions of other TIMP-3 genetic polymorphisms rs9862 and rs11547635 were all in line with the equilibrium (χ2 value: 3.84, p=0.147, d.f.=2 and χ2 value: 0.09, p=0.958, d.f.=2, respectively).
It showed no significant differences for the genotypic distributions of T/T, T/C and C/C in TIMP-3 polymorphism rs9619311 (p=0.620) between patients with cervical neoplasias and control females. The genotypic frequencies of other TIMP-3 SNPs, rs9862 and rs11547635 also revealed no significant differences between patients with cervical neoplasias and control women (p=0.655 and 0.774, respectively; Table 1). After adjusting for age, it still found no significant differences in these three TIMP-3 genetic variants between these patients and control women (p=0.783, 0.618 and 0.789, respectively; Table 1).
Genotypic distributions of three tissue inhibitor of metalloproteinases-3 polymorphisms in patients with cervical neoplasias and normal controls in Taiwanese women
| Genetic variants | Normal controls (n = 300) | Cervical neoplasiasa (n= 220 ) | ORs (95% CIs) | p values | AORs (95% CIs)b | Adjusted p valuesb |
|---|---|---|---|---|---|---|
| rs9619311 | 0.620 | 0.783 | ||||
| T/Tc | 246 | 181 | 1.00 | 1.00 | ||
| T/C | 50 | 38 | 1.03 (0.65-1.64) | 0.891 | 1.06 (0.65-1.71) | 0.829 |
| C/C | 4 | 1 | 0.34 (0.04-3.07) | 0.336 | 0.47 (0.05-4.42) | 0.512 |
| T/Tc | 246 | 181 | 1.00 | 1.00 | ||
| T/C & C/C | 54 | 39 | 0.98 (0.62-1.55) | 0.936 | 1.02 (0.63-1.63) | 0.942 |
| T/T & T/Cc | 296 | 219 | 1.00 | 1.00 | ||
| C/C | 4 | 1 | 0.34 (0.04-3.04) | 0.333 | 0.47 (0.05-4.37) | 0.506 |
| rs9862 | 0.655 | 0.618 | ||||
| C/Cc | 110 | 78 | 1.00 | 1.00 | ||
| C/T | 130 | 98 | 1.06 (0.72-1.57) | 0.759 | 1.10(0.73-1.66) | 0.642 |
| T/T | 57 | 34 | 0.84 (0.50-1.41) | 0.510 | 0.85 (0.50-1.4 6) | 0.558 |
| C/Cc | 110 | 78 | 1.00 | 1.00 | ||
| C/T & T/T | 187 | 132 | 1.00 (0.69-1.44) | 0.981 | 1.03 (0.70-1.50) | 0.900 |
| C/C & C/Tc | 240 | 176 | 1.00 | 1.00 | ||
| T/T | 57 | 34 | 0.81(0.51-1.30) | 0.386 | 0.81 (0.50-1.31) | 0.388 |
| rs11547635 | 0.774 | 0.789 | ||||
| C/Cc | 127 | 86 | 1.00 | 1.00 | ||
| C/T | 127 | 98 | 1.14 (0.78-1.67) | 0.500 | 1.14 (0.77-1.70) | 0.517 |
| T/T | 34 | 23 | 1.00 (0.55-1.81) | 0.997 | 1.00 (0.53-1.87) | 0.997 |
| C/Cc | 127 | 86 | 1.00 | 1.00 | ||
| C/T & T/T | 161 | 121 | 1.11 (0.77-1.59) | 0.572 | 1.11 (0.76-1.63) | 0.586 |
| C/C & C/Tc | 254 | 184 | 1.00 | 1.00 | ||
| T/T | 34 | 23 | 0.93 (0.53-1.64) | 0.811 | 0.93 (0.52-1.69) | 0.820 |
Statistical analysis: logistic regression model or chi-square or Fisher's tests.
aCervical neoplasias consisted of precancerous lesions and invasive cancer of uterine cervix.
bThe adjusted p values and adjusted odds ratios (AORs) and their 95% confident intervals (95% CIs) were defined through logistic regression model after adjusting age.
cUsed as a reference for comparison to define the odds ratios of other genotypic distributions.
While patients with cervical neoplasias group was further subdivided into subgroup of patients with invasive cancer and subgroup of those with precancerous lesions, it still did not reveal significant differences for the genetic frequencies of T/T, T/C and C/C in TIMP-3 genetic polymorphism rs9619311 among invasive cancer, precancerous and control subgroups (p=0.171; Table 2). No significantly different distributions were also found in TIMP-3 genetic polymorphisms rs9862 and rs11547635 (p=0.551 and p=0.969, respectively; Table 2). After adjusting for age, there were still no significant differences in TIMP-3 rs9619311 among them (T/C vs T/T, p=0.137; C/C vs T/T, unavailable, while precancerous lesions subgroup compared with normal control subgroup; T/C vs T/T, p=0.227; C/C vs T/T, 0.838, while invasive cancer subgroup compared with normal control; Table 2). It also found no significant differences in the genotypic frequencies of other TIMP-3 genetic polymorphisms, rs9862 (C/T vs C/C, p=0.909; T/T vs C/C, p=0.145, while precancerous lesions subgroup compared with normal control; C/T vs C/C, p=0.373; T/T vs C/C, 0.494, while invasive cancer subgroup compared with normal control; Table 2) and rs11547635 (C/T vs C/C, p=0.683; T/T vs C/C, p=0.974, while precancerous lesions subgroup compared with normal control; C/T vs C/C, p=0.619; T/T vs C/C, 0.919, while invasive cancer subgroup compared with normal control; Table 2). The risks of invasive cancer and precancerous lesion of uterine cervix were not elevated for these TIMP-3 genetic polymorphisms (Table 2).
Compared with CC, patients with cervical cancer carrying genotypes CT/TT in TIMP-3 rs9862 had a higher risk of developing moderate and poor cell grading (grade 2/3, OR: 3.61, 95% CI: 1.14-12.01, p=0.012; Table 3). There were no associations of TIMP-3 rs9862 with other clinicopathological characteristics. Moreover, there were no relationships among TIMP-3 genetic variants rs9619311, rs11547635 and various clinicopathological parameters.
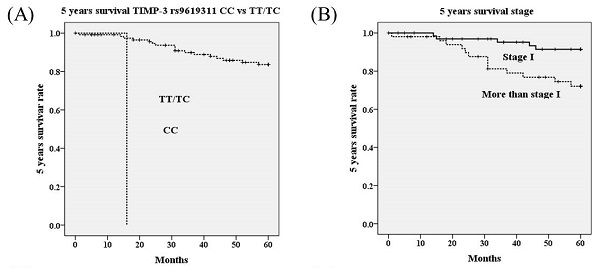
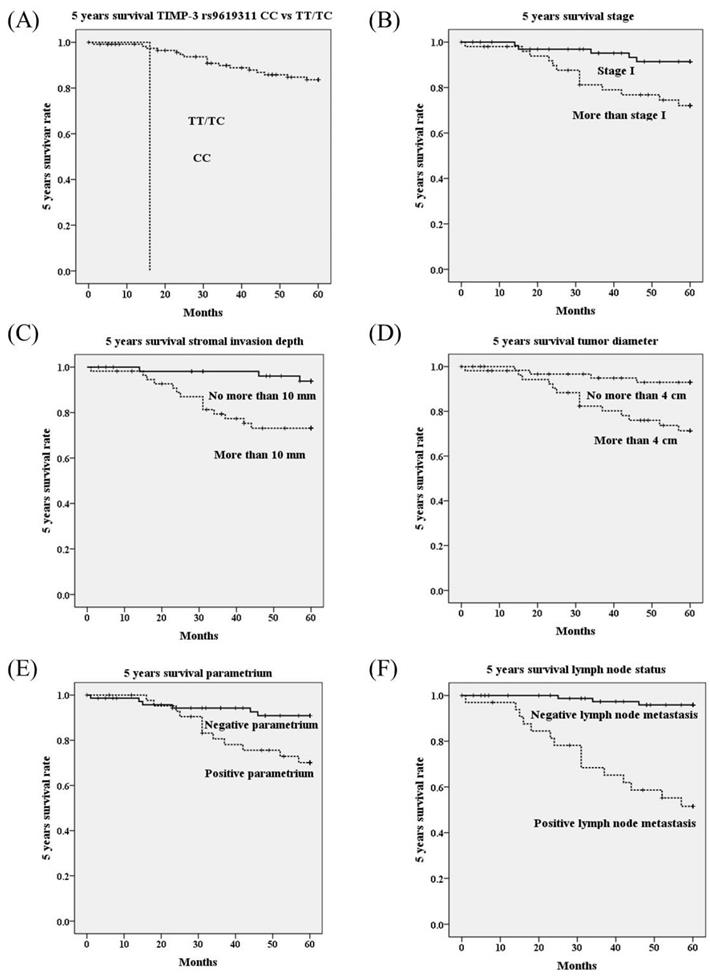
Using univariate analysis, genotype CC in TIMP-3 genetic polymorphism rs9619311 had poorer 5 years survival as compared to genotypes TT/TC (OR=37.05, 95% CI=3.85-356.26; p<0.001; Table 4; Figure 1A). Other TIMP-3 variants rs9862 and rs11547635 were not associated with 5 years survival. Furthermore, it revealed that HRs with poorer 5 years survival could be noted in cervical patients with more advanced stages (≥ stage II; p=0.009; Figure 1B), deep stromal invasion (p=0.003; Figure 1C), large tumor diameter (p=0.004; Figure 1D), positive parametrium invasion (p=0.009; Figure 1E), and positive pelvic lymph node metastasis (p<0.001; Table 4; Figure 1F). Using multivariate analysis, TIMP-3 genetic polymorphisms rs9619311, rs9862 and rs11547635 were not related to 5 years survival. Whereas, only positive lymph node metastasis could significantly predict poorer 5 years survival among clinicopathological variables (HR: 6.84, 95% CI: 1.41-33.11, p=0.017; Table 5).
Genotypic distributions of three tissue inhibitor of metalloproteinases-3 polymorphisms in patients with uterine cervical invasive cancer or precancerous lesion and normal controls in Taiwanese women
| Genetic variants | Normal controls (n = 300) | Pre-cancerous lesions (n = 97) | Invasive cancer (n = 123) | p values | AORs (95% CIs)a | Ad. p values | AORs (95% CIs)b | Ad. p values |
|---|---|---|---|---|---|---|---|---|
| rs9619311 | ||||||||
| T/Tc | 246 | 74 | 107 | 0.171 | 1.00 | 1.00 | ||
| T/C | 50 | 23 | 15 | 1.53 (0.87-2.67) | 0.137 | 0.66 (0.33-1.30) | 0.227 | |
| C/C | 4 | 0 | 1 | u.a. | u.a. | 1.27 (0.13-12.94) | 0.838 | |
| T/Tc | 246 | 74 | 107 | 0.120 | 1.00 | 1.00 | ||
| T/C & C/C | 54 | 23 | 16 | 1.41 (0.81-.046) | 0.219 | 0.68 (0.35-1.31) | 0.247 | |
| T/T & T/Cc | 296 | 97 | 122 | 0.829 | 1.00 | 1.00 | ||
| C/C | 4 | 0 | 1 | u.a. | u.a. | 1.35 (0.13-13.71) | 0.798 | |
| rs9862 | ||||||||
| C/Cc | 110 | 37 | 41 | 0.551 | 1.00 | 1.00 | ||
| C/T | 130 | 45 | 53 | 1.03 (0.62-1.70) | 0.909 | 1.28 (0.75-2.19) | 0.373 | |
| T/T | 57 | 11 | 23 | 0.57 (0.27-1.21) | 0.145 | 1.27 (0.64-2.49) | 0.494 | |
| C/Cc | 110 | 37 | 41 | 0.779 | 1.00 | 1.00 | ||
| C/T & T/T | 187 | 56 | 76 | 0.89 (0.55-1.44) | 0.636 | 1.27 (0.77-2.11) | 0.346 | |
| C/C & C/Tc | 240 | 82 | 94 | 0.233 | 1.00 | 1.00 | ||
| T/T | 57 | 11 | 23 | 0.57 (0.28-1.13) | 0.106 | 1.10 (0.61-2.01) | 0.748 | |
| rs11547635 | ||||||||
| C/Cc | 127 | 38 | 48 | 0.969 | 1.00 | 1.00 | ||
| C/T | 127 | 42 | 56 | 1.11 (0.67-1.84) | 0.683 | 1.14 (0.68-1.91) | 0.619 | |
| T/T | 34 | 10 | 13 | 0.99 (0.45-2.18) | 0.974 | 0.96 (0.43-2.15) | 0.919 | |
| C/Cc | 127 | 38 | 48 | 0.840 | 1.00 | 1.00 | ||
| C/T & T/T | 161 | 52 | 69 | 1.09 (0.67-1.75) | 0.740 | 1.10 (0.68-1.80) | 0.701 | |
| C/C & C/Tc | 254 | 80 | 104 | 0.972 | 1.00 | 1.00 | ||
| T/T | 34 | 10 | 13 | 0.94 (0.44-1.98) | 0.861 | 0.90 (0.42-1.92) | 0.780 |
aAdjusted p values and adjusted odds ratios with their 95% CIs were evaluated using multinomial logistic regression models after adjusting for age between patients with uterine cervical precancerous lesions and control women.
bAdjusted p values and adjusted odds ratios with their 95% CIs were evaluated using multinomial logistic regression models after adjusting for age between patients with uterine cervical invasive cancer and control women.
cUsed as a reference for comparison to assess the odds ratios of other genotypic distributions.
AORs, adjusted odds ratios; 95% CIs, 95% confidence intervals; Ad. p, adjusted p; u.a., unavailable.
Associations between genotypic distributions of tissue inhibitor of metalloproteinases-3 polymorphisms and clinicopathological factors in patients with cervical invasive cancer
| Parametersa | rs9619311 | rs9862 | rs11547635 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTb | TC/CC | TT/TCb | CC | CCb | CT/TT | CC/CTb | TT | CCb | CT/TT | CC/CTb | TT | |
| Clinical stage | ||||||||||||
| stage Ib | 61 | 9 | 70 | 0 | 22 | 47 | 53 | 16 | 29 | 39 | 62 | 6 |
| ≥ stage II | 45 | 7 | 51 | 1 | 19 | 28 | 40 | 7 | 18 | 30 | 40 | 8 |
| P value | 0.922 | 0.426 | 0.345 | 0.271 | 0.578 | 0.202 | ||||||
| Pathologic type | ||||||||||||
| squamous cell carcinomab | 93 | 15 | 107 | 1 | 34 | 70 | 82 | 22 | 44 | 61 | 93 | 12 |
| adenocarcinoma | 14 | 1 | 15 | 0 | 7 | 6 | 12 | 1 | 3 | 9 | 10 | 2 |
| P value | 0.690 | 0.708 | 0.215 | 0.459 | 0.357 | 0.636 | ||||||
| Cell grading | ||||||||||||
| well (grade 1)b | 14 | 5 | 19 | 0 | 11 | 7 | 16 | 2 | 5 | 12 | 13 | 4 |
| moderate & poor (grades 2/3) | 93 | 11 | 103 | 1 | 30 | 69 | 78 | 21 | 42 | 58 | 90 | 10 |
| P value | 0.073 | 1.000 | 0.012* | 0.520 | 0.328 | 0.121 | ||||||
| Stromal invasion depth | ||||||||||||
| ≤10 mmb | 50 | 9 | 59 | 0 | 23 | 35 | 45 | 13 | 20 | 36 | 50 | 6 |
| >10 mm | 51 | 5 | 55 | 1 | 14 | 37 | 41 | 10 | 24 | 29 | 47 | 6 |
| P value | 0.300 | 0.487 | 0.179 | 0.720 | 0.309 | 0.919 | ||||||
| Tumor diameter | ||||||||||||
| ≤ 4 cmb | 56 | 10 | 66 | 0 | 24 | 41 | 53 | 12 | 25 | 39 | 57 | 7 |
| >4 cm | 49 | 6 | 54 | 1 | 17 | 33 | 39 | 11 | 22 | 29 | 44 | 7 |
| P value | 0.493 | 0.455 | 0.746 | 0.638 | 0.659 | 0.650 | ||||||
| Parametrium | ||||||||||||
| no invasionb | 67 | 9 | 76 | 0 | 26 | 48 | 59 | 15 | 27 | 45 | 64 | 8 |
| invasion | 38 | 7 | 44 | 1 | 15 | 26 | 33 | 8 | 20 | 23 | 37 | 6 |
| P value | 0.560 | 0.372 | 0.876 | 0.922 | 0.342 | 0.652 | ||||||
| Vagina | ||||||||||||
| no invasionb | 68 | 10 | 78 | 0 | 27 | 48 | 61 | 14 | 30 | 44 | 66 | 8 |
| invasion | 37 | 6 | 42 | 1 | 14 | 26 | 31 | 9 | 17 | 24 | 35 | 6 |
| P value | 0.860 | 0.355 | 0.915 | 0.625 | 0.923 | 0.563 | ||||||
| Pelvic lymph node | ||||||||||||
| no metastasisb | 75 | 13 | 88 | 0 | 32 | 54 | 72 | 14 | 35 | 49 | 75 | 9 |
| metastasis | 30 | 3 | 32 | 1 | 9 | 20 | 20 | 9 | 12 | 19 | 26 | 5 |
| P value | 0.553 | 0.273 | 0.548 | 0.108 | 0.775 | 0.521 | ||||||
Statistical analyses: chi-square or Fisher's exact tests
*p<0.05
aClinicopathological data of some cases could not be obtained from the patients with cervical invasive cancer because of incomplete medical charts or records.
bAs a reference.
Five years survival rate based on polymorphisms of tissue inhibitor of metalloproteinases-3 (TIMP-3) (A), stage (B), stromal invasion depth (C), tumor diameter (D), parametrium invasion (E) and lymph node status (F) using Kaplan-Meier curve. Significantly worse 5 years survivals are showed in cervical cancer patients with genotype CC than those with TT/TC in TIMP-3 polymorphism rs9619311 (p<0.001 by log rank test), with stage ≥ stage II than stage I (p=0.009), with stromal invasion depth >10 mm than ≤10 mm (p=0.003), with tumor diameter >4 cm than ≤4 cm (p=0.004), with positive parametrium than negative parametrium invasion (p=0.009), with positive lymph than negative lymph node metastasis (p<0.001).
Univariate analysis of genetic polymorphisms of tissue inhibitor of metalloproteinases-3 and clinicopathological variables for 5 years survival in cervical cancer patients
| 5 years survival | ||||
|---|---|---|---|---|
| Variablesa | + | - | P value | HR (95% CIs)c |
| rs9619311 | ||||
| TTb | 88 | 16 | 0.768 | 1.00 |
| TC/CC | 14 | 2 | 0.80 (0.18-3.49) | |
| TT/TCb | 102 | 17 | <0.001* | 1.00 |
| CC | 0 | 1 | 37.05 (3.85-356.26) | |
| rs9862 | ||||
| CCb | 33 | 8 | 0.329 | 1.00 |
| CT/TT | 65 | 8 | 0.62 (0.23-1.65) | |
| CC/CTb | 77 | 13 | 0.995 | 1.00 |
| TT | 19 | 3 | 1.00 (0.28-3.50) | |
| rs11547635 | ||||
| CCb | 41 | 3 | 0.066 | 1.00 |
| CT/TT | 56 | 14 | 3.03 (0.87-10.56) | |
| CC/CTb | 85 | 15 | 0.889 | 1.00 |
| TT | 12 | 2 | 0.90 (0.21-3.94) | |
| Clinical stage | ||||
| stage Ib | 62 | 5 | 0.009* | 1.00 |
| ≥ stage II | 39 | 13 | 3.63 (1.30-10.19) | |
| Pathologic type | ||||
| squamous cell carcinomab | 92 | 14 | 0.160 | 1.00 |
| adenocarcinoma | 10 | 4 | 2.17 (0.72-6.60) | |
| Cell grading | ||||
| well (grade 1)b | 15 | 3 | 0.941 | 1.00 |
| moderate & poor (grades 2/3) | 87 | 15 | 0.95 (0.28-3.30) | |
| Stromal invasion depth | ||||
| ≤ 10 mmb | 53 | 3 | 0.003* | 1.00 |
| > 10 mm | 42 | 14 | 5.29 (1.52-18.42) | |
| Tumour diameter | ||||
| ≤ 4 cmb | 59 | 4 | 0.004* | 1.00 |
| > 4 cm | 41 | 14 | 4.45 (1.47-13.54) | |
| Parametrium | ||||
| no invasionb | 67 | 6 | 0.009* | 1.00 |
| invasion | 33 | 12 | 3.43 (1.29-9.13) | |
| Vagina | ||||
| no invasionb | 66 | 9 | 0.131 | 1.00 |
| invasion | 34 | 9 | 2.01 (0.80-5.07) | |
| Pelvic lymph node | ||||
| no metastasisb | 82 | 3 | <0.001* | 1.00 |
| metastasis | 18 | 15 | 15.72 (4.55-54.40) | |
Statistical analyses: Kaplan-Meier curve model
*p<0.05
aClinicopathological data of some cases could not be obtained from the patients with cervical invasive cancer because of incomplete records of medical chart.
bAs a reference.
cHR, hazard ratio and 95% CI, 95% confidence interval for tissue inhibitor of metalloproteinases-3 genetic polymorphisms rs9619311, rs9862 and rs11547635 and clinicopathological factors, compared to their respective controls.
Survival: +, survival, -, mortality.
Multivariate analysis of genetic polymorphisms of tissue inhibitor of metalloproteinases-3 and clinicopathological variables for 5 years survival in cervical cancer patients
| 5 years survival | ||
|---|---|---|
| Variables | P value | HR & 95% CIb |
| TIMP-3 genetic polymorphisms | ||
| rs9619311 | ||
| TC /CC vs. TTa | 0.741 | 0.72 (0.11-5.00) |
| CC vs. TT & TCa | 0.988 | u.a. |
| rs9862 | ||
| CT/TT vs. CCa | 0.644 | 0.74 (0.21-2.63) |
| TT vs. CC/CT a | 0.774 | 1.28 (0.24-6.98) |
| rs11547635 | ||
| CT/TT vs. CCa | 0.146 | 3.05 (0.68-13.68) |
| TT vs. CC/CTa | 0.406 | 0.43 (0.06-3.18) |
| Clinicopathological characteristics | ||
| Pelvic lymph node | ||
| metastasis vs. no metastasisa | 0.017* | 6.84 (1.41-33.11) |
Statistical analyses: Cox proportional hazard model
*p<0.05
aAs a comparison reference
bHR, hazard ratio and 95% CI, 95% confidence interval for tissue inhibitor of metalloproteinases-3 genetic polymorphisms rs9619311, rs9862 and rs11547635 as well as clinicopathological characteristics, compared to their respective controls.
TIMP-3, tissue inhibitor of metalloproteinases-3; u.a., unavailable.
Discussion
TMIP-3 has its unique binding to the extracellular matrix and is found to inhibit all MMPs, especially MMP-2 and MMP-9 [12, 30, 31]. Decreased TMIP-3 expression has been found in tissues of several types of cancer, as compared to their normal controls [14, 32, 33]. It was also demonstrated to induce apoptosis in cervical cancer [16]. Reduced TMIP-3 expression may be associated with poor outcome, including large tumor size, poor stage and positive metastasis [22, 34, 35]. TIMP-3 genetic variant rs9619311 is at promoter region (cDNA position and nucleotide change, -1296 T/C), rs9862 is at exon region (synonymous SNPs, 249C/T: H83H), and rs11547635 is at exon as well (synonymous SNPs, 261C/T; S87S) [22]. These genetic polymorphisms may influence TIMP-3 expressions and have been demonstrated to be associated with susceptibility and progression of several cancers [20, 21, 36-38]. To date, no study investigates the implications of TIMP-3 genetic polymorphisms in the occurrence of cervical cancer and patient prognosis in Taiwanese women. Therefore, we devised this study to explore the significance of TIMP-3 genetic polymorphisms in the development and progression of cervical cancer.
This study was conducted to associate the genotypic distributions of three TIMP-3 genetic polymorphisms, rs9619311, rs9862 and rs11547635, with uterine cervical carcinogenesis in Taiwanese women. It revealed that there were no significantly different genotypic frequencies in these three TIMP-3 polymorphisms between patients with cervical neoplasias and normal control women. Even after classifying cervical neoplasia patients group into invasive and precancerous subgroups and controlling for age, there were still no statistically different genotypic distributions of these TIMP-3 polymorphisms. TIMP-3 genetic variants, rs9619311, rs9862 and rs11547635, were not found to be related to the susceptibility of cervical cancer. However among these three TIMP-3 variants, Su et al could find that Taiwanese carrying allele T in rs9862 displayed more risk of developing oral squamous cell carcinoma [22]. In addition, Tsai et al. revealed that TIMP-3 rs961033 at promoter region were significantly related to the occurrence of hepatocellular carcinoma among women but not in men [38]. In contrast, genotypic frequencies in TIMP-3 rs9619311 were not associated with the development of bladder cancer [39].
In relating the TIMP-3 genetic variants rs9619311, rs9862 and rs11547635 to the clinicopathological parameters, only women with CT/TT in rs9862 were found to display a higher risk of developing cervical cancer with moderate and poor cell differentiation. Otherwise, no TIMP-3 genotypes in these polymorphisms were demonstrated to be related to the clinicopathological factors. Reduced TIMP-3 expression has been reported to result in large tumor size, advanced stage, and metastasis [22, 34, 35]. Interestingly, Chang et al. revealed that patients carrying T/T in TIMP-3 rs9862 exhibited a higher risk of presenting mutant epidermal growth factor receptor (EGFR), as compared to those with CC, in lung adenocarcinoma. In addition, among male subjects having mutant EGFR, patients carrying allele T in TIMP-3 rs9862 exhibited a higher risk of displaying an advanced stage and positive lymph node metastasis [40].
Because TIMP-3 genetic variants have been reported to be correlated with breast cancer prognosis [41, 42], we further investigated the association of TIMP-3 SNPs with patient survival in cervical cancer. The current study reveals that cervical cancer patients carrying genotypes CC in rs9619311 exhibit a poorer 5 years survival, as compared to those with TT/TC in Taiwanese women, using univariate analysis. But, the low minor allele C frequency, which led to small numbers of homozygote CC women, restricted this conclusion. Other TIMP-3 SNPs were found to be not associated with patient 5 years survival of cervical cancer patients. Bashash et al. found that genetic variants in rs9619311 were not related to patient survival in patients with gastroesophageal junction cancer [36]. In contrast, patients carrying CT/TT displayed a poorer survival in rs9862 even after adjusting for age, disease stage and treatment, as compared to those with CC. However, in multivariate analysis in this current research, it reveals that TIMP-3 polymorphisms rs9619311, rs9862 and rs11547635 are not associated with patient survival in cervical cancer for Taiwanese women after adjusting the clinicopathological variables. Moreover, only pelvic lymph node metastasis was determined to independently predict 5 years survival in cervical cancer patients among these parameters in Taiwan. This finding is reasonable since pelvic lymph node metastasis has been demonstrated to be the most important indicator to predict survival of cervical cancer patients [43].
Although this study is the only research investigating the relationships between TIMP-3 SNPs and cervical cancer, there are several limitations present. First, the study was a hospital-based study and the selection bias may inevitably occur. Second, only central Taiwan subjects were included and residents from other regions were not enrolled. In addition, the studied sample size may not be large enough to draw a statistical significance especially for low subject numbers in precancerous subgroup and low minor allele C frequency in rs9619311, which resulted in small numbers of homozygote CC women, and therefore restricted the external validity of this research. Third, female subjects in the control subgroup were recruited from the outpatient clinic of our hospital for general examination. Routine examination for human papillomavirus (HPV) infection was not popular for studied female individuals even after suggestion by medical staff because of their conservative attitude. The effect of HPV cannot be analyzed. Fourth, since the occurrence ages of cervical precancerous lesions and invasive cancer are inherently different, the age distributions of both diseases are different. Therefore, the logistic regression models were needed to adjust the impact of age. Fifth, we did not collect the tissue samples in the enrolled women, we therefore could not investigate the possible correlation between TIMP-3 polymorphisms and TIMP-3 levels in the women. However, we shall investigate any possible correlation in the future study.
Acknowledgements
This study was supported by Chung Shan Medical University Hospital (CSH-2021-E-001-Y2).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cui N, Hu M, Khalil RA. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1-73
2. Kapoor C, Vaidya S, Wadhwan V, Hitesh, Kaur G, Pathak A. Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther. 2016;12:28-35
3. Laronha H, Caldeira J. Structure and Function of Human Matrix Metalloproteinases. Cells. 2020;9:1076
4. Hua H, Li M, Luo T, Yin Y, Jiang Y. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68:3853-3868
5. Alaseem A, Alhazzani K, Dondapati P, Alobid S, Bishayee A, Rathinavelu A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin Cancer Biol. 2019;56:100-115
6. Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57-83
7. Hsiao YH, Su SC, Lin CW, Chao YH, Yang WE, Yang SF. Pathological and therapeutic aspects of matrix metalloproteinases: implications in childhood leukemia. Cancer Metastasis Rev. 2019;38:829-837
8. Yang JS, Lin CW, Su SC, Yang SF. Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert Opin Drug Metab Toxicol. 2016;12:191-200
9. Su CW, Lin CW, Yang WE, Yang SF. TIMP-3 as a therapeutic target for cancer. Ther Adv Med Oncol. 2019;11:1758835919864247
10. Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter RJ, Yang SF. Cancer metastasis: Mechanisms of inhibition by melatonin. J Pineal Res. 2017;62:12370
11. Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer. 2017;17:38-53
12. English JL, Kassiri Z, Koskivirta I, Atkinson SJ, Di Grappa M, Soloway PD. et al. Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol Chem. 2006;281:10337-10346
13. Anania MC, Sensi M, Radaelli E, Miranda C, Vizioli MG, Pagliardini S. et al. TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene. 2011;30:3011-3023
14. Lin H, Zhang Y, Wang H, Xu D, Meng X, Shao Y. et al. Tissue inhibitor of metalloproteinases-3 transfer suppresses malignant behaviors of colorectal cancer cells. Cancer Gene Ther. 2012;19:845-851
15. Su CW, Chang YC, Chien MH, Hsieh YH, Chen MK, Lin CW. et al. Loss of TIMP3 by promoter methylation of Sp1 binding site promotes oral cancer metastasis. Cell Death Dis. 2019;10:793
16. Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer. 1999;79:1347-1355
17. Hurgobin B, Edwards D. SNP Discovery Using a Pangenome: Has the Single Reference Approach Become Obsolete? Biology (Basel). 2017;6:21
18. Su SC, Hsieh MJ, Lin CW, Chuang CY, Liu YF, Yeh CM. et al. Impact of HOTAIR Gene Polymorphism and Environmental Risk on Oral Cancer. J Dent Res. 2018;97:717-724
19. Yang SF, Lin CW, Chuang CY, Lee YC, Chung WH, Lai HC. et al. Host Genetic Associations with Salivary Microbiome in Oral Cancer. J Dent Res. 2022;101:590-598
20. Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3-22
21. Hua KT, Liu YF, Hsu CL, Cheng TY, Yang CY, Chang JS. et al. 3'UTR polymorphisms of carbonic anhydrase IX determine the miR-34a targeting efficiency and prognosis of hepatocellular carcinoma. Sci Rep. 2017;7:4466
22. Su CW, Huang YW, Chen MK, Su SC, Yang SF, Lin CW. Polymorphisms and Plasma Levels of Tissue Inhibitor of Metalloproteinase-3: Impact on Genetic Susceptibility and Clinical Outcome of Oral Cancer. Medicine (Baltimore). 2015;94:e2092
23. Cubie HA, Campbell C. Cervical cancer screening - The challenges of complete pathways of care in low-income countries: Focus on Malawi. Womens Health (Lond). 2020;16:1745506520914804
24. Hsiao YH, Lin CW, Wang PH, Hsin MC, Yang SF. The Potential of Chinese Herbal Medicines in the Treatment of Cervical Cancer. Integr Cancer Ther. 2019;18:1534735419861693
25. Hsin MC, Hsieh YH, Hsiao YH, Chen PN, Wang PH, Yang SF. Carbonic Anhydrase IX Promotes Human Cervical Cancer Cell Motility by Regulating PFKFB4 Expression. Cancers (Basel). 2021;13:1174
26. Nasiell K, Roger V, Nasiell M. Behavior of mild cervical dysplasia during long-term follow-up. Obstet Gynecol. 1986;67:665-669
27. Martin CM, O'Leary JJ. Histology of cervical intraepithelial neoplasia and the role of biomarkers. Best Pract Res Clin Obstet Gynaecol. 2011;25:605-615
28. Sun YH, Chou YH, Ou CC, Ng SC, Shen HP, Lee YC. et al. Investigation of metastasis-associated in colon cancer-1 genetic variants in the development and clinicopathologcial characteristics of uterine cervical cancer in Taiwanese women. Int J Med Sci. 2020;17:490-497
29. Weng SL, Ng SC, Lee YC, Hsiao YH, Hsu CF, Yang SF. et al. The relationships of genetic polymorphisms of the long noncoding RNA growth arrest-specific transcript 5 with uterine cervical cancer. Int J Med Sci. 2020;17:1187-1195
30. Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44-46:247-254
31. Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuna JM, Perez-Romero BA, Guerrero-Rodriguez JF. et al. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int J Mol Sci. 2020;21:9739
32. Su CW, Su BF, Chiang WL, Yang SF, Chen MK, Lin CW. Plasma levels of the tissue inhibitor matrix metalloproteinase-3 as a potential biomarker in oral cancer progression. Int J Med Sci. 2017;14:37-44
33. Gu X, Fu M, Ding Y, Ni H, Zhang W, Zhu Y. et al. TIMP-3 expression associates with malignant behaviors and predicts favorable survival in HCC. PLoS One. 2014;9:e106161
34. Wu DW, Tsai LH, Chen PM, Lee MC, Wang L, Chen CY. et al. Loss of TIMP-3 promotes tumor invasion via elevated IL-6 production and predicts poor survival and relapse in HPV-infected non-small cell lung cancer. Am J Pathol. 2012;181:1796-1806
35. Yu JL, Lv P, Han J, Zhu X, Hong LL, Zhu WY. et al. Methylated TIMP-3 DNA in body fluids is an independent prognostic factor for gastric cancer. Arch Pathol Lab Med. 2014;138:1466-1473
36. Bashash M, Shah A, Hislop G, Treml M, Bretherick K, Janoo-Gilani R. et al. Genetic polymorphisms at TIMP3 are associated with survival of adenocarcinoma of the gastroesophageal junction. PLoS One. 2013;8:e59157
37. Lei H, Hemminki K, Altieri A, Johansson R, Enquist K, Hallmans G. et al. Promoter polymorphisms in matrix metalloproteinases and their inhibitors: few associations with breast cancer susceptibility and progression. Breast Cancer Res Treat. 2007;103:61-69
38. Tsai HT, Hsieh MJ, Chiou HL, Lee HL, Hsin MC, Liou YS. et al. TIMP-3 -1296 T>C and TIMP-4 -55 T>C gene polymorphisms play a role in the susceptibility of hepatocellular carcinoma among women. Tumour Biol. 2014;35:8999-9007
39. Wieczorek E, Reszka E, Jablonowski Z, Jablonska E, Krol MB, Grzegorczyk A. et al. Genetic polymorphisms in matrix metalloproteinases (MMPs) and tissue inhibitors of MPs (TIMPs), and bladder cancer susceptibility. BJU Int. 2013;112:1207-1214
40. Chang JH, Lai TC, Yang PJ, Shih PC, Yang YC, Lee KL. et al. Associations of TIMP-3 Genetic Polymorphisms with EGFR Statuses and Cancer Clinicopathologic Development in Lung Adenocarcinoma Patients. Int J Mol Sci. 2020;21:8023
41. Beranek M, Kankova K, Muzik J. Identification of novel common polymorphisms in the promoter region of the TIMP-3 gene in Czech population. Mol Cell Probes. 2000;14:265-268
42. Peterson NB, Beeghly-Fadiel A, Gao YT, Long J, Cai Q, Shu XO. et al. Polymorphisms in tissue inhibitors of metalloproteinases-2 and -3 and breast cancer susceptibility and survival. Int J Cancer. 2009;125:844-850
43. Choi KH, Kim JY, Lee DS, Lee YH, Lee SW, Sung S. et al. Clinical impact of boost irradiation to pelvic lymph node in uterine cervical cancer treated with definitive chemoradiotherapy. Medicine (Baltimore). 2018;97:e0517
Author contact
![]() Corresponding author: Po-Hui Wang, MD, PhD, Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo North Road, Taichung, 40201, Taiwan. Tel.: 886-4-24739595 ext. 21721; Fax: 884-4-24738493; E-mail: wang082160com
Corresponding author: Po-Hui Wang, MD, PhD, Institute of Medicine, Chung Shan Medical University, 110, Section 1, Chien-Kuo North Road, Taichung, 40201, Taiwan. Tel.: 886-4-24739595 ext. 21721; Fax: 884-4-24738493; E-mail: wang082160com

 Global reach, higher impact
Global reach, higher impact