3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(12):2743-2751. doi:10.7150/ijms.60517 This issue Cite
Research Paper
Cytokine TGF-β1, TNF-α, IFN-γ and IL‐6 Gene Polymorphisms and Localization of Premalignant Gastric Lesions in Immunohistochemically H. pylori-negative Patients
1. Department of Clinical Science-Internal Medicine, George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureș, Mureș, Romania.
2. Department of Medical Informatics and Biostatistics, Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, Cluj-Napoca, Romania.
3. Genetics Laboratory, Center for Advanced Medical and Pharmaceutical Research, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureș, Târgu Mureș, Romania.
4. Department of Medical Genetics, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureș, Târgu Mureș, Romania.
5. Pathology Department, Emergency County Hospital Targu Mures, Mureș 540139, Romania.
Received 2021-3-16; Accepted 2021-5-11; Published 2021-5-21
Abstract
Background: Cytokines and their gene variants are proven to play a role in pathogenic gastritis and carcinogenesis. The study assesses associations of the cytokine gene polymorphisms with extension of atrophic gastritis/intestinal metaplasia (AGIM) in patients without Helicobacter pylori infection on immunohistochemistry study.
Methods: 224 adult consecutive patients undergoing an upper digestive endoscopy were included and grouped according to localization of AGIM: 37 patients with antrum-limited AGIM, 21 corpus-limited AGIM, 15 extended-AGIM (antrum and corpus) and 151 patients had no AGIM. Medical records of the patients were checked and a structured direct interview was applied in order to collect clinical data, including digestive symptoms. In all cases, IFN-γ +874T>A, TGF-β1 +869T>C, TNF‐α-308G>A and -238G>A, and IL-6 -174C>G polymorphisms were genotyped.
Results: The mean age was significantly higher in the AGIM group, while the comorbidies were similar among patients with different localization of lesions or in patients without AGIM. There were no significant differences in digestive symptoms, nor in the consumption of non-steroidal anti-inflammatory drugs or proton pump inhibitor with the different extensions of AGIM. There was a significant association between oral anticoagulant consumption and localization of AGIM (P = 0.042), frequency being higher among patients with corpus-limited AGIM than those with no AGIM (P = 0.007, adjusted P = 0.041). TGF-β1 +869T>C was less frequent among patients with corpus-limited AGIM (n=7, 33.3%) and extended AGIM (n=5, 33.3%) than in antrum-limited AGIM (n=25, 67.6%). There were no other significant differences regarding variant and wild genotype frequencies of IFN-γ +874T>A (86.5%, 81.0%, 86.7%, p=0.814), TNF‐α-308G>A (35.1%, 28.6%, 53.3%, p=0.48) and IL-6 -174C>G (70.3%. 61.9%, 73.3% p=0.656) among patients with antrum-limited, corpus-limited or extended AGIM. TGF-β1 +869T>C was associated with a decreased risk for corpus-affected AGIM (adjusted odds ratio: 0.42, 95% confidence interval: 0.19-0.93, P = 0.032). The dominant inheritance models no revealed significant association for IFN-γ +874T>A, TNF‐α-308G>A and IL-6 -174C>G gene polymorphism and the risk of localization of AGIM.
Conclusion: TGF-β1 +869T>C gene polymorphism is associated with a decreased risk for corporeal localization of premalignant lesions, while IFN-γ +874T>A, TNF-α-308G>A and IL-6 -174C>G are not associated with the risk for AGIM in immunohistochemically H. pylori negative patients.
Keywords: atrophic gastritis, intestinal metaplasia, cytokine polymorphism, TGF‐β1, TNF‐α, IFN‐γ, IL-6
Introduction
Gastric cancerogenesis is accepted today to be correlated with long-standing chronic inflammation in the stomach mucosa, most frequently due to Helicobacter pylori (H. pylori) infection [1]. Both host and H. pylori genetic factors seem to play a crucial role in gastric inflammation and progression toward cancer [2]. “Gastric cancer phenotype” described as corpus predominant gastritis, atrophic gastritis and/or intestinal metaplasia (AGIM), decreased acid secretion or H. pylori infection (present or previous) increases the risk for cancer [3]. On the other hand, autoimmune atrophic gastritis, a condition diagnosed in the presence of corpus gastric atrophy with antrum sparing, can also lead to the development of adenocarcinoma or neuroendocrine neoplasia [4]. Recent meta-analysis support that single nucleotide polymorphisms may be used for the assessment of genetic predisposition to gastric cancer, with ethnicity, environmental factor and cancer subtype contributing to inconsistency in the results [5]. From among all molecular factors playing a role in progression from inflammation toward preneoplastic gastric lesions and cancer, recent research has focused on the possible influence of different type cytokine gene variants [6]. Recently, it was shown that cytokine gene variants are associated with susceptibility to different types of malignancy in a Romanian population [7].
Experimental data support that the CD4+ T cell-derived interferon-gamma (IFN-γ) provides the key stimulus for the development of gastric premalignant lesions [8]. The IFN‐γ +874T>A (rs2430561) polymorphism influencing IFN-γ expression has not been studied in relation to mucosal gastric lesion [9,10].
The transforming growth factor-β1 (TGF-β1) gene and its protein play an important role in modulating expression of multiple genes, being involved in inflammatory responses in gastric mucosa and cancer progression [11]. Published meta-analysis did not confirm the association between TGF-β1 +869T>C (rs1800470) and 915G>C (rs1800471) polymorphisms and the risk for gastric cancer [12], while another indicated that TGF β1 -509C>T rather than +869T>C can increase the risk for gastric cancer [13]. The functional TGF-β1 +869T>C, rs1800470 polymorphisms have been identified to be associated with expression and the level of plasma TGF-β1 protein [11,14,15].
Tumor necrosis factor-alpha (TNF-α) is a potent immunomodulator and pro-inflammatory cytokine that inhibits gastric acid production, and it is upregulated in the gastric mucosa in response to H. pylori infection [16]. Meta-analysis sustained the association between TNF-α -308G>A (rs1800629) polymorphism and gastric cancer [17]. The variant genotype of TNF-α -308G>A was not associated with gastric atrophy (GA) in European studies [18,19] nor in meta-analysis [20], but has an impact on H. pylori-related gastroduodenal conditions like gastritis, ulcer or cancer [21]. TNF-α -238G>A (rs361525) polymorphism was associated with the increased risk of gastric cancer in Chinese population, not in Caucasians [22,23] and was significantly associated with a high risk of gastritis in an African population [24].
Interleukin-6 (IL-6) is a multifunctional cytokine (endocrine and inflammatory mediator) and its polymorphism and expression seem to influence the susceptibility to various diseases, including gastric conditions related to H. pylori infection [25]. Meta-analysis questioning the influence of IL-6 promoter polymorphism did not reveal increased risk for gastric cancer [26]. A study from Brasilia supported that the G allele frequency of IL-6 -174C>G (rs1800795) was higher in patients with gastric cancer than in patients with chronic gastritis [27], while others did not support any influence on gastric conditions related with H. pylori infection (gastritis, ulcer, adenocarcinoma) [28].
The current concept supports that gastric atrophy can be a result of chronic H. pylori infection or of autoimmune gastritis; in the latter, the sensitized T cells and autoantibodies are the key factors of the pathological process [29]. H. pylori infection and autoimmune atrophic gastritis face overlapping biological characteristics and the germ itself may accelerate progression toward atrophy in individuals with a particular genetic background [36].
To the best of our knowledge there are no published studies investigating the importance of cytokine gene variants with a role in the inflammatory response of gastric mucosa on the extension of AGIM with negative biopsies for active H. pylori related gastritis. Based on all the observations cited above, the present study focused on assessing the associations of the IFN‐γ, TGF‐β1, TNF-α, and IL‐6 gene polymorphisms with a possible role in clinical course of gastric inflammatory response, with the histologic extent of AGIM in patients without active inflammatory cell infiltrate on histology (mononuclear and neutrophilic). We questioned the possible influence of inflammatory cytokine polymorphism on localization or extension of premalignant gastric lesions leading to different susceptibility for gastric cancer, irrespective of triggered mucosal aggression. We present the following article in accordance with the STROBE reporting checklist.
Patients and Methods
Ethical consideration
The Ethical Committee of Targu Mures County Emergency Clinical Hospital (10846/15.04.2019) and of George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures, Romania (282/19.07.2019) approved the study.
Study sample
We conducted a single-center observational study in 224 adult consecutive patients, in whom an upper digestive endoscopy was performed in Medical Clinic II -Targu Mures County Emergency Clinical Hospital. A structured direct interview was applied after the informed consent was obtained. We questioned smoking and alcohol consumption, present symptoms (pain and/or heartburns and/or nausea and/or regurgitation), and some non-recorded data of the past medical history. The medical records of subjects were checked for previous symptoms, diagnosis, investigations or treatments for peptic ulcer disease and/or H. pylori eradication therapies, as well as for concomitant diseases (hypertension, cardiac, respiratory, kidney or liver diseases, stroke, diabetes mellitus, atherosclerosis, dyslipidemia, osteoarticular diseases, other chronic medical conditions) or treatments with potential gastric effect (protective or aggressive).
Patients drinking at least 10 Units (1 Unit=10 ml of pure alcohol) weekly were considered drinkers. Subjects reporting consumption of less than 10 Units of pure alcohol per week were considered non-drinkers. If the patients used to smoke 5 or more cigarettes/d, including recent quitters (last 5 years), they were considered as smokers. Non-steroidal non-aspirin anti-inflammatory drugs (commonly known as NSAIDs) consumption was considered if the patients took regular daily doses of over-the-counter drugs or based on medical prescription, for more than 1 mo. We recorded the use of antiplatelet dose of aspirin (75-125 mg/d) or clopidogrel 75 mg/d for more than 1 mo. Patients were considered on acenocumarolum (ACO) therapy if they used regular doses for a therapeutic international normalized ratio for at least 2 week before endoscopy. Patients were considered exposed to proton pump inhibitors (PPI) (omeprazole, pantoprazole, esomeprazole) if they used regular doses within the last month, irrespective of the type of administration (continuous or on-demand).
Exclusion criteria were: 1. Incomplete set of histological or clinical data; 2. Active H. pylori related gastritis on histology using immunohistochemistry study; 3. Previous gastric surgery; 4. Active bleeding during endoscopy requiring hemostatic therapy; 5. Advanced or end-stage digestive disease (cirrhosis, esophageal varices); 6. Dysplasia or gastric cancer.
Pathology
At least four biopsies (two from the antrum and two from the gastric body, both from the lesser and the greater curvature) were routinely analyzed. The cases with absence of H. pylori infection in all biopsies on microscopy after staining tissues with hematoxylin-eosin, periodic acid Schiff-alcian blue and Giemsa were considered negative. If the germ was present in at least one site, the case was considered H. pylori-positive and it was excluded. If H. pylori infection was suspicioned (abundant inflammatory cells, extensive intestinal metaplasia), an immunohistochemistry study was performed, especially in patients on PPI therapy and the case was also excluded if infection was confirmed. The Updated Sydney System was used to assess the degree of mucosal chronic inflammation and activity, H. pylori infection, glandular atrophy, and intestinal metaplasia. The lack of biopsies from incisura in some patients did not allow us to use de OLGA/OLGIM system to quantify the severity of premalignant lesions. Moreover, we did not intend to study the association of the SNPs, clinical and endoscopic variables with the severity of premalignant lesions, but only with their presence or absence.
In this study, we focused on the presence of AGIM in any part of the stomach. Antrum-limited AGIM was defined as the presence of AGIM in the antrum, with a healthy stomach corpus. Corpus-limited AGIM was defined as the presence of AGIM in the corpus, with a healthy antrum. Extended AGIM was defined as the simultaneous histological presence of AGIM in the antrum and corpus of the stomach. In patients with corpus limited changes, enterocromaffin-like cell hyperplasia and suspicion of autoimmune gastritis, further studies were performed to confirm the diagnosis (anti parietal cell antibody and anti-intrinsic factor, B12 vitamin and folate serum level). Patients with other abnormal histological changes were excluded.
Genetic study
Rapid extraction of genomic DNA from whole blood samples stored in EDTA tubes was performed by using PureLink Genomic DNA Mini Kits (ThermoFisher Scientific, Waltham, MA, United States). For TaqMan SNP genotyping, we used TaqMan Fast Advanced Master Mix and the assay for TNF-α rs361525 and the 7500 Fast Dx Real‐time PCR System (ThermoFisher Scientific). TGF‐β1 rs1800470, TNF-α rs1800629, IFN‐γ rs2430561, and IL‐6 rs1800795 SNPs were evaluated by using the previously described amplification-refractory mutation system (commonly known as the ARMS‐PCR) technique [31].
Statistical analysis
Statistical analysis was performed using the R software (version 3.6.1). Distribution of observed and expected genotypes of studied gene polymorphisms was tested for consistency with Hardy-Weinberg equilibrium and linkage equilibrium using the SNPassoc R package [32]. Demographic variables, such as age, were described by mean and standard deviation, while other clinical categorical factors were shown using absolute frequencies and percentages. Comparisons between demographic and clinical factors among patients with different extensions of AGIM were performed using the Student's t, ANOVA, chi-square or Fisher's exact test.
The differences in genotype frequencies of IFN‐γ, TGF‐β1, TNF‐α, and IL‐6 gene polymorphisms among patients with different extensions of AGIM were tested using chi-square or Fisher's exact test. In the case of a significant result (P-value < 0.05), in order to identify the pattern of differences, we also performed the pairwise comparisons using chi-square or Fisher's exact test, and then the P-values were adjusted using the Benjamini-Hochberg method [33].
Binomial and multinomial logistic regression analysis with adjustment for age, gender, and current smoking were performed to estimate the association between variant genotype of IFN‐γ, TGF‐β1, TNF‐α, and IL‐6 gene polymorphisms and extension of the presence of AGIM. The association between each of the studied gene polymorphisms was expressed in terms of the odds ratio (OR) and the corresponding 95% confidence interval (CI). The estimated ORs were obtained using the VGAM R package [34]. The regression results were considered statistically significant if 95%CI for OR did not contain unity or if two-tailed P-values obtained from Wald z-tests were lower than the significance level of 0.05.
Results
Study sample
Among the 224 consecutive patients included in the study, the frequency of AGIM was 73 (32.6%) cases. The histopathological investigation revealed that the extension of AGIM was the following: 37 (50.7%) patients had antrum-limited AGIM; 21 (28.8%) patients had corpus-limited AGIM; and 15 (20.5%) patients had extended AGIM, involving both antrum and corpus.
The main demographic and clinical variables are presented in Table 1. The mean age of studied patients was 62.3 ± 12.7 years, with range of age varying between a minimum of 20 years to a maximum of 85 years (significant differences found when the group with AGIM was compared to patients with no AGIM; Student's t-test, P = 0.004). We noticed that mean age of patients with AGIM was higher than of patients with no AGIM (65.52 ± 10.47 vs 60.79 ± 13.37 years). The distribution of gender was similar between the groups with and without AGIM (chi-square test, P = 0.776).
Demographic and clinical features in patients with different extensions of atrophic gastritis and/or intestinal metaplasia
| Variable | AGIM in any stomach site, n = 73 (32.6%) | Absence of AGIM, n = 151 (67.4%) | Antrum-limited AGIM, n1 = 37 (16.5%) | Corpus-limited AGIM, n2 = 21 (9.4%) | Extended AGIM, n3 = 15 (6.7%) |
|---|---|---|---|---|---|
| Age in years, mean ± SD | 65.52 ± 10.471 | 60.79 ± 13.37 | 64.51 ± 10.18 | 66.67 ± 10.62 | 66.40 ± 11.42 |
| Gender, male | 34 (46.6) | 67 (44.4) | 15 (40.5) | 11 (52.4) | 8 (53.3) |
| Peptic ulcer history | 47 (64.4) | 95 (62.9) | 23 (62.2) | 14 (66.74) | 10 (66.7) |
| Non-aspirin NSAIDs use | 9 (12.3) | 15 (9.9) | 6 (16.2) | 1 (4.8) | 2 (13.3) |
| Aspirin use | 29 (39.7) | 63 (41.7) | 17 (45.9) | 7 (33.3) | 5 (33.3) |
| ACO use | 20 (27.4)* | 24 (15.9) | 8 (21.6) | 9 (42.9)** | 3 (20.0) |
| Clopidogrel use | 13 (17.8) | 19 (12.6) | 12 (32.4) | 0 (0.0) | 1 (6.7) |
| PPI use | 50 (68.5) | 105 (69.5) | 27 (73.0) | 13 (61.9) | 10 (66.7) |
| Digestive symptoms | |||||
| Abdominal pain | 30 (41.1) | 82 (54.3) | 17 (45.9) | 9 (42.9) | 4 (26.7) |
| Pyrosis | 15 (20.8) | 41 (27.2) | 11 (30.6) | 1 (4.8) | 3 (20.0) |
| Nausea/vomiting | 13 (17.8) | 22 (14.6) | 6 (16.2) | 3 (14.3) | 4 (26.7) |
| Bloating | 21 (28.8) | 34 (22.7) | 14 (37.8) | 4 (19.0) | 3 (20.0) |
| Regurgitation | 3 (4.1) | 8 (5.3) | 1 (2.7) | 1 (4.8) | 1 (6.7) |
| Comorbidities† | 72 (98.6) | 144 (95.4) | 37 (100.0) | 21 (100.0) | 14 (93.3) |
| Current smoking | 9 (12.3)* | 5 (3.3) | 4 (10.8)*** | 3 (14.3)**** | 2 (13.3) |
| Alcohol consumption, > 10 u/wk | 7 (9.6) | 11 (7.3) | 2 (5.4) | 2 (9.5) | 3 (20.0) |
Data are presented as n (%), unless indicated otherwise. *P < 0.05 obtained from Student's t-test/Fisher's exact test, applied to compare the AGIM in any stomach site and no AGIM groups; **P < 0.05 obtained from Fisher's exact test, applied to compare the corpus-limited AGIM and no AGIM groups; † presence of at least one comorbidity as described in Methods; ***P < 0.05 obtained from Fisher's exact test, applied to compare the antrum-limited AGIM and extended AGIM; ****P < 0.05 obtained from Fisher's exact test, applied to compare the corpus-limited AGIM and extended AGIM groups. ACO: Acenocumarolum; AGIM: Atrophic gastritis and/or intestinal metaplasia; NSAID: Non-steroidal non-aspirin anti-inflammatory drug; SD: Standard deviation.
Although we observed that among patients with different histologic extensions of AGIM, the frequency of gastrotoxic drugs consumption (aspirin, non-aspirin NSAIDs) was higher for patients with antrum-limited AGIM (n = 19; 51.4%) versus corpus-limited AGIM (n = 8; 38.1%), versus extended AGIM (n = 7; 46.7%), the observed differences were not statistically significant (chi-square test, P = 0.733). The frequency of gastro-protective (PPI) drugs was similar according to different histologic extensions of AGIM: 105 (69.5%) for no AGIM vs 27 (73.0%) for antrum-limited AGIM vs 13 (61.9%) for corpus-limited AGIM vs 10 (66.7%) for extended AGIM (chi-square test, P = 0.874).
There was a significant association between ACO consumption and localization of AGIM (Fisher's exact test, P = 0.042), with the pairwise comparisons showing that the frequency of ACO consumption was higher among patients with corpus-limited AGIM than those with no AGIM (Fisher's exact test, P = 0.007, adjusted P = 0.041).
There were no significant differences regarding the frequency distribution in any of digestive symptoms for patients with antrum-limited AGIM, corpus-limited and extended AGIM (chi-square test, P = 0.168 for abdominal pain, P = 0.125 for pyrosis; Fisher's exact test, P = 0.624 for nausea and P = 0.895 for regurgitation; chi-square test, P = 0.230 for bloating).
There was a significant association between smoking and distribution pattern of AGIM (Fisher's exact test, P = 0.026), the post-hoc analysis identifying significant differences between patients with extended AGIM and those with antrum-limited AGIM (P = 0.015, adjusted P = 0.091) and those with corpus-limited AGIM (Fisher's exact test, P = 0.032, adjusted P = 0.097).
Hardy-Weinberg equilibrium for IFN-γ, TGF-β1, TNF-α and IL-6 gene polymorphisms
The studied SNPs were tested for the condition of Hardy-Weinberg equilibrium. The results showed that genotype distribution of observed and expected frequency of each SNP did not differ significantly in patients with and without AGIM, all the studied SNPs (excepting TNF-α -238G>A, for which we found only two genotypes) being in agreement with Hardy-Weinberg equilibrium expectation; for the AGIM group and for patients without AGIM: IFN-γ +874T>A: P = 0.1543 and P = 0.6204; TGF-β1 869T>C: P = 0.8533 and P = 1.000; TNF-α -308G>A: P = 0.4437 and P = 0.1332; and IL-6 -174C>G: P = 1.0000 and P = 0.4968). We also tested linkage disequilibrium between TNF-α -308G>A and -238G>A SNPs and found no significant deviation from linkage disequilibrium (P = 0.801, D' = 0.047, r2 = 0.012).
Association of TGF-β1, TNF-α, IFN-γ, and IL-6 gene polymorphisms with histologic extensions of AGIM
Table 2 summarizes the frequencies of studied SNPs' genotypes in relation to the histologic extension of AGIM. There was no significant difference regarding variant genotype and wild-type genotype frequencies among patients with different localization of AGIM, except for TGF-β1 +869T>C gene polymorphism (chi-square test, P = 0.031). In addition, we noticed that the variant genotype TGF-β1 +869T>C gene polymorphism occurred less frequently among patients with corpus-limited AGIM (n = 7, 33.3%) and extended AGIM (n = 5, 33.3%) than those with antrum-limited AGIM (n = 25, 67.6%).
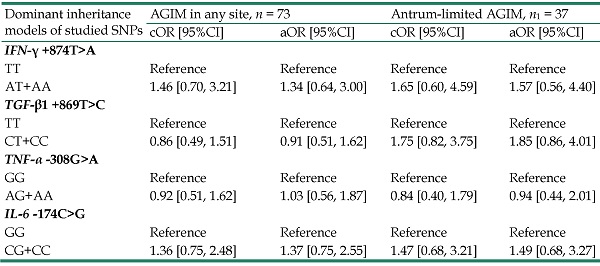
As described in Table 3, the dominant inheritance models showed a significant association only for TGF-β1 +869T>C gene polymorphism with decreased risk of corpus-affected AGIM (adjusted OR= 0.42, 95%CI: 0.19- 0.93, P = 0.032).
Discussion
Our study investigated the environmental factors and their effects, as well as the IFN‐γ, TGF‐β1, TNF‐α and IL‐6 gene polymorphisms of cytokines involved in the immune response, on patients with different extent of AGIM without active H. pylori infection on histology.
Identification of cytokines' functions and their cellular actions has increased during the last years, and our understanding of the link between inflammation and gastric carcinogenesis has advanced greatly. Even though gastritis associated with H. pylori infection mainly involving the antrum is distinct from atrophic autoimmune gastritis (implicating the gastric corpus because the inflammatory process affects the parietal gastric cells), the correlation between the two is still controversial [30]. A recent meta-analysis supported that autoimmune mechanisms might exacerbate H. pylori gastritis in the absence of classical biological features of autoimmune gastritis [35]. We did not intend in our study to clarify the etiology of gastric atrophy or metaplasia, but we started the research in order to investigate possible inflammatory gene polymorphisms that may modulate the increasing cases of H. pylori-negative biopsies in dyspeptic or anemic patients undergoing endoscopy, probably due to unintentional eradication or spontaneous disappearance of the germ in “old” atrophic gastritis [36]. The corpus-limited lesions group include both patients with confirmed autoimmune gastritis or not.
Genotype frequencies of IFN‐γ, TGF‐β1, TNF‐α, and IL‐6 gene polymorphisms in patients with different histologic extensions of AGIM
| Gene polymorphism, n (%) | AGIM in any site [n=73 (32.6)] | Absence of AGIM [n=151 (67.4)] | p-value* | Antrum-limited AGIM [n1=37 (16.5)] | Corpus-limited AGIM [n2=21 (9.4)] | Extended AGIM [n3=15 (6.7)] | p-value** |
|---|---|---|---|---|---|---|---|
| IFN‐γ +874T>A | |||||||
| TT | 11 (15.1) | 31 (20.5) | 0.244 | 5 (13.5) | 4 (19.0) | 2 (13.3) | 0.440 |
| AT | 43 (58.9) | 71 (47.0) | 19 (51.4) | 13 (61.9) | 11 (73.3) | ||
| AA | 19 (26.0) | 49 (32.5) | 13 (35.1) | 4 (19.0) | 2 (13.3) | ||
| AT+AA | 62 (84.9) | 120 (79.5) | 0.326 | 32 (86.5) | 17 (81.0) | 13 (86.7) | 0.814 |
| TGF-β1 +869T>C | |||||||
| TT | 36 (49.3) | 69 (45.7) | 0.747 | 12 (32.4) | 14 (66.7) | 10 (66.7) | 0.123 |
| CT | 31 (42.5) | 65 (43.0) | 21 (56.8) | 5 (23.8) | 5 (73.3) | ||
| CC | 6 (8.2) | 17 (11.3) | 4 (10.8) | 2 (9.5) | 0 (0.0) | ||
| CT+CC | 37 (50.7) | 82 (54.3) | 0.611 | 25 (67.6) | 7 (33.3) | 5 (33.3) | 0.031 |
| TNF-α -308G>A | |||||||
| GG | 46 (63.0) | 92 (60.9) | 0.953 | 24 (64.9) | 15 (71.4) | 7 (46.7) | 0.719 |
| AG | 26 (35.6) | 56 (37.1) | 12 (32.4) | 6 (28.6) | 8 (53.3) | ||
| AA | 1 (1.4) | 3 (2.0) | 1 (2.7) | 0 (0.0) | 0 (0.0) | ||
| AG+AA | 27 (37.0) | 59 (39.1) | 0.763 | 13 (35.1) | 6 (28.6) | 8 (53.3) | 0.481 |
| TNF-α -238G>A | |||||||
| GG | 71 (97.3) | 146 (96.7) | 1.00 | 35 (94.6) | 21 (100.0) | 15 (100.0) | 0.799 |
| AG | 2 (2.7) | 5 (3.3) | 2 (5.4) | 0 (0.0) | 0 (0.0) | ||
| IL-6 -174C>G | |||||||
| GG | 23 (31.5) | 58 (38.4) | 0.598 | 11 (29.7) | 8 (38.1) | 4 (26.7) | 0.555 |
| CG | 37 (50.7) | 68 (45.0) | 20 (54.1) | 11 (52.4) | 6 (40.0) | ||
| GG | 13 (17.8) | 25(16.6) | 6 (16.2) | 2 (9.5) | 5 (33.3) | ||
| CG+CC | 50 (68.5) | 93(61.6) | 0.313 | 26 (70.3) | 13 (61.9) | 11 (73.3) | 0.656 |
*P-values resulted from the comparison of genotype distributions in patients with and without AGIM by chi-square or Fisher's exact tests; **P-values resulted from the comparison of genotype distributions according to the distribution pattern of AGIM by chi-square or Fisher's exact tests. Statistical significance was reached if P-value < 0.05. AGIM: Atrophic gastritis and/or intestinal metaplasia.
Associations between IFN‐γ, TGF‐β1, TNF‐α and IL‐6 gene polymorphisms and extensions of atrophic gastritis and/or intestinal metaplasia
| Dominant inheritance models of studied SNPs | AGIM in any site, n = 73 | Antrum-limited AGIM, n1 = 37 | Corpus-affected (limited+extended) AGIM, n2=36 | |||
|---|---|---|---|---|---|---|
| cOR [95%CI] | aOR [95%CI] | cOR [95%CI] | aOR [95%CI] | cOR [95%CI] | aOR [95%CI] | |
| IFN‐γ +874T>A | ||||||
| TT | Reference | Reference | Reference | Reference | Reference | Reference |
| AT+AA | 1.46 [0.70, 3.21] | 1.34 [0.64, 3.00] | 1.65 [0.60, 4.59] | 1.57 [0.56, 4.40] | 0.97 [0.49, 3.38] | 0.97 [0.43, 3.09] |
| TGF-β1 +869T>C | ||||||
| TT | Reference | Reference | Reference | Reference | Reference | Reference |
| CT+CC | 0.86 [0.49, 1.51] | 0.91 [0.51, 1.62] | 1.75 [0.82, 3.75] | 1.85 [0.86, 4.01] | 0.42 [0.20, 0.90]* | 0.42 [0.19, 0.93]* |
| TNF-α -308G>A | ||||||
| GG | Reference | Reference | Reference | Reference | Reference | Reference |
| AG+AA | 0.92 [0.51, 1.62] | 1.03 [0.56, 1.87] | 0.84 [0.40, 1.79] | 0.94 [0.44, 2.01] | 0.99 [0.47, 2.09] | 1.14 [0.53, 2.47] |
| IL-6 -174C>G | ||||||
| GG | Reference | Reference | Reference | Reference | Reference | Reference |
| CG+CC | 1.36 [0.75, 2.48] | 1.37 [0.75, 2.55] | 1.47 [0.68, 3.21] | 1.49 [0.68, 3.27] | 1.25 [0.58, 2.68] | 1.26 [0.57, 2.79] |
P-values obtained from Wald z-tests of multinomial logistic regression. aOR: Adjusted odds ratio for gender, age older than 60 years, and current smoking; CI: Confidence interval; cOR: Crude odds ratio; AGIM: Atrophic gastritis and/or intestinal metaplasia; SNPs: Single nucleotide polymorphisms.
The frequency of AGIM in the studied sample (32.6%) was three-times higher than the prevalence of intestinal metaplasia reported by Huang et al. [37] in a recent retrospective large American study of 17,710 biopsies, and also higher than that reported in European studies (one in four biopsies of patients undergoing gastroscopy) [38]. Both genetic and environmental factors are accepted to play a role in this discrepancy, including the prevalence of H. pylori infection [39].
There were not significant differences regarding the symptoms or history of ulcer in patients with or without AGIM, nor in patients with different extent of histologic lesions. NSAID or aspirin consumption were not different in the studied groups, while ACO therapy was more frequent in patients with corpus-limited AGIM than in those with no AGIM. As it was reported, the use of ACO was frequently associated with the extension of preneoplastic lesions [40]. Vitamin B12 and folic acid deficiency occurring in corporeal autoimmune gastritis are associated with variable increased homocysteine levels [41], which seem to increase the risk for thrombotic events [42]. Even though the association remains controversial [43], the link between atrophic gastritis and thrombotic risk should be further investigated. Regarding the relation between smoking and histologic extent of AGIM, our findings revealed that smoking was associated with AGIM, as in other similar studies of dyspeptic patients [44], or with endoscopic lesions. The underlying mechanisms seem to be related to the influence on mucosal cell death, proliferation, decreased blood flow, or modulation of the immune responses in gastric mucosa [45].
Our data showed that IFN-γ +874T>A, TNF-α -308G>A and -238G>A, IL-6 -174C>G polymorphisms were not associated with the extent of AGIM in patients without active H. pylori infection immunohistochemically assessed. Similar results were observed in gastric cancer [12, 22, 23,26] and in gastric atrophy in Europeans [18,19, 20] but no data are available for patients without active H. pylori infection.
From among all the polymorphisms studied, only the TGF-β1 +869T>C SNP was associated with localization of premalignant gastric lesions in patients without H. pylori infection. The multifunctional TGF-β1 proteins were proved to control cell growth, proliferation, differentiation and apoptosis and their role on carcinogenesis was extensively studied [11]. In our study, the variant genotype TGF-β1 +869T>C was associated with a protective effect against corporeal localization of AGIM. The findings suggest the possible effect of TGF-β1 +869T>C, rs1800470 polymorphisms on modulation of the host immune response in gastric corpus mucosa. It is accepted today that H. pylori infection might mediate aggression against the proton pump, leading to corporeal atrophic mucosal changes similar to those of primary gastric autoimmunity, disappearing from altered mucosa [1]. This overlapped pathogenic mechanism of gastric corpus atrophy might be influenced by the TGF-β1 +869T>C SNP, modulating the cytokine activity that plays roles in gastric inflammation via regulatory mechanisms.
Our study is the first one questioning the role of cytokine polymorphisms in premalignant gastric lesions in patients with unintentional or spontaneous disappearance of H. pylori infection in gastric biopsies or with autoimmune gastritis, as recent studies underline the important roles for cytokines in regulating corporeal atrophy, hyperplasia, different types of metaplasia, and gastric carcinogenesis [46,47] Our study opens the possible direction of research for developing alternative biologic markers for assessment of risk for premalignant gastric lesions and associations with other medical conditions.
One limitation of the study is the lack of data regarding plasma cytokines (IFN‐γ, TGF‐β1, TNF-α, and IL‐6). The second limitation of our study was no separate estimation of the risk for both corpus-limited and extended AGIM, due to reduced numbers of cases with variant genotypes of the studied SNPs. Although the TNF-α-238G>A polymorphism TGF-β1 +869T>C gene polymorphism was significantly associated with decreased risk of corpus-affected AGIM, the results should be regarded with caution due to the small frequencies of variant genotype and the clinical significance should be retested on a larger sample. The final regression model does not include the TNF-α-238G>A polymorphism due to a lack of cases with the variant genotype and extension of AGIM into the gastric corpus and it was the third limitation of the present study. Further studies should investigate the effect of TGF-β1+869T>C, rs1800470 polymorphisms on chronic gastritis occurrence in a specific population. The fourth limitation was the small sample size, that did not allow for the development of a multivariable logistic model to study the clinical predictors together with IFN‐γ, TGF‐β1, TNF-α, and IL‐6 gene for different localization or extension of AGIM. The small sample size of each group based on AGIM localization was also related to the wide confidence intervals of estimated parameters (cOR, aOR) so further studies with larger sample sizes should be made to get a greater precision of findings and to investigate the ability of multivariable clinical and genetic model to predict the extent of AGIM, as well as the importance of the past or present H. pylori infection.
Conclusions
In patients without active H. pylori gastritis in biopsy samples, the TGF-β1 +869T>C gene polymorphism was associated with a decreased risk for corporeal localization of AGIM. The dominant inheritance models revealed no significant association for IFN-γ +874T>A, TNF‐α-308G>A and IL-6 -174C>G gene polymorphism with the risk of localization of AGIM. Higher consumption of ACO was observed in patients with corpus-limited precancerous lesions, while symptoms were not associated with localization of premalignant lesions. In patients without active H. pylori infection smoking was associated with extended AGIM.
Acknowledgements
Supported by an Internal Research Grant from the University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureș (Nr. 615/12/17.01.2019).
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author contributions
Conception and design: AN, CB and MI; Administrative support: AN, CB, FT, AC, SM; Provision of study materials or patients: AN, CB; Collection and assembly of data: AN, CB, MI, FT, AC; Data analysis and interpretation: MI, AN, CB; Manuscript writing: All authors; Final approval of manuscript: All authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rugge M, Genta RM, Graham DY. et al. Chronicles of a cancer foretold: 35 years of gastric cancer risk assessment. Gut. 2016;65(5):721-725
2. Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150(1):64-78
3. Annibale B, Esposito G, Lahner E. A current clinical overview of atrophic gastritis. Expert Rev Gastroenterol Hepatol. 2020;14(2):93-102
4. Weise F, Vieth M, Reinhold D. et al. Gastric cancer in autoimmune gastritis: A case-control study from the German centers of the staR project on gastric cancer research. United European Gastroenterol J. 2020;8(2):175-184
5. de Brito BB, da Silva FAF, de Melo FF. Role of polymorphisms in genes that encode cytokines and Helicobacter pylori virulence factors in gastric carcinogenesis. World J Clin Oncol. 2018;9(5):83-89
6. Tian J, Liu G, Zuo G. et al. Genetic polymorphisms and gastric cancer risk: a comprehensive review synopsis from meta-analysis and genome-wide association studies. Cancer Biol Med. 2019;16(2):361-389
7. Bănescu C, Tripon F, Trifa AP. et al. Cytokine rs361525, rs1800750, rs1800629, rs1800896, rs1800872, rs1800795, rs1800470, and rs2430561 SNPs in relation with prognostic factors in acute myeloid leukemia. Cancer Med. 2019;8(12):5492-506
8. Sayi A, Kohler E, Hitzler I. et al. A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182(11):7085-101
9. Osaki LH, Bockerstett KA, Wong CF. et al. Interferon-γ directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol. 2019;247(4):513-523
10. Canedo P, Corso G, Pereira F. et al. The interferon gamma receptor 1 (IFNGR1) -56C/T gene polymorphism is associated with increased risk of early gastric carcinoma. Gut. 2008;57(11):1504-8
11. Nianshuang Li, Chuan Xie, Nong-Hua Lu. Transforming growth factor-β: an important mediator in Helicobacter pylori-associated pathogenesis. Front Cell Infect Microbiol. 2015;5:77
12. Chang WW, Zhang L, Su H, Yao Y. An updated meta-analysis of transforming growth factor-β1 gene: three polymorphisms with gastric cancer. Tumour Biol. 2014;35(4):2837-44
13. Zhang CF, Wang ZW, Hou MX. et al. Transforming growth factor β1-509C/T and +869T/C polymorphisms on the risk of upper digestive tract cancer: a meta-analysis based on 10,917 participants. Ann Hum Genet. 2012;76(5):363-76
14. Achyut BR, Ghoshal UC, Moorchung N. et al. Transforming Growth Factor-B1and Matrix Metalloproteinase-7 Promoter Variants Induce Risk for Helicobacter pylori-Associated Gastric Precancerous Lesions. Dna And Cell Biology. 2009;28(6):295-301
15. Zhang P1, Di JZ, Zhu ZZ. et al. Association of transforming growth factor-beta 1 polymorphisms with genetic susceptibility to TNM stage I or II gastric cancer. Jpn J Clin Oncol. 2008;38(12):861-866
16. Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signaling: live or let die. Nat Rev Immunol. 2015;15(6):362-74
17. Yang JP, Hyun MH, Yoon JM. et al. Association between TNF-alpha-308 G/A gene polymorphism and gastric cancer risk: a systematic review and meta-analysis. Cytokine. 2014;70(2):104-14
18. Stubljar D, Jeverica S, Jukic T. et al. The influence of cytokine gene polymorphisms on the risk of developing gastric cancer in patients with Helicobacter pylori infection. Radiol Oncol. 2015;49(3):256-64
19. Gao L, Weck MN, Michel A. et al. Association between chronic atrophic gastritis and serum antibodies to 15 Helicobacter pylori proteins measured by multiplex serology. Cancer Res. 2009;69(7):2973-2980
20. Peleteiro B, Lunet N, Carrilho C. et al. Association between cytokine gene polymorphisms and gastric precancerous lesions: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(3):762-76
21. You W, Lai X, Lv J. et al. Association of tumor necrosis factor-α gene polymorphisms with susceptibility to helicobacter pylori-associated gastroduodenal diseases in the Chinese population. Int J Clin Exp Pathol. 2016;9(12):12836-12842
22. Zhao H, Liu L, Liu B. et al. An updated association between TNF-α -238G/A polymorphism and gastric cancer susceptibility in East Asians. Biosci Rep. 2018 38(6)
23. Xu T, Kong Z, Zhao H. Relationship Between Tumor Necrosis Factor-α rs361525 Polymorphism and Gastric Cancer Risk: A Meta-Analysis Front Physiol. 2018; 9:469.
24. Essadik A, Jouhadi H, Rhouda T. et al. Polymorphisms of Tumor Necrosis Factor Alpha in Moroccan Patients with Gastric Pathology: New Single-Nucleotide Polymorphisms in TNF-α(-193) (G/A). Mediators Inflamm. 2015;2015:143941
25. Leja M, Wex T, Malfertheiner P. Markers for gastric cancer premalignant lesions: where do we go? Dig Dis. 2012;30(3):268-76
26. Yin YW, Sun QQ, Hu AM. et al. Associations between interleukin-6 gene -174 C/G and -572 C/G polymorphisms and the risk of gastric cancer: a meta-analysis. J Surg Oncol. 2012;106(8):987-93
27. Gatti LL, Burbano RR, Zambaldi-Tunes M. et al. Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res. 2007;38(5):551-555
28. Ramis IB, Vianna JS, Gonçalves CV. et al. Polymorphisms of the IL-6, IL-8 and IL-10 genes and the risk of gastric pathology in patients infected with Helicobacter pylori. J Microbiol Immunol Infect. 2017;50(2):153-159
29. El-Zimaity H, Choi WT, Lauwers GY, Riddell R. The differential diagnosis of Helicobacter pylori negative gastritis. Virchows Arch. 2018;473(5):533-550
30. Bizzaro N, Antico A, Villalta D. Autoimmunity and Gastric Cancer. Int J Mol Sci. 2018;19(2):377
31. Daneshmandi S, Pourfathollah AA, Pourpak Z, Heidarnazhad H, Kalvanagh PA. Cytokine gene polymorphism and asthma susceptibility, progress and control level. Mol Biol Rep. 2012;39(2):1845-1853
32. González JR, Armengol L, Guinó E. et al. SNPassoc: SNPs-based whole genome association studies. 2014. R package version 1.9-2. Available from: https://CRAN.R-project.org/package=SNPassoc.
33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing.1995. Journal of the Royal Statistical Society Series B. 57:289-300. Available from: http://www.jstor.org/stable/2346101
34. Yee T. VGAM: Vector Generalized Linear and Additive Models. R package version 1.1-2. URL. 2019 Available from: https://CRAN.R-project.org/package=VGAM
35. Song M, Latorre G, Ivanovic-Zuvic D. et al. Autoimmune Diseases and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Cancer Res Treat. 2019;51(3):841-850
36. Kishikawa H, Ojiro K, Nakamura K. et al. Previous Helicobacter pylori infection-induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter. 2020;25(1):e12669
37. Huang R, Ende A, Singla A. et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc. 2020;91:70-77
38. Trieu JA, Bilal M, Saraireh H. et al. Update on the Diagnosis and Management of Gastric Intestinal Metaplasia in the USA. Dig Dis Sci. 2019;64(5):1079-1088
39. Huang RJ, Choi AY, Truong CD, Yeh MM, Hwang JH. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver. 2019;13(6):596-603
40. Anciuc M, Tripon F, Crauciuc GA. et al. The angiotensinogen gene polymorphism, lifestyle factors, associated diseases and gastric areas of inflammatory and preneoplastic lesions in a Romanian sample of patients. Rev Romana Med Lab. 2019;27(4):401-11
41. Ammouri W, Tazi ZM, Harmouche H. et al. Venous thromboembolism and hyperhomocysteinemia as first manifestation of pernicious anemia: a case series. J Med Case Rep. 2017;11(1):250
42. Ekim M, Ekim H, Yilmaz YK. et al. Study on relationships among deep vein thrombosis, homocysteine and related B group vitamins. Pak J Med Sci. 2015;31(2):398-402
43. Ducros V, Barro C, Yver J. et al. Should Plasma Homocysteine Be Used as a Biomarker of Venous thromboembolism? A Case-Control Study. Clin Appl Thromb Hemost. 2009;15(5):517-22
44. Flores-Luna L, Bravo MM, Kasamatsu E. et al. Risk factors for gastric precancerous and cancers lesions in Latin American counties with difference gastric cancer risk. Cancer Epidemiol. 2019;64:101630
45. Li LF, Chan RL, Lu L. et al. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms. Int J Mol Med. 2014;34(2):372-80
46. Negovan A, Iancu M, Fülöp E. et al. Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions. World J Gastroenterol. 2019;25(30):4105-4124
47. Bockerstett KA, DiPaolo RJ. Regulation of Gastric Carcinogenesis by Inflammatory Cytokines. Cell Mol Gastroenterol Hepatol. 2017;4(1):47-53
Author contact
![]() Corresponding author: Mihaela Iancu, PhD, Department of Medical Informatics and Biostatistics, Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, Cluj-Napoca, Romania, 8 Babeş Street, 400012 Cluj-Napoca, Phone: +40-264-597-256, E-mail: miancuro.
Corresponding author: Mihaela Iancu, PhD, Department of Medical Informatics and Biostatistics, Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, Cluj-Napoca, Romania, 8 Babeş Street, 400012 Cluj-Napoca, Phone: +40-264-597-256, E-mail: miancuro.

 Global reach, higher impact
Global reach, higher impact