ISSN: 1449-1907
Int J Med Sci 2021; 18(10):2245-2250. doi:10.7150/ijms.54996 This issue Cite
Research Paper
Second-line Eribulin in Triple Negative Metastatic Breast Cancer patients. Multicentre Retrospective Study: The TETRIS Trial
1. Division of Medical Oncology 2, IRCCS Regina Elena National Cancer Institute, Rome, Italy.
2. Department of Surgical, Oncological and Oral Sciences, Medical Oncology Unit, University of Palermo, Italy.
3. Department of Surgery, IRCCS Regina Elena National Cancer Institute, Rome, Italy.
4. Department of Radiation Oncology, IRCCS Regina Elena National Cancer Institute, Rome, Italy.
5. Medical Oncology Unit B, Policlinico Umberto I, Rome, Italy.
6. Medical Oncology Unit, Azienda Ospedaliera Universitaria Sant'Andrea, Rome, Italy.
7. Department of Radiological, Oncological and Anatomo-Pathological Sciences, 'Sapienza' University of Rome, Policlinico Umberto I, Rome, Italy.
8. Sbarro Institute for Cancer Research and Molecular Medicine and Center of Biotechnology, College of Science and Technology, Temple University, Philadelphia, Pennsylvania, USA.
9. Medical Oncology, Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, L'Aquila, Italy.
10. Medical Oncology, St. Salvatore Hospital, L'Aquila, Italy
11. Radiation Oncology Unit and Department of Clinical and Experimental Biomedical Sciences “Mario Serio”, Azienda Ospedaliera Universitaria Careggi, University of Florence, Florence, Italy.
12. Division of Oncology, San Giovanni Addolorata Hospital, Rome, Italy.
13. Department of Pathology, Surgery and Oncology, “Mater Salutis” Hospital, ULSS21, Verona, Italy.
14. Oncology Unit, S. Maria Goretti Hospital, Latina, Italy.
15. UO Oncologia Medica I, S. Chiara Hospital, Dipartimento di Oncologia, Dei Trapianti e Delle Nuove Tecnologie, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy.
16. Division of Medical Oncology, Department of Oncology and Hematology, University Hospital of Modena, Modena, Italy.
17. Department of Medical Oncology, “Giovanni Paolo II” Institute, Bari, Italy.
18. Gynecology Oncology Unit, Catholic University of the Sacred Heart, Rome, Italy.
19. Department of Gynecology-Obstetrics and Urology, “Sapienza” University of Rome, Rome, Italy.
20. Department of Oncology, University Campus Biomedico of Rome, Rome, Italy.
21. Department of Medical Oncology, Policlinico Universitario “A. Gemelli”, Rome, Italy.
22. Medical Oncology, La Maddalena Nursing Home, University of Palermo, Palermo, Italy
23. Medical Oncology Unit, Sandro Pertini Hospital, Rome, Italy.
24. Scientific Direction, IRCCS Regina Elena National Cancer Institute, Rome, Italy.
25. Bio-Statistics Unit, IRCCS Regina Elena National Cancer Institute, Rome, Italy.
Abstract
Introduction: Large and consistent evidence supports the use of eribulin mesylate in clinical practice in third or later line treatment of metastatic triple negative breast cancer (mTNBC). Conversely, there is paucity of data on eribulin efficacy in second line treatment.
Methods: We investigated outcomes of 44 mTNBC patients treated from 2013 through 2019 with second line eribulin mesylate in a multicentre retrospective study involving 14 Italian oncologic centres.
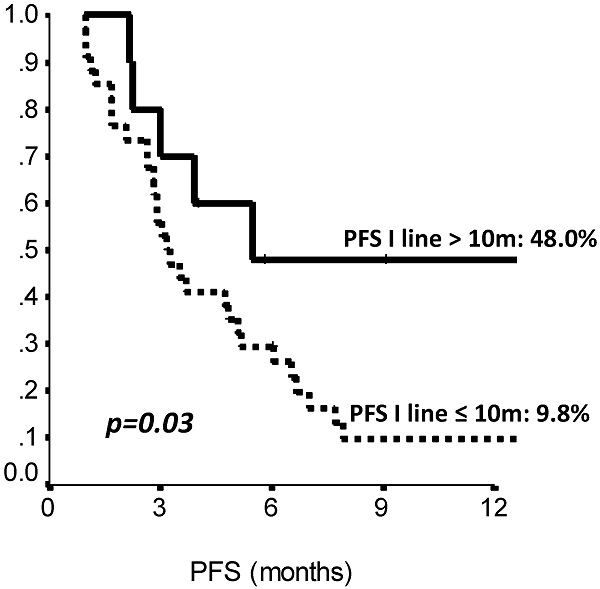
Results: Median age was 51 years, with 11.4% of these patients being metastatic at diagnosis. Median overall survival (OS) and progression free survival (PFS) from eribulin starting were 11.9 (95%CI: 8.4-15.5) and 3.5 months (95%CI: 1.7-5.3), respectively. We observed 8 (18.2%) partial responses and 10 (22.7%) patients had stable disease as best response. A longer PFS on previous first line treatment predicted a better OS (HR=0.87, 95%CI: 0.77-0.99, p= 0.038) and a longer PFS on eribulin treatment (HR=0.92, 95%CI: 0.85-0.98, p=0.018). Progression free survival to eribulin was also favorably influenced by prior adjuvant chemotherapy (HR=0.44, 95%CI: 0.22-0.88, p=0.02). Eribulin was generally well tolerated, with grade 3-4 adverse events being recorded in 15.9% of patients.
Conclusions: The outcomes described for our cohort are consistent with those reported in the pivotal Study301 and subsequent observational studies. Further data from adequately-sized, ad hoc trials on eribulin use in second line for mTNBC are warranted to confirm our findings.
Keywords: eribulin mesylate, triple negative metastatic breast cancer, efficacy outcomes, toxicity outcomes, chemotherapy

 Global reach, higher impact
Global reach, higher impact