3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(4):769-778. doi:10.7150/ijms.71972 This issue Cite
Review
IL-17: an important pathogenic factor in endometriosis
1. Laboratory for Reproductive Immunology, Hospital of Obstetrics and Gynecology, Fudan University, Shanghai 200080, People's Republic of China.
2. NHC Key Lab of Reproduction Regulation (Shanghai Institute for Biomedical and Pharmaceutical Technologies), Hospital of Obstetrics and Gynecology, Fudan University, Shanghai 200080, People's Republic of China.
3. Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Hospital of Obstetrics and Gynecology, Fudan University, Shanghai 200080, People's Republic of China.
Received 2022-2-12; Accepted 2022-3-31; Published 2022-4-11
Abstract
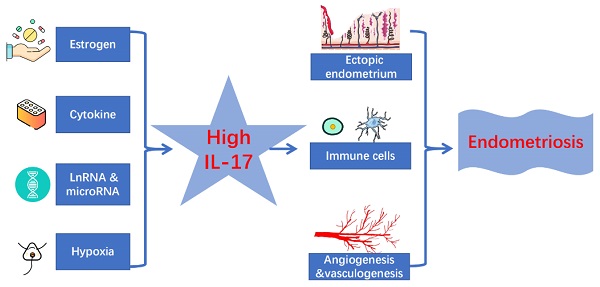
Interleukin-17 (IL-17) is known as a Th17-cell-derived proinflammatory cytokine, which plays a pivotal role in several inflammatory and autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and psoriasis. Emerging evidence has shown that IL-17 is linked to endometriosis, although the etiology of endometriosis is still unknown. The IL-17 expression is up-regulated in serum, peritoneal fluid (PF) and endometriotic lesions from patients with endometriosis but the related regulation mechanisms are complex and obscure. Meanwhile, the specific roles of IL-17 in endometriosis are also worthy of further exploration. Through the integration and summary of literature, we conclude that the secretion of IL-17 increases under the regulation of ectopic microenvironment and other factors, and then IL-17 is deeply involved in endometriosis in the regulation of immune microenvironment, the invasion and growth of ectopic lesions, and so on, which implies its therapeutic value in this disorder.
Keywords: IL-17, endometriosis, cytokine, inflammatory, Th17 cell
Introduction
The IL-17 is secreted by the CD4+ T helper 17 (Th17) cells and regarded as the signature cytokine of a distinct cluster of these cells, which was discovered in 1999 using T-cell clones from the joints of patients with rheumatoid arthritis [1-4]. The development of Th17 cells is distinct from the development of Th1, Th2 and regulatory T cells and requires specific transcription factors and cytokine requirements, such as transforming growth factor-β (TGF-β), combined with IL-6 or IL-21 and the transcription factor, retineic-acid-receptor-related orphan receptor gamma (RORγt). As for their particular expression of the “master” transcription factor RORγt, it is then activated by the IL-12 family cytokine IL-23, and the resulting “IL-23-IL-17 axis” was found to function as a critical driver of autoimmune disease [5, 6, 4, 7-16]. To date, the IL-17 family of cytokines contains 6 structurally related cytokines (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F) that share sequence homology, and their 5 corresponding receptors (IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE) present on the surface of cells. These IL-17 receptors subunits adopt a shared cytoplasmic motif termed a ''SEFIR'' (SEF/ IL-17 receptor), which is analogous to the toll-IL-1 receptor (TIR) domain expressed in toll-like receptor (TLR) and IL-1 receptor family members [10, 17-19]. IL-17A is the first described member of this family and also the best characterized one. It was once believed to be primarily produced by Th17 cells [20, 21, 19]. In fact, IL-17A and IL-17F exhibit high sequence similarity and can form homodimers and heterodimers to signal, and they also signal through the same receptor complex, so they largely share biological functions, with IL-17A being more potent than IL-17F. While the other four IL-17 isoforms only exist as homodimers [3, 22, 18, 23].
Since IL-17 is reported to be conserved in evolution [24-28], its host-protective attributes can play diverse roles both in immune-protection and also immunopathology. As for immune-protective functions, IL-17 exerts its function as a key mediator of mucosal surveillance and barrier integrity through maintaining epithelial integrity, promoting the production of antimicrobial factors and regulating the recruitment and generation of neutrophils [29-35, 22, 36, 37]. However, IL-17 also has been increasingly implicated as a driver of immunopathology in settings of autoimmunity, cancer and chronic inflammation [38-41]. Take IL-17A as an example, it has been recognized in its critical role in the promotion of disease progression, pathogenesis of autoimmune diseases, tumors, mechanical injury, infection, obesity and chronic inflammatory disorders [20, 10, 42, 18, 43, 19].
Endometriosis is a common estrogen-dependent inflammatory gynecological disease and is defined as the presence of functional endometrial glands and stroma outside the uterine cavity.[44-46] This disorder is similar to malignancies in some ways: progressive and invasive growth, a tendency to metastasize and recurrence. Although the physiopathology of endometriosis is not completely understood and several theories have been proposed to explain it, it is well established that this pathogenesis is closely related to the immune system. The defective immune responses may include increased levels of activated peritoneal macrophages and various proinflammatory cytokines, abnormal T- and B-lymphocyte activation, reduced natural killer cell activity, and the production of various autoantibodies [47, 48, 44, 49-56]. All these activities not only fail to effectively clear discarded endometrial tissues but may actually allow development of chronic inflammation and even a hyperinflammatory state, which can help endometrial cells to escape immunosurveillance and also use inflammatory mechanisms to promote their growth within the peritoneal cavity. The amount of misplaced endometrial tissues will in turn overwhelm the resident and recruited immune cells, leading to dysfunctional in their ability [57, 50]. And as is reported in many previous studies, the IL-17 family plays a pivotal role in the pathogenesis of endometriosis [58, 49, 59-61].
In this review, we attempt to outline the roles of IL-17 in endometriosis, present the regulatory mechanism of IL-17 expression in endometriosis, identify the biological function (regulation of ectopic endometrial lesions, recruitment and function regulation of immune cells, and angiogenesis) of IL-17 in endometriosis, and discuss prospects in the potential treatment of these patients as well.
Expression of IL-17 and its receptors
With the deepening of the research on cytokines and endometriosis, elevated levels of IL-17 in endometriosis have been reported and confirmed more widely, especially in the early stages of the disease [62-64, 42, 60, 61]. Further investigations have documented that IL-17 is produced not only by Th17/ThIL-17 cells, but also by activated CD8+ T cells, γδ T cells, NK cells, neutrophils as well as mast cells [65-72]. For the first time, Zhang and his colleagues demonstrated higher IL-17 levels in the PF of patients with endometriosis. Meanwhile, there was a correlation between the concentration of IL-17 in PF and progression of the disease, and the concentrations of IL-17 in PF were significantly higher in the patients with minimal/mild endometriosis than those with moderate/severe endometriosis and those without endometriosis. This study also suggested the concentration of IL-17 in PF was associated with endometriosis-related infertility [61]. In line with Zhang's study, increased amounts of IL-17 was later demonstrated in the PF of women with endometriosis by some other teams [62, 63, 73, 74, 42, 60]. Bungum et al. also found high expression of IL-17E in PF, but unlike Zhang's team, they did not find an association between IL-25 (also called IL-17E) levels and the stage of endometriosis [62]. They speculated that this result could be due to the fact that the inflammatory response seems to be low at more severe stages of endometriosis, just as Salmeri et al. suggested [75]. Besides, some other reports even demonstrated that in the context of endometriosis, IL-17A was elevated in the plasma [76] and PF of women with endometriosis compared to controls and that endometriotic lesions produce IL-17A [42, 60]. Sabbaghi et al. declared that a similar elevation in IL-17A level was observed both in blood serum and follicular fluid (FF) when endometriosis and infertility co-exist [77]. In Ahn's research, though they did not find a significant difference in the PF concentration of IL-17A between women with endometriosis and without disease, they found it in the plasma samples. Additionally, in their study, immunohistochemistry revealed the localization of IL-17A-positive cells in the stroma and surrounding the vasculature in matched eutopic endometrium and ectopic lesion samples from women with endometriosis. Thereout, they suspected it was possible that IL-17A was primarily generated by tissue-resident immune cells and as such may not be detectable in the PF [78].
In addition, Hirata et al. successively reported that endometriotic stromal cells (ESCs) expressed IL-17RA [64] and IL-17RC [79]. In 2008, this group first examined presence of IL-17A-positive cells in endometriotic tissues and Th17 cells in peritoneal fluid mononuclear cells (PFMCs) [64]. Then they also demonstrated expression of IL-17F in mononuclear cells from endometriotic lesions (EMMCs) [79].
A recent study, performed by Gogacz et al., described the increased percentage of Th17 cells in the PF in comparison with peripheral blood (PB) in endometriotic patients. And their data also showed that the percentage of Th17 cells in PF corresponded with the severity of endometriosis. In severe endometriosis, the percentage of Th17 cells in PF was higher than with early (I/II stage) endometriosis [80]. This correlation was also demonstrated by other researchers [81]. Liu et al. further showed that the percentage of IL-17 and Th17 cells were both increased in peritoneal fluid mononuclear cells (PFMCs) of patients with endometriosis [82]. It is also newly demonstrated the abundance of CD8+ T cells and CD56+ NK cells with enriched IL-17 signalling pathway in the eutopic endometria of women with endometriosis [83].
However, there are several papers reporting that they did not find any relationship between the level of IL-17 and endometriosis [84, 85]. But some of them still found IL-17/IL-10 and IL-17/IL-23 ratios were respectively increased in PF and serum samples from endometriosis group. And the increased IL-17/IL-23 ratio was also found in the periphery of endometriosis women, which was explained because IL-17 was antagonized by anti-inflammatory cytokines such as TGF-β1 in the latter stages of the disease (Table 1) [85].
Recent publications about IL-17, IL-17R or Th17 cells in patients with endometriosis and their correlation
| Parameter | Distribution | Correlation | Reference |
|---|---|---|---|
| IL-17 | PF↑ | Negative correlation | 60 |
| IL-17 | PF↑ | No mention | 63, 73, 74 |
| IL-25 | PF↑ | No correlation | 64 |
| IL-17A | Plasma↑, PF↑ | No mention | 43, 61 |
| IL-17 | Serum↑ | No mention | 76 |
| IL-17A | Serum↑, FF↑ | No mention | 77 |
| IL-17A | Plasma↑, PF no difference | No mention | 78 |
| IL-17A-positive cells | Stroma and surrounding the vasculature in matched eutopic endometrium and ectopic lesion samples↑ | No mention | 78 |
| IL-17 | No difference | No correlation | 84 |
| IL-17 | No difference | No correlation | 85 |
| IL-17/IL-10 ratio | PF↑, serum↑ | No mention | 85 |
| IL-17/IL-23 ratio | PF↑, serum↑, periphery↑ | No mention | 85 |
| IL-17F | EMMCs↑ | No mention | 79 |
| IL-17RA | ESCs↑ | No mention | 62 |
| IL-17RC | ESCs↑ | No mention | 79 |
| IL-17A-positive cells | Endometriotic tissues↑ | No mention | 62 |
| TH17 cells | PFMCs↑ | No mention | 62 |
| TH17 cells | PF↑ | Positive correlation | 80, 81 |
| IL-17 + TH17 cells | PFMCs↑ | No mention | 82 |
| CD8+ T cells, CD56+ NK cells, IL-17 signalling pathway | Eutopic endometria↑ | No mention | 83 |
Note: Abbreviations: IL-17, interleukin 17; IL-17R, interleukin 17 receptor; Th17 cells, T helper 17 cells; PF, peritoneal fluid; FF, follicular fluid; EMMCs, mononuclear cells from endometriotic lesions; ESCs, endometriotic stromal cells; PFMCs, peritoneal fluid mononuclear cells.
By considering the above-mentioned facts, it is prevalent that the level of IL-17 rises in endometriosis patients, commonly detected in PF and blood. Due to the effects of anti-inflammatory factors and other unknown factors, this concentration is not necessarily proportional to the progression of the disease. It is possible that continuous dynamic detection of concentration changes will provide more valuable hints, which require further large sample studies. Besides, in view of the differences in sample size, measurement methods and sampling among different studies (sampling at different times of the menstrual cycle, measure differences in samples such as tissues or cells and etc.), there must be inevitable differences in different research results.
Regulatory mechanisms of IL-17 expression in endometriosis
Estrogen
Deena Khan's team found that estrogen not only enhanced the levels of IL-17 and intracellular IL-17+ cells but also upregulated the IL-17-specific transcription factor, RORγt, in activated splenocytes in wide type mice. They also suggested estrogen upregulates IL-17 induction in autoimmune mice [86]. Similarly, Newcomb et al. provided evidence that 17β-estradiol (E2) and progesterone (P4) increased IL-17A production from Th17 cells, by decreasing let-7f miRNA expression and increasing IL-23R expression [87]. While other in vitro studies reported the production of IL-17 was under the negative regulation of E2. These variable findings might be due to different T-cell activation and differentiation protocols [88-93]. Taken together, these findings confirmed that estrogen is involved in the expression of IL-17, although there is no direct evidence that the site of action is endometriosis. Furthermore, Ning's findings showed IL-17A participated in CD68+CD163+ macrophage-stimulated endometrial cancer cell proliferation by regulating the estrogen receptor alpha (Erα) pathway [94]. According to the above analysis, we suppose that estrogen may well be involved in the expression of IL-17 in the microenvironment of endometriosis and prefer it to be a positive moderator, which still warrants further investigation.
Cytokine
It is extensively acknowledged that some cytokines also participate in the production of IL-17 in endometriosis. Bungum et al. proposed that the pathophysiology could involve macrophages producing IL-1β and tumor necrosis factor-α (TNF-α), found in PF from women with endometriosis [95], which stimulates production of Regulated on Activation, Normal T cell Expressed and Secreted (RANTES) and Monocyte Chemotactic Protein-1 (MCP-1) [96, 97]. RANTES and MCP-1 may be responsible for recruiting macrophages, producing Histamine Releasing Factor (HRF), into endometriotic implants. In turn HRF might induce production and release of IL-4 and maybe IL-25 from mast cells [62]. Current evidence largely suggests that IL-23 is responsible for the differentiation and expansion of Th17/ThIL-17 cells, and so it is viewed as an important IL-17 inducer to regulate the expression of IL-17 [98, 99, 35, 100]. Additionally, Chang's group concluded that under the stimulation of IL-6 and TGF-β, signal transducer and activator of transcription 3 (STAT3) may be activated in naive T cells, which further promotes RAR-related orphan receptor C (RORC) and IL-17A transcription, and induces IL-17A production. And IL-27 was considered to induce the IL-10 and IL-17A double-producing Th17 cells in endometriosis. In advanced endometriosis, the formation of a c-Maf, RORγt and Blimp-1 complex triggered by IL-27 contributes to the expansion of IL-10-producing Th17 cells. Hence IL-27 is regarded as a pivotal regulator in endometriotic immune tolerance by triggering Th17 cells to produce IL-10 and IL-17A and promoting the rapid growth and implantation of ectopic lesions [101]. TGF-β1, a kind of anti-inflammatory cytokines with a myriad of functions including cell differentiation, proliferation, migration, angiogenesis and vasorelaxation [102, 103], is found to support both Th17 and Treg cells differentiation in a dose dependent manner. And in higher concentrations, the immune response will shift toward Treg cells [104]. Therefore, Tarokh et al. assumed that higher concentrations of TGF-β1 might regulate the inflammation in the patients via reducing IL-17 concentration in the later stage of endometriosis [85]. In a recent study, the researchers found that treatment with anti-bone morphogenetic protein 1 (anti-BMP1) antibodies dose-dependently increased lesion volume in mice with endometriosis, reduced IL-17 and IL-1β levels [105]. This may suggest that BMP1 is involved in the regulation of IL-17 expression although further study is needed to confirm it. Given all this, the role of cytokines, in the regulation of IL-17 expression in endometriosis is beyond doubt. However, further studies are still needed to determine whether the specific regulatory mechanisms are intersected or the effects of various cytokines are independent. Inhibition of positive factors and promotion of negative factors may help to reduce the concentration of IL-17, thus providing new ideas for the treatment of endometriosis.
LnRNA and microRNA
Gene-level studies on the regulation of IL-17 expression in endometriosis are also under way. Zhi et al. proved that lack of immediate early response gene (IER3, also called IEX-1), belonging to the group of genes rapidly activated during inflammation, promoted Th17 differentiation and then increased IL-17A production [106]. And IER3, predicted by bioinformatics software, is one of the target genes of miR-342-3p which has been found to be highly expressed in serum of woman with EMS [107]. Besides, LncRNA H19 was also successively detected in women with EMS and found lower expression in the eutopic endometrium of women with EMS with its mechanism in reducing the proliferation of ESCs [108, 109]. Recently, Liu et al. first confirmed miR-342-3p could negatively regulate IER3 expression. And they also demonstrated that LncRNA H19 over-expression could decrease IL-17 secretion, suppress Th17 differentiation and ESCs proliferation through inhibiting miR-342-3p [82]. It is plausible that given the limited exposure reported to date, miR-342-3p has a positive promoting effect on IL-17, while LncRNA H19 plays a detrimental role.
Hypoxia
Retrograde menstruation has been proposed and well accepted to be a crucial constituent for the development of the etiology of endometriosis. According to this theory, shed-off endometrial tissues first lost blood supply and may well face hypoxic stress. Consequently, the ectopic lesion in endometriosis is indeed an anoxic environment. Hypoxia-inducible transcription factor-1 (HIF-1), the oxygen-sensitive transcription factor, is key transcriptional regulator of hypoxia-associated genes to adapt to decreased availability of O2 [110]. Dang et al. once observed a higher proportion of IL-17A+ cells in hypoxic compared to normoxic culture conditions. And their results demonstrated that HIF-1 plays a dual role in regulating IL-17A transcriptional activity by directly activating RORγt transcription and then collaborating with RORγt at the IL-17A promoter to recruit p300, thus generating a permissive chromatin structure. Meanwhile, Th17 differentiation is enhanced under hypoxia in a HIF-1α-dependent manner. Therefore, HIF-1α activity is suggested to represent a major mechanism by which the hypoxic conditions associated with inflammation can promote Th17 differentiation [111]. It is also proved that hypoxia and HIF-1α overexpression in in rheumatoid arthritis potentiated RASF-mediated expansion of inflammatory Th1 and Th17 cells, leading to proinflammatory interferon-γ (IFN-γ) and IL-17 production [112]. In addition, it has been found that the abundance of HIF isoforms was mechanistically linked to elevated IL-1β and IL-17 in sarcoidosis [113]. Taken together, IL-17 is regulated by HIF-1 and hypoxia. However, it is a pity that there may be scarce direct evidence that hypoxia or HIF-1 regulates IL-17 expression in endometriosis up to date. Further research is needed on whether the same regulatory mechanism exists in endometriosis.
The biological functions of IL-17 in endometriosis
Regulation of ectopic endometrial lesions
Based on an analysis of the literature, we suspect that the regulation of IL-17 on ectopic endometrial is mainly likely to trigger its invasion, implantation, growth and proliferation during the early stage of disease, that is IL-17 might be more important in the initiation, but not in the later process of endometriosis [64, 114, 85]. Ahn et al. reported that IL-17A mainly promoted proliferation and invasion, and restricted the adhesion of ESCs, thereby accelerating the growth, implantation and dissemination of an ectopic lesion in vitro and in vivo [78], which is also verified by Chang's team [101]. IL-17A stimulated propagation of ESCs may be partially attributable to its mitogenic effect and the increased production of IL-8 induced by IL-17A [64], since IL-8 has been shown to facilitate the proliferation of endometrial stromal cells [115]. And another member of the IL-17 family, IL-17F, had a similar function in stimulating the secretion of IL-8 and the expression of COX2 in ESCs and it is speculated IL-17F may promote endometriosis through these mechanisms [79].
In addition, IL-17 has also been found to enhance both the production and secretion of IL-1β by peritoneal macrophages [116]. It has been reported that IL-1β induces the production of IL-8 and vascular endothelial growth factor (VEGF) [117]. Therefore, elevated concentrations of IL-17 in patients with endometriosis may imply an increased hypervascularisation, leading to possible facilitation of the implantation, proliferation and establishment of early endometriotic lesions [61].
Furthermore, according to Khan's finding, endometriosis is relevant to Treg- and Th17-cell alteration causing survival and implantation of ectopic endometrial lesions in the initial stage of the disorder, with consequent progression toward the advanced stage [73].
Recruitment and function regulation of immune cells
Hirata et al. have shown that one of the pathogeneses of endometriosis by which IL-17A is involved in is the secretion of IL-8 [64, 114, 79]. While pleiotropic functions of IL-8, such as chemoattraction and activation of neutrophils, are clear and suggested to promote endometriosis [118-120]. Additionally, IL-17 can enhance granulopoiesis by stimulation of granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) [121]. Takamura et al. suggested that IL-17A produced by neutrophils stimulates growth-related oncogene-α (Gro-α) secretion from EoSCs, thereby recruiting more neutrophils and inducing perpetuating inflammation in endometriosis [122]. Gro-α is known as a powerful activator of neutrophils in its ability to induce chemotaxis, a rise in intracellular free calcium, exocytosis, and the respiratory burst in neutrophils [123, 124, 120]. While neutrophils are suggested to secrete VEGF under inflammatory milieu, trigger angiogenesis in the early stage of endometriosis, generate reactive oxygen species (ROS) at endometriotic sites and impose oxidative stress that affects the development of endometriosis [125, 126].
Furthermore, macrophages are also thought to be a pivotal player in promoting endometriosis [54]. It is reported that IL-17A is chemotactic for macrophages via its receptor, IL-17RA, and can also induce M2 polarization in lung cancer [127], which is also demonstrated in endometriosis. These researchers propose IL-17A is involved in macrophage recruitment and may be indirectly polarizing SPM into a pathogenic M2 phenotype by first interacting with the endometriotic lesion [42]. Indeed, M2 macrophages have been proved to mediate processes such as extracellular matrix (ECM) reconstruction and vascularization, which are associated with the progression of endometriosis [128-130]. And increased production of TNF caused by the activated macrophages and biological effect of IL-17 on endometrial cells accelerates the occurrence of endometriotic lesions accompanied by infertility and unexplained pelvic pain. Besides, IL-17 has been shown to induce the production of nitric oxide synthase 2 (NOS2) and nitric oxide (NO) by peritoneal macrophages [131]. Recent work suggested that increased levels of IL-17 in PF from the endometriosis patients with infertility may induce the production of NOS2 and NO by peritoneal macrophages, which adversely affect female reproductive system, sperm, embryos, implantation and oviductal function. These abnormalities likely all impact fertility, leading to the infertility or subfertility observed in the patients with endometriosis [61], just like the toxic effects of those increased concentrations of Th1 cytokines such as TNF-α, IL-1β, IL-4, IL-6, IL-12, and IFN-γ in women with endometriosis [132-139]. It is also showed that IL-17 should be inhibited by the emergence of decidual stromal cells and placentation, which enables the evolution of embryo implantation and ongoing pregnancy [140]. Herein, it can be inferred that the elevated IL-17 level is related to poor reproductive outcome not only in chronic endometritis [141] but also in endometriosis.
Overall, IL-17 could be the stimuli that mediates the recruitment and activation of immune cells such as macrophages and neutrophils to facilitate the immune escape of ectopic endometrial cells, promote the progress of endometriosis, and contribute to the unexplained infertility.
Angiogenesis and vasculogenesis
The survival of endometriotic implants on the peritoneal membrane within the peritoneal cavity relies on the establishment of blood supply for the provision of oxygen and nutrients to the developing lesions. And endometriotic lesions are densely vascularized. All these fuel the notion that mechanisms of angiogenesis and/or vasculogenesis may be utilized by endometriosis to establish its own vascular network to sustain its survival [142]. Angiogenesis refers to a complex process of new blood vessel formation from previously existing vessels with endothelial cell proliferation [143, 144]. While vasculogenesis refers to a process of de novo formation of blood vessels arising from migration, proliferation, and incorporation of angioblasts or endothelial progenitor cells (EPCs) from the bone marrow [145].
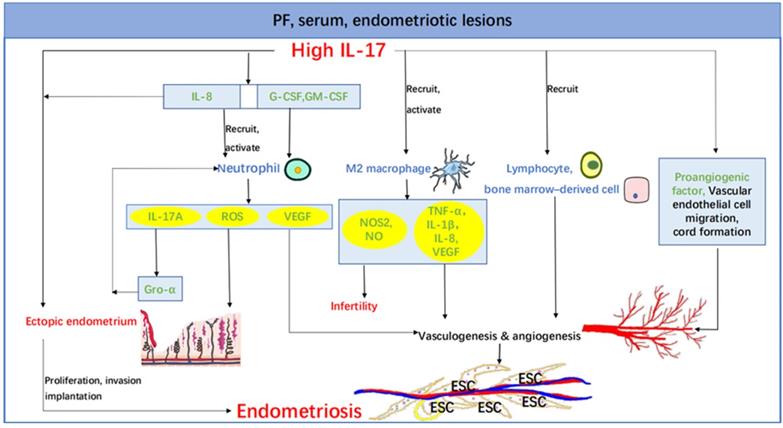
Numasaki et al. reported that IL-17 promotes angiogenesis via inducing elaboration of a variety of proangiogenic factors that lead to the imbalance between angiogenesis activators and inhibitors present within the vascular microenvironment and triggers vasculogenesis via stimulation of vascular endothelial cell migration and cord formation. IL-17, on the other hand, stimulates production of proangiogenic factors in fibroblasts and promotes fibroblast-induced neovessel formation in inflammation [146]. Another study suggests that IL-17A has the potential to enhance vascularization of the lesion through VEGF-and IL-8-mediated pathways. Their data also prove a potential involvement of IL-17A in mediating neoangiogenesis and recruitment of lymphocytes and bone marrow-derived cells to the site of lesion development [78]. Moreover, as mentioned above, IL-17 has the capacity to activate macrophages and neutrophils. Then the secretory products such as TNF-α, IL-8, and VEGF secreted by activated macrophages have the ability to influence each phase of the angiogenic process, including modifying the local extracellular matrix, induction of endothelial cells to migrate or proliferate, and inhibition of vascular growth with formation of differentiated capillaries [147], thus facilitating the proliferation of endometrial cells and subsequently progressing to severe endometriosis. And neutrophils have been shown to release VEGF under inflammatory milieu and promote angiogenesis and the maturation of endometrial blood vessels in the early stage of endometriosis [126]. Taken together, it is probable that IL-17 regulates ectopic lesions to create new blood vessels through both angiogenesis and vasculogenesis, so cutting off IL-17 might have a significant effect on blood supply of endometriotic lesions (Figure 1).
Conclusions and perspectives
In conclusion, under the regulation of estrogen and ectopic microenvironment, such as hypoxia and cytokines, the secretion of IL-17 increases, which may be derived from endometrial stromal cells or from the production of Th17 differentiation. Then IL-17 further leads to the proliferation, growth and invasion of ectopic foci, promotes the immune escape of ectopic foci and the progression of endometriosis by recruiting and inducing M2 macrophage differentiation. In addition, IL-17 may well be involved in endometriosis-related infertility by motivating the secretion of activated macrophages, accentuating inflammation, influencing the stability of fetal-maternal interface and poisoning all stages of pregnancy. So as for the treatment, IL-17 may be placed as a candidate target molecule for novel treatment strategies of endometriosis, especially when endometriosis coexists with infertility. A moderate reduction in estrogen levels, inhibition of IL-17 inducer such as IL-23, IL-27, miR-342-3p and HIF-1, promotion of negative factors such as TGF-β1 and LncRNA H19, all are worth trying to block the source of IL-17 to intervene in this disease. Similarly, intercepting the downstream pathway of IL-17 may also affect the progression of endometriosis through decreasing IL-8 and Gro-α concentrations, normalizing the number of macrophages and neutrophils, and so on. Moreover, it is reported that the concentration of IL-17 is related to the degree of disease, which may be of certain value in evaluating therapeutic effect by the way of continuous monitoring of IL-17 level. However, the value of intervening IL-17A in the treatment of endometriosis is still to be studied.
The role of IL-17 in endometriosis. Increased IL-17 in PF, serum, endometriotic lesions and etc. leads to the proliferation, invasion and implantation of ectopic endometrium partly by recruiting and activating neutrophil and M2 macrophage. And the macrophage secretes NOS2 and NO, giving rise to co-existence of endometriosis and infertility. IL-17 also acts directly on its own and indirectly through IL-8. The promotion of vasculogenesis and angiogenesis is another important role of IL-17, which can act through a variety of pathways, such as stimulation of vascular endothelial cell migration and cord formation, recruitment of lymphocytes and bone marrow-derived cells and inducing elaboration of a variety of proangiogenic factors (e.g., VEGF, IL-1β, TNF-α, and IL-8). Abbreviations: PF, peritoneal fluid; IL, interleukin; G-CSF, colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; NOS2, nitric oxide synthase 2; NO, nitric oxide; Gro-α, growth-related oncogene-α; TNF-α, tumor necrosis factor-α; ESC, endometriotic stromal cell.
Abbreviations
IL-17: Interleukin-17; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; PF: peritoneal fluid; Th17: T helper 17; TGF-β: transforming growth factor-β; RORγt: retineic-acid-receptor-related orphan receptor gamma; NK: natural killer; FF: follicular fluid; ESCs: endometriotic stromal cells; PFMCs: peritoneal fluid mononuclear cells; EMMCs: mononuclear cells from endometriotic lesions; PB: peripheral blood; E2: 17β-estradiol; P4: progesterone; Erα: estrogen receptor alpha; TNF-α: tumor necrosis factor-α; RANTES: Regulated on Activation, Normal T cell Expressed and Secreted; MCP-1: Monocyte Chemotactic Protein-1; HRF: Histamine Releasing Factor; STAT3: signal transducer and activator of transcription 3; RORC: RAR-related orphan receptor C; c-Maf: c-musculoaponeurotic fibrosarcoma; Blimp-1: B lymphocyte-induced maturation protein-1; anti-BMP1: anti-bone morphogenetic protein 1; IER3: immediate early response gene; HIF-1: Hypoxia-inducible transcription factor-1; O2: oxygen; RASF-mediated: rheumatoid arthritis synovial fibroblasts-mediated; IFN-γ: interferon-γ; COX2: Cyclooxygensase-2; VEGF: vascular endothelial growth factor; G-CSF: granulocyte colony-stimulating factor; GM-CSF: granulocyte macrophage colony-stimulating factor; Gro-α: growth-related oncogene-α; EoSCs: endometrioma stromal cells; ROS: reactive oxygen species; SPM: small peritoneal macrophage; ECM: extracellular matrix; NOS2: nitric oxide synthase 2; NO: nitric oxide; EPCs: endothelial progenitor cells.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 81971362, 92057119, 31970798), the Program for Zhuoxue of Fudan University (JIF157602) and the Support Project for Original Personalized Research of Fudan University.
Author Contributions
J.L.S. performed the literature research, wrote the manuscript, and prepared the table. Z.M.Z., M.C., and H.H.S. revised the manuscript. M.Q.L. and J.S. designed and wrote the review, supervised, and critically reviewed the complete manuscript. All authors approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246-51
2. Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999;162:494-502
3. Beringer A, Noack M, Miossec P. IL-17 in Chronic Inflammation: From Discovery to Targeting. Trends Mol Med. 2016;22:230-41
4. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123-32
5. Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nature reviews. Immunology. 2006;6:329-33
6. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-3
7. Whibley N, Gaffen SL. Gut-Busters: IL-17 Ain't Afraid of No IL-23. Immunity. 2015;43:620-2
8. Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566-73
9. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-40
10. McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 Family of Cytokines in Health and Disease. Immunity. 2019;50:892-906
11. McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B. et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford). 2017;56:1993-2003
12. Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454-67
13. Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685-91
14. Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677-88
15. Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6:1069-70
16. O'Connor W Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010;11:471-6
17. Novatchkova M, Leibbrandt A, Werzowa J, Neubüser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226-9
18. Patel DD, Kuchroo VK. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity. 2015;43:1040-51
19. Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248-56
20. Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593-603
21. Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445-56
22. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763-76
23. Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J. et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36-9
24. Buckley KM, Ho ECH, Hibino T, Schrankel CS, Schuh NW, Wang G. et al. IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. Elife. 2017 6
25. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479-89
26. Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T. et al. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794-800
27. Han Q, Das S, Hirano M, Holland SJ, McCurley N, Guo P. et al. Characterization of Lamprey IL-17 Family Members and Their Receptors. J Immunol. 2015;195:5440-51
28. Torchinsky MB, Blander JM. T helper 17 cells: discovery, function, and physiological trigger. Cell Mol Life Sci. 2010;67:1407-21
29. Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353-61
30. Karp DR, Marthandan N, Marsh SG, Ahn C, Arnett FC, Deluca DS. et al. Novel sequence feature variant type analysis of the HLA genetic association in systemic sclerosis. Hum Mol Genet. 2010;19:707-19
31. Kolls JK, McCray PB Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829-35
32. Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y. et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727-38
33. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271-9
34. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519-31
35. Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M. et al. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 2015;43:739-50
36. Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC. et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504-12
37. Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol. 2016;13:418-31
38. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-8
39. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149-62
40. Melnik BC, John SM, Chen W, Plewig G. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br J Dermatol. 2018;179:260-72
41. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668-77
42. Miller JE, Ahn SH, Marks RM, Monsanto SP, Fazleabas AT, Koti M. et al. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front Immunol. 2020;11:108
43. Song X, Qian Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal. 2013;25:2335-47
44. Braun DP, Dmowski WP. Endometriosis: abnormal endometrium and dysfunctional immune response. Curr Opin Obstet Gynecol. 1998;10:365-9
45. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-98
46. Osuga Y. Novel therapeutic strategies for endometriosis: a pathophysiological perspective. Gynecol Obstet Invest. 2008;66(Suppl 1):3-9
47. Morotti M, Vincent K, Becker CM. Mechanisms of pain in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:8-13
48. Berbic M, Fraser IS. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J Reprod Immunol. 2011;88:149-55
49. Saito S, Nakashima A, Ito M, Shima T. Clinical implication of recent advances in our understanding of IL-17 and reproductive immunology. Expert Rev Clin Immunol. 2011;7:649-57
50. Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol. 2011;7:611-26
51. Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed Res Int. 2015;2015:795976
52. Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T. et al. Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol. 2008;60:383-404
53. Kobayashi H, Iwai K, Niiro E, Morioka S, Yamada Y. Fetal programming theory: implication for the understanding of endometriosis. Hum Immunol. 2014;75:208-17
54. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10
55. Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget. 2017;8:7138-47
56. Podgaec S, Rizzo LV, Fernandes LF, Baracat EC, Abrao MS. CD4(+) CD25(high) Foxp3(+) cells increased in the peritoneal fluid of patients with endometriosis. Am J Reprod Immunol. 2012;68:301-8
57. Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139-47
58. Fan YY, Chen HY, Chen W, Liu YN, Fu Y, Wang LN. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol Endocrinol. 2018;34:507-12
59. Mariuzzi L, Domenis R, Orsaria M, Marzinotto S, Londero AP, Bulfoni M. et al. Functional expression of aryl hydrocarbon receptor on mast cells populating human endometriotic tissues. Lab Invest. 2016;96:959-71
60. Sikora J, Smycz-Kubanska M, Mielczarek-Palacz A, Bednarek I, Kondera-Anasz Z. The involvement of multifunctional TGF-beta and related cytokines in pathogenesis of endometriosis. Immunol Lett. 2018;201:31-7
61. Zhang X, Xu H, Lin J, Qian Y, Deng L. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG. 2005;112:1153-5
62. Bungum HF, Nygaard U, Vestergaard C, Martensen PM, Knudsen UB. Increased IL-25 levels in the peritoneal fluid of patients with endometriosis. J Reprod Immunol. 2016;114:6-9
63. Omwandho CO, Konrad L, Halis G, Oehmke F, Tinneberg HR. Role of TGF-betas in normal human endometrium and endometriosis. Hum Reprod. 2010;25:101-9
64. Hirata T, Osuga Y, Hamasaki K, Yoshino O, Ito M, Hasegawa A. et al. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology. 2008;149:1260-7
65. Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J. et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336-40
66. Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467-76
67. Le Jan S, Plée J, Vallerand D, Dupont A, Delanez E, Durlach A. et al. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol. 2014;134:2908-17
68. Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321-30
69. Passos ST, Silver JS, O'Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184:1776-83
70. Rei M, Gonçalves-Sousa N, Lança T, Thompson RG, Mensurado S, Balkwill FR. et al. Murine CD27(-) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014;111:E3562-70
71. Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285-94
72. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331-41
73. Khan KN, Yamamoto K, Fujishita A, Muto H, Koshiba A, Kuroboshi H. et al. Differential Levels of Regulatory T Cells and T-Helper-17 Cells in Women With Early and Advanced Endometriosis. J Clin Endocrinol Metab. 2019;104:4715-29
74. Li C, Lu Z, Bi K, Wang K, Xu Y, Guo P. et al. CD4(+)/CD8(+) mucosa-associated invariant T cells foster the development of endometriosis: a pilot study. Reprod Biol Endocrinol. 2019;17:78
75. Salmeri FM, Laganà AS, Sofo V, Triolo O, Sturlese E, Retto G. et al. Behavior of tumor necrosis factor-α and tumor necrosis factor receptor 1/tumor necrosis factor receptor 2 system in mononuclear cells recovered from peritoneal fluid of women with endometriosis at different stages. Reprod Sci. 2015;22:165-72
76. Rafi U, Ahmad S, Bokhari SS, Iqbal MA, Zia A, Khan MA. et al. Association of Inflammatory Markers/Cytokines with Cardiovascular Risk Manifestation in Patients with Endometriosis. Mediators Inflamm. 2021;2021:3425560
77. Sabbaghi M, Aram R, Roustaei H, Fadavi Islam M, Daneshvar M, Castano AR. et al. IL-17A concentration of seminal plasma and follicular fluid in infertile men and women with various clinical diagnoses. Immunol Invest. 2014;43:617-26
78. Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J Immunol. 2015;195:2591-600
79. Hirata T, Osuga Y, Takamura M, Saito A, Hasegawa A, Koga K. et al. Interleukin-17F increases the secretion of interleukin-8 and the expression of cyclooxygenase 2 in endometriosis. Fertil Steril. 2011;96:113-7
80. Gogacz M, Winkler I, Bojarska-Junak A, Tabarkiewicz J, Semczuk A, Rechberger T. et al. Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J Reprod Immunol. 2016;117:39-44
81. Takamura M, Koga K, Izumi G, Hirata T, Harada M, Hirota Y. et al. Simultaneous Detection and Evaluation of Four Subsets of CD4+ T Lymphocyte in Lesions and Peripheral Blood in Endometriosis. Am J Reprod Immunol. 2015;74:480-6
82. Liu Z, Liu L, Zhong Y, Cai M, Gao J, Tan C. et al. LncRNA H19 over-expression inhibited Th17 cell differentiation to relieve endometriosis through miR-342-3p/IER3 pathway. Cell Biosci. 2019;9:84
83. Wu XG, Chen JJ, Zhou HL, Wu Y, Lin F, Shi J. et al. Identification and Validation of the Signatures of Infiltrating Immune Cells in the Eutopic Endometrium Endometria of Women With Endometriosis. Front Immunol. 2021;12:671201
84. Andreoli CG, Genro VK, Souza CA, Michelon T, Bilibio JP, Scheffel C. et al. T helper (Th)1, Th2, and Th17 interleukin pathways in infertile patients with minimal/mild endometriosis. Fertil Steril. 2011;95:2477-80
85. Tarokh M, Ghaffari Novin M, Poordast T, Tavana Z, Nazarian H, Norouzian M. et al. Serum and Peritoneal Fluid Cytokine Profiles in Infertile Women with Endometriosis. Iran J Immunol. 2019;16:151-62
86. Khan D, Dai R, Karpuzoglu E, Ahmed SA. Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur J Immunol. 2010;40:2549-56
87. Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS. et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1025-34 e11
88. Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L272-8
89. Lélu K, Laffont S, Delpy L, Paulet PE, Périnat T, Tschanz SA. et al. Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386-93
90. Relloso M, Aragoneses-Fenoll L, Lasarte S, Bourgeois C, Romera G, Kuchler K. et al. Estradiol impairs the Th17 immune response against Candida albicans. J Leukoc Biol. 2012;91:159-65
91. Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PloS One. 2012;7:e44552
92. Wang Y, Cela E, Gagnon S, Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res. 2010;11:166
93. Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H. et al. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A. 2012;109:9505-10
94. Ning C, Xie B, Zhang L, Li C, Shan W, Yang B. et al. Infiltrating Macrophages Induce ERα Expression through an IL17A-mediated Epigenetic Mechanism to Sensitize Endometrial Cancer Cells to Estrogen. Cancer Res. 2016;76:1354-66
95. Halme J. Release of tumor necrosis factor-alpha by human peritoneal macrophages in vivo and in vitro. Am J Obstet Gynecol. 1989;161:1718-25
96. Akoum A, Lemay A, Brunet C, Hébert J. Cytokine-induced secretion of monocyte chemotactic protein-1 by human endometriotic cells in culture. The Groupe d'Investigation en Gynécologie. Am J Obstet Gynecol. 1995;172:594-600
97. Hornung D, Klingel K, Dohrn K, Kandolf R, Wallwiener D, Taylor RN. Regulated on Activation, Normal T-Cell-Expressed and -Secreted mRNA Expression in Normal Endometrium and Endometriotic Implants: Assessment of Autocrine/Paracrine Regulation by in situ Hybridization. Am J Pathol. 2001;158:1949-54
98. Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910-4
99. Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218-22
100. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-41
101. Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H. et al. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORγt/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017;8:e2666
102. Kim SK, Henen MA, Hinck AP. Structural biology of betaglycan and endoglin, membrane-bound co-receptors of the TGF-beta family. Exp Biol Med (Maywood). 2019;244:1547-58
103. Schubert CL, Yusuf K. Serum levels of TGF-β1, cytokines, angiogenic, and anti-angiogenic factors in pregnant women who smoke. J Reprod Immunol. 2021;147:103351
104. Kong N, Lan Q, Chen M, Wang J, Shi W, Horwitz DA. et al. Antigen-specific transforming growth factor β-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthritis Rheum. 2012;64:2548-58
105. Han X, Hu F, Chen F, Wang W. The inhibition of bone morphogenetic protein 1 attenuates endometriosis lesions in vivo and in vitro. Arch Gynecol Obstet. 2020;302:415-22
106. Zhi L, Ustyugova IV, Chen X, Zhang Q, Wu MX. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J Immunol. 2012;189:1639-47
107. Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106:402-9
108. Ghazal S, McKinnon B, Zhou J, Mueller M, Men Y, Yang L. et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med. 2015;7:996-1003
109. Korucuoglu U, Biri AA, Konac E, Alp E, Onen IH, Ilhan MN. et al. Expression of the imprinted IGF2 and H19 genes in the endometrium of cases with unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 2010;149:77-81
110. Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537-47
111. Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y. et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772-84
112. Hu F, Mu R, Zhu J, Shi L, Li Y, Liu X. et al. Hypoxia and hypoxia-inducible factor-1α provoke toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann Rheum Dis. 2014;73:928-36
113. Talreja J, Talwar H, Bauerfeld C, Grossman LI, Zhang K, Tranchida P. et al. HIF-1α regulates IL-1β and IL-17 in sarcoidosis. Elife. 2019 8
114. Hirata T, Osuga Y, Takamura M, Kodama A, Hirota Y, Koga K. et al. Recruitment of CCR6-expressing Th17 cells by CCL 20 secreted from IL-1 beta-, TNF-alpha-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology. 2010;151:5468-76
115. Arici A, Seli E, Zeyneloglu HB, Senturk LM, Oral E, Olive DL. Interleukin-8 induces proliferation of endometrial stromal cells: a potential autocrine growth factor. J Clin Endocrinol Metab. 1998;83:1201-5
116. Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int Rev Immunol. 1998;16:541-51
117. Akoum A, Lawson C, McColl S, Villeneuve M. Ectopic endometrial cells express high concentrations of interleukin (IL)-8 in vivo regardless of the menstrual cycle phase and respond to oestradiol by up-regulating IL-1-induced IL-8 expression in vitro. Mol Hum Reprod. 2001;7:859-66
118. Arici A. Local cytokines in endometrial tissue: the role of interleukin-8 in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2002;955:101-406
119. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1-10
120. Terui T. Inflammatory and immune reactions associated with stratum corneum and neutrophils in sterile pustular dermatoses. Tohoku J Exp Med. 2000;190:239-48
121. Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z. et al. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783-9
122. Takamura M, Osuga Y, Izumi G, Yoshino O, Koga K, Saito A. et al. Interleukin-17A is present in neutrophils in endometrioma and stimulates the secretion of growth-regulated oncogene-α (Gro-α) from endometrioma stromal cells. Fertil Steril. 2012;98:1218-24 e1-2
123. Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem. 1993;268:15419-24
124. Glynn PC, Henney EM, Hall IP. Peripheral blood neutrophils are hyperresponsive to IL-8 and Gro-alpha in cryptogenic fibrosing alveolitis. Eur Respir J. 2001;18:522-9
125. Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol Endocrinol. 2009;25:75-81
126. Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147:1278-86
127. Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q. et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7:1091-100
128. Okizaki S, Ito Y, Hosono K, Oba K, Ohkubo H, Amano H. et al. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed Pharmacother. 2015;70:317-25
129. Weber C, Telerman SB, Reimer AS, Sequeira I, Liakath-Ali K, Arwert EN. et al. Macrophage Infiltration and Alternative Activation during Wound Healing Promote MEK1-Induced Skin Carcinogenesis. Cancer Res. 2016;76:805-17
130. Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450-62
131. Miljkovic D, Trajkovic V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004;15:21-32
132. Antsiferova YS, Sotnikova NY, Posiseeva LV, Shor AL. Changes in the T-helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertil Steril. 2005;84:1705-11
133. Othman Eel D, Hornung D, Salem HT, Khalifa EA, El-Metwally TH, Al-Hendy A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;137:240-6
134. Gazvani MR, Christmas S, Quenby S, Kirwan J, Johnson PM, Kingsland CR. Peritoneal fluid concentrations of interleukin-8 in women with endometriosis: relationship to stage of disease. Hum Reprod. 1998;13:1957-61
135. Gómez-Torres MJ, Acién P, Campos A, Velasco I. Embryotoxicity of peritoneal fluid in women with endometriosis. Its relation with cytokines and lymphocyte populations. Hum Reprod. 2002;17:777-81
136. Iwabe T, Harada T, Terakawa N. Role of cytokines in endometriosis-associated infertility. Gynecol Obstet Invest. 2002;53(Suppl 1):19-25
137. Overton C, Fernandez-Shaw S, Hicks B, Barlow D, Starkey P. Peritoneal fluid cytokines and the relationship with endometriosis and pain. Hum Reprod. 1996;11:380-6
138. Podgaec S, Abrao MS, Dias JA Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373-9
139. Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. 1996;174:1522-6
140. Chavan AR, Griffith OW, Stadtmauer DJ, Maziarz J, Pavlicev M, Fishman R. et al. Evolution of Embryo Implantation Was Enabled by the Origin of Decidual Stromal Cells in Eutherian Mammals. Mol Biol Evol. 2021;38:1060-74
141. Wang WJ, Zhang H, Chen ZQ, Zhang W, Liu XM, Fang JY. et al. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17:2
142. Nisolle M, Casanas-Roux F, Anaf V, Mine JM, Donnez J. Morphometric study of the stromal vascularization in peritoneal endometriosis. Fertil Steril. 1993;59:681-4
143. Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671-4
144. Sajib S, Zahra FT, Lionakis MS, German NA, Mikelis CM. Mechanisms of angiogenesis in microbe-regulated inflammatory and neoplastic conditions. Angiogenesis. 2018;21:1-14
145. Vallée A, Guillevin R, Vallée JN. Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas. Rev Neurosci. 2018;29:71-91
146. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T. et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620-7
147. Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E. et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698-704
Author contact
![]() Corresponding authors: Jun Shao, E-mail: junshaoedu.cn; or Ming-Qing Li, E-mail: mqliedu.cn.
Corresponding authors: Jun Shao, E-mail: junshaoedu.cn; or Ming-Qing Li, E-mail: mqliedu.cn.

 Global reach, higher impact
Global reach, higher impact