3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(10):2228-2234. doi:10.7150/ijms.56928 This issue Cite
Research Paper
The Relationship between Lymphocyte Subsets and the Prognosis and Genomic Features of Lung Cancer: A Retrospective Study
1. Center of Gerontology and Geriatrics, West China Hospital, Sichuan University, Chengdu, China.
2. Clinical Research Center for Respiratory Diseases, West China Hospital, Sichuan University, Chengdu, China.
3. Nursing Department, West China Hospital, Sichuan University, Chengdu, China.
4. Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China.
Received 2020-12-8; Accepted 2021-3-15; Published 2021-3-25
Abstract
Background: It has been shown that the prognosis of malignant tumors was closely related to the composition and function of immune system, which was associated with genomic features. However, the prognostic value of peripheral T lymphocyte subsets and its relationship with genomic features in lung cancer has not been analyzed extensively. Therefore, this study was intended to evaluate the relationship between lymphocyte subsets and the prognosis and genomic features of lung cancer.
Methods: 598 lung cancer patients with complete data were included in this study between 2011 and 2018. Kaplan-Meier method and Pearson analyses were conducted to study the prognostic value of CD3+, CD4+, CD8+ T lymphocytes and the rate of CD4/CD8.
Results: Patients with EGFR mutation has lower mean percentage of CD8+ lymphocytes than patients with EGFR wild-type (24.71 versus 26.62, respectively, P=0.041). Patients with high CD3 had better OS than those with low (27 versus 14 months, P=0.002). Patients with higher CD4 and CD4/CD8 rate had longer OS than with lower (27 versus 12 months, P=0.002; 25 versus 9 months, P=0.008, respectively). Patients with high CD8 had poor PFS than low group (6 versus 11 months, P=0.009). There was a negative correlation between CD3+ and CD4+ cells and OS in smoking stage Ⅱ female lung cancer patients (PCC = 0.626, P<0.05; PCC = 0.534, P<0.05, respectively). In stage Ⅰ male lung cancer patients, CD8+T cell is negatively correlated with OS and PFS (PCC = 0.295, P<0.05; PCC = 0.280, P<0.05, respectively)
Conclusions: Lung cancer patients with EGFR mutation had lower percentage of CD8+ lymphocytes. Lymphocyte subsets might be potential prognostic biomarkers of lung cancer, but they are affected by gender and tumor stage.
Keywords: CD3+ T lymphocytes, CD8+ T lymphocytes, EGFR mutation, lung cancer, prognosis.
Background
Lung cancer is one of the most common malignant tumors with the highest mortality rate in the world [1]. It accounts for about a quarter of all cancer deaths [2]. Although the treatment of lung cancer, such as surgery, radiotherapy, chemotherapy and targeted therapy, has made great progress in recent years, the prognosis of lung cancer is still poor because most patients have been diagnosed in the locally advanced stage or in the stage of extensive metastasis [3]. The five-year survival rate for patients with localized lung cancer is about 55%, while for patients with distant metastasis is only 4% [4, 5].
A series of studies have shown that the occurrence, development and prognosis of malignant tumors are closely related to the composition and function of immune system [6, 7]. The systemic effects of tumor can make distant tissues and organs change in favor of metastasis and promote the progress of tumor [8-13]. The metastasis of lung cancer is related to the increase of circulating tumor cells [14], which can be influenced by the change of CD3+, CD4+ cells in peripheral blood [15]. A study about laryngeal squamous cell cancer indicates lower CD4/CD8 ratios are related to recurrent disease and a higher level of CD3 and CD4 is associated with nodal metastasis [16]. The increased risk of lung cancer is associated with low CD4 cell count and low CD4/CD8 ratio [17]. However, the prognostic value of peripheral T lymphocytes subsets in lung cancer has not been proved by studies with large data.
It has been reported that the genomic features of lung cancer were associated with inflammatory tumor-infiltrating lymphocytes [18]. For example, the infiltrating B lymphocyte abundance was higher in the EGFR-mutated patients [19]. However, the relationship between genomic features and circulating lymphocyte subsets was not clear. Hence, our study was aimed to evaluate the relationship between lymphocyte subsets and the prognosis and genomic features of lung cancer.
Methods
Patients and data collection
We retrospectively reviewed the medical records of patients who diagnosed with lung cancer at West China Hospital, Sichuan University, from January 2011 to January 2018. Inclusion criteria were: lung cancer patients, age > 18 years, with enough clinical and blood test data, no previous treatment for lung cancer before diagnosis. Criteria for exclusion from this study were: individuals with other malignancies or combined with immune system diseases, with an infection history or using drugs which could affect their immune system within one month before diagnosis.
Clinical and pathological data of included patients were collected through the medical record management system of West China Hospital. The basic information collected were: age at diagnosis, gender, smoking history, and histology. TNM stage of all patients were restaged according to the 8th International Classification System for Lung Cancer [20]. Peripheral indicators were obtained <1 week prior to the first treatment, including the proportion of CD3+, CD4+, CD8+ T lymphocyte subpopulations and the ratio of CD4/CD8.
EGFR mutation, ALK and ROS-1 rearrangements, and PD-L1 expression testing
Tumor specimens used for epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK), proto-oncogene receptor tyrosine kinas (ROS-1) rearrangements, and programmed death-ligand 1 (PD-L1) expression detection were obtained from surgery, bronchoscopy, computed tomography (CT)-guided biopsy, and pleural effusion. EGFR mutation detection was carried out using Amplification Refractory Mutation System technology as the ADx EGFR Mutations Detection Kit (Amoy Diagnostics, China) had been approved for clinical application by the State Food and Drug Administration in China. ALK and ROS-1 gene testing and PD-L1 expression was conducted on biopsy samples using immunostaining or the fluorescence in situ hybridization (FISH) method.
Statistical analysis
The survival data included overall survival (OS) and progression-free survival (PFS). OS was defined as the time from the start of treatment to death from any cause. PFS was the time from start of treatment to radiographic or clinical progression or death from any cause. Patients who had not progressed or died at the last time of follow-up were censored at the date of the last contact.
X-tile software (Yale University, New Haven, CT, USA) was used to determine the optimal cut-off values of the proportion of CD3, CD4, CD8, CD4/CD8, neutrophil to lymphocyte ratio (NLR)and platelet to lymphocyte ratio (PLR) for predicting lung cancer prognosis[21]. Kaplan-Meier curves were used for the analysis of OS and PFS. The difference between groups were compared by the log-rank test. Canonical Correlation Analysis (Canoco 5.0) and Pearson analysis were used to evaluate the correlation between CD3, CD4, CD8, CD4/CD8, NLR, PLR, and other characteristics of lung cancer and PFS, OS. The comparation of lymphocyte subsets between different genomic features was performed by t-test. Two-tailed P values of <0.05 were considered to define statistical significance. All these statistical analyses were carried out using SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
Patients characteristics
597 lung cancer patients with enough clinical and peripheral blood data were included in this study. The baseline characteristics of these patients were shown in Table 1. Median age of these patients was 61 years old. More than half of patients were male (58.96%) and smokers (53.77%). 397 (66.5%) individuals were diagnosed with adenocarcinoma. The majority of patients were diagnosed at late tumor stage (Ⅲ-Ⅳ, 72.53%). The prevalence of EGFR mutation in tumor cell was 45.55%, ALK rearrangement 8.11%, ROS-1 rearrangement 2.56%, PD-L1 expression positive 59.01%.
The clinical-pathological characteristics of patients (N (%)).
| Age, years | Median | 61 |
|---|---|---|
| Range | 20-87 | |
| Gender | Male | 352 (58.96) |
| Female | 245 (41.04) | |
| Smoking | No | 276 (46.23) |
| Yes | 321 (53.77) | |
| Histology | Adenocarcinoma | 397 (66.50) |
| Squamous carcinoma | 100 (16.75) | |
| Others | 100 (16.75) | |
| Stage | Ⅰ | 103 (17.25) |
| Ⅱ | 61 (10.22) | |
| Ⅲ | 167 (27.97) | |
| Ⅳ | 266 (44.56) | |
| CD3 (%) | Mean | 66.69 |
| Standard deviation | 10.89 | |
| CD4 (%) | Mean | 36.98 |
| Standard deviation | 9.44 | |
| CD8 (%) | Mean | 25.27 |
| Standard deviation | 9.19 | |
| CD4/CD8 | Mean | 1.76 |
| Standard deviation | 1.53 | |
| EGFR mutation | Positive | 169 (45.55%) |
| Negative | 202 (54.45%) | |
| ALK rearrangement | Positive | 36 (8.11%) |
| Negative | 408 (91.89%) | |
| ROS-1 rearrangement | Positive | 9 (2.56%) |
| Negative | 343 (97.44%) | |
| PD-L1 expression | Positive | 95 (59.01%) |
| Negative | 66 (40.99%) |
The percentage of T-lymphocyte subsets in lung cancer patients with different genomic aberrations and PD-L1 expression was shown in Table 2. Patients with EGFR mutation has lower percentage of CD8+ lymphocytes than patients with EGFR wild-type (24.71±8.69 and 26.62±9.23, respectively, P=0.041). There was no significant difference in the proportion of T-lymphocyte subsets between PD-L1 expression positive and negative patients.
Survival outcomes
The mean percentage of pretreatment circulating CD3+, CD4+, CD8+ lymphocytes and CD4/CD8 ratio were 66.69%, 36.98%, 25.27%, and 1.76 respectively. Median NLR was 3.39 and PLR was 138.94. The cut-off values of these indicators for predicting prognosis in lung cancer patients were defined by X-tile software. CD3 was 67.25%, CD4 was 42%, CD8 was 33.9%, CD4/CD8 was 1.085, NLR was 1.9, and PLR was 213.8. Patients were stratified into 2 groups (low group and high group) by the cut-off value of these indicators, when Kaplan-Meier curves and log-rank test were used for the analysis of OS and PFS.
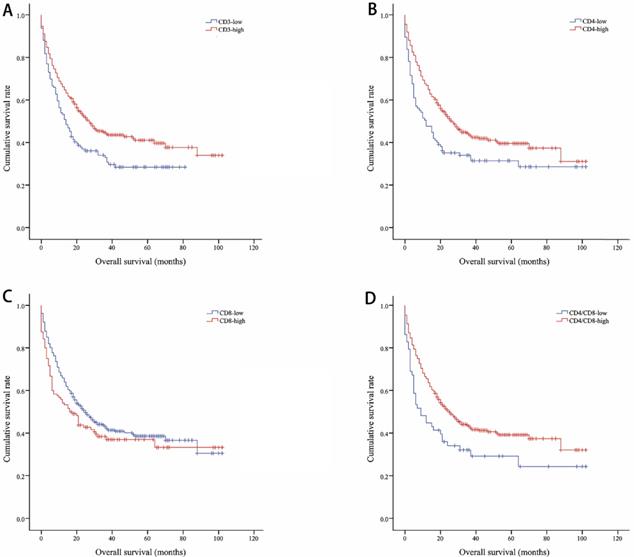
Kaplan-Meier curves showing overall survival by CD3 (A), CD4 (B), CD8 (C), CD4/CD8 (D)
Circulating T-lymphocyte subsets of different patients with lung cancer (mean (±standard deviation)).
| CD3 (%) | P-value | CD4 (%) | P-value | CD8 (%) | P-value | CD4/CD8 | P-value | |
|---|---|---|---|---|---|---|---|---|
| EGFR mutation | 0.368 | 0.389 | 0.041 | 0.938 | ||||
| Positive | 67.26 (±10.90) | 37.72 (±8.77) | 24.71 (±8.69) | 1.80 (±0.98) | ||||
| Negative | 68.25 (±10.28) | 36.92 (±9.11) | 26.62 (±9.23) | 1.78 (±2.59) | ||||
| ALK rearrangement | 0.774 | 0.231 | 0.184 | 0.289 | ||||
| Positive | 67.99 (±9.18) | 35.82 (±8.95) | 27.19 (±8.08) | 1.48 (±0.74) | ||||
| Negative | 66.46 (±10.57) | 37.69 (±8.98) | 25.18 (±8.74) | 1.82 (±1.93) | ||||
| ROS-1 rearrangement | 0.768 | 0.177 | 0.334 | 0.816 | ||||
| Positive | 69.02 (±11.85) | 41.90 (±8.33) | 22.64 (±5.95) | 1.96 (±0.65) | ||||
| Negative | 67.96 (±10.58) | 37.78 (±9.03) | 25.49 (±8.77) | 1.80 (±2.04) | ||||
| PD-L1 expression | 0.834 | 0.308 | 0.075 | 0.069 | ||||
| Positive | 69.58 (±9.29) | 37.70 (±9.75) | 27.50 (±10.46) | 1.58 (±0.722) | ||||
| Negative | 69.94 (±11.72) | 39.19 (±8.64) | 24.79 (±8.68) | 1.80 (±0.792) |
Kaplan-Meier curves showing progression-free survival by CD3 (A), CD4 (B), CD8 (C), CD4/CD8 (D)
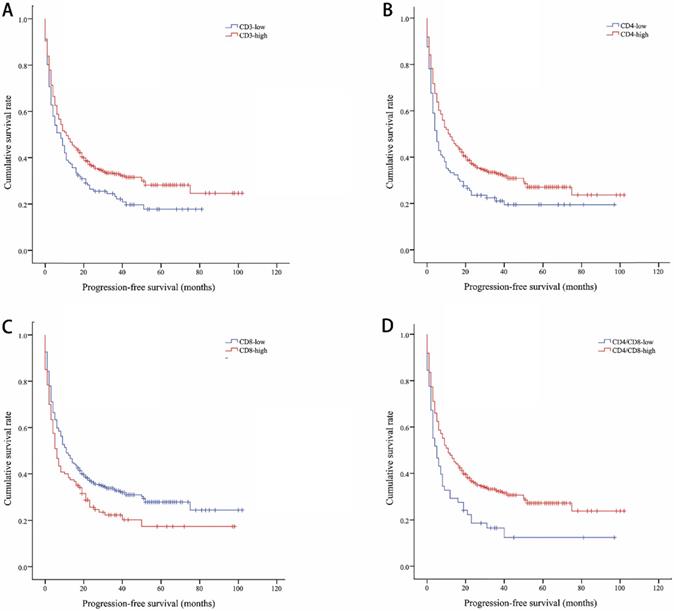
The median OS of all included lung cancer patients was 23 months (95% confidence interval (CI), 18.56-27.44), and the median PFS was 10 months (95%, 7.76-12.24). The OS rates of patients according to CD3, CD4, CD8, and CD4/CD8 were shown in Figure 1. The CD3-high group had higher OS than the CD3-low group, with a median OS of 27 versus 14 months (P=0.002). Patients with higher CD4 shown longer OS (CD4-high versus CD4-low, 27 versus 12 months, P=0.002). The higher rate of CD4/CD8 also had better OS (CD4/CD8-high versus CD4/CD8-low, 25 versus 9 months, P=0.008). However, OS showed no significant difference between CD8-high and CD8-low group (16 versus 25 months, P=0.1). Patients with higher CD3, CD4, or CD4/CD8 rate had longer PFS (11 versus 8 months, P=0.017; 11 versus 5 months, P=0.003; 11 versus 5 months, P=0.002, respectively). Conversely, the CD8-high group had poor PFS than the CD8-low group (6 versus 11 months, P=0.009). The PFS rates of patients according to CD3, CD4, CD8, and CD4/CD8 were shown in Figure 2. The OS and PFS was significant longer in the NLR-low group (OS, 88 versus 19 months, P<0.001; PFS, 26 versus 7 months, P<0.001, respectively), which was also found in the PLR-low group (OS, 29 versus 11 months, P<0.001; PFS, 13 versus 4 months, P<0.001, respectively) (Figure S1).
The correlation between lymphocyte subsets and prognosis
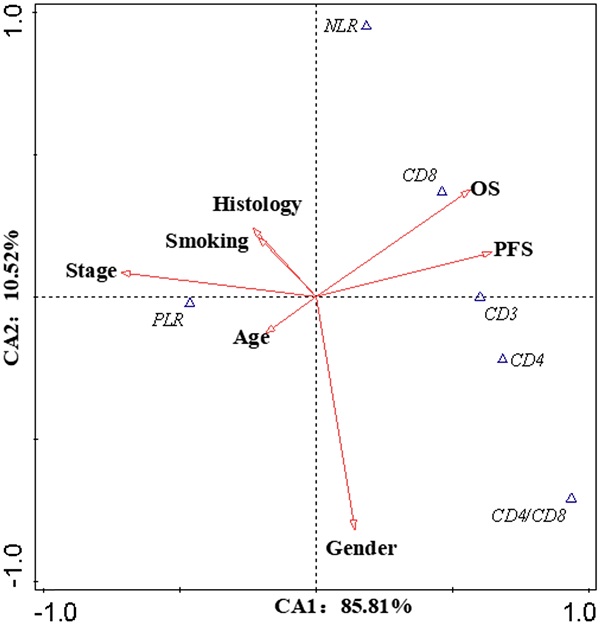
Canonical Correlation Analysis was used to analysis the correlations between lymphocyte subsets, clinical characteristics and prognosis of lung cancer. Four axes were determined via CCA, and the cumulative fitted variations explained by CA1 (85.81%) and CA2 (10.52%) were up to 96.33%, indicating the validity of this analysis. As shown in Figure 3, gender and stage had potential impacts on PFS and OS. Smoking had an impact on the prognosis of female patients. According to clinical characteristic, the patients were divided into different groups to explore the relationship between lymphocyte and prognosis. In non-smoking stage Ⅱ female lung cancer patients, CD8+T cell was positively correlated with OS and PFS (Pearson correlation coefficient (PCC) = 0.587, P<0.05; PCC = 0.586, P<0.05, respectively) (Figure S2). There was a negative correlation between CD3+ and CD4+ cells and OS in smoking stage Ⅱ female lung cancer patients (PCC = 0.626, P<0.05; PCC = 0.534, P<0.05, respectively) (Figure S3). In stage Ⅰ male lung cancer patients, CD8+T cell was negatively correlated with OS and PFS (PCC = 0.295, P<0.05; PCC = 0.280, P<0.05, respectively) (Figure S4). Indicators related to the prognosis of patients with lung cancer were listed in Figure 4.
Discussion
This study indicated that the high percentages of peripheral CD3+, CD4+ T cells and CD4/CD8 ratio were associated with longer OS in lung cancer patients. However, the high baseline proportion of CD8+ T cells was significant predictors of poor PFS. Lung cancer patients with EGFR mutation had lower percentage of CD8+ lymphocytes.
Canonical Correlation Analysis. The canonical correlation analysis (CCA) were performed in this work to further elucidate the interactions among the prognostic biomarkers and the potential impact factors (IF). The values on the axes indicate adjusted explained variations. Triangles mean prognostic biomarkers, arrows mean IF (except OS and PFS). Stage and gender were the dominant IF, which contributed to the variations in CA1 and CA2, respectively. According to the intersection angle between the biomarkers and the IF, CD8 and PLR might be the candidates to estimate the prognosis.
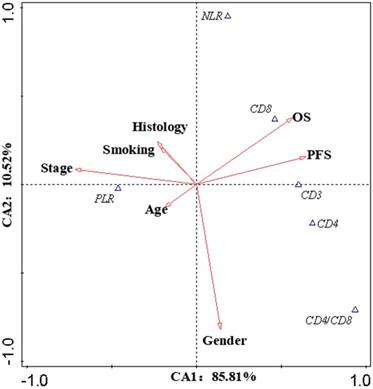
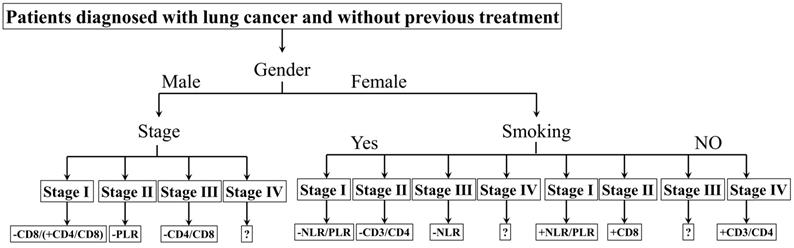
The biomarkers to assess the prognosis. +: positive correlation; -: negative correlation; ?: no appropriate biomarkers in this work. Of male patients, in stage Ⅰ, there was a negative correlation between CD8 and prognosis, a positive correlation between CD4/CD8 and prognosis; in stage Ⅱ, a negative correlation between PLR and prognosis; in stage Ⅲ, a negative correlation between CD4/CD8 and prognosis; in stage Ⅳ, no appropriate biomarker was found. NLR/PLR was negatively correlated with prognosis in smoking stage Ⅰ female patients, while positively in no-smoking stage Ⅰ female patients. CD4/CD8 was negatively correlated with prognosis in smoking stage Ⅱ female patients, while positively in no-smoking stage Ⅳ female patients. There was a negative correlation between NLR and prognosis in smoking stage Ⅲ female patients; a positive correlation between CD8 and prognosis in no-smoking stage Ⅱ female patients. No appropriate biomarker was found in smoking stage Ⅳ and no-smoking stage Ⅲ female patients.
Previous study observed that the risk of lung cancer was associated with low CD4 cell count and low CD4/CD8 ratio [17]. Peripheral CD8+T cells was higher in lung cancer patients compared to the health, while CD3+ and CD4+T cells, and CD4/CD8 ratio was significantly lower [22]. The relationship of lower CD4+/CD8+ ratios and recurrent diseases and higher level of CD3+ and CD4+ cells and nodal metastasis was found in laryngeal squamous cell cancer[16].
In our study, the prevalence of EGFR mutation in tumor cells was 45.55%, ALK rearrangement 8.11%, ROS-1 rearrangement 2.56%, which was similar to the results of other studies [23-25]. The expression of PD-L1 in tumor cells was consistent with previous studies [26, 27]. We found patients with EGFR mutation had lower percentage of circulating CD8+ T cells. A precious study found there was no significant difference in the proportion of tumor infiltration CD8+ T cells between EGFR mutation and EGFR wild-type patients [28]. Future researches were need to clarify the relationship of T lymphocytes and EGFR mutation in lung cancer.
The survival curve of this study showed that peripheral CD4+ T cells but not CD8+ T cells were associated with OS in lung cancer. A previous research also indicated that tumor infiltrating CD4+ T cells, not CD8+ T cells, were associated with favorable prognosis in non-small cell lung cancer [29]. In contrast, an meta-analysis found that CD4+ T cells in tumor compartment had no influence on OS, while CD8+ T cells were associated with better prognosis in terms of OS in non-small cell lung cancer patients [30]. They also found that elevated CD3+ T cells was associated with improved OS, which was constant with our results. Another meta-analysis clarified that high level of CD3+, CD4+, and CD8+ T cells infiltration showed better OS in lung cancer patients [31]. The distant prognosis value of T cells in different sites was needed to be explored by more researches. Increased CD8+CD28+ T cells independently predicted favorable OS and PFS in lung adenocarcinoma, while high levels of CD8+CD28- T cells predicted unfavorable OS and PFS in lung squamous cell carcinoma [32]. The prognosis value was associated with the subtypes of CD8+ T cells. Therefore, the prognostic value of different subtypes of CD4+ and CD8+ T cells in lung cancer were needed to be studied by more researches.
Conclusion
Lung cancer patients with EGFR mutation had lower percentage of CD8+ lymphocytes. High level of peripheral CD3+ T cells predict longer OS in lung cancer patients, while high level of peripheral CD8+ T cells are associated with poor PFS. Lymphocyte subsets might be potential prognostic biomarkers of lung cancer, but they were affected by gender and tumor stage.
Supplementary Material
Supplementary figures and tables.
Abbreviations
EGFR: Epidermal growth factor receptor; OS: Overall survival; PFS: Progression-free survival; ROS-1: Proto-oncogene receptor tyrosine kinase; ALK: Anaplastic lymphoma kinase; PD-L1: Programmed death-ligand 1; HR: Hazard ratio; CI: Confidence interval; PCC: Pearson correlation coefficient.
Acknowledgements
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Committee Approval and Patient Consent
Our study was approved by the ethics committee of West China hospital (IRB:2018/488) and carried out following the local ethical guidelines.
Funding
This work was supported by the fellowship of China Postdoctoral Science Foundation (No. 2020M670054ZX); CAMS Innovation Fund for Medical Science (No: 2019TX310002); Sichuan International/Hong Kong, Macao and Taiwan Science and technology innovation cooperation project: molecular imaging research on targeted treatment of lung cancer (No. 2018hh0161); Sichuan Science and Technology Program (No. 2020YFS0572).
Authorship
Dai Shuiping, Li Weimin, Ren Pengwei, and Yang Lan made substantial contributions to the design of this study. Dai Shuiping, R Pengwei, and Yang Lan acquired the clinical data. Dai Shuiping and Ren Pengwei drafted this manuscript, which was critically revised by Li Weimin.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424
2. Siegel RL, Miller KD. Cancer statistics, 2019; 69: 7-34
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-54
4. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH. et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-89
5. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30
6. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH. et al. The Immune Landscape of Cancer. Immunity. 2018;48:812-30 e14
7. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-70
8. Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102-6
9. Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35-44
10. Oppenheimer SB. Cellular basis of cancer metastasis: A review of fundamentals and new advances. Acta Histochem. 2006;108:327-34
11. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369-75
12. Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289-300
13. Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM. et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906-11
14. O'Flaherty JD, Gray S, Richard D, Fennell D, O'Leary JJ, Blackhall FH. et al. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer. 2012;76:19-25
15. Ye L, Zhang F. Circulating Tumor Cells Were Associated with the Number of T Lymphocyte Subsets and NK Cells in Peripheral Blood in Advanced Non-Small-Cell Lung Cancer. Disease Markers. 2017:5727815.
16. Marchi F, Missale F, Incandela F, Filauro M, Mazzola F, Mora F. et al. Prognostic Significance of Peripheral T-Cell Subsets in Laryngeal Squamous Cell Carcinoma. Laryngoscope investigative otolaryngology. 2019;4:513-9
17. Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D. et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. The Lancet HIV. 2017;4:e67-e73
18. Zhang XC, Wang J, Shao GG, Wang Q, Qu X, Wang B. et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nature communications. 2019;10:1772
19. Wang C, Yin R, Dai J, Gu Y, Cui S, Ma H. et al. Whole-genome sequencing reveals genomic signatures associated with the inflammatory microenvironments in Chinese NSCLC patients. Nat Med. 2018;9:2054
20. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE. et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39-51
21. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-9
22. Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL. et al. Cutting Edge: Regulatory T Cells from Lung Cancer Patients Directly Inhibit Autologous T Cell Proliferation. The Journal of Immunology. 2002;168:4272-6
23. Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175-80
24. Korpanty GJ, Graham DM, Vincent MD, Leighl NB. Biomarkers That Currently Affect Clinical Practice in Lung Cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front Oncol. 2014;4:204
25. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-703
26. Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 Expression in Lung Cancer. J Thorac Oncol. 2016;11:964-75
27. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T. et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935-40
28. Liu SY, Dong ZY, Wu SP, Xie Z, Yan LX, Li YF. et al. Clinical relevance of PD-L1 expression and CD8+ T cells infiltration in patients with EGFR-mutated and ALK-rearranged lung cancer. Lung Cancer. 2018;125:86-92
29. Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S. et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003-9
30. Soo RA, Chen Z, Yan Teng RS, Tan HL, Iacopetta B, Tai BC. et al. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget. 2018;9:24801-20
31. Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J. et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem. 2015;37:1560-71
32. Liu C, Jing W, An N, Li A, Yan W, Zhu H. et al. Prognostic significance of peripheral CD8+CD28+ and CD8+CD28- T cells in advanced non-small cell lung cancer patients treated with chemo(radio)therapy. J Transl Med. 2019;17:344
Author contact
![]() Corresponding author: Weimin Li, E-mail weimin003com
Corresponding author: Weimin Li, E-mail weimin003com

 Global reach, higher impact
Global reach, higher impact