3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(5):924-931. doi:10.7150/ijms.71820 This issue Cite
Research Paper
Epstein-Barr Virus is Associated with Gastric Cancer Precursor: Atrophic Gastritis
Department of Gastroenterology, Tongji Hospital of Tongji Medical College, Huazhong university of Science and Technology, Wuhan 430030 Hubei Province, China
Received 2022-2-8; Accepted 2022-5-15; Published 2022-5-21
Abstract
Background: About 10% of gastric cancer (GC) has been described to be Epstein-Barr virus (EBV) positive. Previous researches have described the association between EBV and GC. However, the association of EBV with atrophic gastritis (AG) is underrecognized. Our study aimed to investigate the relationship between EBV and AG and assess the influence of EBV on gastric function.
Methods: A total of 468 pathologically-confirmed chronic gastritis patients underwent circulating EBV DNA test, include 271 non-atrophic gastritis (NAG) and 197 AG patients.
Results: In this study, H. pylori infection rate was 33.3%, EBV infection rate was 40%, and co-infection rate was 15%. The EBV DNA-positive was significantly associated with AG (P=0.031, OR= 1.509, 95% CI 1.037-2.194), especially in H. pylori-negative subjects (P=0.044, OR=1.619, 95% CI 1.012-2.589). EBV DNA-positive patients had a lower pepsinogen I (PG I) / pepsinogen II (PG II) ratio (PGR) than EBV DNA-negative patients (P=0.0026), especially in the AG subgroup (P=0.0062). There was no significant association between EBV and H. pylori co-infection with increased risk of AG (P>0.05).
Conclusion: EBV infection significantly increased the risk of AG, especially in H. pylori-negative patients. The circulating EBV DNA had a potential in predicting the risk of atrophic gastritis.
Keywords: Helicobacter pylori, Epstein-Barr virus, atrophic gastritis, gastric function
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide, and it is the fourth leading cause of cancer-related death [1]. According to Lauren's classification, two histological subtypes of GC can be distinguished: intestinal and diffuse [2]. About 90% of GC belong to intestinal-type GC, which represents the outcome of the inflammation-atrophic-metaplasia-dysplasia-carcinoma sequence, known as the Correa cascade [3]. GC is a multifactorial disease, where many factors can influence its occurrence and development, include environmental, genetic, and infective factors [4]. Regarding infective factors, Helicobacter pylori (H. pylori) and Epstein-Barr virus (EBV) have been well accepted as class I carcinogens and associated with GC occurrence and development [5].
EBV is a herpesvirus that favors human B lymphocytes [6]. More than 90% of adults have been infected by EBV, and it is asymptomatic in the majority of carriers. EBV is closely associated with a variety of human malignant tumors, such as Burkitt's lymphoma, nasopharyngeal cancer, Hodgkin's disease, and GC, et al. [7] About 10% of GC has been described to be EBV-positive[8]. Many researches have described the association between EBV and GC [9]. Atrophic gastritis (AG) is considered to be gastric precancerous diseases, which are independent risk factors of GC and provide background for the possible development of GC [10]. Previous literature mentioned that the virus is localized in the atrophic epithelium in EBV-positive GC patients [11], and the lesions of EBV-related GC occur in the middle area near the atrophic boundary [12]. EBV infection is also associated with an increased risk of GC and its precursor AG. Most patients who were EBV-positive had moderate AG [13, 14]. These results suggested that EBV plays an important role in the development of GC. However, the association of EBV with AG is underrecognized. Thus, it would be greatly essential to identify the relationship between EBV and gastric precancerous diseases, and be beneficial for GC prevention, treatment, and block the progression of precancerous diseases.
The EBV detection methods include serological tests (anti-VCA IgM, anti-VCA IgG, anti-EBNA 1 IgG, and anti-EA(D) IgG) and circulating EBV DNA [15]. EBV DNA and viral load can be detected by PCR, which has been already applied to examine the association between the EBV DNA and clinical courses of EBV-associated diseases using peripheral blood [16-18]. EBV DNA is more sensitive and specific than serological methods [19]. Based on this background, we applied real-time Q-PCR (RT-qPCR) to detect the copy number of EBV DNA using peripheral blood.
This study aimed to investigate the relationship between EBV with AG and to assess the influence of EBV on gastric function.
Materials and Methods
Study design and patients
This study enrolled 468 patients who underwent upper digestive endoscopy in the department of Gastroenterology of Tongji Hospital (Wuhan, China) from 2016 to 2021. The symptoms include epigastric pain, abdominal distension, heart burn, regurgitation, and nausea. Inclusion criteria for this study: 1) age from 18-80 years; 2) non-atrophic gastritis (NAG) and AG; 3) complete 13C urease breath test (13C-UBT), circulating EBV DNA and gastric function indicators such as pepsinogen I (PG I), pepsinogen II (PG II) and calculate the ratio of pepsinogen I and II (PGR). Exclusion criteria were as follows: 1) the history of gastric dysplasia or cancer, nasopharyngeal carcinoma or other malignant tumors; 2) autoimmune atrophic gastritis; 3) other types of chronic gastritis: such as NSAIDS-related gastritis, Eosinophilic gastritis, Menetrier disease, et al. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committees of Tongji Hospital (TJ-IRB20220534).
Endoscopy and histopathological diagnosis
All subjects underwent gastroscopy with two biopsies taken from the gastric antrum and two biopsies taken from the gastric corpus. The gastroscopy of all patients was performed by experienced endoscopists using an Olympus endoscopy (XQ260, XQ290, Tokyo, Japan). Histopathological diagnosis was performed by experienced digestive pathologists, who observed the changes of atrophic. Gastric inflammation and atrophy were diagnosed according to the Chinese Consensus on Chronic Gastritis (2017, Shanghai, China). Subjects with multiple lesions were diagnosed based on the most severe lesion.
Assessment of H. pylori infection
H. pylori infection was detected by 13C-UBT, which is widely considered as one of the most accurate tests for H. pylori infection detection. Proton pump inhibitors, acid-suppressive drugs, and antibiotics were not permitted for 2 weeks prior to the 13C-UBT. The 13C-UBT was detected using a urea 13C capsule breath test kit (KEADWAY, Shenzhen, China). After fasting 2 hours, the subjects were given a tablet with 13C and collected the breath samples were at 0 min and 30 min. A 13C-Breath Test Analyzer (HeliFANplus, Germany) aided in data analysis. Positivity was defined as a value >4.0, indicating the presence of H. pylori infection.
Quantitative analysis of circulating EBV DNA load
Peripheral venous blood sample (5ml) was collected into EDTA-containing tubes from all subjects. The RT-qPCR system was developed to detect EBV DNA. This was used to identify the Bam HI-W region of the EBV genome. The Bam HI-W consisted of the amplification primers W-44F (5′-CCCAACACTCCACCA CACC-3′) and W-119R (5′-TCTTAGGAGCTGTCCGAGGG-3′) and the dual-labeled fluorescent probe W-67T (5′-[FAM] CACACACTACACACACCCACCCGTCTC [TAMRA]-3′).
Fluorogenic PCR reactions were set up in a reaction volume of 50ul using the TaqMan PCR core Reagent Kit (TGFQ01-001, Targene, Guangzhou, China). Thermal cycling was initiated with a 2-minute denaturation step at 93 °C, followed by 10 cycles at 93 °C for 45 seconds and 55 °C for 1 minute, and 30 cycles at 93 °C for 30 seconds and at 55 °C for 45 seconds. Duplicate samples were analyzed, and the mean quantity of each duplicate was used for further concentration calculations. Multiple negative blanks were included in every analysis. In our study center, a cutoff value of 500 copies/ml was set according to the lower limit of detection. EBV DNA ≥ 500 copies /ml was defined as EBV-positive.
Detection of serum PG I and PG II
Peripheral venous blood sample (5ml) was collected from each eligible subject. Serum PG I and PG II were detected in the Tongji Hospital (Wuhan, China). Serum levels of PG I and PG II were carried out by ELISA (BOTHIT Plc, Helsinki, Finland).
Statistical analysis
SPSS 26.0 was used for the statistical analysis (SPSS, Chicago, IL, USA). Descriptive statistics were based on the total number (%) and expressed as mean ± standard deviation. Chi-squared or Fisher tests were used for quantitative data analysis. To evaluate the risk provided by EBV and H. pylori to AG, the odd rates (ORs) were estimated. The group of EBV and H. pylori double-positive patients was compared with the group infected with only H. pylori or EBV, A two-sided P-value of <0.05 was considered statically significant.
Results
Baseline Characteristics of the Subjects
A total of 468 patients with a median age of 48.1 ± 8.7 years were enrolled in our study. The baseline features of all patients are shown in Table 1. The study subjects were classified as 271 (57.9%) NAG patients and 197 (42.1%) AG patients. H. pylori infection rate was 33.3%, EBV infection rate was 40%, and co-infection rate was 15%.
The baseline characteristics of patients with NAG and AG
| Value | Total (n=468) |
|---|---|
| Age | 48.1 ± 8.7 |
| <50 years | 248 (53.0%) |
| ≥50 years | 220 (47.0%) |
| BMI | 24.8 ± 3.8 |
| Smoking | 91 (19.5%) |
| Drinking | 59 (12.6%) |
| Gender | |
| Male | 333 (71.2%) |
| Female | 135 (28.8%) |
| Gastric lesion | |
| NAG | 271 (57.9%) |
| AG | 197 (42.1%) |
| H. pylori positive | 156 (33.3%) |
| EBV positive | 187 (40%) |
| H. pylori and EBV co-infection | 70 (15.0%) |
H. pylori: Helicobacter pylori; EBV: Epstein-Barr virus; NAG: No-atrophic gastritis; AG: atrophic gastritis.
Distribution Characteristics of Circulating EBV DNA of the Subjects
We analyzed the distribution of circulating EBV DNA based on age, smoking, drinking, gender, H. pylori infection status, and gastritis classification (Table 2). We found that the EBV DNA-positive were correlated with the older group (≥50 years) and AG group (P<0.05). No significant difference was found between EBV and gender, smoking, drinking and H. pylori infection status (P>0.05).
Association between Circulating EBV DNA and AG Risk
We found that the positive rate of EBV DNA was significantly higher in the AG group than NAG group (45.7% vs 35.8%), and EBV DNA-positive could increase the risk of AG by 1.509-fold (P=0.031, 95% CI 1.037-2.194) (Table 2). Stratified analysis was further performed based on age, gender, and H. pylori infection status. We found that the contribution of EBV DNA to AG risk was statistically significant in the H. pylori subgroups, but not in the age and gender subgroups (P>0.05). In the H. pylori-negative subgroups, the risk of AG was increased by 1.619-fold (44.5% vs 33.2%, P=0.044, 95% CI 1.012-2.589) (Table 3).
The characteristics on quantitative of circulating EBV DNA
| Variable | EBV-Negative | EBV-Positive | P-value | OR (95% CI) |
|---|---|---|---|---|
| Age | ||||
| <50 years | 162 (65.3%) | 86 (34.7%) | 0.013 | 1.599 (1.102-2.320) |
| ≥50 years | 119 (54.1%) | 101 (45.9%) | ||
| Smoking | 54 (19.3%) | 37 (19.8%) | 0.894 | 1.032 (0.648-1.646) |
| Drinking | 34 (12.1%) | 25 (13.4%) | 0.696 | 1.117 (0.642-1.941) |
| Gender | ||||
| Male | 196 (58.9%) | 137 (41.1%) | 0.412 | 0.842 (0.557-1.270) |
| Female | 85 (63%) | 50 (37%) | ||
| H. pylori infection status | ||||
| Negative | 195 (62.5%) | 117 (37.5%) | 0.125 | 1.357 (0.919-2.004) |
| Positive | 86 (55.1%) | 70 (44.9%) | ||
| Gastritis classification | ||||
| NAG | 174 (64.2%) | 97 (35.8%) | 0.031 | 1.509 (1.037-2.194) |
| AG | 107 (54.3%) | 90 (45.7%) |
H. pylori: Helicobacter pylori; EBV: Epstein-Barr virus; NAG: No-atrophic gastritis; AG: atrophic gastritis.
Association between Circulating EBV DNA and Pepsinogens
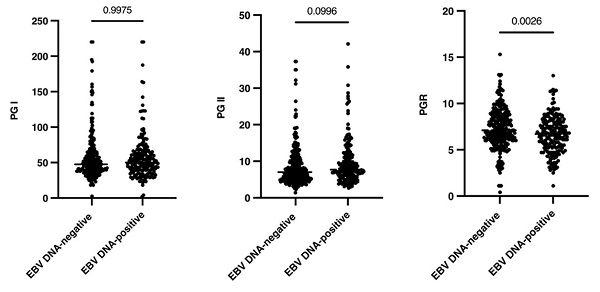
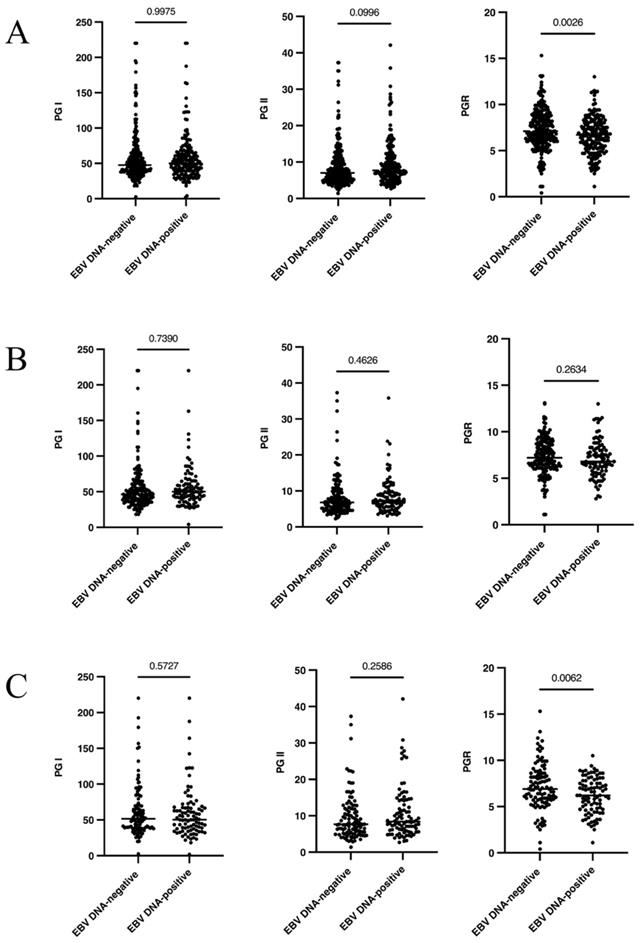
To analyze whether circulating EBV DNA status was associated with gastric function, gastric function indicators were measured in all patients. Compared with EBV DNA-negative patients, the level of serum PGR was significantly decreased in EBV DNA-positive patients (P=0.0026), while no significant difference was observed in PG I and PG II (P>0.05) (Figure 1A). Stratified analysis was further performed based on gastric mucosal status. In the NAG subgroup, the serum gastric function indicators were not significantly variated between EBV DNA-positive with EBV DNA-negative patients (P>0.05) (Figure 1B). In the AG subgroup, the serum PGR also significantly decreased in EBV DNA-positive patients compared with EBV DNA-negative patients (P=0.0062) (Figure 1C).
The association between circulation EBV DNA and gastric function indications (PG I, PG II and PGR) in different EBV carriers. (A): The overall population. (B): In NAG subgroup. (C): In AG subgroup. H. pylori: Helicobacter pylori; EBV: Epstein-Barr virus; NAG: No-atrophic gastritis; AG: atrophic gastritis.
The stratified analysis on association between circulating EBV DNA with AG risk
| Variable | EBV-Negative | EBV-Positive | P-value | OR (95% CI) | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | NAG | 123 (62.8%) | 73 (37.2%) | 0.084 | 1.477 (0.948-2.301) |
| AG | 73 (53.3%) | 64 (46.7%) | |||
| Female | NAG | 51 (68.0%) | 24 (32.0%) | 0.175 | 1.625 (0.803-3.287) |
| AG | 34 (56.7%) | 26 (43.3%) | |||
| Age | |||||
| <50 years | NAG | 109 (68.1%) | 51 (31.9%) | 0.211 | 1.411 (0.822-2.425) |
| AG | 53 (60.2%) | 35 (39.8%) | |||
| ≥50 years | NAG | 65 (58.6%) | 46 (41.4%) | 0.180 | 1.439 (0.845-2.451) |
| AG | 54 (49.5%) | 55 (50.5%) | |||
| H. pylori infection status | |||||
| Negative | NAG | 129 (66.8%) | 64 (33.2%) | 0.044 | 1.619 (1.012-2.589) |
| AG | 66 (55.5%) | 53 (44.5%) | |||
| Positive | NAG | 45 (57.7%) | 33 (42.3%) | 0.520 | 1.231 (0.654-2.315) |
| AG | 41 (52.6%) | 37 (47.4%) |
H. pylori: Helicobacter pylori; EBV: Epstein-Barr virus; NAG: No-atrophic gastritis; AG: atrophic gastritis.
Association between H. pylori/EBV Co-infection and AG Risk
To explore the interaction among AG related infective factors, the relationship between circulating EBV DNA and H. pylori was analyzed. There was no difference between EBV DNA and H. pylori, regardless of whether the overall population or the subgroups (P>0.05) (Table 4). However, we found that EBV DNA-positive increased the risk of AG by 1.619-fold in the H. pylori-negative subgroup (Table 3) and the serum PGR also significantly decreased in EBV DNA-positive patients of the AG subgroup (Figure 1C).
The association between circulating EBV-DNA with H. pylori infection status
| Variable | EBV-Negative | EBV-Positive | P-value |
|---|---|---|---|
| H. pylori infection status | |||
| Negative | 195 (62.5%) | 117 (37.5%) | 0.125 |
| Positive | 86 (55.1%) | 70 (44.9%) | |
| NAG group | |||
| Negative | 129 (66.8%) | 64 (33.2%) | 0.155 |
| Positive | 45 (57.7%) | 33 (42.3%) | |
| AG group | |||
| Negative | 66 (55.5%) | 53 (44.5%) | 0.690 |
| Positive | 41 (52.6%) | 37 (47.4%) |
H. pylori: Helicobacter pylori; EBV: Epstein-Barr virus; NAG: No-atrophic gastritis; AG: atrophic gastritis.
So as to analyze whether there is more risk of getting gastric precancerous disease by infection of the two both pathogens, we compared the group of EBV and H. pylori co-infection (EBV + /H. pylori +) patients with the group infected with only H. pylori or EBV (EBV + /H. pylori - or EBV - /H. pylori +). Whether EBV + /H. pylori + vs EBV + /H. pylori - or EBV + /H. pylori + vs EBV - /H. pylori +, there was no association with AG (P>0.05) (Table 5).
Association between H. pylori/EBV co-infection with AG risk
| NAG | AG | P-value | OR (95% CI) | |
|---|---|---|---|---|
| H. pylori infection status | ||||
| Negative | 193 (71.2%) | 119 (60.4%) | 0.014 | 1.622 (1.100-2.391) |
| Positive | 78 (28.8%) | 78 (39.6%) | ||
| EBV DNA qualitative | ||||
| Negative | 174 (64.2%) | 107 (54.3%) | 0.031 | 1.509 (1.037-2.194) |
| Positive | 97 (35.8%) | 90 (45.7%) | ||
| H. pylori + /EBV + VS H. pylori + /EBV - | 33 (42.3%) | 37 (47.4%) | 0.520 | 1.231 (0.654-2.315) |
| 45 (57.7%) | 41 (52.6%) | |||
| H. pylori + /EBV+ VS H. pylori -/EBV + | 33 (34.0%) | 37 (41.1%) | 0.317 | 1.354 (0.748-2.452) |
| 64 (66.0%) | 53 (58.9%) |
H. pylori: Helicobacter pylori; EBV: Epstein-Barr virus; NAG: No-atrophic gastritis; AG: atrophic gastritis.
Discussion
The prevalence of EBV varied in patients with NAG and AG in the studies, which is directly related to race, geography, and detection methods. Cárdenas-Mondragón et al. [20] found that in patients with chronic gastritis, the infection rate of EBV was 92%, H. pylori was 85.8%, and the co-infection rate was 77.7%; in the gastric precancerous disease, the EBV infection, H. pylori infection and co-infection rate were 97.9%, 89.3%, and 88.2%. A study of EBV detection in Mexico [21] showed that the rate of EBV and H. pylori infection was 69.8% and 48.1%, and the co-infection was 25.4%. In the northern Chinese population [10], the infection rate of EBV was 35.2%, (30.5% with chronic gastritis, 40% with AG, and 38.6% with GC). The incidence of EBV associated gastric cancer is 30.8% in Guangzhou [22]. In our study, we found that the infection rate of EBV in patients was 40.0%, include 35.8% in patients with chronic NAG and 45.7% in patients with AG, which is similar to Chinese studies.
Long lasting inflammation triggers serious damage to the gastric epithelium, increasing the risk to develop precancerous lesions, which in turn increase the risk to end up with a life-threatening GC. More than 90% of GC is related to persistent inflammation. EBV and H. pylori are both considered the main risk factors for chronic inflammatory responses triggering tissue damage [23]. Previous literature mentioned that in EBV-positive gastric carcinoma, the virus was localized in the atrophic epithelium [12]. Nevertheless, Hungermann et al. [24] found evidence that EBV infected epithelial cells of AG mucosa with a relatively low frequency, which is not an early event in GC. Cárdenas-Mondragón et al. [20] study showed that EBV-positive conferred 3.5-fold increased AG risk. Wang et al. [10] found that EBV infection could increase AG risk, especially in the younger and female subgroups. In our study, we found that EBV positive increased the risk of AG by 1.509-fold (P=0.031, 95% CI 1.037-2.194).
There were contradictory results in the effect of EBV and H. pylori co-infection in gastroenterology diseases. Su et al. [25] found that EBV was negatively correlated with H. pylori infection, and there may be antagonism effects between the infection of EBV and H. pylori in GC. Other studies suggested that EBV was positively correlated with H. pylori, EBV and H. pylori co-infection to induce severe inflammation [26, 27], increasing the risk of progression to intestinal-type GC and gastric precancerous diseases [20, 28]. Some studies found that EBV was not associated with H. pylori infection in GC [10, 21, 29]. In our study, there was no significant correlation between EBV and H. pylori (P>0.05). EBV and H. pylori co-infection did not promote the development of gastric mucosa to gastric precancerous disease. Only in H. pylori-negative patients, EBV DNA-positive patients increased AG risk by 1.619-fold. Nerva Dursun et al. [30] also found that atrophy was frequently observed in EBV-positive and H. pylori-negative cases of gastritis.
H. pylori infection was considered to be the prominent cause of GC, which spreads from contaminated food. H. pylori was mainly spread transmitted orally and adhered to the gastric epithelial cells, leading to oxidative stress, toxin, and necrosis of cells, which further lead to chronic inflammation epigenetic modification, and mutation [28]. However, EBV spreads mainly by the oral route through contact with saliva. More than 90% of adults have EBV in the latent stage in B cells [31]. The infected B cells sometimes enter the lytic cycle, producing virus particles that can spread to other hosts. Stable EBV infection and latent EBV gene expression are favorable for promoting the transformation of pre-invasive nasopharyngeal epithelial cells into carcinoma [32]. Moreover, similar to the mechanism of H. pylori induced GC, EBV may indirectly induce chronic inflammation during its viral reactivation cycle by recruiting high levels of immune cell infiltration and thereby promoting tissue damage [33]. Several researched have shown that the cooperation of infectious agents may exacerbate their effect. EBV DNA load in H. pylori-positive individuals was significantly increased, which suggested that H. pylori might play a role in regulating the transformation of EBV to the cleavage stage [34]. H. pylori Cag A was demonstrated associated with the development of GC. Saju et al. [35] discovered that host protein SHP 1 interacts with H. pylori Cag A protein and dephosphorylates Cag A, which antagonized the Cag A oncogenic activity. However, EBV co-infection results in methylation of the host SHP 1 and keeps Cag A phosphorylation and thus may increase the oncogenic potential of Cag A. Fekadu et al. [36] found that H. pylori was exposed to the principal EBV receptor, CD21, in negative gastric epithelial cells, which could induce the expression of EBV receptors EphA2 and NMHC-IIA, and promote the EBV infection. And EphA2 or NMHC-IIA siRNA knockdown, EBV infection was significantly decreased. These results suggest that EBV and H. pylori may have some synergistic effects on the development of GC. In the future, there should be some studies and experiments with a more rigorous design, to confirm the mechanism of how EBV and/or H. pylori infection interacts.
Pepsinogen (PG) and gastrin (G17) are effective indicators that reflect gastric function and screen of GC and its precursor [37]. In our study, we explored the relationship between EBV infection and gastric function. We found that the serum PGR in EBV DNA-positive patients was decreased (P<0.05), while other indicators showed no significant difference (P>0.05). We further found that in the AG subgroup, the serum PGR in the EBV DNA-positive group was lower than that in the EBV DNA-negative group (P<0.05). Su et al. [25] found that EBV-positive patients had significantly higher levels of PGI and PGR than EBV-negative patients (P<0.05). Wang et al.[10] found that the level of PGR in the EBV-positive patients was significantly lower than that of EBV-negative patients (P<0.05), while other indicators showed no significant difference (P>0.05). And the serum PGR in the EBV DNA-positive group was lower in the AG subgroup (P<0.05). The differences in the results may be caused by the different diagnostic criteria and detection methods of EBV in different institutes. Reduction in the PGR demonstrated a strong association with the development of AG and GC. In our study, we found preliminary evidence that EBV DNA was correlated with a low PGR, especially in AG subgroup. These results may provide research clues for confirming that the EBV infection may promote the occurrence of AG.
There were also some research limitations in this study. Firstly, geographical differences in EBV positive had been observed. Our study was a single-center study, we should enroll more patients from different hospitals and regions for research in the future. Second, we enrolled every patient with NAG or AG who came to the institute for diagnosis in a convenient sample instead of paired selection. Lastly, in situ hybridization (ISH) of tissue, a gold benchmark in the determination of EBV infection, was lacking in this study. We should comprehensively evaluate EBV infection, include circulating EBV DNA, multiple specific antibodies for EBV antigens, and ISH, et al.
Conclusion
Given the above, this study showed that the positive EBV DNA was associated with an increased risk of gastric precancerous disease, which was more notable in H. pylori-negative individuals than H. pylori-positive individuals. EBV DNA-positive patients demonstrated a lower serum PGR, which was more distinct in AG patients.
Acknowledgements
This work was supported by the National Natural Science Foundation China [81672392] and the Provincial Natural Science Foundation of Hubei [2015CFA071].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49
2. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020 21
3. Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58-60
4. Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk factors for gastric cancer: a systematic review. Asian Pac J Cancer Prev. 2018;19:591-603
5. Rugge M, Genta RM, Di Mario F, El-Omar EM, El-Serag HB, Fassan M. et al. Gastric cancer as preventable disease. Clin Gastroenterol Hepatol. 2017;15:1833-43
6. Wu WK, Yu J, Chan MT, To KF, Cheng AS. Combinatorial epigenetic deregulation by Helicobacter pylori and Epstein-Barr virus infections in gastric tumourigenesis. J Pathol. 2016;239:245-9
7. Chen XZ, Chen H, Castro FA, Hu JK, Brenner H. Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine (Baltimore). 2015;94:e792
8. Shannon-Lowe C, Rickinson A. The global landscape of EBV-associated tumors. Front Oncol. 2019;9:713
9. Uozaki H, Fukayama M. Epstein-Barr virus and gastric carcinoma-viral carcinogenesis through epigenetic mechanisms. Int J Clin Exp Pathol. 2008;1:198-216
10. Wang Z, Lv Z, Ding H, Xu Q, Sun L, Jing J. et al. Role of serum EBV-VCA IgG detection in assessing gastric cancer risk and prognosis in northern Chinese population. Cancer Med. 2018;7:5760-74
11. Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G. et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-88
12. Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000;53:255-61
13. Hirano A, Yanai H, Shimizu N, Okamoto T, Matsubara Y, Yamamoto K. et al. Evaluation of Epstein-barr virus DNA load in gastric mucosa with chronic atrophic gastritis using a real-time quantitative PCR assay. Int J Gastrointest Cancer. 2003;34:87-94
14. Kartika AV, Iizasa H, Ding D, Kanehiro Y, Tajima Y, Kaji S. et al. Application of biopsy samples used for Helicobacter pylori urease test to predict Epstein-Barr virus-associated cancer. Microorganisms. 2020;8:926
15. Liu W, Chen G, Gong X, Wang Y, Zheng Y, Liao X. et al. The diagnostic value of EBV-DNA and EBV-related antibodies detection for nasopharyngeal carcinoma: a meta-analysis. Cancer Cell Int. 2021;21:164
16. Trevisiol C, Gion M, Vaona A, Fabricio ASC, Roca E, Licitra L. et al. The appropriate use of circulating EBV-DNA in nasopharyngeal carcinoma: comprehensive clinical practice guidelines evaluation. Oral Oncol. 2021;114:105128
17. He Y, Yang D, Zhou T, Xue W, Zhang J, Li F. et al. Epstein-Barr virus DNA loads in the peripheral blood cells predict the survival of locoregionally-advanced nasopharyngeal carcinoma patients. Cancer Biol Med. 2021;18:888-99
18. Shoda K, Ichikawa D, Fujita Y, Masuda K, Hiramoto H, Hamada J. et al. Clinical utility of circulating cell-free Epstein-Barr virus DNA in patients with gastric cancer. Oncotarget. 2017;8:28796-804
19. Lam WKJ, Chan KCA, Lo YMD. Plasma Epstein-Barr virus DNA as an archetypal circulating tumour DNA marker. J Pathol. 2019;247:641-9
20. Cárdenas-Mondragón MG, Torres J, Flores-Luna L, Camorlinga-Ponce M, Carreón-Talavera R, Gomez-Delgado A. et al. Case-control study of Epstein-Barr virus and Helicobacter pylori serology in Latin American patients with gastric disease. Br J Cancer. 2015;112:1866-73
21. Del Moral-Hernández O, Castañón-Sánchez CA, Reyes-Navarrete S, Martínez-Carrillo DN, Betancourt-Linares R, Jiménez-Wences H. et al. Multiple infections by EBV, HCMV and Helicobacter pylori are highly frequent in patients with chronic gastritis and gastric cancer from southwest Mexico: an observational study. Medicine (Baltimore). 2019;98:e14124
22. Chen JN, Jiang Y, Li HG, Ding YG, Fan XJ, Xiao L. et al. Epstein-Barr virus genome polymorphisms of Epstein-Barr virus-associated gastric carcinoma in gastric remnant carcinoma in Guangzhou, southern China, an endemic area of nasopharyngeal carcinoma. Virus Res. 2011;160:191-9
23. Martínez-López JL, Torres J, Camorlinga-Ponce M, Mantilla A, Leal YA, Fuentes-Pananá EM. Evidence of Epstein-Barr virus association with gastric cancer and non-atrophic gastritis. Viruses. 2014;6:301-18
24. Hungermann D, Müller S, Spieker T, Lisner R, Niedobitek G, Herbst H. Low prevalence of latently Epstein-Barr virus-infected cells in chronic gastritis. Microsc Res Tech. 2001;53:409-13
25. Su X, Ye Z, Wang Z, Long Y, Qiu M, He C. Epstein-Barr virus infection associated with pepsinogens and Helicobacter pylori infection in patients with gastric cancer. Virus Res. 2018;256:1-5
26. Camargo MC, Kim KM, Matsuo K, Torres J, Liao LM, Morgan DR. et al. Anti-Helicobacter pylori antibody profiles in Epstein-Barr virus (EBV)-positive and EBV-negative gastric cancer. Helicobacter. 2016;21:153-7
27. Cárdenas-Mondragón MG, Carreón-Talavera R, Camorlinga-Ponce M, Gomez-Delgado A, Torres J, Fuentes-Pananá EM. Epstein Barr virus and Helicobacter pylori co-infection are positively associated with severe gastritis in pediatric patients. PLoS One. 2013;8:e62850
28. Singh S, Jha HC. Status of Epstein-Barr virus coinfection with Helicobacter pylori in gastric cancer. J Oncol. 2017;2017:3456264
29. Kim Y, Shin A, Gwack J, Ko KP, Kim CS, Park SK. et al. Epstein-Barr virus antibody level and gastric cancer risk in Korea: a nested case-control study. Br J Cancer. 2009;101:526-9
30. Dursun N, Hacıhasanoğlu E, Okçu O, Paşaoğlu E, Leblebici C. Epstein-Barr virus infection in patients with chronic gastritis without Helicobacter pylori infection. Turk J Gastroenterol. 2020;31:205-10
31. Farrell PJ. Epstein-Barr virus and cancer. Annu Rev Pathol. 2019;14:29-53
32. Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017 372
33. Morales-Sanchez A, Fuentes-Panana EM. Epstein-Barr virus-associated gastric cancer and potential mechanisms of oncogenesis. Curr Cancer Drug Targets. 2017;17:534-54
34. Shukla SK, Prasad KN, Tripathi A, Singh A, Saxena A, Ghoshal UC. et al. Epstein-Barr virus DNA load and its association with Helicobacter pylori infection in gastroduodenal diseases. Braz J Infect Dis. 2011;15:583-90
35. Saju P, Murata-Kamiya N, Hayashi T, Senda Y, Nagase L, Noda S. et al. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nat Microbiol. 2016;1:16026
36. Fekadu S, Kanehiro Y, Kartika AV, Hamada K, Sakurai N, Mizote T. et al. Gastric epithelial attachment of Helicobacter pylori induces EphA2 and NMHC-IIA receptors for Epstein-Barr virus. Cancer Sci. 2021;112:4799-811
37. Tu H, Sun L, Dong X, Gong Y, Xu Q, Jing J. et al. A serological biopsy using five stomach-specific circulating biomarkers for gastric cancer risk assessment: a multi-phase study. Am J Gastroenterol. 2017;112:704-15
Author contact
![]() Corresponding author: Jiazhi Liao, Email address: liaojiazhitjmu.edu.cn; Department of Gastroenterology, Tongji Hospital of Tongji Medical College, Huazhong university of Science and Technology, Wuhan 430030 Hubei Province, China
Corresponding author: Jiazhi Liao, Email address: liaojiazhitjmu.edu.cn; Department of Gastroenterology, Tongji Hospital of Tongji Medical College, Huazhong university of Science and Technology, Wuhan 430030 Hubei Province, China

 Global reach, higher impact
Global reach, higher impact