3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(5):932-940. doi:10.7150/ijms.72497 This issue Cite
Research Paper
HAT2CH2 score performance predicting neurologic events after cardiac implantable electronic device
Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Received 2022-3-1; Accepted 2022-5-17; Published 2022-5-21
Abstract
Objectives: The HAT2CH2 score has been evaluated for predicting new-onset atrial fibrillation in several clinical conditions, but never for adverse neurologic events. We aimed to evaluate the effectiveness of HAT2CH2 score in predicting neurologic events in patients with cardiac implantable electronic device (CIED), comparing with atrial high-rate episodes (AHRE).
Methods: This case-control study enrolled 314 consecutive patients aged 18 years or older with CIED implantation between January 2015 and April 2021. Patient data were analyzed retrospectively. The primary endpoint was subsequent neurologic events (NE) after implantation. AHRE was defined as > 175 bpm (Medtronic®) lasting ≥ 30 seconds. Variables associated with independent risk of NE were identified using multivariate Cox regression analysis with time-dependent covariates.
Results: Patients' median age was 73 years and 61.8% of them were male. During follow-up (median 32 months), 18 NE occurred (incidence rate 2.15/100 patient-years, 95% CI 1.32-4.30). Multiple Cox regression analysis showed that the HAT2CH2 score (HR 2.424, 95% CI 1.683 - 3.492, p < 0.001) was an independent predictor for NE. Optimal HAT2CH2 score cutoff value was 3 with highest Youden index (AUC, 0.923; 95% CI, 0.881-0.966; p < 0.001). Both AHRE ≥ 1 minute and HAT2CH2 score ≥ 3 had the highest AUC of the receiver-operating characteristic (0.898, 95% CI, 0.831-0.965, p < 0.001). Significant increase was observed in NE occurrence rates using the HAT2CH2 score (p < 0.001).
Conclusion: The HAT2CH2 score and episodes of AHRE lasting ≥ 1 minute are independent risk factors for NE in patients with CIED.
Keywords: Atrial high-rate episodes, cardiac implantable electronic device, HAT2CH2 score, neurologic events
Introduction
The HAT2CH2 score, which comprises hypertension <1 point>, age >75 years <1 point>, stroke or transient ischemic attack <2 points>, chronic obstructive pulmonary disease [COPD] <1 point>, and heart failure <2 points>, was first developed in 2010 to identify patients likely to progress to sustained forms of atrial fibrillation (AF) in the near future [1]. Since then, numerous studies have been conducted to examine the prediction of AF in several clinical conditions, including post ablation for atrial flutter [2], cancer patients [3], after coronary bypass surgery [4], patients receiving electric cardioversion [5], and emergency-department patients [6]. Recently, the C2HEST score [7] and mCHEST score [8] were also evaluated for predicting new AF, which revealed acceptable discriminating power. However, the predictive performance of the HAT2CH2 score, C2HEST score and mCHEST score for new-onset atrial fibrillation and neurologic events (NE) in patients with cardiac implantable electronic device (CIED) was rarely evaluated.
The latest European Society of Cardiology (ESC) guidelines regarding non-valvular AF [9] state that AHRE > 5-6 minutes and > 180 bpm detected by CIED increase the risk for NE and clearly recommend that AHRE should be closely monitored and treated. The CHA2DS2-VASc score is also recommended for risk stratification of NE [10]. However, the utility of CHA2DS2-VASc in non-valvular AF patients is debated and controversial, primarily because it is a vascular scoring system, which does not incorporate AF-related parameters [10]. Meta-analysis showed that the discriminative power of the CHA2DS2-VASc score was only modest in predicting NE, and results were similar in the presence or absence of non-valvular AF [10]. HASBLED score is recommended to use as a bleeding risk score [9], however, never to be used to predict NE. Also, the performance of several scoring systems (CHA2DS2-VASc score, HAT2CH2 score, C2HEST score and mCHEST score) and the optimal cutoff for AHRE duration to predict subsequent NE in patients with CIED have not been well studied previously. Therefore, more accurate NE prediction models are still needed for the non-valvular AF population and patients with CIED.
The present study aimed to investigate the performance of the HAT2CH2 score in predicting NE compared to AHRE in minutes and other scoring systems (CHA2DS2-VASc score, HASBLED score, C2HEST score and mCHEST score) in patients with CIED and without a history of AF.
Methods
Consecutive patients aged 18 years or older who underwent CIED implantation (Medtronic®: dual chamber pacemaker, dual chamber implantable cardioverter defibrillator, cardiac resynchronization therapy-pacing, and cardiac resynchronization therapy-defibrillator) in the Cardiology Department of National Cheng Kung University Hospital from January 2015 to April 2021 were included. All patient data were retrieved from hospital records and were analyzed retrospectively.
Ethical considerations
The protocol for this cohort study was reviewed and approved by the ethics committee of National Cheng Kung University Hospital and was conducted according to guidelines of the International Conference on Harmonization for Good Clinical Practice (B-ER-108-278). All included patients provided signed informed consent at the time of their implantation procedures for data to be recorded for later publication in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Patient and Public Involvement statement
Patients and the public did not involve in the design, or conduct, or reporting, or dissemination plans of the research.
Data collection and definitions
Patients' medical history and data of co-morbidities and echocardiographic parameters were collected from chart records for retrospective evaluation during the implantation date and as the baseline data. Diabetes mellitus was defined by the presence of symptoms and casual plasma glucose concentration ≥ 200 mg/dL, fasting plasma glucose concentration ≥ 126 mg/dL, 2-hour plasma glucose concentration ≥ 200 mg/dL from a 75-g oral glucose tolerance test, or taking antidiabetic medication. Hypertension was defined as in-office systolic blood pressure values ≥ 140 mmHg and/or diastolic blood pressure values ≥ 90 mmHg or taking antihypertensive medication. Dyslipidemia was defined as low-density lipoprotein ≥ 140 mg/dL, high-density lipoprotein < 40 mg/dL, triglycerides ≥ 150 mg/dL, or taking medication for dyslipidemia. Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/ min / 1.73 m2 for at least 3 months.
AHRE were extracted from the devices via telemetry performed at each office visit (3~6 months). AHRE electrograms were reviewed by at least one experienced electrophysiologist, who carefully considered the possibility that AHRE included lead noise or artifacts, far-field R-waves, paroxysmal supraventricular tachycardia and visually confirmed AF in the detected AHRE. Atrial sensitivity was programmed to 0.3 mV with bipolar sensing of Medtronic devices. AHRE was defined as heart rate >175 bpm and at least 30 seconds of atrial tachyarrhythmia recorded by the devices on any day during the study period. If patients had multiple AHRE, the longest AHRE duration was recorded. The duration between data extraction and device implantation was similar in every subject. No consistent increase of AHRE in different extraction after device implantation was found.
The primary endpoint was the occurrence of NE after the date of CIED implantation, including stroke or TIA diagnosed by experienced neurologists. For each outcome, only the first event of that outcome in a specific subject was included. For the composite outcome, only the first event in a given patient was included.
Statistical analysis
Categorical variables are presented as percentages and continuous variables as means and standard deviations (mean ± SD) for normally distributed values or medians and interquartile interval (IQI) for non-normally distributed values. The normal distribution for continuous variables was assessed using the Kolmogorov-Smirnov method. Pearson's chi-square test or Fisher's exact test was used to determine differences in baseline characteristics for categorical variables, and a two-sample student's t-test or Mann-Whitney U-test was used to analyze continuous variables. Survival was estimated by the Kaplan-Meier method, and differences in survival were evaluated with the log-rank test. Multivariable Cox regression analysis was used to identify variables associated with NE occurrence, reported as hazard ratios (HR) with 95% confidence intervals (CI). If the p value for any factor in univariable analysis was < 0.05, the parameter was entered into multivariable analysis. Because CHA2DS2-VASc scores, HASBLED scores, and HAT2CH2 scores overlapped many factors in univariate analysis, they were used as independent factors in multivariable Cox regression analysis. We assured that the proportional hazard equation was met in each variables using log minus log plot, which each curve did not meet during the observation periods. Indicators of CHA2DS2-VASc scores, HASBLED scores, and HAT2CH2 scores were used separately as time-dependent covariates in Cox proportional hazards regression, to compute hazard ratios (HR) and adjusted HR in multivariable models including clinical variables, such as prior stroke, diabetes, hyperlipidemia, and AHRE ≥ 1 minute. We used the linear regression analysis to address the multicollinearity. A tolerance of less than 0.20 or 0.10 and/or a VIF (variance inflation factor) of 5 or 10 and above indicates a multicollinearity problem. We assured that each variable selection had no multicollinearity problem. The receiver-operating characteristic (ROC) area under the curve (AUC) for AHRE and the HAT2CH2 score and the associated 95% confidence intervals (CI) were evaluated for associations with future NE after CIED implantation. The optimal cutoff values with the highest Youden index were chosen based on the results of ROC curve analysis and used to evaluate the associated values of AHRE in minutes and HAT2CH2 score for determining NE. For all comparisons, p < 0.05 was considered statistically significant. All data were analyzed using SPSS statistical package version 23.0 (SPSS Inc. Chicago, IL, USA).
Results
Between January 1, 2014 and April, 2021, a total of 453 consecutive patients receiving Medtronic CIED transplantation at National Cheng Kung University Hospital were recruited initially. Patients with previous AF (n=139) were excluded. The final analysis included data of 314 patients, of whom 18 had experienced NE.
The median follow-up period was 32 months after implantation of CIEDs. Table 1 shows patients' baseline demographic and clinical characteristics based on the occurrence of NE or not. Patients' median age was 73 years and 61.8% of patients were men. Types of CIEDs included dual chamber pacemaker (220, 70.1%), dual chamber ICD (66, 21.0%), CRTP (23, 7.3%) and CRTD (5, 1.6%). The most common indication for CIED implantation was sick sinus syndrome (44.9%), followed by atrioventricular block (25.2%) and ventricular tachyarrhythmia (29.9%) (Table 1). Overall atrial pacing median percentages (25.0%) and ventricular pacing median percentages (1.9%) were noted. High percentages of hypertension (80.6%), hyperlipidemia (76.8%), and diabetes (45.2%) suggest relatively high risk of NE for the entire study cohort. Components of NE, incidence rates, and 95% CI are reported in Table 2. Overall, the total number of NE was 18 (incidence rate (IR) 2.15/100 patient-years, 95% CI 1.32-4.30) (Table 2).
Univariable analysis and multivariable Cox regression analysis to identify independent predictors of NE
Univariable analysis revealed that prior stroke, diabetes mellitus, hyperlipidemia, AHRE in minutes, AHRE ≥ 1 minute, CHA2DS2-VASc score, HAS-BLED score, and HAT2CH2 score were significantly associated with NE occurrence (Table 1). Multivariable Cox regression analysis using model 1-3 in Table 3 (including CHA2DS2-VASc score, HASBLED score, and HAT2CH2 score as confounders) showed that only HAT2CH2 score (HR 2.424, 95% CI 1.683 - 3.492, p < 0.001) was independently associated with NE. When we used the all variables in CHA2DS2-VASc score and HAT2CH2 score as confounders, only prior stroke or TIA (HR 11.726, 95% CI 3.649 - 37.678, p < 0.001) was the independent factor of NE (data not shown). We also showed that the HAT2CH2 score was an independent predictor for new onset AF in Supplementary Table 1 and 2.
ROC-AUC determination of AHRE and HAT2CH2 score cutoff values as predictive factors for future NE and survival analysis
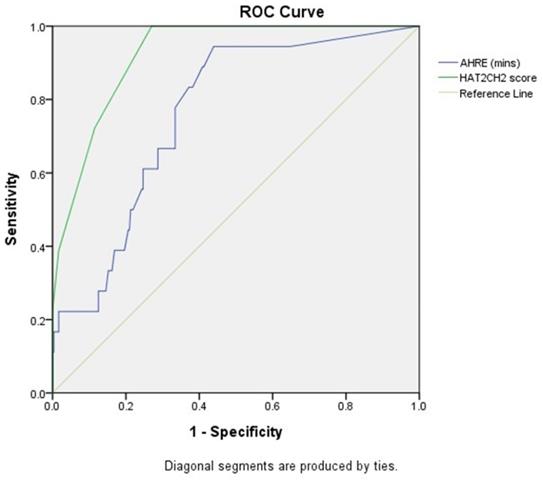
The optimal AHRE cutoff value predictive of future NE was 1 minute with the highest Youden index (sensitivity, 94.4%; specificity, 56.1%; AUC, 0.761; 95% CI, 0.664-0.857; p < 0.001) (Fig. 1). The optimal HAT2CH2 score cutoff value predictive of future NE was 3 with the highest Youden index (sensitivity, 100.0%; specificity, 73.0%; AUC, 0.923; 95% CI, 0.881-0.966; p < 0.001) (Fig. 1).
Baseline Characteristics of the Overall Study Group and with/without neurologic events
| Variables | All Patients (n=314) | Neurologic events | Univariate p-value | |
|---|---|---|---|---|
| Yes (n=18) | No (n=296) | |||
| Age (years) | 73 (62-81) | 75 (68-83) | 72 (61-81) | 0.153 |
| Gender | 0.212 | |||
| Male | 194(61.8%) | 14(77.8%) | 180(60.8%) | |
| Female | 120(38.2%) | 4(22.2%) | 116(39.2%) | |
| BMIa (kg/m2) | 24.6(22.5-26.3) | 23.9(22.6-25.9) | 24.6(22.4-26.3) | 0.616 |
| Device type | 0.318 | |||
| Dual chamber PMb | 220(70.1%) | 16(88.9%) | 204(68.9%) | |
| Dual chamber ICDc | 66(21.0%) | 1(5.6%) | 65(22.0%) | |
| CRTPd | 23(7.3%) | 1(5.6%) | 22(7.4%) | |
| CRTDe | 5(1.6%) | 0(0.0%) | 5(1.7%) | |
| Primary Indication | 0.178 | |||
| Sinus node dysfunction | 141(44.9%) | 13(72.2%) | 128(43.2%) | |
| Atrioventricular block | 79(25.2%) | 3(16.7%) | 76(25.7%) | |
| Heart failure/VTf/VFg | 94(29.9%) | 2(11.2%) | 92(9.1%) | |
| Atrial pacing (%) | 25.0 (5.8-71.4) | 18.5(1.2-87.9) | 25.2(6.1-70.6) | 0.922 |
| Ventricular pacing (%) | 1.9 (0.2-98.3) | 14.4(0.2-75.1) | 1.6(0.2-98.4) | 0.522 |
| Hypertension | 253(80.6%) | 17(94.4%) | 236(79.7%) | 0.215 |
| Diabetes mellitus | 142(45.2%) | 14(77.8%) | 128(43.2%) | 0.006 |
| Hyperlipidemia | 241(76.8%) | 18(100.0%) | 223(75.3%) | 0.010 |
| Chronic obstructive pulmonary disease | 14 (4.5%) | 1(5.6%) | 13(4.4%) | 0.570 |
| Prior stroke | 19(6.1%) | 6(33.3%) | 13(4.4%) | <0.001 |
| Prior myocardial infarction | 57(18.2%) | 5(27.8%) | 52(17.6%) | 0.275 |
| Heart failure | 0.462 | |||
| Preserved LVEFh | 44(14.0%) | 2(11.1%) | 42(14.2%) | |
| Reduced LVEFh | 68(21.7%) | 6(33.3%) | 62(20.9%) | |
| None | 202(64.3%) | 10(55.6%) | 192(64.9%) | |
| Chronic kidney disease | 108(34.4%) | 9(50.0%) | 99(33.4%) | 0.151 |
| Chronic liver disease | 15(4.8%) | 1(5.6%) | 14(4.7%) | 0.596 |
| Thyroid disease | 22 (7.0%) | 1(5.6%) | 21(7.1%) | 0.950 |
| Hemoglobin (mg/dL) | 12.0(11.013.0) | 11.6(10.0-12.2) | 12.0(11.0-13.0) | 0.184 |
| Platelet | 206 (175-229) | 201(156-244) | 206(181-225) | 0.738 |
| Echo parameters | ||||
| LVEFh (%) | 66 (53.8-73.0) | 60.0 (44.3-72.0) | 66.0 (54.0-73.0) | 0.242 |
| Mitral E/e' | 11.0 (8.0-13.6) | 11.0 (9.7-14.3) | 11.0 (8.0-13.4) | 0.614 |
| LAi diameter (cm) | 3.8 (3.2-4.1) | 3.8 (3.5-4.4) | 3.8 (3.2-4.1) | 0529 |
| RVj systolic function (s', m/s) | 12.0 (11.0-13.6) | 12.0 (10.8-14.0) | 12.0 (11.0-14.0) | 0.849 |
| Drug prescribed at baseline | ||||
| Antiplatelets | 121(38.5%) | 12(66.7%) | 109(36.8%) | 0.012 |
| Anticoagulants | 30(9.6%) | 1(5.6%) | 29(9.8%) | 1.000 |
| Beta blockers | 122(38.9%) | 6(33.3%) | 116(39.2%) | 0.621 |
| Ivabradine | 25(8.0%) | 3(16.7%) | 22(7.4%) | 0.164 |
| Amiodarone | 58(18.5%) | 2(11.1%) | 56(18.9%) | 0.543 |
| Dronedarone | 4(1.3%) | 2(11.1%) | 2(0.7%) | 0.017 |
| Flecainide | 1(0.3%) | 0(0.0%) | 1(0.3%) | 1.000 |
| Propafenone | 13(4.1%) | 0(0.0%) | 13(4.4%) | 1.000 |
| Digoxin | 5(1.6%) | 0(0.0%) | 5(1.7%) | 1.000 |
| non-DHP CCBsk | 12(3.8%) | 0(0.0%) | 12(4.1%) | 1.000 |
| RAASl inhibitors | 141(45.0%) | 7(38.9%) | 134(45.4%) | 0.589 |
| Diuretics | 47(15.0%) | 4(22.2%) | 43(14.5%) | 0.325 |
| Statins | 121(38.5%) | 6(33.3%) | 115(38.9%) | 0.640 |
| Metformin | 50(15.9%) | 3(16.7%) | 47(15.9%) | 1.000 |
| SGLT2m inhibitors | 13(4.1%) | 0(0.0%) | 13(4.4%) | 1.000 |
| Follow-up duration (months) | 32 (16-52) | 23.5 (11.8-44.5) | 34.0 (16.0-52.0) | 0.069 |
| CHA2DS2-VASc scoren | 3 (2-4) | 4 (3-5) | 3 (2-4) | 0.005 |
| HAS-BLED scoreo | 2 (1-3) | 3 (2-3) | 2 (1-3) | 0.002 |
| C2HEST scorep | 3 (1-3) | 3 (1-4) | 3 (1-3) | 0.114 |
| mC2HEST scoreq | 3 (2-3) | 3(2-4) | 3(1-3) | 0.099 |
| HAT2CH2 scorer | 2 (1-3) | 4 (3-5) | 2 (1-3) | <0.001 |
| AHREs Duration (minutes) | 0.9(0.0-30.0) | 45.0(3.5-12893.7) | 0.6(0.0-14.1) | <0.001 |
| AHRE ≥ 1 minute | 147 (46.8%) | 17(94.4%) | 130(43.9%) | <0.001 |
| AHRE ≥ 2 minutes | 127 (40.4%) | 15(83.3%) | 112(37.8%) | <0.001 |
Data are presented as medians (interquartile interval) or n (%). Non-parametric continuous variables, as assessed using the Kolmogorov-Smirnov method, were analyzed using the Mann-Whitney U test. Statistical significance is set at p < 0.05.
aBMI = body mass index
bPM = pacemaker
cICD = implantable cardioverter defibrillator
dCRTP = cardiac resynchronization therapy pacemaker
eCRTD = cardiac resynchronization therapy defibrillator
fVT = ventricular tachycardia
gVF = ventricular fibrillation
hLVEF = left ventricular ejection fraction
iLA = left atrium
jRV = right ventricle
knon-DHP CCBs = non-dihydropyridine calcium channel blockers
lRAAS = renin-angiotensin-aldosterone system
m SGLT2 = sodium glucose co-transporters 2
n CHA2DS2-Vasc score = Range from 0 to 9. History of heart failure, hypertension, diabetes, vascular disease, age 65-74 years, and female sex each is calculated as 1 point; 75 years or older and prior stroke, TIA, or thromboembolism each is calculated as 2 points.
oHASBLED score = Range from 0 to 9. Point score is calculated as 1 point each for hypertension, abnormal kidney function, abnormal liver function, prior stroke, prior bleeding or bleeding predisposition, labile international normalized ratio (INR), older than 65 years, medication usage predisposing to bleeding, and alcohol use.
pC2HEST score = Range from 0 to 8. C2: CAD/COPD (1 point each); H: hypertension (1 point); E: elderly (age ≥ 75 years, 2 points); S: systolic HF (2 points); and T: thyroid disease (hyperthyroidism, 1 point).
qmC2HEST score = Range from 0 to 8. C2: CAD/COPD (1 point each); H: hypertension (1 point); E: elderly (age 65~74 years, 1 point; age ≥ 75 years, 2 points); S: systolic HF (2 points); and T: thyroid disease (hyperthyroidism, 1 point).
rHAT2CH2 score = Range from 0 to 7. Hypertension, 1 point; age >75 years, 1 point; stroke or transient ischemic attack, 2 points; chronic obstructive pulmonary disease, 1 point; heart failure, 2 points.
sAHRE = atrial high-rate episodes
Types and incidences of neurologic events
| Types of neurologic events | Number | Incidence rate (100 patient-years) | 95% CIa |
|---|---|---|---|
| Transient ischemic attack | 11 | 1.31 | 0.81-2.63 |
| Ischemic stroke | 7 | 0.84 | 0.51-1.67 |
| Total events | 18 | 2.15 | 1.32-4.30 |
aCI = confidence intervals
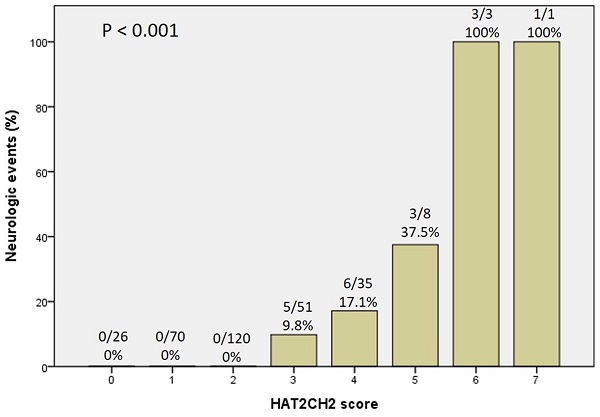
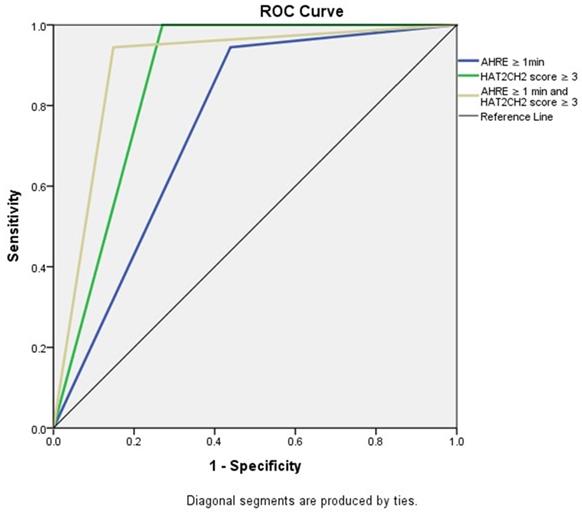
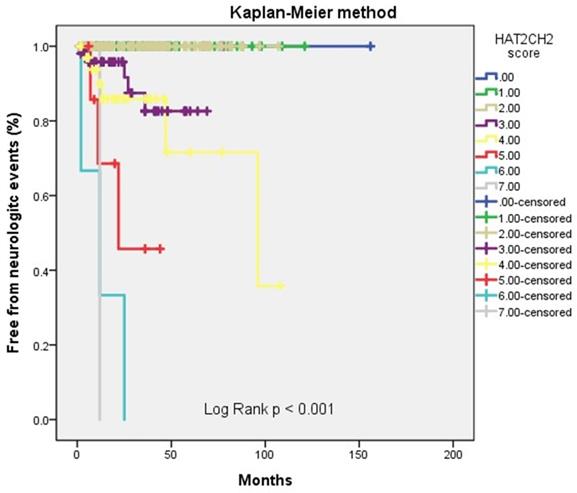
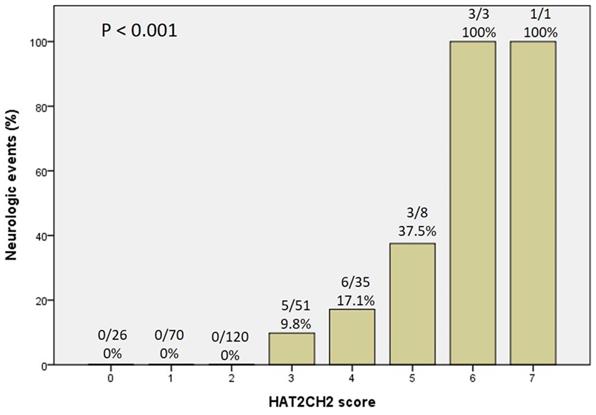
We further used the AHRE ≥ 1 minute and HAT2CH2 score ≥ 3 to analyze the added value of one be to the other, which showed that patients with both AHRE ≥ 1 minute and HAT2CH2 score ≥ 3 had the highest area of 0.898, 95% CI, 0.831-0.965, p < 0.001, compared to only AHRE ≥ 1 minute: area of 0.753, 95% CI, 0.665-0.840, p < 0.001; only HAT2CH2 score ≥ 3: area of 0.865, 95% CI, 0.817-0.912, p < 0.001 (Fig. 2). Kaplan-Meier curves depict the different accumulative survival rates free from NE for HAT2CH2 score groups from 0-7. The survival curve shows that patients with HAT2CH2 scores of 4-7 had higher risk for NE development compared with those with HAT2CH2 scores of 0-3 (log-rank test, P < 0.001) (Fig. 3). The NE occurrence percentage increased significantly with HAT2CH2 scores from 0-2 (0%), 3 (9.8%), 4 (17.1%), 5 (37.5%), to 6-7 (100%) (p < 0.001) (Fig. 4). Included patients could be further classified as very low risk with HAT2CH2 scores of 0-2, low risk with HAT2CH2 scores of 3, medium risk with HAT2CH2 scores of 4, high risk with HAT2CH2 scores of 5, and very high risk with HAT2CH2 scores of 6-7.
Discussion
The main finding of the present study is that the HAT2CH2 score and AHRE are significantly and independently associated with NE in a Taiwanese population with CIED and no history of AF. The optimal cutoff value of the HAT2CH2 score for subsequent NE was 3 and AHRE was 1 minute. These results suggest that comprehensive assessment of the HAT2CH2 score and AHRE in patients with CIEDs is essential to support early, aggressive therapy to prevent NE.
The leading scoring systems for predicting subsequent NE after CIED implantation include the CHA2DS2-VASc score, HAT2CH2 score, C2HEST score and mCHEST score, often used in conjunction with the optimal cutoff for AHRE duration. Of these, the CHA2DS2-VASc score is a well-known and guideline-recommended measure (9) for NE risk prediction. However, recent studies [10,11] have reported that the CHA2DS2-VASc score has insufficient discriminative power for NE prediction. The meta-analysis of Siddiqi et al. [10] reviewed and analyzed 9 studies of patients (n = 453747) with non-valvular AF and 10 studies of patients (n = 138262) without non-valvular AF and found only a modest discriminative power using C-statistics. Similarly, Hu et al. [11] used the Taiwan Health Insurance Research Database to select and evaluate a large group of patients with venous thromboembolism (n= 56996), finding that the area under the curve of ROC of CHA2DS2-VASc score for predicting NE was 0.66, which was also modest.
Multivariable Cox regression analysis of neurologic events
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Prior stroke (yes) | 8.535 | 2.660-27.385 | <0.001 | 9.166 | 3.099-27.111 | <0.001 | 2.780 | 0.869-8.891 | 0.085 |
| Diabetes mellitus (yes) | 2.617 | 0.764-8.967 | 0.126 | 2.715 | 0.840-8.779 | 0.095 | 1.286 | 0.401-4.123 | 0.673 |
| Hyperlipidemia (yes) | 1.918 | 0.000-1.177 | 0.971 | 1.137 | 0.000-1.175 | 0.957 | 1.761 | 0.000-3.200 | 0.960 |
| AHRE ≥ 1 minute | 17.020 | 2.235-129.6 | 0.006 | 17.686 | 2.298-136.08 | 0.006 | 5.937 | 0.736-47.863 | 0.094 |
| CHA2DS2-VASc score | 1.168 | 0.760-1.797 | 0.479 | ||||||
| HAS-BLED score | 1.223 | 0.739-2.025 | 0.434 | ||||||
| HAT2CH2 score | 2.424 | 1.683-3.492 | <0.001 | ||||||
Receiver-operating characteristic curve analysis of atrial high-rate episodes (minutes) and HAT2CH2 score in patients with CIED with subsequent neurologic events. Atrial high-rate episodes (minutes): optimal cutoff value with the highest Youden index, 1 minute; sensitivity, 94.4%; specificity, 56.1%; AUC, 0.761; 95% CI, 0.664-0.857; p < 0.001. HAT2CH2 score: optimal cutoff value with the highest Youden index, 3; sensitivity, 100.0%; specificity, 73.0%; AUC, 0.923; 95% CI, 0.881-0.966; p < 0.001.
Receiver-operating characteristic curve analysis of AHRE ≥ 1 minute, HAT2CH2 score ≥ 3, and AHRE ≥ 1 minute and HAT2CH2 score ≥ 3 for neurologic events. The patients with both AHRE ≥ 1 minute and HAT2CH2 score ≥ 3 had the highest area of 0.898, 95% CI: 0.831-0.965, p < 0.001.
Kaplan-Meier curves depict the accumulative survival rates free from neurologic events regarding HAT2CH2 score (0-7, log-rank p < 0.001).
Neurologic events rate significantly increased with the HATCH score.
In the present study, the area under the curve of ROC curve was 0.92, indicating a highly discriminative measure. To the best of our knowledge, the present study is the first to reveal that the HAT2CH2 score is superior to the CHA2DS2-VASc score, C2HEST score and mCHEST score in the reliable prediction of NE in patients with CIED. Further, results of the present study support our proposed risk stratification system for NE as follows: HAT2CH2 scores of 0-2 indicate very low risk, HAT2CH2 scores of 3 indicate low risk, HAT2CH2 scores of 4 indicate medium risk, HAT2CH2 scores of 5 indicate high risk, and HAT2CH2 scores of 6-7 indicate very high risk. In future study, an external validation population is needed to ensure HAT2CH2 score accuracy in predicting NE in patients with CIED.
Results of the present study also demonstrated that the HAT2CH2 score (AUC of ROC 0.65) independently predicts new onset AF in this study population (data not shown). The HAT2CH2 score includes COPD as one point, rather than diabetes or vascular disease as one point in the CHA2DS2-VASc score, which highlights the varied impact of different diseases in NE in patients with CIED based on our study results. COPD-related systemic inflammation and oxidative stress may promote platelet hyperactivity and cerebral vascular dysfunction [12], and COPD increases the risk of NE, independent of other shared risk factors of cardiovascular disease [13]. Smoking has been recognized as a major causative factor for COPD [14] and also a leading cause of NE [15]. Additional prospective studies are required to elucidate the possible mechanisms underlying COPD-related NE risk, and then to identify effective preventive interventions.
Different cutoff values of AHRE have been proposed previously. One Japanese study even demonstrated that AHRE lasting ≥ 30 seconds was associated with increased risk of stroke [16]. That study enrolled 348 patients who received CIED from Medtronic (atrial rate > 175 beats/minutes), Abbott (atrial rate > 190 beats/minutes), and Biotronik (atrial rate > 200 beats/minutes). Atrial sensitivity was programmed to 0.5 mV with bipolar sensing. Another study [17] enrolled 355 patients who received dual chamber pacemakers from Medtronic (atrial rate > 175 beats/minutes) and Biotronik (atrial rate > 200 beats/minutes) and atrial sensitivity was programmed to 0.3 mV with Medtronic bipolar sensing and 0.2 mV with Bieotronic bipolar sensing. That study also revealed that AHRE ≥ 2 minutes increased risk of NE. In the present study, all 314 patients included had been implanted with Medtronic CIED to prevent the different default settings for AHRE detection from affecting multivariate analysis; we also did the multivariate analysis using AHRE ≥ 2 minutes but the HR was lower than AHRE ≥ 1 minute (data not shown), so we concluded that the optimal AHRE cutoff was 1 minute for subsequent NE after CIED implantation. Even though the latest guidelines [9] recommend that AHRE > 5-6 minutes and > 180 bpm detected by CIED increase the risk for NE, we still suggest that physicians should confirm the default setting of AHRE after patients have undergone CIED implantation.
Limitations
The present study has several limitations. First, this was an observational study with a relatively small number of patients with CIED in a hospital setting, and all patients were Taiwanese. As a result, causality cannot be inferred between AHRE and NE, and the presence of confounding factors cannot be denied. Also, the results may not be generalizable to other populations or locations. Second, this study did not investigate heart rhythms at the time of NE onset, which may not give a complete picture of individual patient conditions. Third, in this retrospective analysis of patient data, we could not confirm that patients had started anticoagulants due to CIED-detected AHRE, although these patients were not excluded because no significant differences were found between anticoagulants use and presence (1, 5.6%) or absence (29, 9.8%) of NE (p = 1.000), as shown in Table 1. Prospective multicenter studies with larger samples are required to confirm results of the present study. Fourth, we did not evaluate the bleeding events after the patients receiving anti-thrombotic therapy for index NE. Every physician should measure the HASBLED score to know the bleeding risk. Fifth, we did not analyze the possible competing hazards (such as death) in this study. Sixth, we did not record the history of the anti-thrombotic drugs during follow-up periods, which may be a bias in such as a retrospective study. Finally, the major limitation was retrospective, relative small case numbers, and low rates of outcome (only 18 events). The fact is that the study could not make definite conclusions.
Conclusions
The HAT2CH2 score and episodes of AHRE lasting ≥ 1 minute are independent risk factors for NE in patients with CIED during mid-term follow-up. When AHRE ≥ 1 minute is detected in patients with CIED, long-term monitoring is advisable to detect clinical AF as well as performing comprehensive assessment of NE risk using the HAT2CH2 score. Results of the present study suggest that early detection of AHRE ≥ 1 minute and calculation of the HAT2CH2 score in patients with CIED may be warranted to support early, aggressive therapy to prevent NE.
Abbreviations
AF: atrial fibrillation; AHRE: atrial high-rate episodes; AMI: acute myocardial infarction; CIED: cardiac implantable electronic device; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; NE: neurologic events; TIA: transient ischemic attacks.
Supplementary Material
Supplementary tables.
Acknowledgements
The authors would like to thank Convergence CT for assistance with English editing of the manuscript.
Source of Funding
The authors would like to thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under contract MOST 110-2218-E-006-017 and MOST 110-2218-E-006-015.
Author Contributions
Conception and design: J-YC; data acquisition: T-WC, W-DL; data analysis and interpretation: J-YC; statistical analysis: J-YC; drafting and finalizing the article: J-YC; critical revision of the article for important intellectual content: J-YC.
IRB information
Approved by the Institutional Review Board of National Cheng Kung University Hospital (B-ER-108-278).
Competing Interests
The authors have declared that no competing interest exists.
References
1. de Vos CB, Pisters R, Nieuwlaat R. et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2021;55(8):725-731
2. Chen K, Bai R, Deng W. et al. HATCH score in the prediction of new-onset atrial fibrillation after catheter ablation of typical atrial flutter. Heart Rhythm. 2015;12(7):1483-1489
3. Hu WS, Lin CL. Comparison of CHA 2 DS 2-VASc, CHADS 2 and HATCH scores for the prediction of new-onset atrial fibrillation in cancer patients: A nationwide cohort study of 760,339 study participants with competing risk analysis. Atherosclerosis. 2017;266:205-211
4. Emren V, Aldemir M, Duygu H. et al. Usefulness of HATCH score as a predictor of atrial fibrillation after coronary artery bypass graft. Kardiol Pol. 2016;74(8):749-753
5. Emren SV, Kocabaş U, Duygu H. et al. The role of HATCH score in predicting the success rate of sinus rhythm following electrical cardioversion of atrial fibrillation. Kardiol Pol. 2016;74(9):978-984
6. Barrett TW, Self WH, Wasserman BS, McNaughton CD, Darbar D. Evaluating the HATCH score for predicting progression to sustained atrial fibrillation in ED patients with new atrial fibrillation. Am J Emerg Med. 2013;31(5):792-797
7. Li YG, Pastori D, Farcomeni A. et al. A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian Subjects: Derivation in 471,446 Chinese Subjects, With Internal Validation and External Application in 451. 199 Korean Subjects. Chest. 2019;155(3):510-518
8. Li YG, Bai J, Zhou G. et al. Refining age stratum of the C2HEST score for predicting incident atrial fibrillation in a hospital-based Chinese population. Eur J Intern Med. 2021 S0953-6205(21)00137-0
9. Hindricks G, Potpara T, Dagres N, ESC Scientific Document Group. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373-498
10. Siddiqi TJ, Usman MS, Shahid I. et al. Utility of the CHA2DS2-VASc score for predicting ischaemic stroke in patients with or without atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol. 2021 zwab018
11. Hu WS, Lin CL. The predictive role of CHA2DS2-VASc score between venous thromboembolism and ischemic stroke: a large-scale cohort study. J Hypertens. 2018;36(3):628-633
12. Austin V, Crack PJ, Bozinovski S, Miller AA, Vlahos R. COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond). 2016;130(13):1039-1050
13. Kim YR, Hwang IC, Lee YJ, Ham EB, Park DK, Kim S. Stroke risk among patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Clinics (Sao Paulo). 2018;73:e177
14. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765-773
15. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8(7):917-932
16. Nakano M, Kondo Y, Nakano M. et al. Impact of atrial high-rate episodes on the risk of future stroke. J Cardiol. 2019;74(2):144-149
17. Lu WD, Chen JY. The optimal cutoff of atrial high-rate episodes for neurological events in patients with dual chamber permanent pacemakers. Clin Cardiol. 2021;44(6):871-879
Author contact
![]() Corresponding author: Ju-Yi Chen, MD, PhD, Professor of Medicine, Division of Cardiology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, 138 Sheng-Li Road, Tainan 704, Taiwan. E-mail: juyincku.edu.tw; Tel: 886-6-235-3535 ext. 2383; Fax: 886-6-275-3834
Corresponding author: Ju-Yi Chen, MD, PhD, Professor of Medicine, Division of Cardiology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, 138 Sheng-Li Road, Tainan 704, Taiwan. E-mail: juyincku.edu.tw; Tel: 886-6-235-3535 ext. 2383; Fax: 886-6-275-3834

 Global reach, higher impact
Global reach, higher impact