3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(6):1003-1012. doi:10.7150/ijms.71971 This issue Cite
Research Paper
Nomograms based on lactate dehydrogenase to albumin ratio for predicting survival in colorectal cancer
1. Department of Ultrasound Imaging, Renmin Hospital of Wuhan University, Wuhan, China; 430061
2. Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; 430022
*Contributed equally to this manuscript.
Received 2022-2-12; Accepted 2022-5-13; Published 2022-5-29
Abstract
Purpose: We aimed to determine if lactate dehydrogenase to albumin ratio (LAR) might play a prognostic role for patients with operable colorectal cancer (CRC).
Patients and Methods: 1334 operable CRC patients in Wuhan Union Hospital Between July 2013 and September 2017 were enrolled in this study and were randomly appointed them into training (n=954) and validation (n=380) sets. The relationship between LAR and overall survival (OS) and disease-free survival (DFS) were determined by restricted cubic splines (RCS) with Cox regression models. LAR was then divided into three categories based on the RCS and compared to the well-known TNM stage system. Finally, survival nomograms were developed by compounding the LAR and other clinical factors.
Results: Baseline LAR values and the all-cause mortality were U shaped, which slowly decreased until around 4.50 and then started to increase rapidly when the LAR ranged from 4.50-6.68 and then became flat thereafter (P for non-linearity <0.001). LAR was superior to TNM stage for OS as well as DFS and LAR plus TNM stage could add more net benefit than clinical model alone. Moreover, the survival nomograms based on LAR achieved great predictive ability for OS and DFS in operable CRC patients.
Conclusions: LAR could be served as a reliable prognostic factor for OS as well as DFS, with more accurate prognostic prediction than current TNM stage for patients with operable CRC.
Keywords: colorectal cancer, lactate dehydrogenase to albumin ratio, prognosis, inflammation, survival nomogram
Introduction
Colorectal cancer (CRC) ranks as one of the most common solid tumors in patients all over the world (1-4). Despite great prognoses in our intelligence on its risk, development and especially in the field of surgical treatment and chemoradiotherapy, CRC remains a frustrating tumor without an optimistic long-term prognosis (5-7). Hence, determination of a great prognostic predictor with easily obtained and relatively good accuracy for a better risk stratification is essential for clinicians.
Lactate dehydrogenase (LDH) is a considerable indicator for liver in clinical practice and has been advocated to play a momentous role in immune condition in several cancers so as to CRC (8-13). Serum albumin is also a readily accostable element for nutrition and proposed to have prognostic importance for different tumor patients (14, 15). Therefore, the combination of them, LDH to serum albumin ratio (LAR), was recommended to be a good prognostic ratio for patients with cancers (16-18). However, limited study investigated the link between LAR and CRC patients (19). Therefore, in this study, we initially assessed the link between LAR and the outcomes of CRC patients. Then, a comparison between LAR and TNM stage system of predictive ability was also made. Finally, the survival nomograms based on LAR were built and verified in this study.
Materials and methods
Study population
We consecutively enrolled a CRC database with 3500 patients at the Wuhan Union Hospital from July 2013 and September 2017, as previously reported (20). In short, CRC patients without infective disease who did not receive anti-inflammatory agents prior to the surgical excision were studied. Patients with missing measurements of serum LDH or albumin before surgery treatments were also excluded. Finally, a total of 1334 CRC patients were studied and were randomly specified to the test (n=954) and verified sets (n=380).
This retrospective study was performed according to the Helsinki Declaration and all patients were asked to offers their informed consents. Moreover, the study was authorized by the Ethics Administration Office of our hospital.
Data collection
Demographic data as well as other clinical information were automatically obtained from this platform. Initial laboratory results prior to the surgery treatment were also obtained. In addition, follow-up visits were implemented every 3 months in the first two years and twice a year in the following third to fifth years.
The primary outcome of this study was overall survival (OS) while the secondary outcome was disease-free survival (DFS).
The LAR was computed by initial serum LDH (U/L) /serum albumin (g/L).
Statistical analyses
SPSS 23.0 and R 3.3.1 software were utilized for all analyses. Continuous variables were presented as means with standard deviations (SD) or interquartile ranges, and as frequencies along with percentages for binary variables. Multivariable Cox proportional hazards regression models were utilized to assess hazard ratios of overall mortality for LAR concentration. Firstly, we utilized restricted cubic spline (RCS) models fitted for Cox models with 5 knots at the 5th, 35th, 50th, 65th, and 95th percentiles of LAR. Through the RCS analysis, the cut-off values of the LAR based on OS were obtained, and then this continuous variable were translate to ternary variables in accordance with the cut-off values. Then, receiver operating characteristic (ROC) analyses, integrated discrimination index (IDI) and net reclassification improvement (NRI) were also exploited to assess the accuracy of LAR and TNM stage for clinical outcomes. Furthermore, LAR and other clinical features were also grouped to develop survival nomograms for OS and DFS. The discrimination and calibration were assessed by time-dependent ROC (td-ROC) curves and calibration curves, respectively. Finally, the decision curve analysis (DCA) was also utilized to assess the clinical benefits of the nomograms for OS and DFS. P < 0.05 at both sides represents that the difference is of statistical significance.
Results
Patient clinical features
A total of 1334 patients with CRC (803 men and 531 women) met the inclusion criterion were finally studied and patients were randomly assigned to the test (n=954) and verified sets (n=380). Furthermore, all variables in the test and verified sets were comparable in this study (Table 1).
Clinicopathological characteristics of all patients
| Characteristics | Training set (n=954) | Validation set (n=380) | P value |
|---|---|---|---|
| Age (years) (mean (SD)) | 58.5±11.9 | 58.2±12.4 | 0.685 |
| Sex, male, n (%) | 557 (58.4) | 246 (64.7) | 0.038 |
| BMI, kg/m2 (mean (SD)) | 22.8±2.9 | 22.7±2.9 | 0.758 |
| Smoking, n (%) | 220 (23.1) | 97 (25.5) | 0.377 |
| Family history of cancer, n (%) | 83 (8.7) | 34 (8.9) | 0.971 |
| Primary site, n (%) | 0.992 | ||
| Left colon | 238 (24.9) | 96 (25.3) | |
| Right colon | 225 (23.6) | 89 (23.4) | |
| Rectum | 491 (51.5) | 195 (51.3) | |
| Histological grade, n (%) | 0.276 | ||
| Well differentiated | 135 (14.2) | 66 (17.4) | |
| Moderately differentiated | 683 (71.6) | 257 (67.6) | |
| Poorly differentiated | 136 (14.3) | 57 (15.0) | |
| Tumor size, n (%) | 0.267 | ||
| <2cm | 53 (5.6) | 14 (3.7) | |
| 2-5cm | 672 (70.4) | 265 (69.7) | |
| ≥5cm | 229 (24.0) | 101 (26.6) | |
| Perineural invasion, n (%) | 0.05 | ||
| Yes | 187 (19.6) | 84 (22.1) | |
| No | 767 (80.4) | 296 (77.9) | |
| T stage, n (%) | 0.478 | ||
| T1 | 69 (7.2) | 21 (5.5) | |
| T2 | 158 (16.6) | 59 (15.5) | |
| T3 | 546 (57.2) | 217 (57.1) | |
| T4 | 181 (19.0) | 83 (21.9) | |
| N stage, n (%) | 0.903 | ||
| N1 | 550 (57.7) | 222 (58.4) | |
| N2 | 254 (26.6) | 102 (26.8) | |
| N3 | 150 (15.7) | 56 (14.7) | |
| TNM stage, n (%) | 0.708 | ||
| Stage I | 168 (17.6) | 60 (15.8) | |
| Stage II | 370 (38.8) | 159 (41.8) | |
| Stage III | 405 (42.5) | 158 (41.6) | |
| Stage IV | 11 (1.2) | 3 (0.8) | |
| Adjuvant chemotherapy, n (%) | 0.698 | ||
| Yes | 490 (51.4) | 190 (50.0) | |
| No | 464 (48.6) | 190 (50.0) | |
| Post radiotherapy, n (%) | 0.059 | ||
| Yes | 39 (4.1) | 24 (6.3) | |
| No | 915 (95.9) | 356 (93.8) | |
| Laboratory results, median (IQR) | |||
| WBC, ×109/L | 6.0 (5.0, 7.0) | 6.0 (5.0, 7.0) | 0.137 |
| HGB, g/dL | 121.5 (104.0, 136.0) | 120.0 (102.8, 134.0) | 0.309 |
| PLT, ×109/L | 221.5 (178.0, 277.0) | 220.0 (172.0, 277.0) | 0.655 |
| Albumin, g/L | 40.0 (36.0, 43.0) | 40.0 (36.8, 43.0) | 0.907 |
| Bilirubin, mmol/L | 11.0 (8.0, 15.0) | 11.0 (8.0, 14.0) | 0.794 |
| ALP, U/L | 74.0 (62.0, 89.0) | 73.0 (60.0, 85.0) | 0.121 |
| LDH, U/L | 184.0 (157.0, 195.0) | 182.0 (157.3, 191.0) | 0.894 |
| LAR, U/g | 4.5 (3.9, 5.2) | 4.5 (3.9, 5.2) | 0.830 |
| Creatinine, umol/L | 69.0 (59.0, 80.8) | 70.0 (61.0, 82.0) | 0.207 |
| Urea nitrogen, mmol/L | 5.0 (4.0, 6.0) | 5.0 (4.0, 6.0) | 0.374 |
| CEA, ng/mL | 4.0 (2.0, 8.0) | 4.0 (2.0, 9.0) | 0.834 |
| CA125, U/mL | 12.0 (8.0, 18.0) | 12.0 (8.0, 18.0) | 0.983 |
| CA199, U/mL | 8.5 (4.0, 22.8) | 8.0 (3.0, 20.0) | 0.262 |
| Overall survival months | 21.9 (14.0, 33.4) | 21.9 (13.7, 32.0) | 0.433 |
| Disease-free survival months | 21.2 (13.4, 32.9) | 21.3 (13.4, 31.7) | 0.482 |
| Death, n (%) | 114 (11.9) | 42 (11.1) | 0.715 |
| Recurrence, n (%) | 119 (12.5) | 46 (12.1) | 0.926 |
BMI, body mass index, IQR, interquartile range, WBC, white blood count, HGB, hemoglobin, PLT, platelet, ALP, alkaline phosphatase, LDH, lactate dehydrogenase, LAR, lactate dehydrogenase to albumin ratio, CEA, carcino-embryonic antigen, CA125, carbohydrate antigen 125.
LAR and the risk of death and recurrence
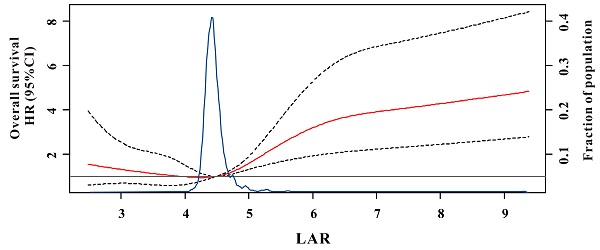
During the median of 21.9 months (ranges 0.2-79.0 months) for OS and 21.2 months (ranges 0.2-75.0 months) for DFS follow-up, we found 114 (11.9%) deaths and 119 (12.5%) recurrences in the test set. RCS showed a U-curved link between LAR and OS after adjusted confounding indexes (Figure 1). In Figure 2, the risk of all-cause mortality was slowly decreasing until around 4.50 of predicted OS and then started to increase rapidly when the LAR was ranged from 4.50-6.68 and then became flat thereafter (P for non-linearity <0.001). Moreover, a similar result had also been conducted for the relationship between LAR and DFS in the test cohort (Supplemental Figure 1). Therefore, according to the results of RCS, we classified CRC patients into three categories based on the values of LAR: low-risk (<4.5), intermediate (4.5-6.68) and high-risk group (>6.68).
The comparison of LAR and TNM staging system
Compared with patients in the low-risk group, patients with CRC in the high-risk or intermediate groups revealed worse OS and DFS in the training cohort (Figure 2A and E) so as to in the verified set (Figure 2C and G). Similarly, TNM system showed significantly different survival probability between stages in the training set (Figure 2B and F) and in the verified cohort (Figure 2D and H). Moreover, the accuracy of LAR for OS was 0.708 and 0.714, respectively. Similarly, LAR also obtained relatively good capability for DFS (AUC=0.706, 0.712, respectively, Table 2). Meanwhile, the AUCs of the TNM stage system for OS were 0.642 and 0.653, respectively, while for DFS, AUCs were 0.644 and 0.676, respectively. Furthermore, as described in Table 2, the addition of LAR significantly improved the risk reclassification (as measured using NRI and IDI) of OS as well as DFS compared to the TNM stage system. Therefore, LAR demonstrated better predicting performance of the OS and DFS than TNM stage system in patients with CRC.
Multivariable adjusted hazard ratios (HR) for overall survival according to levels of lactate dehydrogenase to albumin ratio (LAR) on a continuous scale. Solid red lines are multivariable adjusted HR, with dashed black lines showing 95% confidence intervals derived from restricted cubic spline regressions with three knots. Reference lines for no association are indicated by the solid gray lines at a hazard ratio of 1.0. Dashed blue curves show the fraction of the population with different levels of LAR.
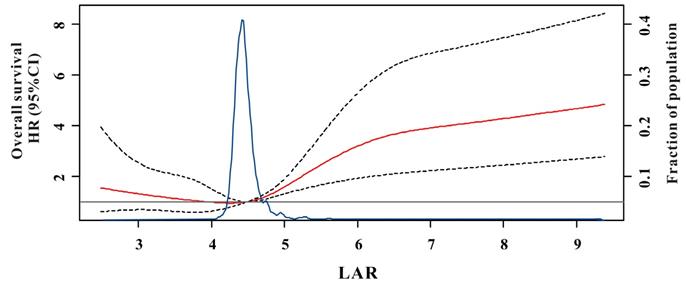
Kaplan-Meier survival curves for overall survival (OS) (A-D) and disease-free survival (DFS) (E-H) of the test cohort and the verified cohort in different models. LAR is divided into high-risk group, intermediate, and low-risk group in the training cohort (A and C) and in the validation cohort (E and G); TNM staging system is divided into stage I, stage II and stage III/IV in the training cohort (B and D) and in the validation cohort (F and H).
NRI and IDI analyses for risk reclassification of overall survival and disease-free survival
| Outcome | AUC | IDI | NRIa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensibility (%) | Specificity (%) | Biomarker | Biomarker+ clinical model | clinical modelb | Value (95%CI) | P Value | Value (95%CI) | P Value | |
| In training set | |||||||||
| For OS | |||||||||
| LAR | 60.4 | 79.6 | 0.708 | 0.807 | 0.797 | 0.013 (0.002-0.026) | 0.039 | 0.100 (0.030-0.170) | 0.005 |
| TNM stage | 65.8 | 59.4 | 0.642 | 0.799 | 0.003 (-0.002-0.008) | 0.232 | 0.056 (0.002-0.111) | 0.042 | |
| LAR+TNM | 60.3 | 80.8 | 0.728 | 0.810 | 0.017 (0.003-0.031) | 0.020 | 0.112 (0.038-0.185) | 0.003 | |
| For DFS | |||||||||
| LAR | 58.9 | 75.5 | 0.706 | 0.776 | 0.784 | 0.017 (0.003-0.032) | 0.020 | 0.109 (0.031-0.188) | 0.006 |
| TNM stage | 63.0 | 59.2 | 0.644 | 0.762 | 0.009 (0.001-0.017) | 0.027 | 0.095 (0.021-0.169) | 0.012 | |
| LAR+TNM | 59.6 | 82.3 | 0.734 | 0.795 | 0.027 (0.011-0.044) | <0.001 | 0.163 (0.080-0.247) | <0.001 | |
| In validation set | |||||||||
| For OS | |||||||||
| LAR | 57.1 | 80.6 | 0.714 | 0.826 | 0.812 | 0.011 (0.004-0.017) | 0.031 | 0.103 (0.040-0.305) | 0.011 |
| TNM stage | 63.3 | 62.7 | 0.653 | 0.815 | -0.001 (-0.017-0.014) | 0.862 | -0.006 (-0.018-0.006) | 0.317 | |
| LAR+TNM | 64.3 | 84.7 | 0.734 | 0.833 | 0.016 (0.005-0.037) | 0.024 | 0.164 (0.032-0.296) | 0.015 | |
| For DFS | |||||||||
| LAR | 56.4 | 79.7 | 0.712 | 0.813 | 0.794 | 0.019 (0.006-0.047 | 0.009 | 0.118 (0.052-0.265) | 0.004 |
| TNM stage | 66.4 | 67.9 | 0.676 | 0.803 | 0.008 (-0.014-0.030) | 0.464 | -0.011 (-0.109-0.087) | 0.822 | |
| LAR+TNM | 67.0 | 86.4 | 0.746 | 0.821 | 0.033 (0.007-0.059) | 0.005 | 0.173 (0.048-0.338) | 0.009 | |
AUC, area under the receiver-operating characteristic curve, IDI, integrated discrimination improvement, NRI, Net reclassification index, OS, overall survival, LAR, lactate dehydrogenase to albumin ratio, DFS, disease-free survival.
aThe NRI is calculated through two-way category by using the event rate of overall survival and disease-free survival.bThe clinical model for predicting overall survival and disease-free survival are composed of age, gender, BMI, smoking, family history of cancer, primary site, tumor size, grade, T stage, N stage, M stage, perineural invasion, chemotherapy, radiotherapy, and laboratory results except for LAR and albumin.
Construction and validation of survival nomograms
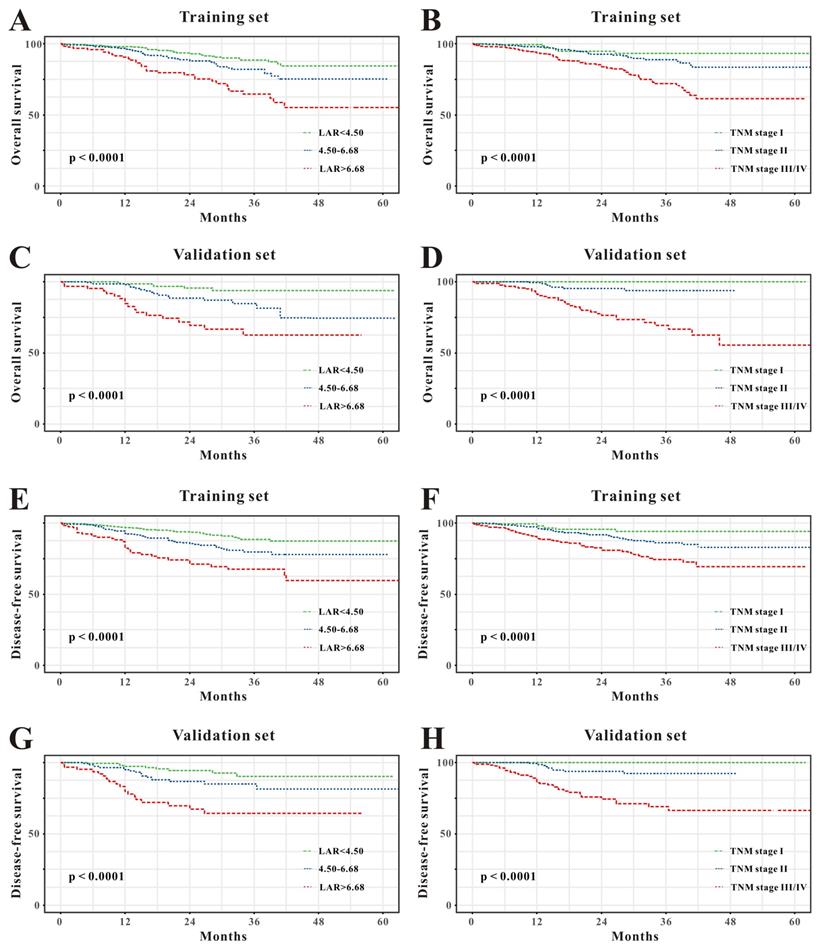
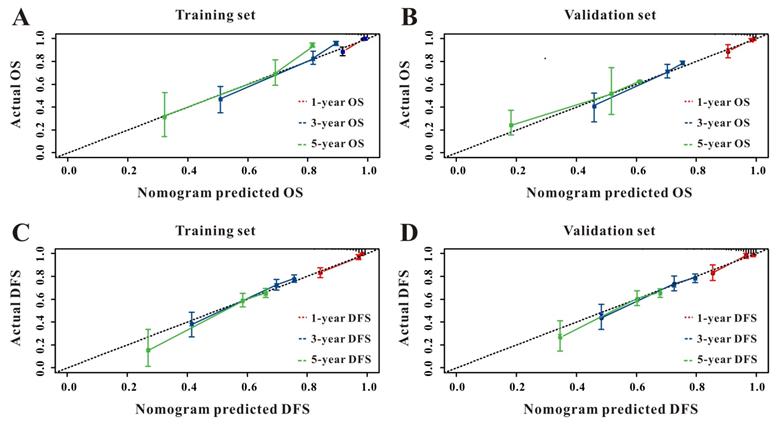
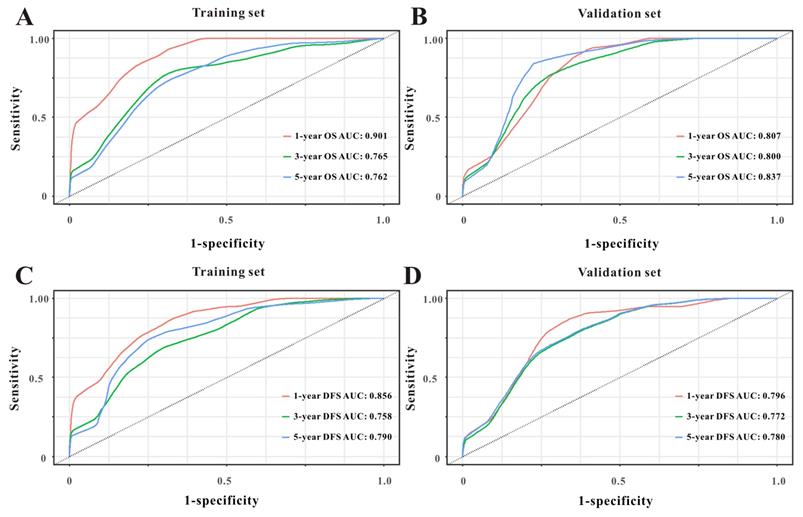
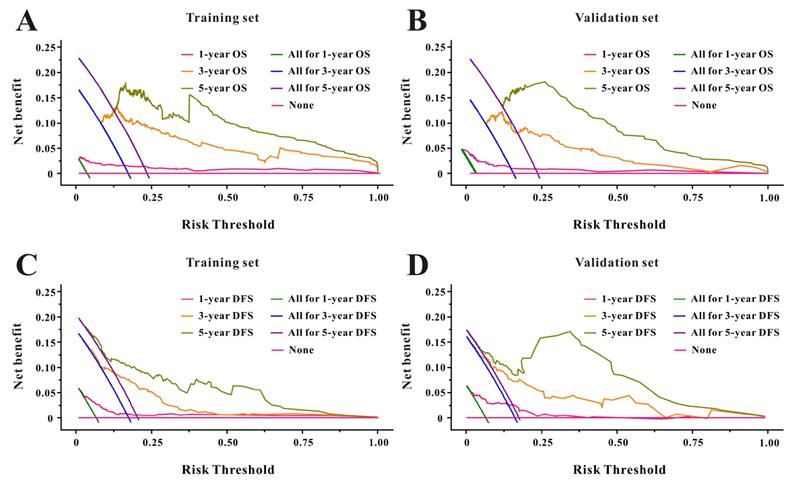
Based on the multivariate Cox results for OS (Supplemental Table 1), seven variables were finally included in the OS nomogram: age, LAR, T stage, perineural invasion, CEA, CA125 and chemotherapy (Figure 3A). The calibration curves (Figure 4A-B) revealed that the prognostic nomogram possessed responsible reproducibility. What's more, td-ROC analyses were also utilized to assess the fatidic value for OS and DFS. In the evaluation of the 1-year, 3-year and 5-year survival rates, the predictive power measured by AUCs were 0.901, 0.765 and 0.762 in the test set (Figure 5A), and 0.807, 0.800 and 0.837 in the verified set (Figure 5B), respectively. According to the DCA, when the threshold probability for a patient was within the range of 0-100%, the nomogram added more net benefit than the “treat all” or “treat none” strategies both in the test cohort and in the verified cohort for 1-, 3-, 5-year OS (Figure 6A-B), which indicated that the prognostic nomogram could be clinical usefulness for OS with CRC patients.
As for DFS (Supplemental Table 2), six informative variables (age, LAR, TNM stage, perineural invasion, CEA, total bilirubin) were eventually incorporated into the establishment of the DFS nomogram (Figure 3B). First, the calibration curves (Figure 4C-D) indicating that the prognostic nomogram for DFS possessed reliable repeatability. Then, in the assessment of the 1-year, 3-year and 5-year survival rates, the predictive power of the survival nomogram as measured by AUCs were 0.856, 0.758 and 0.790 in the test set (Figure 5C), and 0.796, 0.772 and 0.780 in the verified set (Figure 5D), respectively. Finally, the nomogram added more net benefit than the “treat all” or “treat none” strategies both in the test cohort and in the verified cohort for DFS (Figure 6C-D) according to DCA curves, which indicated that the survival nomogram could be clinical usefulness for DFS for CRC patients.
Discussion
In this retrospective study of 1334 individuals, we demonstrated a U-shaped relationship between LAR and all-cause mortality and recurrence in CRC patients who received surgical excision treatment. Based on the results of RCS, LAR values were converted into ternary variables and compared with the current TNM stage system, LAR was superior to TNM stage for predicting OS and DFS and LAR plus TNM stage could added more net benefit for OS and DFS than clinical model alone. Moreover, the survival nomograms incorporating LAR and other clinical features reached the much higher predictive value in predicting OS and DFS in CRC patients than any other single factor. Therefore, our study concluded that the LAR could serve as a reliable prognostic marker for OS and DFS in patients with operable CRC.
Despite recent advancement in the diagnosis and treatment of them, CRC remains to be the second most common reason for cancer death. Even with radical resection as well as neoadjuvant chemoradiotherapy, the rates of 5-year OS for CRC patients remain pessimistic, with approximately 70% for stage II and 60% for stage III (21). Hence, it's important for clinicians to determine CRC patients at high risk of mortality with easily accessible and cost-effective biomarker.
It's well known that inflammation plays a significant role in the initial, development and prognosis of cancer patients and thus inflammation-based methods could be applied to assess the prognosis of cancer patients (22, 23). Though hematoxylin- eosin (H&E)-stained slices based on the morphology characteristics of inflammatory cells is reliable to evaluate the cancer-associated inflammation, it is inconvenient and invasive (24). Hence, most clinicians had concentrated on the link between inflammatory indexes and prognosis of CRC patients (25-27). Among them, LAR might be one of the most relevant biomarkers for CRC patients.
Evaluation of overall survival (A) and disease-free survival (B) associated nomograms for operable patients with CRC.
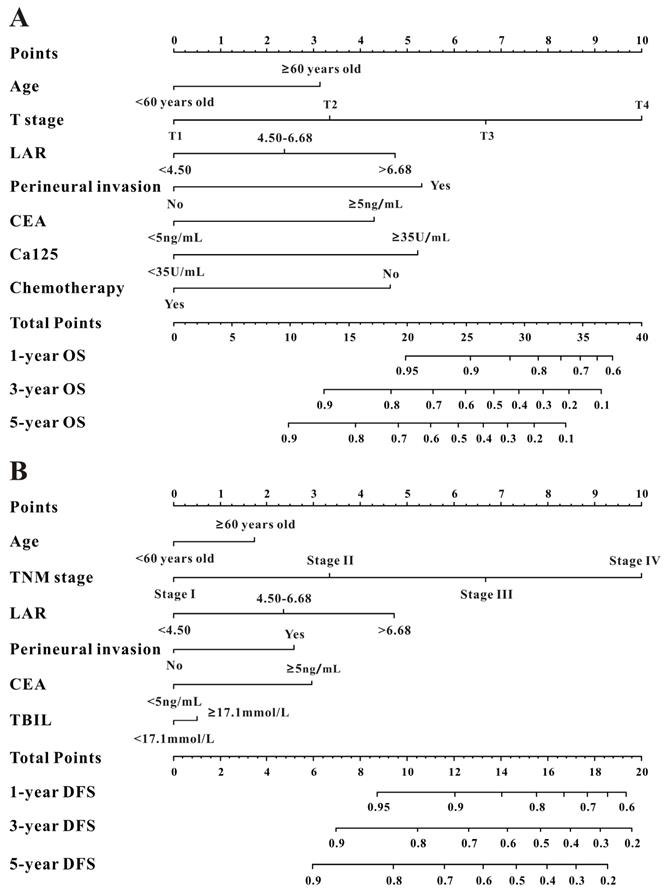
The calibration curves for predicting OS in CRC patients at 1-, 3-, and 5-year in the test set (A) and at 1-, 3-, and 5-year in the verified set (B). The calibration curves for predicting DFS in CRC patients at 1-, 3-, and 5-year in the test set (C) and at 1-, 3-, and 5-year in the verified set (D).
Time-dependent ROC curves from the nomograms for the prediction of OS and DFS in the test (A, C) and verified (B, D) sets, respectively.
Decision curve analysis of LAR for overall survival and disease-free survival in CRC patients to detect its clinical usefulness in the training set (A, C), and in the validation set (B, D), respectively.
The link between LAR and clinical outcomes of cancer patients has been conducted in several studies (16-18). Peng et al. revealed that the LAR played an important role for poor OS (HR=1.60, 95%CI 1.23-2.10, P=0.001) and progression-free survival (HR=1.42, 95%CI 1.10-1.85, P=0.008) in a retrospective study of 1661 patients with nasopharyngeal carcinoma when the cutoff value was reached at 4.04 (28). Feng et al. divided LAR into two categories based on X-tile and concluded that LAR is a helpful potential prognostic biomarker for surgical esophageal squamous cell carcinoma patients with the optimal cut-off value was 5.5 (29). Moreover, a recent retrospective study with 295 CRC patients undergoing curative resection revealed that higher LAR (≥ 52.7) was importantly combined with worse OS and DFS (19). Similarly, in this study, we firstly found a U shaped relationship between baseline LAR and prognosis of CRC patients, in addition, we divided the LAR into three categories and compared with the currently TNM system, LAR was superior to TNM stage for predicting OS and DFS and LAR plus TNM stage could added more net benefit for OS and DFS than clinical model alone.
LAR is the ratio of LDH concentration and serum albumin value. LDH had also been proposed to reflect tumor growth, invasive potential and immune suppression (30, 31) and had been demonstrated to have interpersonal link with prognosis in several cancers especially gastrointestinal tract cancers (32-34). In cancer patients, low level of serum albumin means the status of malnutrition, which may lead to decreased synthesis and lateral leakage and thus serum albumin had also been proposed to have interpersonal link with the prognosis of several malignant tumors (35, 36). LDH and serum albumin are clinical availability and the combination of them, LAR, is not only a marker of inflammation but also reflect nutritional condition, however, limited data is accessible for the combination of LDH and serum albumin for the prognosis of patients with CRC. In the current study, we firstly found an non-linear relationship between LAR levels and the prognosis of CRC patients undergoing curative resection. Moreover, when we combined LAR with other significant variables to create survival nomograms, we found that the survival nomograms obtained great predictive ability in the prediction of OS or DFS, implying that LAR may act as a new prognostic factor for patients with CRC.
There are some limitations in this study. Firstly, this was a retrospective study carried out in a single center and lacks external verification. Secondly, we only collected the initial levels of LAR prior to surgical treatment and dynamic monitoring of LAR during hospital stay may be more precise in this way. Finally, we did not conduct the gene profiling related to inflammatory pathways considering the lack of related medical records. Hence, large scale prospective cohort studies are needed in more patients with different cancer species to determine the broader independent predictive effects of LAR and to external verify our survival nomograms.
Conclusions
Our study firstly found the non-linear relationship between LAR and the prognosis of CRC patients and demonstrated that LAR seems to be a promising marker of survival outcomes in operable CRC patients. LAR outperforms the well-known TNM stage system and LAR plus TNM stage could added more net benefit for OS and DFS than clinical model alone. Moreover, nomograms based on LAR for predicting OS and DFS in CRC may serve as a clinically personalized tool to provide reliable prognostic information for the greatest survival benefits for CRC patients through layered management.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This study was supported by the Nature and Science Foundation of China (No. 81971624) and Wuhan Science and Technology Bureau Application Foundation Frontier (2019020701011477).
Ethics statement
This study protocol was reviewed and successfully approved prior to the initiation of the research by the Ethics Administration Office (No. 2018-S377).
Author contributions
YH and YZ drafted this manuscript. YH, YC, HW, YY, RJ, and QG performed the data analysis. YH and QZ conceived of the study and participated in its design and coordination. YH and QZ contributed to the interpretation. All authors discussed the results and commented on the manuscript. All authors contributed to the article and approved the submitted version.
Data availability statement
All data in our study are available from the corresponding author upon reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Goding SA, Fedewa SA, Butterly LF, Anderson JC. et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-64
2. Keum N, Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-32
3. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK. et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179-85
4. Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ. et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820-6
5. Araghi M, Soerjomataram I, Jenkins M, Brierley J, Morris E, Bray F. et al. Global trends in colorectal cancer mortality: Projections to the year 2035. Int J Cancer. 2019;144:2992-3000
6. Gini A, Jansen E, Zielonke N, Meester R, Senore C, Anttila A. et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: A systematic review. Eur J Cancer. 2020;127:224-35
7. Cao F, Li F, Shi L, Zhang L, Ma T, Zhang G. Mortality trends of colorectal cancer among overweight patients at the global and national levels. Int J Colorectal Dis. 2019;34:1689-95
8. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19:353-63
9. Zhang X, Guo M, Fan J, Lv Z, Huang Q, Han J. et al. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark. 2016;16:415-23
10. de Jong C, Deneer V, Kelder JC, Ruven H, Egberts T, Herder G. Association between serum biomarkers CEA and LDH and response in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Thorac Cancer. 2020;11:1790-800
11. Philipp AB, Nagel D, Stieber P, Lamerz R, Thalhammer I, Herbst A. et al. Circulating cell-free methylated DNA and lactate dehydrogenase release in colorectal cancer. Bmc Cancer. 2014;14:245
12. Wang N, Liu F, Xi W, Jiang J, Xu Y, Guan B. et al. Development and validation of risk and prognostic nomograms for bone metastases in Chinese advanced colorectal cancer patients. Ann Transl Med. 2021;9:875
13. Bar J, Spencer S, Morgan S, Brooks L, Cunningham D, Robertson J. et al. Correlation of lactate dehydrogenase isoenzyme profile with outcome in patients with advanced colorectal cancer treated with chemotherapy and bevacizumab or cediranib: Retrospective analysis of the HORIZON I study. Clin Colorectal Cancer. 2014;13:46-53
14. Xie H, Wei L, Tang S, Gan J. Prognostic value of pretreatment Albumin-to-Alkaline phosphatase ratio in cancer: A Meta-Analysis. Biomed Res Int. 2020 6661097
15. Xu Y, Liang F, Chen Y, Wang Z, Zhong H, Tang W. Novel model to predict the prognosis of patients with stage II-III colon cancer. Biomed Res Int. 2020 8812974
16. Gao S, Wu M, Chen Y, Lou W, Zhou G, Li J. et al. Lactic dehydrogenase to albumin ratio in prediction of unresectable pancreatic cancer with intervention chemotherapy. Future Oncol. 2018;14:1377-86
17. Aday U, Tatli F, Akpulat FV, Inan M, Kafadar MT, Bilge H. et al. Prognostic significance of pretreatment serum lactate dehydrogenase-to-albumin ratio in gastric cancer. Contemp Oncol (Pozn). 2020;24:145-9
18. Nakazawa N, Sohda M, Yamaguchi A, Watanabe T, Saito H, Ubukata Y. et al. An elevated serum lactate dehydrogenase-to-albumin ratio is a useful poor prognostic predictor of nivolumab in patients with gastric cancer. Anticancer Res. 2021;41:3925-31
19. Aday U, Boyuk A, Akkoc H. The prognostic significance of serum lactate dehydrogenase-to-albumin ratio in colorectal cancer. Ann Surg Treat Res. 2020;99:161-70
20. Cao Y, Deng S, Yan L, Gu J, Mao F, Xue Y. et al. An oxidative stress Index-Based score for prognostic prediction in colorectal cancer patients undergoing surgery. Oxid Med Cell Longev. 2021 6693707
21. Hari DM, Leung AM, Lee JH, Sim MS, Vuong B, Chiu CG. et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: Do the complex modifications improve prognostic assessment? J Am Coll Surg. 2013;217:181-90
22. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-503
23. Lucas C, Barnich N, Nguyen H. Microbiota, inflammation and colorectal cancer. Int J Mol Sci. 2017 18
24. van Verschuer VM, Hooning MJ, van Baare-Georgieva RD, Hollestelle A, Timmermans AM, Koppert LB. et al. Tumor-associated inflammation as a potential prognostic tool in BRCA1/2-associated breast cancer. Hum Pathol. 2015;46:182-90
25. Deng Q, Geng Y, Zhao L, Li R, Zhang Z, Li K. et al. NLRP3 inflammasomes in macrophages drive colorectal cancer metastasis to the liver. Cancer Lett. 2019;442:21-30
26. Ratjen I, Shivappa N, Schafmayer C, Burmeister G, Nothlings U, Hampe J. et al. Association between the dietary inflammatory index and all-cause mortality in colorectal cancer long-term survivors. Int J Cancer. 2019;144:1292-301
27. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ. et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-72
28. Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram based on lactate Dehydrogenase-to-Albumin ratio (LAR) and Platelet-to-Lymphocyte ratio (PLR) for predicting survival in nasopharyngeal carcinoma. J Inflamm Res. 2021;14:4019-33
29. Feng JF, Wang L, Yang X, Jiang YH. Prognostic value of lactate dehydrogenase to albumin ratio (LAR) in patients with resectable esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:7243-51
30. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19:353-63
31. de la Cruz-Lopez KG, Castro-Munoz LJ, Reyes-Hernandez DO, Garcia-Carranca A, Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143
32. Wei Y, Xu H, Dai J, Peng J, Wang W, Xia L. et al. Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. Biomed Res Int. 2018 1804086
33. Aday U, Tatli F, Akpulat FV, Inan M, Kafadar MT, Bilge H. et al. Prognostic significance of pretreatment serum lactate dehydrogenase-to-albumin ratio in gastric cancer. Contemp Oncol (Pozn). 2020;24:145-9
34. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D. et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced Non-Small cell lung cancer. Jama Oncol. 2018;4:351-7
35. Ikeda S, Yoshioka H, Ikeo S, Morita M, Sone N, Niwa T. et al. Serum albumin level as a potential marker for deciding chemotherapy or best supportive care in elderly, advanced non-small cell lung cancer patients with poor performance status. Bmc Cancer. 2017;17:797
36. Ni XF, Wu J, Ji M, Shao YJ, Xu B, Jiang JT. et al. Effect of C-reactive protein/albumin ratio on prognosis in advanced non-small-cell lung cancer. Asia Pac J Clin Oncol. 2018;14:402-9
Author contact
![]() Corresponding author: Qing Zhou, Department of Ultrasound Imaging, Renmin Hospital of Wuhan University, 99 Ziyang Road, Wuhan, China, 430060, Email: qingzhou128com, Tel: +86027-88041911
Corresponding author: Qing Zhou, Department of Ultrasound Imaging, Renmin Hospital of Wuhan University, 99 Ziyang Road, Wuhan, China, 430060, Email: qingzhou128com, Tel: +86027-88041911

 Global reach, higher impact
Global reach, higher impact