3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(12):2673-2688. doi:10.7150/ijms.58147 This issue Cite
Review
Repurposing New Use for Old Drug Chloroquine against Metabolic Syndrome: A Review on Animal and Human Evidence
Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Bandar Tun Razak, 56000 Cheras, Kuala Lumpur, Malaysia.
Received 2021-1-13; Accepted 2021-5-4; Published 2021-5-13
Abstract
Chloroquine (CQ) and hydroxychloroquine (HCQ) are traditional anti-malarial drugs that have been repurposed for new therapeutic uses in many diseases due to their simple usage and cost-effectiveness. The pleiotropic effects of CQ and HCQ in regulating blood pressure, glucose homeostasis, lipid, and carbohydrate metabolism have been previously described in vivo and in humans, thus suggesting their role in metabolic syndrome (MetS) prevention. The anti-hyperglycaemic, anti-hyperlipidaemic, cardioprotective, anti-hypertensive, and anti-obesity effects of CQ and HCQ might be elicited through reduction of inflammatory response and oxidative stress, improvement of endothelial function, activation of insulin signalling pathway, inhibition of lipogenesis and autophagy, as well as regulation of adipokines and apoptosis. In conclusion, the current state of knowledge supported the repurposing of CQ and HCQ usage in the management of MetS.
Keywords: diabetes, dyslipidaemia, hypertension, immunomodulation, inflammation, obesity
Introduction
Chloroquine (CQ) and hydroxychloroquine (HCQ), first approved for medical use in 1949 and 1955 respectively, have been widely recognised for its effectiveness in the treatment of malaria and autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE). They exert anti-plasmodial effect against Plasmodium parasites by inhibiting the chemical polymerisation of toxic heme released during proteolysis of haemoglobin in the parasites' digestive vacuole [1]. Autoimmune diseases are caused by dysfunction of immune system that attacks and damages healthy tissues instead of foreign invaders. Both CQ and HCQ possess anti-inflammatory and immunomodulatory actions which account for their immunosuppressive capability during autoimmune disorders, mainly through (a) the inhibition of lysosomal enzyme activity that blocks the antigen presentation and (b) the suppression of Toll-like receptor (TLR) signalling pathway to reduce the synthesis of pro-inflammatory cytokines [2]. Research advancement also revealed the anti-viral properties of CQ and HCQ to fight against rabies [3], polio [4], Chikungunya [5], dengue [6,7], Zika [8], herpes simplex [9], human immunodeficiency [10], hepatitis C [11], and influenza [12] viruses. Most recently, CQ and HCQ have received much attention due to the purported efficacy as promising candidates to treat the novel coronavirus (COVID-19) [13]. Apart from that, these anti-malarial drugs have been also reported to have pleiotropic effects in multifarious non-rheumatoid conditions such as infectious diseases, coagulopathies, bone disorders, malignancies, atherosclerosis, diabetes, disordered lipid metabolism, and hypertension [14,15]. The latter three conditions are closely associated with metabolic syndrome (MetS).
Metabolic syndrome is a complex medical condition with co-existence of three out of five abnormalities that predispose an individual to a higher risk of cardiovascular disease and stroke [16]. The risk factors for MetS include increased waist circumference, blood pressure (BP), fasting blood glucose (FBG), triglycerides (TG) and reduced high-density lipoprotein cholesterol (HDL-C). A close relationship between MetS as well as malaria, rheumatic disorders, and virus infections has been previously elucidated whereby the presence of obesity, diabetes, hypertension, and/or dyslipidaemia can aggravate these diseases. There were more death cases and higher severity of malaria in diabetic patients than non-diabetics [17]. A cross-sectional study in North India reported a higher prevalence of MetS in human immunodeficiency virus-positive patients [18]. Another cross-sectional study assessed the prevalence of MetS in adult patients with SLE in Argentina. The findings concluded a significantly higher prevalence of MetS and frequency of arterial hypertension in the SLE patients than the control subjects. The use of HCQ was negatively associated with MetS (odds ratio: 0.13, 95% confidence interval: 0.03-0.68), indicating that HCQ was protective against the presence of MetS [19]. Hence, the repurposing of CQ and HCQ as a treatment for MetS remains clinically significant.
This review focused on summarising the potential role of CQ and HCQ in preventing MetS and its associated medical conditions. The underlying molecular mechanisms governing the positive effects of CQ and HCQ are also highlighted. We hope to provide information for healthcare providers and researchers about the possibility of expanding the clinical applicability for CQ and HCQ.
The anti-hyperglycaemic action of chloroquine
Evidence from animal studies
In vivo studies investigating the hypoglycaemic effects of CQ or HCQ have been conducted since several decades ago using diabetic and healthy animals (Table 1). Streptozotocin (STZ) is an anti-neoplastic agent that damages the insulin-producing beta cells of the pancreas, thus it is commonly used in the induction of type 1 diabetes mellitus in animals [20]. In one of the earlier studies, Asamoah and colleagues pre-treated female Sprague-Dawley rats with CQ (20 mg/kg) twice a week for 12 weeks via intramuscular (i.m.) injection and followed by induction of diabetes using 50 mg/kg STZ through intravenous (i.v.) injection. Results from this study indicated that CQ lowered FBG, glycated haemoglobin (HbA1c), glycated plasma protein and increased plasma immunoreactive insulin in the diabetic rats [21]. Using male Sprague-Dawley rats, Emami and co-authors intraperitoneally (i.p.) injected the animals with STZ to induce diabetes and orally supplemented them with HCQ (80, 120 or 160 mg/kg/day) for 10 days. Treatment with HCQ showed dose-dependent effects in lowering serum glucose and increasing serum insulin [22]. In another study, the STZ-induced diabetic rats were orally treated with 200 mg/kg/week HCQ for four weeks. Reductions of FBG, oral glucose tolerance test (OGTT) as well as increases of insulin level and β-cell function were observed in the animals. Histologically, the islets of Langerhans showed preserved structure, intact cellular component, minimal hyaline deposition, and absence of inflammatory cells. Immunohistochemical results revealed that the islets of Langerhans of HCQ-treated diabetic rats exhibited more intense insulin expressing cells and less predominant glucagon expressing cells [23]. However, a study by Yuan et al. showed opposite outcome whereby administration of CQ (60 mg/kg/day, i.p.) for 14 days did not have any effect on blood glucose level in STZ-induced male mice [24]. The negligible effects observed in this study might be due to the difference in treatment dose, duration and mode of administration. Alloxan is a hydrophilic glucose analogue with preferential toxicity towards pancreatic beta cells, which is also used to induce insulin-dependent diabetes mellitus [25]. Pareek et al. utilized a diabetic rat model induced by alloxan monohydrate to investigate the effect of HCQ on blood glucose. Similar to most of the studies using STZ-induced diabetic animals, they also reported a fall in glucose level in diabetic mice following HCQ administration [26].
Apart from that, researchers have investigated the anti-hyperglycaemic actions of CQ and HCQ in diet-induced animal models. Halaby et al. induced insulin resistance in male Wistar rats using a high-fat diet containing 35% lard. After three months of induction, the animals were intraperitoneally administered with 3.5 mg/kg CQ twice per week for one month. As compared to the negative controls, the CQ-treated animals had higher glucose uptake in L6 muscle cells [27]. Subsequently, another group of researchers pointed out that oral treatment with HCQ (6.5 mg/kg/day) for 12 weeks lowered FBG, OGTT, insulin, homeostatic model assessment for insulin resistance (HOMA-IR), and raised β-cell function in rats fed with high-fat diet containing 46% fat, 20.3% protein, and 24% carbohydrate. Histological findings from this study also showed that the structure of islets of Langerhans was preserved, cellular component was intact as well as hyaline deposition and inflammatory cell infiltration were absent in the CQ-treated animals. Regression of islets of Langerhans expansion, mild reaction of insulin-secreting cells and more abundant glucagon expressing cells were also seen in the high-fat diet-fed animals with HCQ treatment [28]. More recently, Qiao and co-researchers found that pre-treatment of HCQ (40 mg/kg/day) via intraperitoneal injection to male C57BL/6J mice before subjected the animals with high-fat diet significantly reduced intraperitoneal glucose tolerance test (IPGTT), insulin, insulin tolerance test (ITT), HOMA-IR as well as increased homeostatic model assessment for insulin sensitivity (HOMA-IS) and glucose uptake in HepG2 cells [29].
In a genetically diabetic (db/db) mouse model, oral treatment with 50 mg/kg HCQ did not cause a significant change in glucose level as compared to the control group after one week. However, the level of insulin in the HCQ-treated mice was increased [30]. The diabetic characteristics of db/db mouse model are derived from single autosomal recessive mutation in leptin receptor gene on chromosome 4, whereby the defective leptin receptor results in excessive extracellular leptin but lack of intracellular leptin [31]. The impairment of leptin signalling perturbs the physiological processes of glucose utilisation, energy expenditure and insulin sensitivity [32]. Thus, it has been postulated that HCQ might be inefficient to alleviate insulin resistance caused by attenuation of leptin signalling in the leptin receptor-deficient mice. In normal animals, studies found both positive and negligible effects of CQ and HCQ on parameters related to hyperglycaemia. Intramuscular injection of CQ (20 mg/kg) thrice weekly into female normal Sprague-Dawley rats resulted in the decreases in FBG, HbA1c and increase in insulin level [21]. In contrast, a study by Emami et al. described that oral HCQ (160 mg/kg) treatment for 10 days did not influence FBG and IPGTT in healthy rats [22]. The reduction of glucose level might be minimal owing to the normal glycaemic status in healthy animals. Findings from these studies suggested that CQ and HCQ might exert lesser glycaemic control in genetically diabetic animals and normal healthy animals.
Taken together, most of the studies indicated the positive effects of CQ and HCQ in hindering of hyperglycaemic condition in animals except for few studies. The heterogeneous outcomes might be attributed to the variation in treatment dose, duration, route of administration and type of animal model.
The anti-hyperglycaemic effects of CQ and HCQ in animal studies
| Types of animal | Types of induction | Treatment, dose, route and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|---|
| Female Sprague-Dawley rats | STZ (50 mg/kg, i.v.) | CQ (20 mg/kg, twice weekly, i.m., 12 weeks) | FBG: ↓, HbA1c: ↓, glycated plasma protein: ↓, insulin: ↑ | - | [21] |
| - | CQ (20 mg/kg, thrice weekly, i.m., 20 weeks) | FBG: ↓, HbA1c: ↓, insulin: ↑ | - | ||
| Adult male Sprague-Dawley rats | STZ (60 mg/kg, i.p.) | HCQ (80, 120 or 160 mg/kg/day, oral, 10 days) | Glucose: ↓ | - | [22] |
| - | HCQ (160 mg/kg/day, oral, 10 days | FBG: ↔ | - | ||
| Adult Sprague-Dawley rats | STZ (27.5 mg/kg, i.p.) | HCQ (200 mg/kg/week, oral, 4 weeks) | FBG: ↓, OGTT: ↓, insulin: ↑, β-cell function: ↑ | IL-1β: ↓, IL-6: ↓, TNF-α: ↓, TGF-β1: ↓, MCP-1: ↓, IL-10: ↔, caspase-3: ↓, Bcl-2: ↑ | [23] |
| Male C57BL mice | STZ (60 mg/kg, i.p.) | CQ (60 mg/kg/day, i.p., 14 days) | Glucose: ↔ | LC3-II: ↑, p62: ↑, Beclin1: ↔, autophagic vacuoles: ↓ | [24] |
| Male Wistar rats | Alloxan monohydrate (90 mg/kg, i.p.) | HCQ (200 mg/kg/day, oral, 9 days) | Glucose: ↓ | - | [26] |
| Male Wistar rats | High-fat diet (35% lard) | CQ (3.5 mg/kg, twice per week, i.p., 1 month) | Glucose uptake in muscle cells: ↑ | p-Akt: ↑, p-JNK: ↔, p-GSK3β: ↑, glycogen synthase activity: ↑ | [27] |
| Adult Sprague-Dawley rats | High-fat diet (46% fat, 20.3% protein, 24% carbohydrate) | HCQ (6.5 mg/kg/day, oral, 12 weeks) | FBG: ↓, OGTT: ↓, insulin: ↓, HOMA-IR: ↓, β-cell function: ↑ | E-selectin: ↓, ICAM-1: ↓, VCAM-1: ↓, leptin: ↓, resistin: ↓, visfatin: ↓, adiponectin: ↑, lipocalin-2: ↓ | [28] |
| Male C57BL/6J mice | High-fat diet (60% fat) | HCQ (40 mg/kg/day, pre-treatment, i.p., 17 weeks) | IPGTT: ↓, insulin: ↓, ITT: ↓, HOMA-IR: ↓, HOMA-IS: ↑, glucose uptake in HepG2 cells: ↑ | IL-1β: ↓, IL-6: ↓, TNF-α: ↓, MCP-1: ↓, CD68: ↓, Arg1: ↓, p-Akt: ↑, p-IRS1: ↑ | [29] |
| Male db/db mice | - | HCQ (50 mg/kg/day, oral, 7 days) | Insulin: ↑, glucose: ↔ | IGF-1: ↔, p-Akt: ↑, p-mTOR: ↔, p-S6: ↔ | [30] |
Abbreviations: Arg1, arginase 1; Bcl-2, B-cell lymphoma 2; CD, cluster of differentiation; CQ, chloroquine; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; HCQ, hydroxychloroquine; HepG2, human liver cancer cell line; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-IS, homeostatic model assessment for insulin sensitivity; ICAM-1, intercellular adhesion molecule 1; IGF-1, insulin growth factor 1; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IL-10, interleukin-10; i.m., intramuscular; i.p., intraperitoneal; IPGTT, intraperitoneal glucose tolerance test; ITT, insulin tolerance test; MCP-1, monocyte chemoattractant protein-1; OGTT, oral glucose tolerance test; p-Akt, phosphorylated protein kinase B; p-GSK3β, phosphorylated glycogen synthase kinase-3 beta; p-IRS1, phosphorylated insulin receptor substrate 1; p-JNK, phosphorylated c-Jun N-terminal kinase; p-mTOR, phosphorylated mammalian target of rapamycin; STZ, streptozotocin; TC, total cholesterol; TG, triglyceride; TGF-β1, transforming growth factor-beta 1; TNF-α, tumour necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule 1; ↑, increase/stimulate; ↓, decrease/inhibit; ↔, no change.
The anti-hyperglycaemic effects of CQ and HCQ in animal studies
| Study population | Treatment, dose and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|
| Adults with newly diagnosed rheumatoid arthritis and no diabetes (n=1127, aged ≥18 years) | HCQ (6.5 mg/kg or 400 mg/day) | Risk of diabetes: ↓ | - | [33] |
| SLE patients with newly diagnosed diabetes mellitus (n=221) | HCQ (cumulative dose ≥ 129 g) | Risk of diabetes: ↓ | - | [34] |
| Patients with prediabetes (n=20; aged 45.9 ± 7.32 years) | HCQ (6.5 mg/kg/day, 12 weeks) | Insulin: ↑, OGTT: ↓ | - | [35] |
| Patients with diabetes mellitus (n=45, aged 61 ± 13 years) | HCQ (dose not mentioned, >12 weeks) | HbA1c: ↓ | - | [36] |
| Patients with T2DM (n=10; aged 43-61 years) | CQ (250 mg, four times daily, 3 days) | FBG: ↓, fasting plasma insulin: ↑ | - | [37,38] |
| Patients with T2DM (n=135; aged 18-65 years) | HCQ (400 mg/day, 24 weeks) | HbA1c: ↓, FBG: ↓, postprandial glucose: ↓ | - | [39] |
| Sulfonylurea-refractory patients with poorly controlled T2DM (n=69; aged 35-80 years) | HCQ (300 mg/day, 6 months) | HbA1c: ↓, glucose tolerance: ↑ | - | [40] |
| Patients with T2DM failing metformin and sulfonylurea (n=15; aged 18-75 years) | HCQ (400 mg/day, 4 months) | HbA1c: ↓, FBG: ↓ | - | [41] |
| Obese, non-diabetic subjects (n=13; aged 24-71 years) | HCQ (6.5 mg/kg/day, 6 weeks) | ISI: ↑, HOMA-IR: ↓ | CRP: ↔, IL-6: ↔ | [42] |
| Overweight or obese subjects with one or more markers of insulin resistance (n=17; aged 50.1 ± 14.5 years) | HCQ (400 mg/day, oral, 13 ± 1 weeks) | Insulin sensitivity: ↑, β-cell function: ↑, FBG: ↓, HbA1c: ↓ | Adiponectin: ↑ | [43] |
| Patients with primary dyslipidaemia (n=127; aged 49.21 ± 9.58 years) | HCQ (200 mg/day) + atorvastatin (10 mg/day), 24 weeks | HbA1c: ↓, FBG: ↓ | hs-CRP: ↓ | [44] |
| Patients with MetS (n=25; aged 18-60 years) | Placebo (3 weeks) → CQ (80 mg/week, 3 weeks) → CQ (80 mg/day, 3 weeks) → CQ (250 mg/day, 3 weeks) | Hepatic glucose production: ↓, hepatic insulin sensitivity: ↑, FBG: ↓, OGTT: ↔ | TNF-α: ↓, CRP: ↔, leptin: ↔, adiponectin: ↔ | [45] |
| Patients with MetS (n=56; aged 18-70 years) | CQ (80 mg/day, 1 year) | OGTT: ↔, HOMA-IR: ↔, ISI: ↔ | p-JNK: ↓ | |
| Women with SLE (n=71; aged 49.8 ± 9.9 years) | HCQ (400 mg, duration not mentioned) | FBG: ↓, HOMA-IR: ↓ | - | [46] |
| Women with rheumatoid arthritis (n=31; aged 56.5 ± 9.0 years) | HCQ (200 mg, duration not mentioned) | FBG: ↓ | - | |
| Patients with SLE or rheumatoid arthritis(n=26; aged 46 ± 16 years) | HCQ (mean daily dose: 284.6 ± 67.5 mg, 5-6 months) | HbA1c: ↓ | - | [47] |
| Patients with rheumatoid arthritis without diabetes (n= 23; aged 56 ± 11.4 years) | HCQ (6.5 mg/kg/day, 8 weeks) | ISI: ↔, HOMA-IR: ↔ | - | [48] |
Abbreviations: CQ, chloroquine; CRP, C-reactive protein; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; HCQ, hydroxychloroquine; HOMA-IR, homeostatic model assessment for insulin resistance; hs-CRP, high sensitivity C-reactive protein; IL-6, interleukin-6; ISI, insulin sensitivity index; MetS, metabolic syndrome; OGTT, oral glucose tolerance test; p-JNK, phosphorylated c-Jun N-terminal kinase; SLE, systemic lupus erythematosus; TNF-α, tumour necrosis factor-alpha; T2DM, type 2 diabetes mellitus; ↑, increase/stimulate; ↓, decrease/inhibit; ↔, no change.
Evidence from human studies
Numerous human studies have been performed to investigate the relationship between CQ or HCQ use and risk of diabetes as well as the effects of CQ or HCQ in preventing hyperglycaemia. These studies were conducted in patients displaying various types of medical conditions such as type 2 diabetes mellitus (T2DM), obesity, MetS, dyslipidaemia, rheumatoid arthritis and SLE (Table 2).
In a retrospective cohort consisting of 1127 adults aged 18 years and above with newly diagnosed rheumatoid arthritis and no diabetes, Bili and colleagues classified the patients into two subgroups (ever users and never users of HCQ) to examine the relationship between HCQ use and incidence of diabetes. The hazard ratio (HR) for diabetes incident among HCQ users was 0.29 [95% confidence interval (CI) 0.09-0.95], reiterating the potential of HCQ in reducing the risk of diabetes in rheumatoid arthritis patients [33]. A nationwide population-based cohort study was implemented to study the association between HCQ use and risk of diabetes mellitus in SLE patients. This study enrolled a total of 221 SLE patients with newly diagnosed diabetes mellitus and they were divided into those had taken or had never taken HCQ. The data collected from this study demonstrated that the use of HCQ was associated with a lower risk of diabetes mellitus incident, with the reported HR for diabetes incident among HCQ users was 0.26 [95% CI 0.18-0.37] [34].
In prediabetic patients (n=20; aged 45.9 ± 7.32 years), a randomised double-blinded controlled trial revealed an increase in insulin level and a decrease in OGTT after 12 weeks of HCQ initiation at the dose of 6.5 mg/kg/day [35]. In diabetic patients (n=45; aged 61 ± 13 years), there was a significantly greater reduction in HbA1c after 12 months of HCQ treatment [36]. Studies conducted by Powrie and co-authors determined the effects of three days oral CQ administration on FBG and fasting plasma insulin among patients with non-insulin-dependent diabetes mellitus (NIDDM) (n=10; aged 43 - 61 years). CQ caused a decrease in FBG and an increase in fasting plasma insulin in these patients [37,38]. Pareek et al. carried out a double-blind study to test the efficacy of HCQ as a treatment of hyperglycaemia in the Indian population. Patients with uncontrolled T2DM (n=135; mean age 52.60 ± 8.55 years) receiving 400 mg HCQ daily for 24 weeks exhibited lower HbA1c, FBG and postprandial glucose at week 12 and week 24 as compared to baseline [39]. Gerstein et al. designed a randomised trial to assess the glucose-lowering efficacy and responsiveness of HCQ in sulfonylurea-refractory T2DM patients (n=69; mean age: 58 ± 9.6 years). Participants were given 300 mg HCQ daily. The level of HbA1c was decreased and glucose tolerance was improved after 6 months of supplementation [40]. More recently, another randomised controlled trial determined the effects of HCQ as an anti-hyperglycaemic agent in T2DM patients failing metformin and sulfonylurea (n=15; aged 18-75 years). It was noted that the HbA1c and FBG levels were significantly reduced throughout the study period of four months [41].
Mercer et al. conducted a longitudinal study to determine the effects of HCQ (6.5 mg/kg/day) treatment for six weeks on insulin resistance, insulin sensitivity and pancreatic β-cell secretion of insulin in obese non-diabetic subjects (n=13; mean age: 49 ± 15 years). The parameters were measured at three time points including week 0 (before HCQ treatment), week 6 (at the end of HCQ treatment) and week 12 (6 weeks after HCQ treatment). There was an increase in insulin sensitivity index (ISI) and a decrease in HOMA-IR after six weeks of HCQ therapy. However, these parameters returned toward baseline at week 12 [42]. Later on, a randomised, double-blind, parallel-arm trial was done on overweight or obese subjects with one or more markers of insulin resistance aged 18 years and above (n=17) to study the anti-diabetogenic effects of HCQ (400 mg/day). After the intervention, the participants had positive changes in insulin sensitivity and β-cell function as well as negative changes in FBG and HbA1c [43].
Comparable outcomes were observed when a combination of HCQ (200 mg) and atorvastatin (10 mg) was given to the patients with primary dyslipidaemia (n=127; aged 49.21 ± 9.58 years). The levels of HbA1c and FBG were lowered in these patients [44]. Recently, a comprehensive study consisting of two clinical trials of CQ was conducted in people with MetS. In the first trial, 25 patients aged 18-60 years were evaluated and intervention of CQ was provided in dose escalation. The MetS subjects were given placebo capsules for 21 days (first phase), 80 mg of CQ weekly for 21 days (second phase), 80 mg of CQ daily for 21 days (third phase) and 250 mg of CQ daily for 21 days (fourth phase). A washout period of five to seven weeks was applied after each phase. Results from this trial showed significant suppression of hepatic glucose production, decrease in fasting glucose and increase in hepatic insulin sensitivity [45]. In the second trial, 107 patients were included and randomly assigned to placebo (n=51) and 80 mg/day CQ (n=56) for a year. However, the findings showed no effect of CQ in OGTT, HOMA-IR and ISI between the two intervention arms. Their study suggested that long-term treatment with a lower dose of CQ might not be clinically useful for glucose level control [45].
Both CQ and HCQ are prescription for the treatment of SLE and rheumatoid arthritis, thus researchers measured the glycaemic change in these patients after receiving CQ or HCQ. A cross-sectional study recruited non-diabetic women with SLE (n=149) or rheumatoid arthritis (n=177). Among these subjects, 48% of SLE patients and 18% of rheumatoid arthritis patients took HCQ medication at the dose of 400 mg and 200 mg, respectively. The data derived from this study showed that serum glucose was lower in HCQ user than non-users in both SLE and rheumatoid arthritis patients. Lower HOMA-IR was also seen in rheumatoid arthritis patients receiving HCQ [46]. In Japanese population, it was also found that patients with SLE or rheumatoid arthritis (n=26; aged 46 ± 16 years) prescribed with HCQ with mean daily dose of 284.6 ± 67.5 mg had lower HbA1c after five and six months of HCQ [47]. On the contrary, distinct findings were obtained from a randomised, blinded crossover trial. In this study, patients with rheumatoid arthritis without diabetes (n=23; aged 56 ± 11.4 years) were randomised to two groups. One group was allocated to receive 8 weeks of 6.5 mg/kg/day HCQ followed by 8 weeks of placebo whereas another group was assigned to placebo followed by HCQ for the same duration. No statistically significance was observed in ISI and HOMA-IR during HCQ administration as compared to placebo. The authors postulated that a longer treatment duration might allow an apparent anti-hyperglycaemic effect of HCQ in rheumatoid arthritis patients. Besides, observable effect of HCQ in improving insulin and glucose metabolism was not detected in this study as the rheumatoid arthritis patients had normal glycaemic status [48].
In line with in vivo studies, most of the human studies indicated that CQ and HCQ users potentially had a lower risk of diabetes, improved insulin sensitivity and glucose tolerance, increased β-cell function, and reduced glucose production in the liver. However, long-term treatment with a lower dose of CQ seemed to have no effect in glycaemic control.
The anti-hyperlipidaemic action of chloroquine
Evidence from animal studies
Several preclinical experiments have investigated the anti-hyperlipidaemic actions of CQ and HCQ (Table 3). Favourable effects of HCQ on lipid profile was reported in STZ-induced diabetic rats. Four weeks oral supplementation of 200 mg/kg/day HCQ reduced the levels of total cholesterol (TC), TG, free fatty acid (FFA), low-density lipoprotein cholesterol (LDL-C) and increased HDL-C in the serum of diabetic animals [23]. In high-fat diet-fed mice, HCQ (40 mg/kg/day, i.p.) regulated lipid metabolism by reducing TG, TC, FFA and LDL-C in serum in relative to the mice fed with standard chow diet after 17 weeks. The increased amount of TG and TC in liver of high-fat diet-fed mice was also prevented by treatment with HCQ [29]. In another study using animals fed with a low protein diet, it was worthy to note that serum TC and TG were lowered after eight weeks of oral CQ (5 mg/kg) administration [49]. In contrast with these findings, supplementation of 50 mg/kg HCQ daily for seven days failed to lower the levels of TG, TC and FFA levels in male db/db mice [30]. In short, evidence from animal studies suggested that CQ and HCQ might be promising agents in improving lipid profile in animals, but a short duration of treatment may be insufficient to induce these changes.
Evidence from human studies
The anti-hyperlipidaemic actions of CQ and HCQ have been widely explored in various study populations displaying MetS-associated medical conditions and autoimmune diseases (Table 4). Treatment with CQ and HCQ at the dose between 250-400 mg decreased TC, TG, LDL-C, apolipoprotein B (apoB) and the ratio of apoB to apolipoprotein A-1 (apoA-1) but did not affect non-esterified fatty acids (NEFA) and HDL-C levels in T2DM patients [37,39]. A study by Gerstein et al. delineated that HCQ (300 mg/day) reduced LDL-C in T2DM patients showing poor glycaemic control on maximal doses of sulfonylurea after six months of treatment [40]. Another recent randomised trial also found that four months supplementation of HCQ at 400 mg lowered TC and non-HDL-C in patients with T2DM who are refractory to sulfonylureas [41]. In patients with primary dyslipidaemia receiving a 24-week course of HCQ (200 mg) and atorvastatin (10 mg) in combination, reductions of TC, TG, LDL-C and non-HDL-C were detected [44]. McGill and co-researchers conducted two clinical trials of CQ in people with MetS. The first trial indicated the lowering of TC, non-HDL-C and LDL-C after intervention with dose escalation of CQ from 80 mg/week for three weeks, followed by 80 mg/day for subsequent three weeks and 250 mg/day for another three weeks. Similar observations were observed in a second trial whereby the participants were treated with 80 mg/day for one year [45]. However, one studies indicated paradoxical outcome. An open-label longitudinal study indicated no change in the levels of TC, TG, HDL-C and LDL-C in obese and non-diabetic subjects (n=13; aged 24 - 71 years) administered with 6.5 mg/kg/day HCQ for six weeks. The small sample size and short treatment period for HCQ might contribute to such distinct findings [42].
The anti-hyperlipidaemic effects of CQ and HCQ in animal studies
| Types of animal | Types of induction | Treatment, dose, route and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|---|
| Adult Sprague-Dawley rats | STZ (27.5 mg/kg, i.p.) | HCQ (200 mg/kg/week, oral, 4 weeks) | TG: ↓, TC: ↓, FFA: ↓, LDL-C: ↓, HDL-C: ↑ | IL-1β: ↓, IL-6: ↓, TNF-α: ↓, TGF-β1: ↓, MCP-1: ↓, IL-10: ↔ | [23] |
| Male C57BL/6J mice | High-fat diet (60% fat) | HCQ (40 mg/kg/day, pre-treatment, i.p., 17 weeks) | Serum TG: ↓, TC: ↓, LDL-C: ↓, FFA: ↓, HDL-C: ↔ Liver TG: ↓, TC: ↓ | Inflammation IL-1β: ↓, IL-6: ↓, TNF-α: ↓, MCP-1: ↓, CD68: ↓, Arg1: ↓ Lipid metabolism CPT1α: ↑, CPT1β: ↑, PPAR-γ: ↓, Mgat-1: ↓, SREBP1c: ↓, ChREBP: ↓, ACC: ↓, FAS: ↓ | [29] |
| Male Wistar rats | Low protein diet | CQ (5 mg/kg, 3 days/week, oral, 8 weeks) | TC: ↓, TG: ↓ | - | [49] |
| Male db/db mice | - | HCQ (50 mg/kg/day, oral, 7 days) | FFA: ↔, TG: ↔, TC: ↔, TC (heart): ↔ | IGF-1: ↔, p-Akt: ↑, p-mTOR: ↔, p-S6: ↔ | [30] |
Abbreviations: ACC, acetyl-CoA carboxylase; Arg1, arginase 1; CD, cluster of differentiation; ChREBP, carbohydrate response element binding protein; CPT1α, carnitine palmitoyltransferase 1 alpha; CPT1β, carnitine palmitoyltransferase 1 beta; CQ, chloroquine; FAS; fatty acid synthase; FFA, free fatty acids; HCQ, hydroxychloroquine; HDL-C, high-density lipoprotein cholesterol; IGF-1, insulin growth factor 1; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IL-10, interleukin-10; i.p., intraperitoneal; LDL-C, low-density lipoprotein cholesterol; MCP-1, monocyte chemoattractant protein-1; Mgat-1, monoacylglycerol-O-acyltransferase; p-Akt, phosphorylated protein kinase B; p-mTOR, phosphorylated mammalian target of rapamycin; PPAR-γ, peroxisome proliferator-activated receptor-gamma; SREBP1c, sterol regulatory element-binding transcription factor 1; STZ, streptozotocin; TC, total cholesterol; TG, triglyceride; TGF-β1, transforming growth factor-beta 1; TNF-α, tumour necrosis factor-alpha; ↑, increase/stimulate; ↓, decrease/inhibit; ↔, no change.
The anti-hyperlipidaemic effects of CQ and HCQ in human studies
| Study population | Treatment, dose and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|
| Patients with T2DM (n=10; aged 43-61 years) | CQ (250 mg, four times daily, 3 days) | TC: ↓, LDL-C: ↓, apoB: ↓, apoB/apoA-1 ratio: ↓, NEFA: ↔ | - | [37] |
| Patients with T2DM (n=135; aged 18-65 years) | HCQ (400 mg/day, 24 weeks) | TC: ↓, TG: ↓, LDL-C: ↓, HDL-C: ↔ | - | [39] |
| Sulfonylurea-refractory patients with poorly controlled T2DM (n=69; aged 35-80 years) | HCQ (300 mg/day, 6 months) | LDL-C: ↓ | - | [40] |
| Patients with T2DM failing metformin and sulfonylurea (n=15; aged 18-75 years) | HCQ (400 mg/day, 4 months) | TC: ↓, non-HDL-C: ↓, TG: ↔, LDL-C: ↔, HDL-C: ↔ | - | [41] |
| Patients with primary dyslipidaemia (n=127; aged 49.21 ± 9.58 years) | HCQ (200 mg/day) + atorvastatin (10 mg/day), 24 weeks | TC: ↓, TG: ↓, LDL-C: ↓, non-HDL-C: ↓, HDL-C: ↔ | hs-CRP: ↓ | [44] |
| Patients with MetS (n=25; aged 18-60 years) | Placebo (3 weeks) → CQ (80 mg/week, 3 weeks) → CQ (80 mg/day, 3 weeks) → CQ (250 mg/day, 3 weeks) | TC: ↓, non-HDL-C: ↓, LDL-C: ↓, TG: ↔, HDL-C: ↔, NEFA: ↔ | TNF-α: ↓, CRP: ↔, leptin: ↔, adiponectin: ↔ | [45] |
| Patients with MetS (n=56; aged 18-70 years) | CQ (80 mg/day, 1 year) | TC; ↓, non-HDL-C: ↓, LDL-C: ↓ | p-JNK: ↓ | |
| Obese, non-diabetic subjects (n=13; aged 24-71 years) | HCQ (6.5 mg/kg/day, 6 weeks) | TC: ↔, TG: ↔, HDL-C: ↔, LDL-C: ↔ | CRP: ↔, IL-6: ↔ | [42] |
| Patients with SLE (n=51; aged ≥18 years) | HCQ (dose and duration not mentioned) | Dyslipidaemia: ↓ | - | [53] |
| Patients with SLE (n=24; aged 37.2 ± 16.0 years) | HCQ (200 mg/day) - 3 months | TC: ↓, LDL-C: ↓, frequency of dyslipidaemia: ↓ | - | [54] |
| Female patients with SLE (n=17) | HCQ (400 or 800 mg/day) - 3 months | TC: ↓, TG: ↓, VLDL-C: ↓, LDL-C: ↔, HDL-C: ↔, non-HDL-C: ↓, TC/HDL-C ratio: ↓, LDL-C/HDL-C ratio: ↓ | - | [55] |
| Patients with SLE (n=34; aged 48.7 ± 13.3 years) | HCQ (standard dose) | TC: ↔, HDL-C: ↔ | - | [56] |
| Chinese patients with mild or inactive SLE (n=44; aged 38.9 ± 7.9 years) | HCQ (244 ± 86 mg/day, duration not mentioned) | TG: ↔, TC: ↔, HDL-C: ↔, LDL-C: ↔ | apoA-1: ↔, apoB: ↔, lipoprotein A: ↔ | [57] |
| Patients with rheumatoid arthritis without diabetes (n= 23; aged 56 ± 11.4 years) | HCQ (6.5 mg/kg/day, 8 weeks) | TC: ↓, LDL-C: ↓, TG: ↔, HDL-C: ↔ | - | [48] |
| Male and female patients with rheumatoid arthritis (n=150; aged 65.8 ± 10.0 years) | HCQ (dose not mentioned, >3 months) | TG: ↓, TC: ↓, LDL-C: ↓, HDL-C: ↔, HDL-C/LDL-C: ↑, TC/HDL-C: ↓ | - | [58] |
| Patients with rheumatoid arthritis (n=256; aged 54-72 years) | HCQ (6.5 mg/kg/day, median exposure time: 1.98 years) | TG: ↓, TC: ↓, LDL-C: ↓, HDL-C: ↑, LDL-C/HDL-C: ↓, TC/HDL-C: ↓ | - | [59] |
| Patients with rheumatoid arthritis (n=254; aged 57.2 ± 11.1 years) | HCQ (dose and duration not mentioned) | TG: ↓, TC: ↓, LDL-C: ↓, HDL-C: ↑, TC/HDL-C: ↓, LDL-C/HDL-C: ↓ | - | [60] |
| Patients with early rheumatoid arthritis (n=6130; aged 45.45 ± 12.12 years) | HCQ (dose and duration not mentioned) | TC: ↓, LDL-C: ↓, HDL-C: ↑ | - | [61] |
| Patients with early rheumatoid arthritis (n=119; aged 48.66 ± 11.98 years) | Methotrexate + sulfasalazine + HCQ - 24, 48 and 102 weeks | TC: ↓, LDL-C: ↓, HDL-C: ↑ | - | [62] |
| Patients with early rheumatoid arthritis (n=103; aged 49.3 ± 12.5 years) | Methotrexate + sulfasalazine + HCQ - 24, 48 and 102 weeks | - | PON-1 activity: ↑, apoA-1: ↑, HDL inflammatory index: ↓, MPO: ↓ | [73] |
| Patients with SLE or rheumatoid arthritis (n=26; aged 46 ± 16 years) | HCQ (mean daily dose: 284.6 ± 67.5 mg, 5 - 6 months) | TG: ↓, HDL-C: ↔, LDL-C: ↓, non-HDL-C: ↓ | - | [47] |
| Female patients with Sjögren syndrome (n=71; aged >18 years) | HCQ | TC: ↓, HDL-C: ↑, atherogenic index: ↓ | - | [64] |
Abbreviations: apoA-1, apolipoprotein A-1; apoB, apolipoprotein B; CQ, chloroquine; CRP, C-reactive protein; HCQ, hydroxychloroquine; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein; IL-6, interleukin-6; LDL-C, low-density lipoprotein cholesterol; MetS, metabolic syndrome; MPO, myeloperoxidase; NEFA, non-esterified fatty acids; p-JNK, phosphorylated c-Jun N-terminal kinase; PON-1, paraoxonase 1; SBP, systolic blood pressure; SLE, systemic lupus erythematosus; TC, total cholesterol; TG, triglyceride; TNF-α, tumour necrosis factor-alpha; T2DM, type 2 diabetes mellitus; VLDL-C, very low-density lipoprotein cholesterol; ↑, increase/stimulate; ↓, decrease/inhibit; ↔, no change.
Dyslipidaemia is highly prevalent in patients with active autoimmune diseases, such as SLE [50], rheumatoid arthritis [51] and primary Sjögren's syndrome [52]. Inconsistent lipid-reducing effects of CQ and HCQ were reported in patients with SLE. In a cross-sectional study, the use of HCQ was negatively associated with development of dyslipidaemia in patients with SLE [relative risk (RR): 0.18; 95% CI: 0.36-5.09] [53]. The lipid-lowering effect of 200 mg/day HCQ was noted in SLE patients, observed by reduced TC, LDL-C and frequency of dyslipidaemia after three months of treatment [54]. A double-blind, randomised, placebo-controlled, pilot study by Kavanaugh et al. also revealed the alteration of lipoprotein profile [evidenced by decreased TC, TG, very low-density lipoprotein cholesterol (VLDL-C), non-HDL-C, TC/HDL-C ratio and LDL-C/HDL-C ratio] after treated with daily doses of 400 and 800 mg HCQ for three months in SLE patients [55]. Nonetheless, two groups of researchers found that CQ and HCQ intakes did not distinguish serum lipid profile in SLE patients as compared to the non-treated control group. In Brazilian population, there was no difference in TC and HDL-C levels between users and non-users of CQ. The authors suggested that the heterogeneity of clinical outcomes might be due to lipid profile was not adjusted for SLE disease activity [56]. In SLE patients of Chinese population, researchers were unable to demonstrate significant changes in fasting serum levels of TG, TC, HDL-C and LDL-C between the subjects receiving and not receiving 200-400 mg HCQ daily. These findings could be probably due to the two groups of patients were with mild or inactive disease whereby they had a relatively low level of lipids even not on HCQ treatment [57].
On the other hand, potent anti-hyperlipidaemic activity was consistently reported in rheumatoid arthritis patients. In a study with small sample size consisting of 23 rheumatoid arthritis patients without diabetes (aged 56 ± 11.4 years), Solomon and friends found that the patients allocated to received HCQ (6.5 mg/kg/day) had lower TC and LDL-C after eight weeks [48]. In larger study populations, several groups of researchers described comparable findings whereby HCQ use was associated with improved lipid profile (as shown by lowered TG, TC, LDL-C and elevated HDL-C) in individuals with rheumatoid arthritis [58-60]. A retrospective cohort study demonstrated a direct relationship between HCQ use and lower risk of hyperlipidaemia among early rheumatoid arthritis patients (HR: 0.75; 95% CI: 0.58-0.98) [61]. The use of a triple therapy consisting of methotrexate, sulfasalazine and HCQ was also associated with improved cholesterol profiles over two-year follow-up in early rheumatoid arthritis patients [62]. The levels of TC, LDL-C was reduced but HDL-C was increased after individual HCQ administration and its combination with methotrexate and sulfasalazine [61,62]. However, the most likely interaction between methotrexate and CQ is the reduction of methotrexate bioavailability when co-administered with CQ [63]. In a study recruiting SLE patients (n=22) and rheumatoid arthritis (n=4) patients prescribing HCQ for more than a month, the lipid profile of these patients improved significantly visualised by the lowering of TG, LDL-C and non-HDL-C [47]. In addition, significant decreases in TC, atherogenic index and increase in HDL-C were also noted in female patients with Sjögren's syndrome (n=71; aged 64 ± 11 years) receiving HCQ [64].
To sum up, the hypolipidaemic effects of HCQ were suggested based on the currently available evidence. Short treatment duration may be insufficient to induce improvement in lipid profile. In addition, CQ and HCQ may not be able to affect serum lipid levels significantly in patients with mild or inactive autoimmune diseases.
The cardioprotective and anti-hypertensive actions of chloroquine
Several studies were conducted by investigators to evaluate the cardioprotective and hypotensive effects of CQ and HCQ in animals (Table 5) and humans (Table 6). Yuan et al. found an improvement of diastolic cardiac function in STZ-induced diabetic mice after intraperitoneally administered with 60 mg/kg CQ for 14 days [24]. A single monocrotaline (60 mg/kg, s.c.) injection was exposed to adult male Sprague-Dawley rats to induce pulmonary arterial hypertension. The rats were then injected (i.p.) with CQ (20 or 50 mg/kg) or HCQ (50 mg/kg) for 20 days. Lower right ventricular systolic pressure (RVSP), higher cardiac output and contractility were detected in animals after the administration [65]. Recently, two studies were done by the same group of researchers to evaluate the anti-hypertensive effect of CQ using adult and young spontaneous hypertensive rats, a genetic animal model with essential hypertension. Therapeutic CQ at the dose of 40 mg/kg/day (i.p.) for 21 days significantly reduced systolic blood pressure (SBP) in spontaneous hypertensive rats of two different ages [66,67]. In humans, McGill et al. designed a trial to administer CQ dose escalations (80 mg/week for three weeks followed by 80 mg/day for three weeks and 250 mg/day for three weeks) in MetS patients. The levels of SBP, diastolic blood pressure (DBP) and mean arterial pressure (MAP) were unaffected at the end of study as compared to baseline. However, CQ treatment at 80 mg/day over a year lowered DBP and MAP in MetS subjects [45]. In another study, Baker et al. reported a decline in SBP and DBP of patients with prevalent rheumatoid arthritis after initiation of HCQ (dose not mentioned) for 6 months [68]. These human studies indicated that long-term administration of CQ and HCQ potentially exhibited anti-hypertensive effects in humans. Although there were not many studies investigated the cardioprotective and anti-hypertensive role of CQ and HCQ, but the findings demonstrated positive outcomes that require further clarification in other animal models and human populations.
The anti-obesity action of chloroquine
In animals, two studies reported positive effect whereas one study reported negligible effect on body weight and fat mass reduction (Table 7). Obesity was induced in C57BL/6 mice using a high-fat diet and pre-treated with HCQ (40 mg/kg/day, i.p.) for 17 weeks. The treatment significantly reduced body weight gain and fat mass in these mice [29]. In line with this study, the findings were supported by McCarthy et al. whereby body weight was reduced in adult spontaneous hypertensive rats treated with CQ (40 mg/kg/day, i.p.) for 21 days [67]. Nonetheless, a higher dose of CQ (60 mg/kg/day) provided intraperitoneally to the STZ-induced diabetic mice for 14 days did not change body weight in relative to those treated with vehicle [24]. The reduction of body weight was not seen in this study which might be due to the shorter treatment period of CQ. There was only one human study reporting the effects of HCQ (600 mg/day) in combination with doxycycline (100 mg, twice a day) on body weight in healthy individuals and Q fever endocarditis patients (Table 8). The body weight of the subjects increased after one-year treatment. The authors suggested that abnormal weight gain was the side effect of long-term HCQ and doxycycline treatment [69]. However, it is difficult to justify whether the adverse effect was derived from HCQ or doxycycline when they were given as a combination therapy. In short, there was only a paucity of scientific studies evaluating the effects of CQ and HCQ on body weight. The measurement of waist circumference should be performed to claim the anti-obesity effect of CQ and HCQ as increased waist circumference is one of the criteria for MetS. More studies are also warranted to validate whether weight gain is the consequence for long-term administration of CQ and HCQ.
The cardioprotective and anti-hypertensive effects of CQ and HCQ in animal studies
| Types of animal | Types of induction | Treatment, dose, route and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|---|
| Male C57BL mice | STZ (60 mg/kg, i.p.) | CQ (60 mg/kg/day, i.p., 14 days) | Diastolic cardiac function: ↑ | LC3-II: ↑, p62: ↑, Beclin1: ↔, autophagic vacuoles: ↓ | [24] |
| Male Sprague-Dawley rats | Monocrotaline (60 mg/kg) | CQ (20 or 50 mg/kg/day, i.p., 20 days) | RVSP: ↓, cardiac output: ↑, cardiac contractility: ↑ | p62: ↑, Ki67: ↓, TUNEL-positive cells: ↑, BMPR-II: ↑ | [65] |
| HCQ (50 mg/kg/day, i.p., 20 days) | RVSP: ↓ | - | |||
| Young spontaneous hypertensive rats | - | CQ (40 mg/kg/day, i.p., 21 days) | SBP: ↓ | MyD88: ↓, TRAF6: ↓, p-NF-κB: ↓, immune cell recruitment and infiltration into the vasculature: ↓ | [66] |
| Adult spontaneous hypertensive rats | - | CQ (40 mg/kg/day, i.p., 21 days) | - | TLR8: ↑, MyD88: ↑, IRAK: ↑, p-NF-κB: ↑ | |
| Adult spontaneous hypertensive rats | - | CQ (40 mg/kg/day, i.p., 21 days) | SBP: ↓ | COX enzymes: ↓, ROS: ↓, nitric oxide: ↑, MMP: ↓ | [67] |
Abbreviations: BMPR-II, bone morphogenetic protein receptor type II; COX, cyclooxygenase; CQ, chloroquine; HCQ, hydroxychloroquine; i.p., intraperitoneal; IRAK, IL-1 receptor-associated kinase; MMP, matrix metallopeptidase; MyD88; myeloid differentiation primary response 88; p-NF-κB, phosphorylated nuclear factor-kappa B; ROS, reactive oxygen species; RVSP, right ventricular systolic pressure; STZ, streptozotocin; TLR8, Toll-like receptor 8; TRAF6, tumour necrosis factor receptor-associated factor 6; ↑, increase/stimulate; ↓, decrease/inhibit; ↔, no change.
The cardioprotective and anti-hypertensive effects of CQ and HCQ in human studies
| Study population | Treatment, dose and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|
| Patients with MetS (n=25; aged 18-60 years) | Placebo (3 weeks) → CQ (80 mg/week, 3 weeks) → CQ (80 mg/day, 3 weeks) → CQ (250 mg/day, 3 weeks) | BP: ↔ | TNF-α: ↓, CRP: ↔, leptin: ↔, adiponectin: ↔ | [45] |
| Patients with MetS (n=56; aged 18-70 years) | CQ (80 mg/day, 1 year) | DBP: ↓, MAP: ↓ | p-JNK: ↓ | |
| Patients with prevalent rheumatoid arthritis (n=7,147; aged 63 years) | HCQ (dose not mentioned) - 6 months | SBP: ↓, DBP: ↓ | - | [68] |
Abbreviations: BP, blood pressure; CQ, chloroquine; CRP, C-reactive protein; DBP, diastolic blood pressure; HCQ, hydroxychloroquine; MAP, mean arterial pressure; MetS, metabolic syndrome; p-JNK, phosphorylated c-Jun N-terminal kinase; SBP, systolic blood pressure; TNF-α, tumour necrosis factor-alpha; ↑, increase/stimulate; ↓, decrease/inhibit; ↔, no change.
The anti-obesity effects of CQ and HCQ in animal studies
| Types of animal | Types of induction | Treatment, dose, route and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|---|
| Male C57BL/6J mice | High-fat diet (60% fat) | HCQ (40 mg/kg/day, pre-treatment, i.p., 17 weeks) | Weight gain: ↓, fat mass: ↓ | Inflammation IL-1β: ↓, IL-6: ↓, TNF-α: ↓, MCP-1: ↓, CD68: ↓, Arg1: ↓ Lipid metabolism CPT1α: ↑, CPT1β: ↑, PPAR-γ: ↓, Mgat-1: ↓, SREBP1c: ↓, ChREBP: ↓, ACC: ↓, FAS: ↓ | [29] |
| Adult spontaneous hypertensive rats | - | CQ (40 mg/kg/day, i.p., 21 days) | Body weight: ↓ | COX enzymes: ↓, ROS: ↓, nitric oxide: ↑, MMP: ↓ | [67] |
| Male C57BL mice | STZ (60 mg/kg, i.p.) | CQ (60 mg/kg/day, i.p., 14 days) | Body weight: ↔ | LC3-II: ↑, p62: ↑, Beclin1: ↔, autophagic vacuoles: ↓ | [24] |
Abbreviations: ACC, acetyl-CoA carboxylase; Arg1, arginase 1; CD, cluster of differentiation; ChREBP, carbohydrate response element binding protein; COX, cyclooxygenase; CPT1α, carnitine palmitoyltransferase 1 alpha; CPT1β, carnitine palmitoyltransferase 1 beta; CQ, chloroquine; FAS; fatty acid synthase; HCQ, hydroxychloroquine; IL-1β, interleukin-1 beta; IL-6, interleukin-6; i.p., intraperitoneal; MCP-1, monocyte chemoattractant protein-1; Mgat-1, monoacylglycerol-O-acyltransferase; MMP, matrix metallopeptidase; PPAR-γ, peroxisome proliferator-activated receptor-gamma; ROS, reactive oxygen species; SREBP1c, sterol regulatory element-binding transcription factor 1; STZ, streptozotocin; TNF-α, tumour necrosis factor-alpha; ↑, increase/stimulate; ↓, decrease/inhibit; ↔, no change.
The anti-obesity effects of CQ and HCQ in human studies
| Study population | Treatment, dose and duration | Research outcomes | Mechanism of action | Reference |
|---|---|---|---|---|
| Healthy controls (n=34) and Q fever endocarditis patients (n=48) (mean age: 57 ± 15 years) | Doxycycline (100 mg, twice a day) + HCQ (600 mg daily) | Body weight: ↑ | - | [69] |
Abbreviations: HCQ, hydroxychloroquine; ↑, increase.
The underlying mechanisms of chloroquine for the prevention of MetS-associated medical conditions
The underlying molecular mechanisms of CQ and HCQ in preventing MetS-associated abnormalities have been uncovered by investigators in recent years. Treatment with CQ and HCQ regulated inflammatory response, oxidative stress, endothelial function, insulin signalling, lipogenesis, autophagy, adipokines, and apoptosis during MetS.
Both CQ and HCQ are commonly used as non-steroidal anti-inflammatory drugs to treat autoimmune diseases, suggesting their potent anti-inflammatory property. Interestingly, chronic low-grade inflammation is the hallmark in the pathogenesis of MetS and its individual medical conditions. Previous animal and human studies have explored the anti-inflammatory and immunomodulatory roles of CQ and HCQ in MetS-related conditions. Abdel-Hamid & El-Firgany found reductions in pro-inflammatory cytokines [interleukin-1 beta (IL-1β), interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), transforming growth factor-beta 1 (TGF-β1) and monocyte chemoattractant protein-1 (MCP-1)] and no change in anti-inflammatory cytokine [interleukin-10 (IL-10)] in STZ-induced diabetic rats after four weeks of oral HCQ administration [23]. In high-fat diet-induced obese mice, HCQ decreased the hepatic levels of macrophage-specific genes [cluster of differentiation 68 (CD68) and arginase 1 (Arg1)] and pro-inflammatory genes (IL-1β, TNF-α and MCP-1). Serum levels of inflammatory mediators were also lowered in the HCQ-treated obese mice [29]. Treatment of CQ in frequency and dose escalations from 80 mg/week to 250 mg/day for nine weeks helped in reducing the production of TNF-α but did not affect C-reactive protein (CRP) level in patients with MetS [45]. The combination of HCQ (200 mg daily) and atorvastatin (10 mg daily) successfully lowered high sensitivity C-reactive protein (hs-CRP) among Indian patients with dyslipidaemia [44]. However, a study by Mercer et al. was not in accordance with findings from other groups. They found no significant difference in the levels of CRP and IL-6 in the obese non-diabetic subjects after 6 and 12 weeks of HCQ treatment as compared to their baseline readings. The distinct outcomes might be attributed to the small sample size in this study [42].
The mechanism of action governing the anti-inflammatory property of CQ and HCQ might be mediated through the myeloid differentiation primary response 88 (MyD88)-dependent signalling (also known as the TLR signalling). This signalling pathway is an innate immune system that recognises and responds to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) to coordinate inflammation during MetS [70]. McCarthy et al. investigated the effects of CQ on TLR signalling pathway in young and adult spontaneous hypertensive rats. The expressions of MyD88 and tumour necrosis factor receptor-associated factor 6 (TRAF6) were downregulated by CQ, which eventually decreased phosphorylation of nuclear factor-kappa B (NF-κB), prevented recruitment and infiltration of immune cells into the vasculature as well as lowered SBP. These observations were found in young but not in adult spontaneous hypertensive rats. The authors suggested that the downstream TLR-signalling proteins might be more sensitive to CQ treatment in the young animals, therefore a higher dose of CQ might be needed to exert immunomodulatory effects in the adult animals [66].
High-density lipoprotein (HDL) exerts potent protective effects on cardiovascular system. There are multiple heterogeneous protein components physically associated with HDL such as paraoxonase 1 (PON-1), apoA-1, lecithin cholesterol acyltransferase (LCAT) and platelet-activating factor acetylhydrolase (PAF-AH) [71]. HDL acts as an anti-inflammatory particle under physiological condition, but it becomes non-protective and pro-inflammatory in the state of active or chronic inflammation mainly attributed to the changes in the level and function of its associated proteins. PON-1 can bind to enzyme myeloperoxidase (MPO), inhibiting the onset of lipid peroxidation. ApoA-1 helps to solubilise lipid component, reverse cholesterol transport and promote cholesterol efflux from tissue to the liver for excretion [72]. Besides, apoA-1 also acts as a co-factor for LCAT, an enzyme that functions to catalyse the esterification of free cholesterol to cholesteryl ester. The effects of HCQ on HDL has been investigated. HCQ combined with methotrexate and sulfasalazine increased PON-1 activity and apoA-1 but decreased HDL inflammatory index and MPO with concomitant improvement of lipid profile in the patients with early rheumatoid arthritis [73]. However, it was rather challenging to justify the observed positive effects were derived from HCQ as the intervention was given as combined therapy. In another study, Tam et al. reported that HCQ had a negligible effect on apolipoproteins in Chinese patients with mild or inactive SLE [57]. The discrepancy observed in this study might be due to the level of apolipoprotein did not change significantly in mild or inactive conditions of SLE. In addition, the duration of HCQ treatment was not mentioned in this study.
Endothelial dysfunction has been implicated in the pathogenesis of T2DM, insulin resistance, hypertension, hyperlipidaemia, and atherosclerosis, thus it is closely associated with MetS. A reduction in nitric oxide (NO) bioavailability resulted from decreased NO production and/or increased reactive oxygen species (ROS) is involved in the pathophysiology of endothelial dysfunction. In another word, the induction of oxidative stress is also the precursor for endothelial dysfunction. Another mechanism involved in endothelial dysfunction is cyclooxygenase (COX) activity. The stimulation of COX-1 transforms arachidonic acid to endoperoxides, followed by the conversion to various types of prostaglandin depending on their respective synthase. The formation of thromboxane A2 predominates under pathological conditions, which targets thromboxane-prostanoid receptor on smooth muscle cells and activates vasoconstriction [74]. Using adult spontaneous hypertensive rats administering with CQ, the hypertensive vascular dysfunction in animals was ameliorated via inhibition of COX enzymes, reduction of ROS generation, improvement of NO bioavailability and reduction of matrix metallopeptidase (MMP) enzymes [67].
CQ and HCQ exert their anti-hyperglycaemic effects by inducing physiological changes in pancreatic beta cells. Previous study has demonstrated that CQ and HCQ are a known inhibitor for Kir6.2 [75], an adenosine triphosphate-sensitive potassium channel expressed in many different tissues such as pancreatic beta cells, cardiomyocytes, neurons, and lymphocytes [76-79]. The downregulation of Kir6.2 was associated to an increase of insulin secretion in pancreatic beta cell lines [80]. Hence, CQ and HCQ potentially increase insulin secretion by interacting with Kir6.2 channels in pancreatic beta cells, resulting in hypoglycaemia.
Apart from that, the insulin signalling pathway plays a central role in maintaining glucose and lipid metabolism. The presence of insulin and insulin growth factor-1 (IGF-1) is recognised by their respective tyrosine kinase receptors [insulin receptor and insulin growth factor-1 receptor (IGF-1R)], leading to activation of phosphatidylinositol 3‑kinase (PI3K) / protein kinase B (Akt) signalling pathway. Insulin receptor substrate-1 (IRS-1) is recruited and activated upon the ligand-receptor interaction, which further activates and phosphorylates PI3K converting phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). Subsequently, PIP3 recruits phosphoinositide-dependent kinase 1 (PDK1) to phosphorylate Akt. The activation of Akt phosphorylates a series of downstream targets such as and glycogen synthase kinase-3 beta (GSK3β), Forkhead box O (Foxo) and mammalian target of rapamycin (mTOR) that regulate glucose transport, glucose uptake, gluconeogenesis, glycogen synthesis and lipid synthesis [81]. The hypoglycaemic action of CQ and HCQ has been reported to mediate through the modulation of insulin signalling pathway. Administration of CQ via injection (i.p.) stimulated glucose uptake and glycogen synthesis in muscle cells of rats adopted with high-fat diet via phosphorylation of Akt (activation) and GSK3β (inhibition). As a result, the glycogen synthase activity was enhanced [27]. Wang et al. also verified that supplementation of HCQ in male mice displaying diabetic phenotype resulted in increased insulin level and Akt phosphorylation [30]. A recent study by Qiao et al. revealed that the activation of Akt and IRS-1 was detected in liver tissue of male obese mice provided with a high-fat diet and treated with HCQ [29]. Findings from these studies summarised the knowledge about the role of CQ and HCQ in activating insulin signalling pathway.
Several molecular mechanisms are implicated in orchestrating lipid metabolism during MetS conditions. Firstly, the activation of peroxisome proliferator-activated receptor-gamma (PPAR-γ) promotes the expression monoacylglycerol-O-acyltransferase (Mgat-1), an enzyme essential for TG synthesis and enhances hepatic lipid accumulation [82]. Secondly, the upregulation of sterol regulatory element-binding transcription factor 1 (SREBP1c) and carbohydrate response element-binding protein (ChREBP) enhances the expression of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), facilitating fatty acid biosynthesis and inhibiting fatty acid β-oxidation. Thirdly, the downregulation of carnitine palmitoyltransferase 1 alpha (CPT1α) and carnitine palmitoyltransferase 1 beta (CPT1β) results in the suppression of fatty acid β-oxidation [83]. The widely established anti-hyperlipidaemic effects of CQ and HCQ suggested their possible role in modulating lipid metabolism. A recent study reported that HCQ inhibited de novo lipogenesis. The expression of PPAR-γ and Mgat-1 (the downstream target), lipogenic genes (SREBP1c and ChREBP), lipid enzymes (FAS and ACC) were downregulated whereas the expression of β-oxidation genes (CPT1α and CPT1β) were upregulated in obese mice fed with high-fat-diet [29].
Adipokines are predominantly secreted by adipose tissue. Dysregulated adipokine levels contribute to the development of various pathological conditions. MetS-related disturbances are positively associated with leptin [84], resistin [85], visfatin [86] but negatively associated with adiponectin [87]. On the other hand, lipocalin-2 has both beneficial and detrimental roles in metabolic abnormalities whereby it exerts insulin-sensitising, hepatoprotective, anti- and pro-inflammatory actions [88]. In addition, it is evident that these adipokines regulate the expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, which have direct effects on endothelial function, vascular homeostasis and atherogenesis [89]. In vivo studies found that HCQ corrected the impaired serum level of adipokines (visualised by raised adiponectin and lipocalin-2 as well as lowered leptin, resistin and visfatin) in the high-fat diet rats after 12 weeks. Along with the changes of adipokine level, significant decreases in serum level of soluble adhesive molecules (E-selectin, ICAM-1 and VCAM-1) were also observed in these animals, suggesting the role of HCQ in relieving endothelial stress [28]. A randomised, double-blind, parallel-arm trial pinpointed higher adiponectin level in overweight or obese subjects after HCQ supplementation for 14 weeks but not after placebo supplementation [43]. However, a shorter period of CQ treatment (9 weeks) supplemented in dose escalation failed to induce similar changes in leptin and adiponectin levels in patients with MetS [45].
Autophagy is a process of degenerating cells and tissues by clearing old damaged components, misfolded proteins, and intracellular pathogens. Autophagy machinery is initiated with phagophore formation regulated by class III PI3K complex consisting of beclin-1. Next, phagophore membrane is extended and selected cytoplasmic components for degradation tagged by sequestosome-1 (p62) are captured forming an autophagosome. The internalised p62 is degraded, hence higher expression of p62 indicates lower autophagic process. The second step is facilitated by LC3-II protein and various autophagy-related genes (Atg). Lastly, autophagosome is fused with lysosome leading to proteolytic degradation of engulfed molecules [90]. Autophagy can be activated under pathological conditions such as type 1 diabetic condition, which is often associated with apoptosis and fibrosis [24]. A study by Yuan et al. pointed out that CQ improved cardiac diastolic function by inhibiting autophagy in STZ-induced diabetic mice (evidenced by the decreases in autophagolysosomes, cardiomyocyte apoptosis, cardiac fibrosis, LC3-II/LC3-I protein ratio and increase in p62 expression) [24]. Likewise, in a rat model of pulmonary arterial hypertension induced by monocrotaline, the accumulation of p62 protein level was found after CQ injection suggesting that CQ is an inhibitor for autophagy [65].
Apoptosis is a process of programmed cell death mainly regulated by two main branches of signalling pathways, the intrinsic (mitochondrial) and extrinsic (death receptor-mediated) apoptotic pathway, depending on the source of stresses either derived from intracellular or extracellular environment. The intrinsic pathway is triggered by intracellular stressors (such as DNA damage, oxidative stress, and oxygen deprivation), resulting in mitochondrial dysfunction, release of cytochrome c and subsequent activation of caspase-9 and caspase-3. The mitochondrial outer membrane permeabilisation (MOMP) is influenced by several anti-apoptotic genes [B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-XL)] and pro-apoptotic genes [Bcl-2-associated X protein (Bax) and Bcl-2 homologous antagonist/killer (Bak)]. Meanwhile, the extrinsic pathway is initiated upon the ligation between death ligands and their respective death receptors, leading to the formation of death-inducing signalling complex (DISC), recruitment of Fas-associated death domain (FADD), activation caspase-8 and caspase-3 [91]. Previous studies by two groups of researchers demonstrated that CQ and HCQ suppressed apoptosis of pancreatic β-cells in diabetic condition but stimulated apoptosis of pulmonary artery smooth muscle cells in hypertensive condition. HCQ induced a significant decrease in cleaved caspase-3 and increase in Bcl-2 in pancreatic islet of Langerhans of diabetic rats as compared to the non-treated diabetic groups [23]. On the other hand, Long et al. reported a reduction in the number of Ki67-positive cells and an increase in TUNEL-positive cells in hypertensive rats treated with CQ, indicating the decrease in cell proliferation and increase in apoptosis of pulmonary artery smooth muscle cells [65].
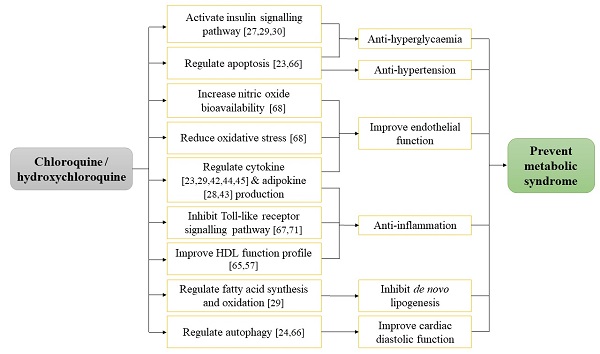
A summary on the mechanism of action of CQ and HCQ in improving MetS-associated conditions has been depicted (Figure 1).
Perspectives
The intervention dose and duration for CQ and HCQ varies depending on the diseases. A total of 25 mg/kg CQ given in a 3-day course is prescribed to treat malaria caused by Plasmodium vivax [92]. For rheumatic diseases, the well-tolerated doses for CQ and HCQ are 200-400 mg/day with duration of 24-36 weeks which result in reduced join pain and improved functional response [14,93]. The recommended regimen for administration of CQ and HCQ in patients with COVID-19 is 500 mg daily with duration of 7 days [94]. Based on the studies included in this review, the dosage of CQ and HCQ commonly used in humans with MetS-associated conditions was 6.5 mg/kg or not exceeding 400 mg/day in adults via oral administration. There was only one study that has tested the anti-hyperlipidaemic effects of 800 mg/day HCQ in patients with SLE [55]. The suggested treatment duration for CQ and HCQ ranged from three days to six months, with only few studies with exposure time of approximately two years. On the other hand, the dose for CQ and HCQ treatment in animals ranged from 7 mg/kg/week to 200 mg/kg/day. The human equivalent dose for the highest dose is approximately 2.3 g for a 60 kg adult, which has exceeded the well-tolerated dose in human therefore it was considered as not clinically useful.
The understanding on pharmacokinetics of CQ and HCQ is also a crucial clinical consideration for their possible application in MetS-associated conditions. The oral absorption of CQ and HCQ is rapid and almost complete following ingestion. They possess an estimate bioavailability of 70 - 80% and large volume of distribution in the blood, resulting in a long half-life for the two drugs. Both CQ and HCQ are metabolised by several members of cytochrome P450 family, such as CYP1A2, CYP2C8, CYP2C19, CYP2D6, CYP3A4, and CYP3A5 [95]. Furthermore, CQ and HCQ can inhibit CYP2D6 activity thus suppressing the metabolism of their own and other drugs metabolised by the enzyme. This suggests the potential clinical interaction with medications that are the predominant substrates of CYP2D6 enzyme.
The proper weight-based daily dosing of CQ and HCQ is crucial to prevent the development of potential adverse events in humans. In a randomised, double-blind, placebo-controlled trial investigating the effects of CQ (500 mg/day, 14-29 days) on breast tumour cellular proliferation in breast cancer patients, the patients receiving CQ developed visual symptoms (such as blurriness and light sensitivity), auditory symptom (tinnitus), muscle weakness, dry mouth, dizziness, fatigue and gastrointestinal upset (such as abdominal cramp, nausea, and diarrhoea) [96]. The incidence of gastrointestinal disturbance has been reported as a brand-related side effect [97]. Long-term use (more than 6 years) and higher daily administration dose (more than 400 mg) of HCQ have been previously proven as the predictors of retinopathy, an ophthalmologic disorder in humans [98]. The occurrence of renal toxicity among anti-malarial drugs is rare yet the main consideration is the use of CQ and HCQ in patients with concomitant renal impairment [99]. Hence, patients with renal impairment [estimated glomerular filtration rate (eGFR) ≤ 30 mL/min/1.73 m2] are excluded from clinical trials related to CQ and HCQ [100]. CQ and HCQ are known to be blockers of the potassium channel. The blockade on potassium channel inhibits potassium current, leading to delayed repolarisation of cardiac myocytes, prolonged cardiac action potential duration and QT interval on electrocardiogram [101]. High dose of CQ has been reported to be well-tolerated but it caused QT interval prolongation similar to those receiving standard-dose CQ under clinical conditions [102]. In preclinical models, decreased aortic output was observed in rats whereas reduced heart function and heart rate were noted in ventricular cardiomyocytes after CQ treatment [103]. Thus, the potential toxicity of CQ and HCQ on the cardiac muscle and large QT syndrome at low doses should not be neglected.
The mechanism of action of CQ and HCQ in improving MetS conditions.
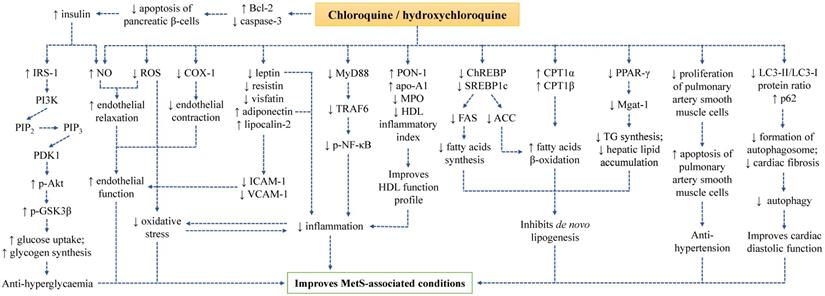
In view of the potential lack of efficiency of CQ and HCQ at short treatment period and the presence of toxicity at higher dose, future studies are warranted to evaluate the optimum treatment dose and duration to ensure the efficacy and safety profile of CQ and HCQ, particularly in subjects with MetS. The combination of lifestyle modification by adopting balanced diet and physical activities with pharmacological treatment is recommended to ensure successful management of MetS-related conditions. The current evidence documented the capability of CQ or HCQ in improving glucose tolerance, insulin sensitivity, lipid profile, cardiac function, reducing BP and weight gain in most of the pre-clinical and human studies. The anti-hyperglycaemic and anti-hyperlipidaemic actions of CQ and HCQ were notable but there was limited literature showing their anti-hypertensive and anti-obesity effects, thus require further investigations to validate the claims. These beneficial outcomes supported the repurposing of CQ and HCQ against MetS initiation and progression despite their current uses as anti-malarial [104], anti-rheumatic [105] and anti-viral drugs [106].
To our knowledge, it is believed that patients with underlying health conditions such as MetS-related abnormalities have an increasingly rapid and severe progression for malaria, autoimmune diseases, virus infections, and vice versa. Obesity, in combination with additional metabolic risk factors such as diabetes, hypertension, and dyslipidaemia were strongly correlated with severe malaria [107]. A comprehensive systematic review and meta-analysis reported that SLE patients had higher odds for the risk of high FBG, BP, TG, waist circumference, and low HDL-C [108]. Moreover, the prevalence of MetS was higher in rheumatoid arthritis patients than in control subjects [109]. With the recent outbreak of COVID-19, patients with comorbidities related to MetS are more likely to develop severe and worsening disease course [110]. In this context, the management of MetS should be considered concurrently in the treatment strategies for these diseases. Herein, CQ and HCQ may be a better treatment option for patients with of malaria, autoimmune diseases, and virus infections who are at risk of developing MetS as well as for MetS patients who are at risk of experiencing severe disease state.
Conclusion
In conclusion, CQ and HCQ have a wide range of biological activities that contribute to their usefulness and effectiveness in the prevention of MetS. The dose and duration of medications need to be paid careful attention. Lower dose and shorter duration may be insufficient to exert beneficial effects whereas higher dose and longer duration may induce adverse symptoms. Besides, the potential adverse reactions of CQ and HCQ to the eyes, ears, muscles, gastrointestinal tract, and heart should be under careful consideration when these drugs are prescribed. Evidence and suggestion from this review awaits further validation from human clinical trials to assess the effects of CQ and HCQ supplementation particularly in MetS subjects to distinctly approve them as a promising agent to improve MetS.
Acknowledgements
The author would like to thank Universiti Kebangsaan Malaysia for the support via FF-2020-366 grant.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sullivan DJ, Gluzman IY, Russell DG. et al. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA. 1996;93:11865
2. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16:155-166
3. Tsiang H, Superti F. Ammonium chloride and chloroquine inhibit rabies virus infection in neuroblastoma cells. Arch. Virol. 1984;81:377-382
4. Kronenberger P, Vrijsen R, Boeyé A. Chloroquine induces empty capsid formation during poliovirus eclipse. J. Virol. 1991;65:7008-11
5. Khan M, Santhosh SR, Tiwari M. et al. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J. Med. Virol. 2010;82:817-24
6. Farias KJS, Machado PRL, de Almeida Junior RF. et al. Chloroquine interferes with dengue-2 virus replication in U937 cells. Microbiol. Immunol. 2014;58:318-326
7. Tricou V, Minh NN, Van TP. et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl. Trop. Dis. 2010;4:e785
8. Shiryaev SA, Mesci P, Pinto A. et al. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci. Rep. 2017;7:15771
9. Lima TLC, Feitosa RdC, Dos Santos-Silva E. et al. Improving Encapsulation of Hydrophilic Chloroquine Diphosphate into Biodegradable Nanoparticles: A Promising Approach against Herpes Virus Simplex-1 Infection. Pharmaceutics. 2018;10:255
10. Savarino A, Gennero L, Chen HC. et al. Anti-HIV effects of chloroquine: mechanisms of inhibition and spectrum of activity. Aids. 2001;15:2221-9
11. Mizui T, Yamashina S, Tanida I. et al. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J. Gastroenterol. 2010;45:195-203
12. Yan Y, Zou Z, Sun Y. et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300-302
13. Cortegiani A, Ingoglia G, Ippolito M. et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279-283
14. Haładyj E, Sikora M, Felis-Giemza A. et al. Antimalarials - are they effective and safe in rheumatic diseases? Reumatologia. 2018;56:164-173
15. Wu K, Zhang Q, Wu X. et al. Chloroquine is a potent pulmonary vasodilator that attenuates hypoxia-induced pulmonary hypertension. Br. J. Pharmacol. 2017;174:4155-4172
16. Alberti KG, Eckel RH, Grundy SM. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-5
17. Thatoi PK. Diabetes and Severe Malaria—Clinical Profile and Outcome. Diabetes. 2018;67:2388-PUB
18. Theengh DP, Yadav P, Jain AK. et al. Assessment of metabolic syndrome in HIV-infected individuals. Indian J. Sex. Transm. Dis. AIDS. 2017;38:152-156
19. Bellomio V, Spindler A, Lucero E. et al. Metabolic syndrome in Argentinean patients with systemic lupus erythematosus. Lupus. 2009;18:1019-1025
20. Graham ML, Janecek JL, Kittredge JA. et al. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp. Med. 2011;61:356-360
21. Asamoah KA, Furman BL, Robb DA. Attenuation of streptozotocin-induced diabetes in rats by pretreatment with chloroquine. Clin. Sci. 1989;76:137-41
22. Emami J, Gerstein HC, Pasutto FM. et al. Insulin-sparing effect of hydroxychloroquine in diabetic rats is concentration dependent. Can. J. Physiol. Pharmacol. 1999;77:118-23
23. Abdel-Hamid AAM, El-Firgany AEL. Hydroxychloroquine hindering of diabetic isletopathy carries its signature on the inflammatory cytokines. J. Mol. Histol. 2016;47:183-93
24. Yuan X, Xiao YC, Zhang GP. et al. Chloroquine improves left ventricle diastolic function in streptozotocin-induced diabetic mice. Drug Des. Devel. Ther. 2016;10:2729-37
25. Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina. 2017;53:365-374
26. Pareek A, Yeole PG, Tenpe CR. et al. Effect of atorvastatin and hydroxychloroquine combination on blood glucose in alloxan-induced diabetic rats. Indian J. Pharmacol. 2009;41:125-8
27. Halaby MJ, Kastein BK, Yang DQ. Chloroquine stimulates glucose uptake and glycogen synthase in muscle cells through activation of Akt. Biochem. Biophys. Res. Commun. 2013;435:708-713
28. Abdel-Hamid AAM, Firgany AEL. Favorable outcomes of hydroxychloroquine in insulin resistance may be accomplished by adjustment of the endothelial dysfunction as well as the skewed balance of adipokines. Acta Histochem. 2016;118:560-573
29. Qiao X, Zhou ZC, Niu R. et al. Hydroxychloroquine Improves Obesity-Associated Insulin Resistance and Hepatic Steatosis by Regulating Lipid Metabolism. Front. Pharmacol. 2019;10:855
30. Wang R, Xi L, Kukreja RC. PDE5 Inhibitor Tadalafil and Hydroxychloroquine Cotreatment Provides Synergistic Protection against Type 2 Diabetes and Myocardial Infarction in Mice. J. Pharmacol. Exp. Ther. 2017;361:29-38
31. Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131-45
32. Wong SK, Chin KY, Soelaiman I-N. Leptin, adiponectin and insulin as regulators for energy metabolism in a rat model of metabolic syndrome. Sains Malaysiana. 2019;48:2701-2707
33. Bili A, Sartorius JA, Kirchner HL. et al. Hydroxychloroquine use and decreased risk of diabetes in rheumatoid arthritis patients. J. Clin. Rheumatol. 2011;17:115-20
34. Chen YM, Lin CH, Lan TH. et al. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: a population-based cohort study. Rheumatology. 2015;54:1244-9
35. Sheikhbahaie F, Amini M, Gharipour M. et al. The effect of hydroxychloroquine on glucose control and insulin resistance in the prediabetes condition. Adv. Biomed. Res. 2016;5:145
36. Rekedal LR, Massarotti E, Garg R. et al. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Rheum. 2010;62:3569-73
37. Powrie JK, Shojaee-Moradie F, Watts GF. et al. Effects of chloroquine on the dyslipidemia of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42:415-9
38. Powrie JK, Smith GD, Shojaee-Moradie F. et al. Mode of action of chloroquine in patients with non-insulin-dependent diabetes mellitus. Am. J. Physiol. 1991;260:E897-904
39. Pareek A, Chandurkar N, Thomas N. et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr. Med. Res. Opin. 2014;30:1257-66
40. Gerstein HC, Thorpe KE, Taylor DW. et al. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas-a randomized trial. Diabetes Res. Clin. Pract. 2002;55:209-19
41. Hsia SH, Duran P, Lee ML. et al. Randomized controlled trial comparing hydroxychloroquine with pioglitazone as third-line agents in type 2 diabetic patients failing metformin plus a sulfonylurea: A pilot study. J. Diabetes. 2020;12:91-94
42. Mercer E, Rekedal L, Garg R. et al. Hydroxychloroquine improves insulin sensitivity in obese non-diabetic individuals. Arthritis Res. Ther. 2012;14:R135
43. Wasko MC, McClure CK, Kelsey SF. et al. Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: a randomised trial. Diabetologia. 2015;58:2336-43
44. Pareek A, Chandurkar N, Thulaseedharan NK. et al. Efficacy and safety of fixed dose combination of atorvastatin and hydroxychloroquine: a randomized, double-blind comparison with atorvastatin alone among Indian patients with dyslipidemia. Curr. Med. Res. Opin. 2015;31:2105-17
45. McGill JB, Johnson M, Hurst S. et al. Low dose chloroquine decreases insulin resistance in human metabolic syndrome but does not reduce carotid intima-media thickness. Diabetol. Metab. Syndr. 2019;11:61
46. Penn SK, Kao AH, Schott LL. et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J. Rheumatol. 2010;37:1136-42
47. Masui Y, Yanai H, Hiraga K. et al. Effects of anti-malarial drug, hydroxychloroquine, on glucose and lipid metabolism in Japanese population. J. Clin. Endocrinol. Metab. 2019;9:159-164
48. Solomon DH, Garg R, Lu B. et al. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis Care Res. 2014;66:1246-51
49. Nwoha PU, Aire TA. Reduced level of serum cholesterol in low protein-fed Wistar rats administered gossypol and chloroquine. Contraception. 1995;52:261-5
50. Yuan J, Li LI, Wang Z. et al. Dyslipidemia in patients with systemic lupus erythematosus: Association with disease activity and B-type natriuretic peptide levels. Biomed. Rep. 2016;4:68-72
51. Erum U, Ahsan T, Khowaja D. Lipid abnormalities in patients with Rheumatoid Arthritis. Pak. J. Med. Sci. 2017;33:227-230
52. Ramos-Casals M, Brito-Zerón P, Sisó A. et al. High prevalence of serum metabolic alterations in primary Sjögren's syndrome: influence on clinical and immunological expression. J. Rheumatol. 2007;34:754-61
53. Batún Garrido JADJ, Radillo Alba HA, Hernández Núñez É. et al. Dyslipidaemia and atherogenic risk in patients with systemic lupus erythematosus. Med. Clin. 2016;147:63-66
54. Cairoli E, Rebella M, Danese N. et al. Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: a longitudinal evaluation of the lipid-lowering effect. Lupus. 2012;21:1178-82
55. Kavanaugh A, Adams-Huet B, Jain R. et al. Hydroxychloroquine effects on lipoprotein profiles (the HELP trial): A double-blind, randomized, placebo-controlled, pilot study in patients with systemic lupus erythematosus. J. Clin. Rheumatol. 1997;3:3-8
56. Rossoni C, Bisi MC, Keiserman MW. et al. Antimalarials and cholesterol profile of patients with systemic lupus erythematosus. Rev. Bras. Reumatol. 2011;51:383-4 386-7
57. Tam LS, Li EK, Lam CW. et al. Hydroxychloroquine has no significant effect on lipids and apolipoproteins in Chinese systemic lupus erythematosus patients with mild or inactive disease. Lupus. 2000;9:413-6
58. Kerr G, Aujero M, Richards J. et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res. 2014;66:1619-26
59. Morris SJ, Wasko MC, Antohe JL. et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res. 2011;63:530-4
60. Restrepo JF, Del Rincon I, Molina E. et al. Use of Hydroxychloroquine Is Associated With Improved Lipid Profile in Rheumatoid Arthritis Patients. J. Clin. Rheumatol. 2017;23:144-148
61. Desai RJ, Eddings W, Liao KP. et al. Disease-modifying antirheumatic drug use and the risk of incident hyperlipidemia in patients with early rheumatoid arthritis: A retrospective cohort study. Arthritis Care Res. 2015;67:457-466
62. Charles-Schoeman C, Wang X, Lee YY. et al. Association of Triple Therapy with Improvement in Cholesterol Profiles over Two-Year Followup in the Treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheumatol. 2016;68:577-586
63. Seideman P, Albertioni F, Beck O. et al. Chloroquine reduces the bioavailability of methotrexate in patients with rheumatoid arthritis. A possible mechanism of reduced hepatotoxicity. Arthritis Rheum. 1994;37:830-3
64. Migkos MP, Markatseli TE, Iliou C. et al. Effect of hydroxychloroquine on the lipid profile of patients with Sjögren syndrome. J. Rheumatol. 2014;41:902-8
65. Long L, Yang X, Southwood M. et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ. Res. 2013;112:1159-70
66. McCarthy CG, Wenceslau CF, Goulopoulou S. et al. Chloroquine Suppresses the Development of Hypertension in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2017;30:173-181
67. McCarthy CG, Wenceslau CF, Goulopoulou S. et al. Autoimmune therapeutic chloroquine lowers blood pressure and improves endothelial function in spontaneously hypertensive rats. Pharmacol. Res. 2016;113:384-394
68. Baker JF, Sauer B, Teng CC. et al. Initiation of Disease-Modifying Therapies in Rheumatoid Arthritis Is Associated With Changes in Blood Pressure. J. Clin. Rheumatol. 2018;24:203-209
69. Angelakis E, Million M, Kankoe S. et al. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob. Agents Chemother. 2014;58:3342-3347
70. Wong SK, Chin KY, Ima-Nirwana S. Toll-like Receptor as a Molecular Link between Metabolic Syndrome and Inflammation: A Review. Curr. Drug Targets. 2019;20:1264-1280
71. González FEM, Ponce-RuÍz N, Rojas-GarcÍa AE. et al. PON1 concentration and high-density lipoprotein characteristics as cardiovascular biomarkers. Arch. Med. Sci, Atheroscler. Dis. 2019;4:e47-e54
72. Mangaraj M, Nanda R, Panda S. Apolipoprotein A-I: A Molecule of Diverse Function. Indian J. Clin. Biochem. 2016;31:253-259
73. Charles-Schoeman C, Yin Lee Y, Shahbazian A. et al. Improvement of High-Density Lipoprotein Function in Patients With Early Rheumatoid Arthritis Treated With Methotrexate Monotherapy or Combination Therapies in a Randomized Controlled Trial. Arthritis Rheumatol. 2017;69:46-57
74. Virdis A, Colucci R, Fornai M. et al. Cyclooxygenase-1 is involved in endothelial dysfunction of mesenteric small arteries from angiotensin II-infused mice. Hypertension. 2007;49:679-86
75. Ponce-Balbuena D, Rodríguez-Menchaca AA, López-Izquierdo A. et al. Molecular mechanisms of chloroquine inhibition of heterologously expressed Kir6.2/SUR2A channels. Molecular pharmacology. 2012;82:803-813
76. Girard CA, Shimomura K, Proks P. et al. Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes. Pflugers Arch. 2006;453:323-32
77. Hong M, Bao L, Kefaloyianni E. et al. Heterogeneity of ATP-sensitive K+ channels in cardiac myocytes: enrichment at the intercalated disk. The Journal of biological chemistry. 2012;287:41258-41267
78. Macfarlane WM, O'Brien RE, Barnes PD. et al. Sulfonylurea receptor 1 and Kir6.2 expression in the novel human insulin-secreting cell line NES2Y. Diabetes. 2000;49:953-60
79. Thomzig A, Laube G, Prüss H. et al. Pore-forming subunits of K-ATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. J Comp Neurol. 2005;484:313-30
80. Chen F, Zheng D, Xu Y. et al. Down-regulation of Kir6.2 affects calcium influx and insulin secretion in HIT-T15 cells. J Pediatr Endocrinol Metab. 2010;23:709-17
81. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014;6:a009191
82. Yu JH, Lee YJ, Kim HJ. et al. Monoacylglycerol O-acyltransferase 1 is regulated by peroxisome proliferator-activated receptor γ in human hepatocytes and increases lipid accumulation. Biochem. Biophys. Res. Commun. 2015;460:715-20
83. Valenzuela R, Videla LA. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011;2:644-8
84. Wang L-H, Liu Y-C, Wang J-H. et al. Serum leptin level positively correlates with metabolic syndrome among elderly Taiwanese. Ci Ji Yi Xue Za Zhi. 2017;29:159-164
85. Makni E, Moalla W, Benezzeddine-Boussaidi L. et al. Correlation of resistin with inflammatory and cardiometabolic markers in obese adolescents with and without metabolic syndrome. Obes. Facts. 2013;6:393-404
86. Abushahla HS, Bulatova N, Kasabri V. et al. Correlates of ghrelin and visfatin in metabolic syndrome patients with and without prediabetes. Int. J. of Diabetes Dev. Ctries. 2019;39:82-93
87. Sparrenberger K, Sbaraini M, Cureau FV. et al. Higher adiponectin concentrations are associated with reduced metabolic syndrome risk independently of weight status in Brazilian adolescents. Diabetol. Metab. Syndr. 2019;11:40
88. Bhusal A, Rahman MH, Lee WH. et al. Paradoxical role of lipocalin-2 in metabolic disorders and neurological complications. Biochem. Pharmacol. 2019;169:113626
89. Ntaios G, Gatselis NK, Makaritsis K. et al. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis. 2013;227:216-21
90. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3-12
91. Loreto C, La Rocca G, Anzalone R. et al. The Role of Intrinsic Pathway in Apoptosis Activation and Progression in Peyronie's Disease. BioMed Res. Int. 2014;2014:616149
92. World Health Organization, Guidelines for the treatment of malaria. 2015: World Health Organization
93. Mota LM, Cruz BA, Brenol CV. et al. Guidelines for the drug treatment of rheumatoid arthritis. Rev Bras Reumatol. 2013;53:158-83
94. Karalis V, Ismailos G, Karatza E. Chloroquine dosage regimens in patients with COVID-19: Safety risks and optimization using simulations. Saf Sci. 2020;129:104842
95. Smit C, Peeters MYM, van den Anker JN. et al. Chloroquine for SARS-CoV-2: Implications of Its Unique Pharmacokinetic and Safety Properties. Clin Pharmacokinet. 2020;59:659-669
96. Arnaout A, Robertson SJ, Pond GR. et al. A randomized, double-blind, window of opportunity trial evaluating the effects of chloroquine in breast cancer patients. Breast Cancer Res. Treat. 2019;178:327-335
97. Srinivasa A, Tosounidou S, Gordon C. Increased Incidence of Gastrointestinal Side Effects in Patients Taking Hydroxychloroquine: A Brand-related Issue? J. Rheumatol. 2017;44:398
98. Jorge A, Ung C, Young LH. et al. Hydroxychloroquine retinopathy - implications of research advances for rheumatology care. Nat. Rev. Rheumatol. 2018;14:693-703
99. Wiwanitkit V. Antimalarial drug and renal toxicity. J. Nephropharmacol. 2015;5:11-12
100. Chen Y, Shen T, Zhong L. et al. Research Progress of Chloroquine and Hydroxychloroquine on the COVID-19 and Their Potential Risks in Clinic Use. Front. Pharmacol. 2020 11
101. Zhang X-l, Li Z-m, Ye J-t. et al. Pharmacological and cardiovascular perspectives on the treatment of COVID-19 with chloroquine derivatives. Acta Pharmacologica Sinica. 2020:1-10
102. Ursing J, Rombo L, Eksborg S. et al. High-Dose Chloroquine for Uncomplicated Plasmodium falciparum Malaria Is Well Tolerated and Causes Similar QT Interval Prolongation as Standard-Dose Chloroquine in Children. Antimicrob Agents Chemother. 2020 64
103. Blignaut M, Espach Y, van Vuuren M. et al. Revisiting the Cardiotoxic Effect of Chloroquine. Cardiovasc Drugs Ther. 2019;33:1-11
104. de Sena LWP, Mello AGNC, Ferreira MVD. et al. Doses of chloroquine in the treatment of malaria by Plasmodium vivax in patients between 2 and 14 years of age from the Brazilian Amazon basin. Malar. J. 2019;18:439
105. Dos Reis Neto ET, Kakehasi AM, de Medeiros Pinheiro M. et al. Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv. Rheumatol. 2020;60:32-32
106. Devaux CA, Rolain J-M, Colson P. et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55:105938-105938
107. Wyss K, Wångdahl A, Vesterlund M. et al. Obesity and Diabetes as Risk Factors for Severe Plasmodium falciparum Malaria: Results From a Swedish Nationwide Study. Clin. Infect. Dis. 2017;65:949-958
108. Hallajzadeh J, Khoramdad M, Izadi N. et al. The association between metabolic syndrome and its components with systemic lupus erythematosus: a comprehensive systematic review and meta-analysis of observational studies. Lupus. 2018;27:899-912
109. Chung CP, Oeser A, Solus JF. et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196:756-63
110. Costa FF, Rosário WR, Ribeiro Farias AC. et al. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabetes Metab. Syndr. 2020;14:809-814
Author contact
![]() Corresponding author: Dr. Wong Sok Kuan, Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Bandar Tun Razak, 56000 Cheras, Kuala Lumpur, Malaysia. E-mail: jocylnwskcom; Contact number: +6012-3977116.
Corresponding author: Dr. Wong Sok Kuan, Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Bandar Tun Razak, 56000 Cheras, Kuala Lumpur, Malaysia. E-mail: jocylnwskcom; Contact number: +6012-3977116.

 Global reach, higher impact
Global reach, higher impact