3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(13):2890-2896. doi:10.7150/ijms.58043 This issue Cite
Research Paper
Role of Unfolded Protein Response and Endoplasmic Reticulum-Associated Degradation by Repeated Exposure to Inhalation Anesthetics in Caenorhabditis elegans
Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea.
Received 2021-1-10; Accepted 2021-5-19; Published 2021-6-1
Abstract
Background: When an imbalance occurs between the demand and capacity for protein folding, unfolded proteins accumulate in the endoplasmic reticulum (ER) lumen and activate the unfolded protein response (UPR). In addition, unfolded proteins are cleared from the ER lumen for ubiquitination and subsequent cytosolic proteasomal degradation, which is termed as the ER-associated degradation (ERAD) pathway. This study focused on changes in the UPR and ERAD pathways induced by the repeated inhalation anesthetic exposure in Caenorhabditis elegans.
Methods: Depending on repeated isoflurane exposure, C. elegans was classified into the control or isoflurane group. To evaluate the expression of a specific gene, RNA was extracted from adult worms in each group and real-time polymerase chain reaction was performed. Ubiquitinated protein levels were measured using western blotting, and behavioral changes were evaluated by chemotaxis assay using various mutant strains.
Results: Isoflurane upregulated the expression of ire-1 and pek-1 whereas the expression of atf-6 was unaffected. The expression of both sel-1 and sel-11 was decreased by isoflurane exposure, possibly indicating the inhibition of retro-translocation. The expression of cdc-48.1 and cdc-48.2 was decreased and higher ubiquitinated protein levels were observed in the isoflurane group than in the control, suggesting that deubiquitination and degradation of misfolded proteins were interrupted. The chemotaxis indices of ire-1, pek-1, sel-1, and sel-11 mutants decreased significantly compared to N2, and they were not suppressed further even after the repeated isoflurane exposure.
Conclusion: Repeated isoflurane exposure caused significant ER stress in C. elegans. Following the increase in UPR, the ERAD pathway was disrupted by repeated isoflurane exposure and ubiquitinated proteins was accumulated subsequently. UPR and ERAD pathways are potential modifiable neuroprotection targets against anesthesia-induced neurotoxicity.
Keywords: Caenorhabditis elegans, endoplasmic reticulum-associated degradation pathway, inhalation anesthetics, unfolded protein response
Introduction
General anesthesia is an essential practice for various surgeries, and inhalation anesthetics are commonly used for general anesthesia, either alone or in combination with other drugs. Over the past decade, extensive pre-clinical researches have consistently shown that anesthetic exposure during early post-natal period can cause the neurotoxicity leading to the behavioral or cognitive defects [1-3]. However, it is still controversial whether the animal studies can translate to clinical field. Recent three studies [4-6] including large population stated that developmental neurotoxicity may not exist in single brief anesthetic exposure during early life. In 2016, the U.S. Food and Drug Administration issued a warning regarding anesthetic and sedative agents describing the potential risk of anesthesia-induced neurotoxicity (AIN) in children aged below 3 years, particularly when they are exposed to these drugs over a long term or repeatedly [7].
Diverse mechanisms related to cell death, growth factor signaling, mitochondria, N-methyl D-aspartate, γ-aminobutyric acid, or the endoplasmic reticulum (ER) have been postulated as an underlying process of AIN [8]. In addition, the mammalian target of rapamycin signaling pathway [9] and brain-derived neurotrophic factor were known to be modulated by anesthesia in developing nervous system [10]. However, the precise mechanism underlying AIN has not yet been determined, and this study focused on ER stress and the following changes in the unfolded protein response (UPR) responses and ER-associated degradation (ERAD) pathway after repeated inhalation anesthetic exposure.
The ER is an intracellular organelle that facilitates the folding and maturation of protein molecules and their transport to the Golgi apparatus. The release of excessive Ca2+ from the ER and the relationship of ryanodine receptors have been studied as a causative factor of anesthesia-induced ER stress [11-13]. Excessive cytosolic Ca2+ changes the protein-folding environment in the ER, leading to ER stress [14]. When an imbalance occurs between the demand and capacity for protein folding, unfolded or misfolded proteins accumulate in the ER lumen and activate UPR [15]. In addition, misfolded proteins are cleared from the ER lumen for ubiquitination and subsequent cytosolic proteasomal degradation, which is termed as ERAD pathway [16]. This process controls the quality and quantity of proteins.
Caenorhabditis elegans contains several orthologous genes involved in the human UPR and ERAD pathways, and they were evaluated in several conditions such as aging or neurodegenerative diseases [17, 18]. This study evaluated changes in the UPR and ERAD pathways induced by the repeated inhalation anesthetic exposure in Caenorhabditis elegans.
Methods
Caenorhabditis elegans strains and anesthesia exposure
Wild-type N2, zcls4[hsp-4::GFP]V, ire-1(ok799) II, pek-1(ok275) X, atf-6(ok551) X, sel-1(mg547) V, cdc-48.1(tm544) II, and cdc-48.2(tm659) II C. elegans were obtained from the Caenorhabditis Genetics Center (Minneapolis, MN, USA). RNA interference (RNAi) was performed when the sel-11 strain was used. Culturing, synchronization, anesthesia, and chemotaxis assay were performed according to the methods described in our previous paper [19]. Isoflurane was used for the anesthesia of C. elegans, and was administered four times during each stage from L1 to L4. The concentration of isoflurane was the 99.9% effective immobilizing dose, which was determined by pilot examination and used in our previous study [19]. Depending on isoflurane exposure, the worms were classified into the control or isoflurane group.
RNA preparation and real-time polymerase chain reaction (PCR)
To evaluate the expression of a specific gene, RNA was extracted from adult worms in each group. After collecting the worm pellet, it was ground to a fine frozen powder using liquid nitrogen. After adding a RLT butter, ethanol, and RW1 buffer sequentially to the frozen powdered worm, purified RNA was extracted using RNeasy mini spin column. Finally, RNase-free water was added to the extracted RNA, which was frozen at -80°C until use. Using a NanoDrop (ND-2000, Thermo Fisher Scientific, MA, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, USA), the ratios of absorbance at 260-280 nm (OD 260/280) and at 260-230 nm (OD 260/230) were determined and the quality-controlled RNA (OD 260/280 >1.5 and OD 260/230 >1.0) was used for real-time polymerase chain reaction. After first-strand cDNA synthesis using Maxima H minus First strand cDNA Synthesis Kit (Thermo Fisher Scientific, MA, USA), real-time PCR was performed using the cDNA, each gene-specific primer (Table 1), and Power SYBT Green PCR Master mix (Applied Biosystems, MA, USA). Pan-actin was used as a reference gene and the ΔCT was calculated.
Western blotting
C. elegans were washed with S-basal buffer three times and lysed in cold RIPA lysis buffer (BRA0500, BIOMAX; 25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 1% sodium dodecyl sulfate) containing 100 mM iodoacetamide. Protein concentrations were measured using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, MA, USA). Equal amounts of target proteins, normalized to the actin level, in 2X Laemmli sample buffer (Bio-Rad, CA, USA) were heated at 95 °C for 5 min. Proteins were resolved by 8-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking with 5% skim milk in a Tris-buttered saline with0.1% Tween® 20 buffer (20 mM Tris, 500 mM sodium chloride, and 0.1% Tween 20, pH 7.5) for 1 h at 20 °C, the membranes were incubated overnight at 4 °C with anti-ubiquitin antibody (1:1000; Abcam, Cambridge, UK) and anti-actin antibody (1:5000; Abcam, Cambridge, UK). After secondary antibody treatment for 1 h at 20 °C, the membranes were developed using an enhanced chemiluminescence kit (DG-W250, DoGen, Korea). Horseradish peroxidase-conjugated secondary antibodies (sc-516102) were obtained from Santa Cruz Biotechnology (Texas, USA).
Forward and Reverse Primer Sequences for Real-Time PCR
| Primer | Sequence |
|---|---|
| ire-1 | |
| Forward | ACAATGGCTAGTCAGCGAGG |
| Reverse | CTTCTGGAGCAATCCAGCCA |
| pek-1 | |
| Forward | TGACATTGACACCGACGAGG |
| Reverse | TGCCCGATGACCTTCTTGAC |
| atf-6 | |
| Forward | ATCGTTGCTCCTGCCTAGTG |
| Reverse | TCAATTGGCCAGTCCCTGTC |
| sel-1 | |
| Forward | GTGGACGAGGGCTCAATCAA |
| Reverse | AATGCATCGGCACTTCCTGA |
| sel-11 | |
| Forward | GCGTCTTCCACACCAACAAC |
| Reverse | CCTAGAAGACGTGCTAGGCG |
| cdc-48.1 | |
| Forward | TGCTCACAATGTGGTTCGGA |
| Reverse | GAACAACACGCAAGGAGCAG |
| cdc-48.2 | |
| Forward | GAGAAGCGTATCGTCTCGCA |
| Reverse | TTAGTAGCGGCGATCACGAC |
| pan-actin | |
| Forward | TCGGTATGGGACAGAAGGAC |
| Reverse | CATCCCAGTTGGTGACGATA |
Fluorescence imaging
In zcls4[hsp-4::GFP]V strain, green fluorescence protein (GFP) expression was measured using a Zeiss LSM 710 confocal microscope system (Oberkochen, Germany). L4 stage worms were mounted on agar pad after immobilizing them by sodium azide.
Chemotaxis assay
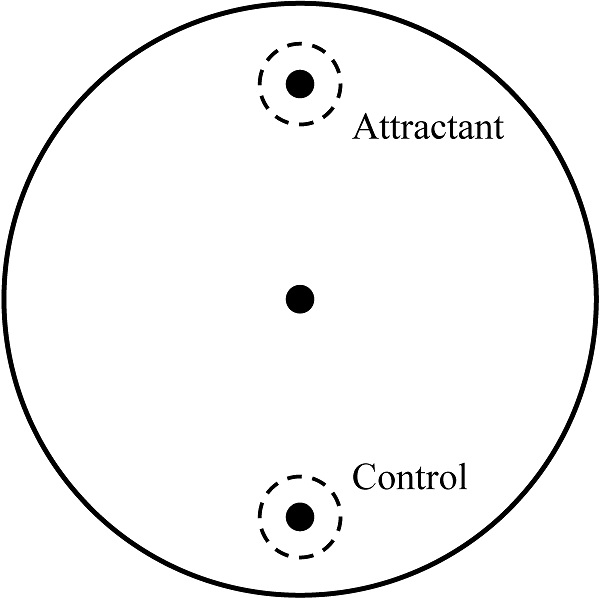
Chemotaxis assay was performed by one experimenter blind to condition to confirm the abnormal behavioral pattern when C. elegans reached the young adult stage as described in our previous study [19]. About 50 young adult worms, which were washed by S-basal buffer, were transferred to the center of the chemotaxis plates (Fig. 1). After 1 h, the number of the worms on each side was counted and chemotaxis index was calculated using the equation: (number of A point - number of C point)/total number of worms  100 (%). Chemotaxis assay was performed three times and all batches included 3 plates in each group.
100 (%). Chemotaxis assay was performed three times and all batches included 3 plates in each group.
Chemotaxis assay plate. A 9-cm petri plate covered with nutrient growth medium was used for chemotaxis assay. OP50 was used for attractant, and control site was blank. Before placing worm pellet in the center, 1 μl of 1 M sodium azide was dropped in both sites to immobilize worms when they reached there. Number of worms in a circle within 1.5-cm radius at each point and in other zone was counted and used for chemotaxis index calculation.
Statistics
Data are presented as the mean and standard deviation. Due to the small sample size, nonparametric test, Mann-Whitney U test, was used to determine the significance of differences between the two groups. Statistical analyses were performed using SPSS (version 21.0; IBM Co., Armonk, NY, USA), and P values of < 0.05 were considered to indicate significant differences.
Results
HSP-4, a heat shock protein in C. elegans, is used to monitor ER stress and it is homologous to binding immunoglobulin protein (BiP) in mammals. The ER-specific heat shock protein HSP-4 reporter (Phsp-4::gfp) was upregulated in L1 larvae after isoflurane exposure, as reported previously [20]. After repeated isoflurane exposure during the developmental period, the expression of hsp-4::GFP was increased (Fig. S1). Real-time PCR was performed to validate the expression of hsp-4. In isoflurane-exposed worms, the expression of hsp-4 was induced (1.4 ± 0.1 in isoflurane group compared to the normalized control group; P <0.001).
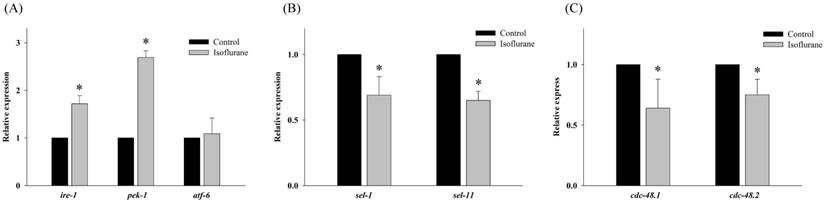
To determine the effects of isoflurane on the regulation of the UPR and ERAD pathway in AIN, the expression of related genes was evaluated by real-time PCR under the same conditions. Further, ire-1, pek-1, and atf-6 correspond to inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) in humans and are related to UPR in C. elegans. Isoflurane upregulated the expression of ire-1 and pek-1 (P <0.001 in both); however, the expression of atf-6 (P = 0.726) remained unaffected (Fig. 2A).
Expression of genes related to the unfolded protein response and endoplasmic reticulum associated degradation. (A) Isoflurane upregulated the expression of ire-1 and pek-1; however, the expression of atf-6 remained unaffected. (B) The expression of both sel-1 and sel-11 was decreased by isoflurane exposure. (C) The expression of cdc-48.1 and cdc-48.2 was decreased by isoflurane exposure. All batches included 3 plates in each group and same assay was performed three times. Error bar, standard deviation; *P<0.001.
Western blotting for ubiquitinated proteins. (A, B) Higher levels of ubiquitinated protein were observed in the isoflurane group. All batches included 3 plates in each group and same assay was performed three times. Error bar, standard deviation; *P<0.001.
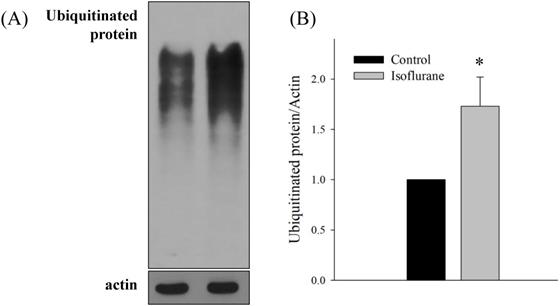
Both sel-1 and sel-11 are orthologs of human SEL-1L and HRD1, which are related to the ERAD pathway and induced by ER stress. Interestingly, the expression of both sel-1 and sel-11 was decreased by isoflurane exposure (P <0.001 in both) (Fig. 2B). Polyubiquitin chains bind to proteins destined for degradation to serve as a degradation signal. Both cdc-48.1 and cdc-48.2 are orthologs of P97, which facilitates the degradation of large amounts of misfolded proteins. The expression of cdc-48.1 and cdc-48.2 was decreased by isoflurane exposure (P <0.001 in both) (Fig. 2C). The decreased expression of sel-1, sel-11, cdc-48.1 and cdc-48.2 suggests that the ERAD pathway was inhibited. Therefore, the levels of ubiquitinated proteins were investigated by western blotting with an anti-ubiquitin antibody. Higher levels of ubiquitinated protein were observed in the isoflurane group than in the control group (P <0.001), suggesting that ERAD was interrupted by isoflurane (Fig. 3).
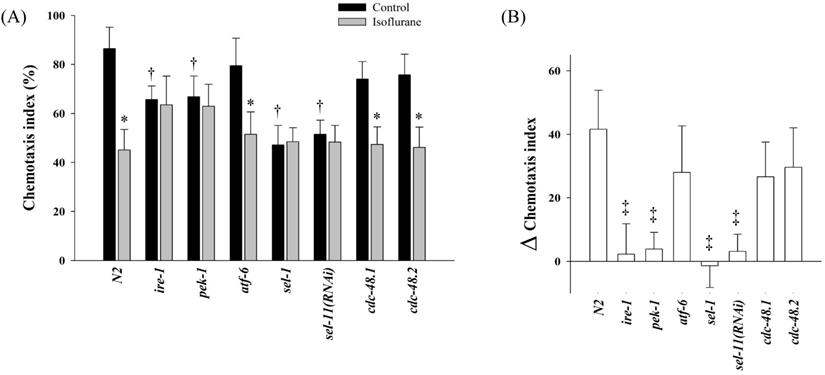
The chemotaxis index of N2 was 86.4 ± 8.8% in the control group, whereas it was 45.1 ± 8.4% in the isoflurane group (P = 0.001). In several mutant strains related UPR and ERAD, the chemotaxis indices were measured (Fig. 4). In the control groups, the chemotaxis indices of ire-1, pek-1, sel-1, and sel-11(RNAi) decreased significantly compared to those of N2 (P < 0.001). The chemotaxis indices were not suppressed further even after the repeated isoflurane exposure in these four mutants.
Discussion
We found that repeated exposure to isoflurane induced the expression of ER chaperones and UPR in C. elegans, indicating an increase of ER stress. To the best our knowledge, it is observed for the first time that ubiquitinated proteins were accumulated considerably with a disrupted ERAD pathway in the isoflurane group than in the control group.
BiP is an ER chaperone present on the ER membrane in the absence of ER stress. However, it dissociates from the ER membrane and binds to misfolded proteins during ER stress [21]. In addition, three membrane proteins, namely PERK, ATF6, and IRE1, are activated and initiate the UPR signaling pathway [22].
Chemotaxis index. In several mutant strains related unfolded protein response and endoplasmic reticulum-associated degradation, the chemotaxis indices were measured (A) and the difference between the control and isoflurane group was calculated in each strains (B). All batches included 3 plates in each group and same assay was performed three times. Error bar, standard deviation; *P <0.05 vs. control in each strain; †P = 0.001 vs. N2 control group; ‡P <0.001 vs. N2.
In C. elegans, hsp-4, pek-1, atf-6, and ire-1 are the homologues of human BiP, PERK, ATF6, and IRE1, respectively. It is known that hsp-4 is induced under ER stress in C. elegans and their UPR is very similar to that of humans [23]. Higher expression of hsp-4 after repeated exposure to isoflurane indicates that isoflurane induces ER stress in C. elegans; a similar result was observed in a previous study [20]. Interestingly, two major UPR regulators, pek-1 and ire-1, were induced in isoflurane-exposed C. elegans, whereas atf-6 was not affected. We could not determine the exact cause of the gene expression mismatch among the three UPR regulators; however, the activation processes differed among the three UPR genes. A previous study reported that IRE1 and PERK have a similar luminal domain and detect unfolded proteins through the same mechanism [24]. IRE1 and PERK exist in an inactive state by binding with BiP in the absence of ER stress. However, ER stress causes BiP to dissociate from the luminal domains of IRE1 and PERK, resulting in their oligomerization and autophosphorylation [25]. In contrast, ER stress causes the translocation of ATF6 from the ER to the Golgi, where it is cleaved to its active form [26] that becomes dominant in the absence of ER stress [27]. Thus, cleaved atf-6 might not be detected by real-time PCR, which caused the discrepancy in gene expression of the three UPR regulators in this study.
Misfolded proteins are retrotranslocated from the ER lumen into the cytosol, and they are recognized and cleared by the ERAD pathway to maintain ER homeostasis [28]. HRD1 and SEL-1L, the most representative ERAD complex, correspond to sel-11 and sel-1 in C. elegans, respectively. Previously, deletion of HRD1 or SEL1L in mice was reported to cause embryonic or premature death [29-31]. The pathobiological role of the E3 ubiquitin ligase HRD1 and its adaptor protein SEL1L was previously evaluated, and their importance was demonstrated in neurodegenerative diseases. Both were proposed to be involved in the pathogenesis of and identified as therapeutic targets against Parkinson's disease [32, 33]. HRD1 was also involved in the accumulation of the amyloid precursor protein and the subsequent production of amyloid β, which is linked to Alzheimer's disease [34-36]. Polymorphism in SEL1L may also be a susceptibility factor for Alzheimer's disease [37]. According to our results, the expression of sel-11 and sel-1 was suppressed by repeated isoflurane exposure, which might have increased the hsp-4::GFP expression sequestrated to the misfolded protein in the ER lumen.
Retrotranslocated misfolded proteins are modified with ubiquitin, and p97 (also known as valosin-containing protein) guides these proteins to the proteasome for degradation [38]. P97 is a component of the ERAD pathway and its gene deletion can have fatal consequences [39]. The orthologs of p97 are cdc-48.1 and cdc-48.2 in C. elegans, and repeated isoflurane-associated decreases in these genes induced aggregation of polyubiquitin-conjugated proteins. The defective ubiquitin-proteasome system interrupts the degradation of retrotranslocated misfolded proteins via the proteasome. Accumulation and aggregation of neurotoxic proteins by dysregulation of the ubiquitin-proteasome system is known to be associated with numerous neurodegenerative diseases [40]. Particularly, polyglutamine aggregation causes several neurodegenerative diseases including Huntington's or Machado-Joseph disease, and p97 homologs have been reported to play a protective role in polyglutamine aggregates [18, 41]. Although we could not identify whether the accumulated ubiquitinated proteins act as toxic aggregates, we found that repeated isoflurane exposure might interrupt the elimination of aggregate formation in C. elegans. A previous study showed that inhalation anesthetic can induce neuronal protein aggregation and mislocalization [42].
Behavioral change was evaluated by chemotaxis assay in our study. The significant decrease in chemotaxis index after repeated isoflurane exposure in wild-type N2 might be caused by increased ER stress and defective ERAD. UPR and ERAD pathway essentially play a role in the stress response, and the aforementioned results were observed in the nervous system as well as in the non-neuronal tissue throughout the entire body of C. elegans; however, they are finally involved in maintaining a variety of physiological conditions in a normal state [17, 23, 43]. Thus, it might be said that developmental repeated exposure of C. elegans to isoflurane worsen the chemotaxis index.
Interestingly, several mutants showed different patterns in chemotaxis indices. Loss of ire-1 or pek-1 was known to affect various basal physiology, such as secretory-protein metabolism, longevity, or development [43-45]; therefore, the basal chemotaxis indices of ire-1 and pek-1 mutants seemed to decrease in the control group compared to that of N2. Unlike ire-1 or pek-1, the atf-6 mutants were known to have extended lifespan without sensitivity to proteotoxic stress [46, 47], and the results of chemotaxis index were not different from that of N2. The other two mutants, sel-1and sel-11, also showed different chemotaxis indices from those of N2. Loss of sel-1 or sel-11 is able to induce ER stress [48] or lead to behavioral defects [49], respectively, which appeared to decrease chemotaxis index in each control group of sel-1 and sel-11 compared to the N2 control. Moreover, repeated isoflurane no longer suppressed the chemotaxis indices further in ire-1, pek-1, sel-1, and sel-11, which could be interpreted that those four genes might be significantly affected by repeated isoflurane exposure during developmental period in C. elegans. Both cdc-48.1 and cdc-48.2 were known to act redundantly in the elimination of misfolded protein from ER [50, 51]; thus, either cdc-48.1 or cdc-48.2 single mutant did not seem to present any difference from the N2 in their chemotaxis assay.
This study had several limitations. First, it is unclear if suppression of each gene may result in decreased levels of each protein. We could not investigate all protein levels involved in the UPR and ERAD pathway because of antibody unavailability for C. elegans. Furthermore, we did not investigate the phosphorylation of each UPR gene or subsequent initiation of downstream signaling events. Finally, this study was conducted in C. elegans. Although this model is valuable for studying certain signaling pathways and cellular processes, which have been well-conserved in humans, it is unclear whether our conclusion can be extrapolated to humans. Despite these limitations, our findings revealed that repeated isoflurane exposure leads to defective expression of genes associated with the UPR and ERAD pathways. Studies are needed to determine how this abnormal gene expression influences potential neurodegenerative consequences by anesthetic agents.
In conclusion, we showed that repeated isoflurane exposure caused significant ER stress in C. elegans. Following the increase in UPR, the ERAD pathway was disrupted by repeated isoflurane exposure and ubiquitinated proteins was accumulated subsequently. UPR and ERAD pathways are potential vulnerable neuroprotective targets against anesthesia-induced neurotoxicity.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding statement
This study was supported by grant No. 02-2020-007 from the SNUBH Research Fund.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lin EP, Lee JR, Lee CS, Deng M, Loepke AW. Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicol Teratol. 2017;60:117-28
2. Andropoulos DB. Effect of Anesthesia on the Developing Brain: Infant and Fetus. Fetal Diagn Ther. 2018;43:1-11
3. Walters JL, Paule MG. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol. 2017;60:2-23
4. Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC. et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA. 2016;315:2312-20
5. McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G. et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393:664-77
6. Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ. et al. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology. 2018;129:89-105
7. FDA US. FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. 2016.
8. Jackson WM, Gray CD, Jiang D, Schaefer ML, Connor C, Mintz CD. Molecular Mechanisms of Anesthetic Neurotoxicity: A Review of the Current Literature. J Neurosurg Anesthesiol. 2016;28:361-72
9. Ju X, Ryu MJ, Cui J, Lee Y, Park S, Hong B. et al. The mTOR Inhibitor Rapamycin Prevents General Anesthesia-Induced Changes in Synaptic Transmission and Mitochondrial Respiration in Late Postnatal Mice. Front Cell Neurosci. 2020;14:4
10. Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603-15
11. Jevtovic-Todorovic V, Boscolo A, Sanchez V, Lunardi N. Anesthesia-induced developmental neurodegeneration: the role of neuronal organelles. Front Neurol. 2012;3:141
12. Jevtovic-Todorovic V. Exposure of Developing Brain to General Anesthesia: What Is the Animal Evidence? Anesthesiology. 2018;128:832-9
13. Wang H, Dong Y, Zhang J, Xu Z, Wang G, Swain CA. et al. Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br J Anaesth. 2014;113:695-707
14. Krebs J, Agellon LB, Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem Biophys Res Commun. 2015;460:114-21
15. Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29-63
16. Ruggiano A, Foresti O, Carvalho P. Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol. 2014;204:869-79
17. Xu Y, Park Y. Application of Caenorhabditis elegans for Research on Endoplasmic Reticulum Stress. Prev Nutr Food Sci. 2018;23:275-81
18. Yamanaka K, Okubo Y, Suzaki T, Ogura T. Analysis of the two p97/VCP/Cdc48p proteins of Caenorhabditis elegans and their suppression of polyglutamine-induced protein aggregation. J Struct Biol. 2004;146:242-50
19. Do SH, Lee SY, Na HS. The effect of repeated isoflurane exposure on serine synthesis pathway during the developmental period in Caenorhabditis elegans. Neurotoxicology. 2019;71:132-7
20. Na HS, Brockway NL, Gentry KR, Opheim E, Sedensky MM, Morgan PG. The genetics of isoflurane-induced developmental neurotoxicity. Neurotoxicol Teratol. 2017;60:40-9
21. Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71-92
22. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326-35
23. Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A. et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893-903
24. Carrara M, Prischi F, Nowak PR, Ali MM. Crystal structures reveal transient PERK luminal domain tetramerization in endoplasmic reticulum stress signaling. EMBO J. 2015;34:1589-600
25. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326-32
26. Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99-111
27. Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem. 2002;277:13045-52
28. Qi L, Tsai B, Arvan P. New Insights into the Physiological Role of Endoplasmic Reticulum-Associated Degradation. Trends Cell Biol. 2017;27:430-40
29. Francisco AB, Singh R, Li S, Vani AK, Yang L, Munroe RJ. et al. Deficiency of suppressor enhancer Lin12 1 like (SEL1L) in mice leads to systemic endoplasmic reticulum stress and embryonic lethality. J Biol Chem. 2010;285:13694-703
30. Yagishita N, Ohneda K, Amano T, Yamasaki S, Sugiura A, Tsuchimochi K. et al. Essential role of synoviolin in embryogenesis. J Biol Chem. 2005;280:7909-16
31. Sun S, Shi G, Han X, Francisco AB, Ji Y, Mendonca N. et al. Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival. Proc Natl Acad Sci U S A. 2014;111:E582-91
32. Omura T, Kaneko M, Okuma Y, Matsubara K, Nomura Y. Endoplasmic reticulum stress and Parkinson's disease: the role of HRD1 in averting apoptosis in neurodegenerative disease. Oxid Med Cell Longev. 2013;2013:239854
33. Omura T, Matsuda H, Nomura L, Imai S, Denda M, Nakagawa S. et al. Ubiquitin ligase HMG-CoA reductase degradation 1 (HRD1) prevents cell death in a cellular model of Parkinson's disease. Biochem Biophys Res Commun. 2018;506:516-21
34. Kaneko M, Koike H, Saito R, Kitamura Y, Okuma Y, Nomura Y. Loss of HRD1-mediated protein degradation causes amyloid precursor protein accumulation and amyloid-beta generation. J Neurosci. 2010;30:3924-32
35. Saito R, Kaneko M, Kitamura Y, Takata K, Kawada K, Okuma Y. et al. Effects of oxidative stress on the solubility of HRD1, a ubiquitin ligase implicated in Alzheimer's disease. PLoS One. 2014;9:e94576
36. Kaneko M, Okuma Y, Nomura Y. Molecular approaches to the treatment, prophylaxis, and diagnosis of Alzheimer's disease: possible involvement of HRD1, a novel molecule related to endoplasmic reticulum stress, in Alzheimer's disease. J Pharmacol Sci. 2012;118:325-30
37. Saltini G, Dominici R, Lovati C, Cattaneo M, Michelini S, Malferrari G. et al. A novel polymorphism in SEL1L confers susceptibility to Alzheimer's disease. Neurosci Lett. 2006;398:53-8
38. Kloppsteck P, Ewens CA, Forster A, Zhang X, Freemont PS. Regulation of p97 in the ubiquitin-proteasome system by the UBX protein-family. Biochim Biophys Acta. 2012;1823:125-9
39. Muller JM, Deinhardt K, Rosewell I, Warren G, Shima DT. Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem Biophys Res Commun. 2007;354:459-65
40. Zheng Q, Huang T, Zhang L, Zhou Y, Luo H, Xu H. et al. Dysregulation of Ubiquitin-Proteasome System in Neurodegenerative Diseases. Front Aging Neurosci. 2016;8:303
41. Nishikori S, Yamanaka K, Sakurai T, Esaki M, Ogura T. p97 Homologs from Caenorhabditis elegans, CDC-48.1 and CDC-48.2, suppress the aggregate formation of huntingtin exon1 containing expanded polyQ repeat. Genes Cells. 2008;13:827-38
42. Coghlan M, Richards E, Shaik S, Rossi P, Vanama RB, Ahmadi S. et al. Inhalational Anesthetics Induce Neuronal Protein Aggregation and Affect ER Trafficking. Sci Rep. 2018;8:5275
43. Richardson CE, Kinkel S, Kim DH. Physiological IRE-1-XBP-1 and PEK-1 signaling in Caenorhabditis elegans larval development and immunity. PLoS Genet. 2011;7:e1002391
44. Safra M, Ben-Hamo S, Kenyon C, Henis-Korenblit S. The ire-1 ER stress-response pathway is required for normal secretory-protein metabolism in C. elegans. J Cell Sci. 2013;126:4136-46
45. Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M. et al. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A. 2010;107:9730-5
46. Burkewitz K, Feng G, Dutta S, Kelley CA, Steinbaugh M, Cram EJ. et al. Atf-6 Regulates Lifespan through ER-Mitochondrial Calcium Homeostasis. Cell Rep. 2020;32:108125
47. Bischof LJ, Kao CY, Los FC, Gonzalez MR, Shen Z, Briggs SP. et al. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 2008;4:e1000176
48. Urano F, Calfon M, Yoneda T, Yun C, Kiraly M, Clark SG. et al. A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol. 2002;158:639-46
49. Waldherr SM, Strovas TJ, Vadset TA, Liachko NF, Kraemer BC. Constitutive XBP-1s-mediated activation of the endoplasmic reticulum unfolded protein response protects against pathological tau. Nat Commun. 2019;10:4443
50. Mouysset J, Kahler C, Hoppe T. A conserved role of Caenorhabditis elegans CDC-48 in ER-associated protein degradation. J Struct Biol. 2006;156:41-9
51. Sasagawa Y, Yamanaka K, Ogura T. ER E3 ubiquitin ligase HRD-1 and its specific partner chaperone BiP play important roles in ERAD and developmental growth in Caenorhabditis elegans. Genes Cells. 2007;12:1063-73
Author contact
![]() Corresponding author: Hyo-Seok Na, Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, Seongnam, Gyeonggi-do, 13620, Republic of Korea. Tel: 82-31-787-7507; Fax: 82-31-787-4063; E-mail: hsknanacom.
Corresponding author: Hyo-Seok Na, Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, Seongnam, Gyeonggi-do, 13620, Republic of Korea. Tel: 82-31-787-7507; Fax: 82-31-787-4063; E-mail: hsknanacom.

 Global reach, higher impact
Global reach, higher impact