3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(9):1966-1974. doi:10.7150/ijms.53743 This issue Cite
Research Paper
Development and validation a simple model for identify malignant ascites
1. Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, China.
2. Department of Oncology, The Fifth Hospital of Wuhan, Wuhan, Hubei, 430050, China.
3. Department of Pathology, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, China.
Received 2020-9-25; Accepted 2021-2-1; Published 2021-3-3
Abstract
The differential diagnosis of benign ascites and malignant ascites is incredibly challenging for clinicians. This research aimed to develop a user-friendly predictive model to discriminate malignant ascites from non-malignant ascites through easy-to-obtain clinical parameters. All patients with new-onset ascites fluid were recruited from January 2014 to December 2018. The medical records of 317 patients with ascites for various reasons in Renmin Hospital of Wuhan University were collected and reviewed retrospectively. Thirty-six parameters were included and selected using univariate logistic regression, multivariate logistic regression, and receiver operating characteristic (ROC) curve analyses to establish a mathematical model for differential diagnosis, and its diagnostic performance was validated in the other groups. Age, cholesterol, hypersensitivity C-reactive protein (hs-CRP) in serum, ascitic fluid adenosine deaminase (AF ADA), ascitic fluid lactate dehydrogenase (AF LDH) involvement in a 5-marker model. With a cut-off level of 0.83, the sensitivity, specificity, accuracy, and area under the ROC of the model for identifying malignant ascites in the development dataset were 84.7%, 88.8%, 87.6%, and 0.874 (95% confidence interval [CI], 0.822-0.926), respectively, and 80.9%, 82.6%, 81.5%, and 0.863 (95% CI,0.817-0.913) in the validation dataset, respectively. The diagnostic model has a similar high diagnostic performance in both the development and validation datasets. The mathematical diagnostic model based on the five markers is a user-friendly method to differentiate malignant ascites from benign ascites with high efficiency.
Keywords: diagnosis, differential, ascites, carcinoma, logistic models
Introduction
Ascites fluid is a common clinical syndrome, which can be divided into benign and malignant ascites for various reasons. When disorders, such as heart failure, renal failure, liver cirrhosis, and hypoproteinemia, affect the peritoneum or protein balance, the pathological fluid, called ascites, accumulates in the peritoneal cavity [1]. Malignant ascites is the result of cancer and account for nearly 10% of all ascites cases occurring in related to various tumors, especially breast, ovary, stomach, pancreas, and colon cancer, and presents a challenging clinical problem in some cases [2]. However, the distinction between the two types of ascites fluid is not only the basis of diagnosis but also a prerequisite for formulating a treatment plan.
Differential diagnosis of benign and malignant ascites can rely on certain precise findings such as appropriate ascites fluid serum analysis, cytological examination, laparoscopy, and the symptoms of the patients. Previous studies have shown that several biochemical indicators, such as total protein [3], serum-ascites albumin gradient (SAAG) [4], and various tumor markers [5, 6], are vital for the differential diagnosis of benign and malignant ascites. However, they all have their own limitations. The use of a predominant beneficial tool, SAAG, is also a matter of debate in etiological diagnosis [7]. The cytology analysis positive rate is only 30-50%, although it has proven to be the gold standard for identifying malignant ascites [8]. The positive rate of peritoneal biopsy is relatively high, but it is not easy for patients to accept because of its invasive nature. Furthermore, the procedures that lead to a definitive diagnosis could result in a delay that threatens the adequate management of the disease and its outcomes [9]. The search for novel biochemical markers in serum and/or ascitic fluid (AF) is still under investigation. Yun et al. [10] reported that exosomal miRNAs offer a novel biomarker for discriminating gastric cancer ascites from non-malignant ascites. However, like other new biomarkers, this assay is not accessible, especially in hospitals in developing countries. Hence, continuous work should be taken to design a simple, cost-effective, and less invasive method to provide accurate information about the etiology of ascites.
In this study, to address the disadvantages of previous studies, we aimed to explore the diagnostic power of easily attainable demographic features and laboratory indicators of patients with ascites to establish a diagnostic model for identifying malignant ascites with high efficiency.
Materials and Methods
Patient selection
We performed a retrospective review of the medical data of all patients with new-onset ascites admitted to Renmin Hospital of Wuhan University from January 2014 to December 2018. The participants in this study were recruited with the following criteria: (a) patients with newly developed ascites, (b) patients who underwent diagnostic abdominal paracentesis and (c) patients who were informed and agreed to participate in the study. Individuals were excluded from the study based on the following criteria: (a) patients who had received peritoneal dialysis treatment, (b) patients with bloody ascites caused by trauma or surgery, (c) patients with ascites of unknown etiology, (d) patients with missing information, and (e) patients with signs of sepsis.
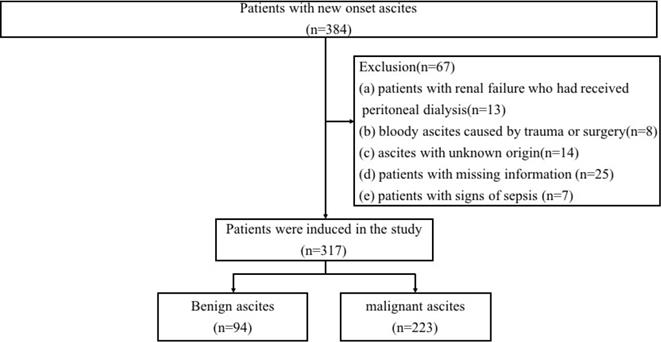
In total, 317 participants were enrolled in our study (94 patients with benign ascites and 223 patients with malignant ascites), and 67 subjects were excluded. The flow of patient selection is shown in Figure 1. Ethical approval for this study was obtained from the Ethics Committee of Renmin Hospital of Wuhan University in Hubei Province (WDRY2019-K014). All participants signed written informed consent after the study protocol was thoroughly explained, and this study was conducted in accordance with the Helsinki Declaration.
Data collection
All patients underwent abdominal paracentesis and blood withdrawal in the fasting state before initiating any treatment, such as administration of intravenous fluids or diuretics or chemotherapy. The medical records of each patient were reviewed in a structured manner to obtain the patients' demographic characteristics and laboratory data. In serum laboratory evaluations, white blood cells (WBCs), neutrophil (Neu), lymphocyte (Lym), red blood cells, hemoglobin, red blood cell distribution width-standard deviation (PDW-SD), platelets, platelet distribution width (PDW), alkaline phosphatase (ALP), total protein (TP), albumin, chloride, total cholesterol (TCh), hypersensitivity C-reactive protein (hs-CRP), C-reactive protein (CRP), lactate dehydrogenase (LDH), alpha-fetoprotein (AFP), cancer antigen 125 (CA125), cancer antigen19-9 (CA19-9), and carcinoembryonic antigen (CEA) during the initial diagnostic period were evaluated.
Flowchart of patient inclusion.
In the laboratory evaluation of ascites fluid, karyocyte count, Neu, Lym, ascitic fluid total protein (AFTP), ascitic fluid adenosine deaminase (AF ADA), glucose, chloride, and LDH were also reviewed. In addition, serum-ascites total protein gradient, ratio of chloride in serum and ascites, ratio of LDH in serum and ascites, platelet-to-lymphocyte ratio (PLR) in serum, and neutrophil-to-lymphocyte ratio (NLR) in both serum and ascites were evaluated.
Diagnostic criteria
Three independent researchers with unknown etiology of ascites reviewed patient data to achieve a definitive diagnosis. If the diagnoses were inconsistent, the case was eliminated. Each case should have complete data and a clear diagnosis. The 317 patients were distributed under a well-established diagnosis basis and were divided into two groups: benign ascites group and malignant ascites group.
Patients in the malignant ascites group met at least one diagnosis criteria as follows: (1) the results of ascites fluid cytology were positive; (2) patients had a diagnostic peritoneal biopsy and obtained a positive result; (3) demonstration of a primary tumor or clinical evidence of tumor dissemination (e.g., lung metastases) and exclusion of other potential causes for the ascites.
The etiology of benign ascites included the following criteria: cirrhosis, nephrotic syndrome, spontaneous bacterial peritonitis, cardiac ascites, tuberculous peritonitis, pancreatic ascites; the well-established clinical criteria of the diagnosis of these diseases were described in previous studies [11-13]. All the benign ascites were diagnosed by clinical and laboratory tests and without any tumor signs.
Statistical analysis
All statistical analyses were performed using the SPSS software (version 21.0, IBM). All continuous variables were expressed as mean ± SD. Differences in continuous variables were assessed using the Student's t-test, while the chi-square test or Fisher's exact test was used for categorical data, as appropriate. To develop a diagnostic model, the 317 patients were randomly divided into two groups, a development dataset (60% of study subjects) or validation dataset (40% of study subjects). In the development group, all P-values less than 0.05 were considered for inclusion in univariate logistic regression analyses. Developing a more concise equation, receiver operating characteristic (ROC) analysis was performed (if the area under the ROC curve was ≥ 0.6) to determine which variables were used in further multivariable logistic regression analyses. The final diagnostic equation was then created by the β-coefficients of the multivariate logistic regression analysis, and the predicted performance was validated in the validation group. ROC curve analysis helped determine the optimal cut-off points for continuous variables based on their highest diagnostic accuracy. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated based on ROC analysis.
Results
Patient characteristics
In this study, clinical data from 384 patients with new-onset ascites were obtained for further study (Figure 1). The summary statistics of the etiology classification for the 317 included patients is shown in Table 1. They were first divided into two groups (benign and malignant) after stratifying according to disease type. A total of 94 patients were diagnosed with benign ascites. Liver causes accounted for 34.0% of cases, followed by peritoneal tuberculosis (13.9%) and congestive heart failure (12.8%). Among the 223 patients of the malignant ascites group, gastrointestinal tumor was the major cause of malignant ascites, which accounted for nearly 29.6% (66/223), followed by ovarian cancer, which accounted for 14.3% (32/223).
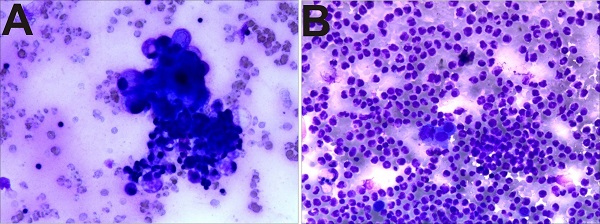
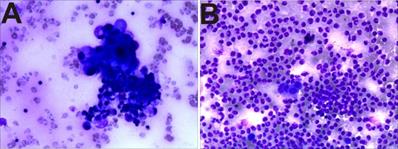
There were 102 cases of pathological cells positive in the 223 cases of malignant ascites (Figure 2A). The pathological cells of the benign group (94 cases) were all negative (Figure 2B), that is, the sensitivity of diagnosis of malignant ascites was 45.7%, specificity was 100%, and accuracy was 61.8%.
Pathological biopsy images showing (A) malignant ascites: adenocarcinoma cells, (B) benign ascites: neutrophils and mesothelial cells.
Univariate analysis for discrimination between benign and malignant ascites
A total of 36 parameters were collected from the enrolled 317 patients, comprising two demographic features and 34 serum or AF laboratory parameters (Tables 2A, 2B). Comparisons between patients with benign and malignant ascites were made using the Student's t-test, and the results showed that 19 variables were significantly different. The malignant ascites and benign ascites (benign group: 47.5% female, 52.5% male vs. malignant group: 58.2% female, 41.8% male; P > 0.05) was not significantly different between sexes based on univariate analyses. In addition, the results demonstrated that patients with malignant ascites had a significantly older mean age (benign group 55.68 ± 17.22 vs. malignant group 62.57 ± 11.60, P < 0.05) than patients with benign ascites.
The aetiology of ascites in the patients enrolled in our study
| Aetiology | Benign group (n=94) | Malignant group (n=223) | Total (n=317) |
|---|---|---|---|
| Cirrhosis | 32 | 0 | 32 |
| Cardiac ascites | 12 | 0 | 12 |
| Tuberculous peritonitis | 13 | 0 | 13 |
| Miscellaneous | 37 | 0 | 37 |
| Liver Cancer | 0 | 31 | 31 |
| Pancreatic Cancer | 0 | 22 | 22 |
| Gastric Cancer | 0 | 44 | 44 |
| Colorectal Cancer | 0 | 24 | 24 |
| Ovarian Cancer | 0 | 32 | 32 |
| Metastatic peritoneal carcinoma | 0 | 16 | 16 |
| Other Cancers | 0 | 54 | 54 |
Among the serum laboratory parameters, WBC, TCh, hs-CRP, CA125, and CA19-9 were significantly higher than those assessed in the benign group. In contrast, the data analysis showed that the Cl levels in the benign group were significantly higher than those in the malignant group (Table 2A). In ascites, LDH levels were considerably higher in the malignant group. Moreover, Lym and ADA levels were also statistically different between the benign and malignant groups (Table 2B). For proportional indicators, serum-ascites total protein gradient was significantly higher in the benign group; in contrast, serum PLR was higher in the malignant group (Table 2B).
Demographic and serum laboratory features of patients with benign and malignant ascites
| Demographic and laboratory features of patients | Benign group (n=94) | Malignant group (n=223) | P value |
|---|---|---|---|
| Age, y | 54.48±18.32 | 62.07±12.63 | 0.006 |
| Sex, male: female | 45:49 | 111:112 | 0.168 |
| WBCs (×109/L) | 6.20±3.38 | 7.02±2.84 | 0.006 |
| Neu (×109/L) | 4.55±2.91 | 8.103±27.63 | 0.228 |
| Lym (×109/L) | 1.02±0.71 | 2.04±8.97 | 0.294 |
| RBC (×1012/L) | 3.71±1.01 | 3.83±0.73 | 0.08 |
| Hb (g/L) | 108.63±27.53 | 113.11±19.93 | 0.043 |
| RDW-SD | 50.36±9.93 | 48.65±9.92 | 0.105 |
| PLT (×106/L) | 199.48±117.82 | 283.65±146.40 | P<0.001 |
| PDW | 12.71±2.85 | 12.13±2.43 | 0.070 |
| ALP (U/L) | 151.17±217.38 | 131.47±150.71 | 0.443 |
| TP (g/L) | 63.26±7.1 | 63.47±7.52 | 0.991 |
| ALB (g/L) | 33.91±5.38 | 35.19±4.87 | 0.106 |
| Cl (mmol/L) | 103.54±5.94 | 102.71±4.98 | 0.032 |
| TCh (mmol/L) | 3.42±1.00 | 4.31±1.50 | P<0.001 |
| hs-CRP (mg/L) | 39.41±42.76 | 53.70±51.52 | P<0.001 |
| CRP (mg/L) | 34.46±36.48 | 47.38±47.10 | 0.043 |
| LDH (U/L) | 272.30±153.91 | 330.58±244.66 | 0.022 |
| AFP (ng/mL) | 2661.91±13586.12 | 1535.51±9741.86 | 0.094 |
| CA125 (U/L) | 513.91±546.45 | 1159.46±2112.09 | P<0.001 |
| CA19-9 (U/L) | 35.11±143.57 | 1242.98±5775.03 | 0.028 |
| CEA (ng/mL) | 34.03±145.00 | 102.96±661.30 | 0.581 |
CRP, hypersensitivity C-reactive protein; CRP, C-reactive protein; LDH, lactate dehydrogenase; AFP, alpha-fetoprotein assay; CA125, cancer antigen 125; CA19-9, cancer antigen 19-9; CEA, carcinoembryonic antigen.
*Data are presented as mean (±) standard deviation.
Ascitic fluid laboratory features and other parameter of patients with benign and malignant ascites
| Demographic and laboratory features of patients | Benign group (n=94) | Malignant group (n=223) | P value |
|---|---|---|---|
| Karyocyte count | 0.94±1.71 | 1.00±1.48 | 0.642 |
| Neu% | 15.46±19.96 | 20.19±17.64 | 0.04 |
| Lym% | 77.42±23.00 | 66.58±19.79 | P<0.001 |
| AFTP (g/L) | 30.00±1703 | 36.74±14.36 | 0.004 |
| ADA (U/L) | 16.72±23.10 | 8.98±10.12 | 0.02 |
| Glu (mmol/L) | 8.22±11.08 | 6.37±2.31 | 0.112 |
| Cl (mmol/L) | 126.51±168.00 | 107.32±5.55 | 0.36 |
| LDH (U/L) | 186.73±189.43 | 424.44±507.28 | P<0.001 |
| Serum-ascites total protein gradient | 32.44±23.08 | 26.74±14.74 | 0.009 |
| Serum-to-ascites LDH ration | 2.54±6.65 | 1.04±0.87 | 0.583 |
| Serum-to-ascites Cl ration | 0.90±0.25 | 4.61±45.74 | 0.688 |
| NLR of serum | 5.37±3.76 | 6.52±2.34 | 0.028 |
| NLR of ascites | 7.07±6.84 | 8.01±3.32 | 0.600 |
| PLR of serum | 302.83±234.91 | 348.34±223.54 | P<0.01 |
Neu, neutrophil; Lym, lymphocyte; AFTP, ascitic fluid total protein (AFTP); ADA, adenosine deaminase (ADA); Glu, glucose; CL, chloride; LDH, lactate dehydrogenase; NLR, neutrophils-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
*Data are presented as mean (±) standard deviation.
Univariate logistic regression analysis of significant variables in the development set
| Variable | B | S. E | Wald | P value | OR (95%CI) |
|---|---|---|---|---|---|
| Age | 0.036 | 0.012 | 9.422 | 0.002 | 1.036 (1.013-1.060) |
| WBCs | 0.169 | 0.063 | 7.128 | 0.008 | 1.184 (1.046-1.341) |
| Hb | 0.015 | 0.007 | 4.023 | 0.325 | 1.015 (1.000-1.030) |
| PLT | 0.005 | 0.001 | 11.676 | 0.383 | 1.005 (1.002-1.008) |
| Cl | -0.073 | 0.034 | 4.447 | 0.020 | 0.930 (0.869-0.995) |
| TC | 0.688 | 0.189 | 13.232 | <0.001 | 1.990 (1.373-2.883) |
| hs-CRP | 0.012 | 0.004 | 8.941 | 0.003 | 1.012 (1.004-1.020) |
| CRP | 0.009 | 0.005 | 4.262 | 0.454 | 1.009 (1.000-1.018) |
| LDH | 0.002 | 0.001 | 3.538 | 0.414 | 1.002 (1.000-1.004) |
| CA125 | 0.001 | 0.001 | 5.939 | 0.011 | 1.001 (1.000-1.001) |
| CA199 | 0.003 | 0.001 | 5.432 | 0.003 | 1.674 (1.000-1.005) |
| AF Neu% | 0.021 | 0.010 | 4.230 | 0.060 | 1.021 (1.001-1.042) |
| AF lym% | -0.034 | 0.010 | 12.902 | <0.001 | 0.966 (0.949-0.985) |
| AF TP (g/L) | 0.033 | 0.011 | 10.250 | 0.441 | 1.035 (1.013-1.056) |
| AF ADA (U/L) | -0.027 | 0.010 | 6.607 | <0.001 | 0.974 (0.954-0.994) |
| AF LDH (U/L) | 0.003 | 0.001 | 13.776 | <0.001 | 1.003 (1.001-1.005) |
| Serum-ascites total protein gradient | -0.032 | 0.010 | 9.823 | <0.001 | 0.969 (0.950-0.988) |
| Serum NLR | 0.064 | 0.037 | 3.023 | 0.082 | 1.066 (0.992-1.146) |
| Serum PLR | 0.002 | 0.001 | 4.869 | 0.027 | 1.002 (1.000-1.003) |
AF: ascitic fluid.
Development of diagnostic models to discriminate malignant ascites from benign ascites
For analytic purposes, the enrolled patients were randomly divided into two groups: the development dataset (60% of study subjects) or validation dataset (40% of study subjects). The development dataset comprised 193 cases (59 benign ascites patients and 134 malignant ascites patients), and the validation dataset included 124 cases (35 patients with benign ascites and 89 with malignant ascites). No significant difference was found between mean age (60.50 ± 14.07 vs. 59.45 ± 15.13 years; P = 0.856) and distribution of males and females (Males: Females, 87:106 vs. 52:72; P = 0.846) between the development and validation groups. Then, the development dataset was used to create a diagnostic model. The list of screened variables (P < 0.05) was selected as a candidate marker to differentiate malignant ascites from benign ascites using univariate logistic regression analysis (Table 3). Further, parameters with higher diagnostic values (AUC ≥ 0.6) such as age, WBC, TCh, hs-CRP, AF ADA, and AF LDH were selected as diagnostic scoring model markers in the development set. After the multivariable binary logistic regression analysis was applied, the WBC count was eliminated. Thus, age, TCh, hs-CRP, AF ADA, and AF LDH remained for the first model in the development set. Furthermore, two vital markers (CA125 (AUC = 0.597) and CA199 (AUC = 0.594)) were also incorporated into the predictive model (the second model) because of their significance in clinical practice (Table 4).
Diagnostic values of the variables used to differentiate benign and malignant ascites in the development set
| Variable | AUC (95%CI) | P value (logistic regression) |
|---|---|---|
| Age | 0.614 (0.520-0.705) | 0.036 |
| WBC | 0.643 (0.552-0.733) | 0.577 |
| Cl | 0.406 (0.314-0.497) | 0.966 |
| TC | 0.689 (0.605-0.773) | 0.023 |
| hs-CRP | 0.616 (0.535-0.698) | 0.016 |
| CA125 | 0.597 (0.535-0.695) | 0.126 |
| CA199 | 0.594 (0.512-0.676) | 0.178 |
| lym% (ascites) | 0.287 (0.201-0.373) | 0.633 |
| ADA (U/L) (ascites) | 0.624 (0.520-0.728) | <0.001 |
| LDH (U/L) (ascites) | 0.712 (0.634-0.789) | 0.005 |
| Serum-ascites total protein gradient | 0.401 (0.305-0.496) | 0.05 |
| PLR | 0.595 (0.476-0.697) | 0.640 |
AUC: Area under ROC curve;
Bold values signify seven diagnostic values among area under the ROC ≥0.6.
The following mathematic models were used to estimate the probability of developing malignant ascites (a higher score implies a higher likelihood of malignant ascites). The predictive performance of the model was evaluated by AUC.
5-markers risk score = 1/[1 + e-(-4.909 + 0.037 × age + 0.638 × TCh + 0.011 × hs-CRP - 0.088 × ADA + 0.006 × LDH)]
7-markers risk score = 1/[1 + e-(-4.985 + 0.036 × age + 0.515 × TCh + 0.001 × hs-CRP - 0.083 × ADA + 0.005 × LDH + 0.001 × CA125 + 0.002 × CA19-9)]
• Code used for the equations:
1. P, predictive value;
2. e, natural logarithm.
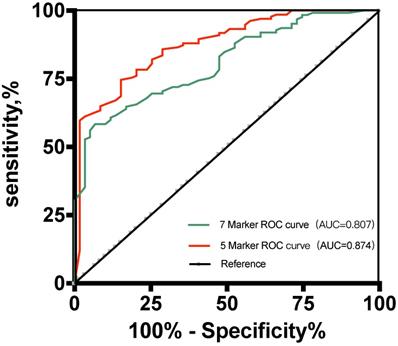
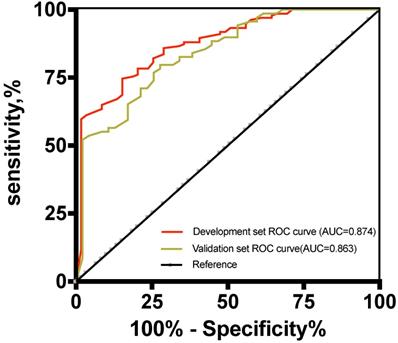
The results showed that the 5-marker predictive model reached an AUC of 0.874 (95% confidence interval [CI], 0.822-0.926) (Figure 3). Under the predicted threshold of 0.83, the sensitivity, specificity, PPV, NPV, PLR, NLR, and accuracy were 84.7%, 88.8%, 92.9%, 76.9%, 7.56%, 0.17%, and 87.6%, respectively, in the 5-marker scoring system. Moreover, we analyzed the performance of single parameters and 7-marker model in discriminating these two conditions. As expected, the AUC of the single variable and 7-marker model (AUC = 0.807) was lower than the AUC of the 5-marker model (Figure 3).
Receiver operating characteristic curves for the two predictive models for the development set.
Validation of the diagnostic model
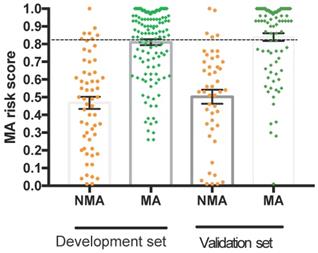
The risk score of the 5-marker predictive model was validated using the validation dataset. ROC analysis showed that the AUC of the predictive model was 0.863 (95% CI, 0.771-0.887) (Figure 4), which was not significantly different between the two datasets. In the validation set, the performance parameters were as follows: sensitivity, 80.9%; specificity, 82.6%; PPV, 92.3%; NPV, 80.5%; PLR, 4.65; NLR, 0.23; the ability to correctly classify the two conditions was 81.5% with a cut-off point of 0.83. All validity indexes listed in the results of the training set and validation set for the 5-marker predictive model were similar, and the predictive model showed good diagnostic efficiency in both datasets. As shown in Figure 4, when the cut-off point was 0.83, the proportions of false-negative malignant ascitic patients and false-positive benign ascitic patients were 15/134 and 9/59 in the development set and 17/89 and 6/35 in the validation set, respectively (Figure 5).
Receiver operating characteristic curves for the development and validation datasets.
Dot plots of the 5-marker malignant ascites risk score in differentiating benign and malignant ascites by datasets. Error bars indicate the mean and standard error of the mean. The dotted line represents the cut-off value for predicting malignant ascites at 0.83. NMA: non-malignant ascites; MA: malignant ascites.
Discussion
Malignant ascites is a common complication associated with a wide variety of neoplasms, including liver, pancreatic, gastric, colorectal, and ovarian cancers. Stukan et al. [14] reported that malignant ascites is a sign of advanced cancer and poor prognosis, requiring timely, appropriate treatment. Therefore, the first and foremost goal of this study was to facilitate the early diagnosis of ascites to improve the short mean survival duration of patients with malignant ascites [15]. Traditionally, differential diagnosis can be made based on certain specific findings such as AF cytology and diagnostic peritoneal biopsy. Unfortunately, these methods have limitations such as invasiveness and limited diagnostic efficacy [16]. In this study, the sensitivity of cytology was 45.7%, and this result was also unsatisfactory.
To date, several serum or AF markers have been studied, including vascular endothelial growth factor [17], endostatin [18], and several cytokines [19]. Despite the higher diagnostic value, these new markers were inaccessible in public clinical settings. Moreover, the value of using a single parameter to distinguish these two conditions is absolutely limited in clinical practice because of low sensitivity or specificity, while the combination of several markers to construct a mathematical model will significantly improve the diagnostic accuracy. Therefore, 36 parameters of 317 enrolled patients were collected with nearly all forms of ascites. A 5-marker predictive model was then established using multivariate regression analysis in the development set, while the diagnostic performance was evaluated in the validation set. To our knowledge, this is the first study to develop a simple, user-friendly predictive model based on the most ordinary clinical parameters to identify malignant ascites.
All easily available clinical and laboratory features were analyzed in this study. The serological examination is the basic examination that all patients will undergo when admitted to the hospital. On univariate analysis of the serum laboratory features in our study, TCh and hs-CRP were included as features favoring malignant ascites. In recent years, the relationship between inflammation and tumors has gradually become a research focus [20]. CRP is an acute-phase reactant that increases during acute or chronic inflammation and infections [21, 22]. hs-CRP is more sensitive than CRP as an inflammatory marker and is known to be elevated in chronic liver diseases and spontaneous bacterial peritonitis. Abdel-Razik et al. [1] studied 398 consecutive patients with ascites and found that CRP was a useful marker for discriminating between malignancy-related and benign ascites. Wiese et al. [23] showed that proinflammatory (hs-CRP) markers can predict the severity of liver disease, cardiac and hemodynamic changes, and long-term survival outcomes. However, little research has been conducted on the role of hs-CRP in distinguishing between benign and malignant ascites. The results of our present study suggest that the diagnostic significance of hs-CRP for identifying malignant ascites was superior to that of CRP. NLR and PLR also reflect the level of systemic inflammation in the body, which is significantly related to cell destruction caused by tumors [24]. Previous studies have confirmed that NLR and PLR help predict the prognosis of tumors [25-27], but few studies have explored the function of NLR and PLR in diagnosing malignant ascites. We first found significant differences in PLR between benign and malignant ascites, although this was not included in this predictive model.
Abnormal lipid metabolism has been reported in various studies to be closely related to the development of tumors [28] and will increase the risk of cancers [29, 30]. In addition, Banerjee et al. [31] showed that cholesterol in AF, as well as in serum, can be used as diagnostic markers to evaluate the nature of ascites. A study conducted by Farwell et al. [32] also suggested that increased levels of serum cholesterol indicate a higher risk for total and high-grade prostate cancer. The cholesterol in serum is a valuable parameter for patients diagnosed in our study, similar to previous findings [19, 33]. The underlying causes of cholesterol elevation in serum were unidentified, which merit further investigation, but this should not confine its scientific value.
Abdominal paracentesis is likely the fastest and most cost-effective way to diagnose the cause of ascites. The clinical diagnostic efficiency of AF ADA and LDH in the etiological diagnosis of unknown origin ascites is reportedly satisfactory [34-36]. There is a large body of work on the function of tumor markers, which showed that it helped identify malignant ascites [6, 27, 37]. However, AFP and CEA levels were not significant (P > 0.05) in our study, and they were not included in the predictive model. The difference between our study and previous studies may be attributed to the different causes of benign ascites in the previous study and our study. The results showed that the percentage of liver cirrhosis and miscellaneous lesions (34.04%, 39.36%, respectively) in our study was higher than that in previous studies (16.12%, 9.68%) [38]. Liver cirrhosis also leads to elevated AFP levels. Previous studies also demonstrated that the CEA of AF does not seem specific enough to diagnose malignancy-related ascites [39, 40]. Similar to our results, the sensitivity and specificity of CEA were 97% and 50%, respectively. The results of our study also showed that the 7-marker model has a lower diagnostic ability than the 5-marker model, which may be related to the reason mentioned above. Although a significant increase in CA125 and CA19-9 is considered a valuable indicator for cancer diagnosis [41, 42], with continuous science development, many scholars have found that CA125 and CA199 has a varying degree of increase and significance in the diagnosis of non-malignant diseases, such as heart failure [43], active tuberculosis [44], inflammatory bowel disease [45], liver cirrhosis [46], and other diseases. In addition, previous studies reported no significant differences in laboratory values, such as WBCs, platelets, and some other variables between malignant and benign ascites, which were similar to those of our results [47].
Numerous studies have attempted to identify a more effective method for the early diagnosis of malignant ascites. However, to date, no sensitive and convenient method for differentiating ascites has been determined. Thus, a combination of the selected valuable parameters to establish a mathematical model may help solve this problem. In 2000, Alexandrakis et al. [8] reported a scoring model including TP, LDH, TNF-α, C4, and haptoglobin, with an accuracy of 89% in the overall date and 70% in the cross-validation date. Tian et al. [48] also calculated scores for diagnosing malignant ascites, including innovative features-effusion CEA, effusion tumor cells (ETC), and ETC cluster count, with an AUC of the training dataset and validation dataset of 0.939 and 0.948, respectively. We also developed a predictive score model for the same purpose. The indicators included in our study are particularly common variables and highly clinically useful. In contrast, with similar diagnostic power, previous models may include disadvantages of high cost, the need for additional examinations, and trained personnel. Moreover, results using this predictive model for a patient with unknown etiology of ascites can be determined easily by entering each value into the risk score formula by calculation.
Our study has several limitations. First, some potentially important markers for malignant diagnoses, such as erythrocyte sedimentation rate (ESR) and AF CEA, were not evaluated in our study, as only some included patients underwent that test. Nevertheless, previous studies have shown that ESR showed no significant difference between benign and malignant ascites [49]. Second, this is a single-center, retrospective study with a limited number of patients. The prevalence of ascites in this study may not reflect the prevalence of ascites in the Chinese population. Therefore, further studies with a larger sample size from multiple centers are needed to validate this predictive model. Thus, it is necessary to reassess the predictive models if they are used in a different population.
In summary, we have established a user-friendly and reliable 5-marker predictive model for classifying ascites into subtypes (malignant or benign). We believe that this simple mathematical model could be a useful diagnostic aid to distinguish malignant from benign ascites in clinical practice.
Abbreviations
AF: ascitic fluid; ROC: receiver operating characteristic; hs-CRP: hypersensitivity C-reactive protein; WBCs: white blood cells; Neu: neutrophil; Lym: lymphocyte; RBCs: red blood cells; PDW-SD: red blood cell distribution width-standard deviation; PDW: platelet distribution width; ALP: alkaline phosphatase; TP: total protein; Tch: total cholesterol; LDH: lactate dehydrogenase; AFP: alpha-fetoprotein assay; CA125: cancer antigen 125; CA19-9: cancer antigen 19-9; CEA: carcinoembryonic antigen; AFTP: ascitic fluid total protein; ADA: adenosine deaminase; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; VEGF: vascular endothelial growth factor; NMA: non-malignant ascites; MA: malignant ascites.
Acknowledgements
We thank these participants who willingly and generously provided information and samples.
Funding information
This work was supported by the National Natural Science Foundation of China (Grant 81572426).
Author Contributions
YYG initiated the hypothesis and organized the studies; YYG, XLP, NZ and JL analyzed the data and contributed to edited the manuscript; YYG and ST participated in reviewing and modifying the manuscript. All authors have equally involved in study design and drafting of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Abdel-Razik A, Eldars W, Elhelaly R. et al. C-reactive protein and insulin-like growth factor-1 in differential diagnosis of ascites. J Gastroenterol Hepatol. 2016;31:1868-73
2. Becker G, Galandi D, Blum HE. Malignant ascites: systematic review and guideline for treatment. European journal of cancer (Oxford, England: 1990). 2006;42:589-97
3. Zhu S, Du L, Xu D. et al. Ascitic fluid total protein, a useful marker in non-portal hypertensive ascites. J Gastroenterol Hepatol. 2019
4. Jiang CF, Shi B, Shi J. et al. New proposal for the serum ascites albumin gradient cut-off value in Chinese ascitic patients. Diagn Pathol. 2013;8:143
5. Teng D, Wu K, Sun Y. et al. Significant increased CA199 levels in acute pancreatitis patients predicts the presence of pancreatic cancer. Oncotarget. 2018;9:12745-53
6. Feng F, Tian Y, Xu G. et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC cancer. 2017;17:737
7. Du L, Zhu S, Lu Z. et al. Ascitic cholesterol is superior to serum-ascites albumin gradient in the detection of non-portal hypertensive ascites and the diagnosis of mixed ascites. Aliment Pharmacol Ther. 2019;49:91-8
8. Alexandrakis MG, Moschandrea JA, Koulocheri SA. et al. Discrimination between malignant and nonmalignant ascites using serum and ascitic fluid proteins in a multivariate analysis model. Dig Dis Sci. 2000;45:500-8
9. Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nature reviews Cancer. 2013;13:273-82
10. Yun J, Han SB, Kim HJ. et al. Exosomal miR-181b-5p Downregulation in Ascites Serves as a Potential Diagnostic Biomarker for Gastric Cancer-associated Malignant Ascites. Journal of gastric cancer. 2019;19:301-14
11. Vaid U, Kane GC. Tuberculous Peritonitis. Microbiology spectrum. 2017 5
12. Papatheodoridi M, Cholongitas E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. Curr Pharm Des. 2018;24:4574-86
13. Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroenterol. 2011;17:1237-48
14. Stukan M. Drainage of malignant ascites: patient selection and perspectives. Cancer management and research. 2017;9:115-30
15. Garrison RN, Kaelin LD, Galloway RH. et al. Malignant ascites. Clinical and experimental observations. Annals of surgery. 1986;203:644-51
16. Yuksel I, Karaahmet F, Coskun Y. et al. Significance of serum and ascitic fluid C-reactive protein in differential diagnosis of benign and malignant ascites. Dig Dis Sci. 2014;59:2588-93
17. Zhan N, Dong WG, Wang J. The clinical significance of vascular endothelial growth factor in malignant ascites. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:3719-25
18. Cheng D, Liang B, Kong H. Clinical significance of vascular endothelial growth factor and endostatin levels in the differential diagnosis of malignant and benign ascites. Medical oncology (Northwood, London, England). 2012;29:1397-402
19. Gulyas M, Kaposi AD, Elek G. et al. Value of carcinoembryonic antigen (CEA) and cholesterol assays of ascitic fluid in cases of inconclusive cytology. Journal of clinical pathology. 2001;54:831-5
20. Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27-41
21. Blann AD, Byrne GJ, Baildam AD. Increased soluble intercellular adhesion molecule-1, breast cancer and the acute phase response. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2002;13:165-8
22. Jabs WJ, Busse M, Kruger S. et al. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney international. 2005;68:2103-10
23. Wiese S, Mortensen C, Gotze JP. et al. Cardiac and proinflammatory markers predict prognosis in cirrhosis. Liver international: official journal of the International Association for the Study of the Liver. 2014;34:e19-30
24. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237-49
25. Liang Y, Zheng G, Yin W. et al. Significance of EGFR and PTEN Expression and PLR and NLR for Predicting the Prognosis of Epithelioid Malignant Peritoneal Mesothelioma. Gastroenterol Res Pract. 2019;2019:7103915
26. Sidana A, Kadakia M, Friend JC. et al. Determinants and prognostic implications of malignant ascites in metastatic papillary renal cancer. Urologic oncology. 2017;35:114 e9- e14
27. Yuan C, Yang K, Tang H. et al. Diagnostic values of serum tumor markers Cyfra21-1, SCCAg, ferritin, CEA, CA19-9, and AFP in oral/oropharyngeal squamous cell carcinoma. OncoTargets and therapy. 2016;9:3381-6
28. Estirado C, Ceccato A, Guerrero M. et al. Microorganisms resistant to conventional antimicrobials in acute exacerbations of chronic obstructive pulmonary disease. Respir Res. 2018;19:119
29. Jiang SS, Weng DS, Jiang L. et al. The clinical significance of preoperative serum cholesterol and high-density lipoprotein-cholesterol levels in hepatocellular carcinoma. J Cancer. 2016;7:626-32
30. Zhang D, Xi Y, Feng Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: a systematic review and meta-analysis of observational epidemiologic studies. Eur J Cancer Prev. 2020
31. Banerjee M, Singh R, Arora MM. et al. Biomarkers of malignant ascites-a myth or reality. Medical journal, Armed Forces India. 2011;67:108-12
32. Farwell WR, D'Avolio LW, Scranton RE. et al. Statins and prostate cancer diagnosis and grade in a veterans population. Journal of the National Cancer Institute. 2011;103:885-92
33. Gerbes AL, Jungst D. Role of cholesterol determination in ascitic fluid analysis. Hepatology (Baltimore, Md). 2009;50:1320
34. Kang SJ, Kim JW, Baek JH. et al. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J Gastroenterol. 2012;18:2837-43
35. Gerbes AL, Jungst D, Xie YN. et al. Ascitic fluid analysis for the differentiation of malignancy-related and nonmalignant ascites. Proposal of a diagnostic sequence. Cancer. 1991;68:1808-14
36. Kucukgoz Gulec U, Paydas S, Guzel AB. et al. Comparative analysis of CA 125, ferritin, beta-2 microglobulin, lactic dehydrogenase levels in serum and peritoneal fluid in patients with ovarian neoplasia. Medical oncology (Northwood, London, England). 2012;29:2937-43
37. Luo G, Liu C, Guo M. et al. Potential Biomarkers in Lewis Negative Patients With Pancreatic Cancer. Annals of surgery. 2017;265:800-5
38. Zhu FL, Ling AS, Wei Q. et al. Tumor markers in serum and ascites in the diagnosis of benign and malignant ascites. Asian Pacific journal of cancer prevention: APJCP. 2015;16:719-22
39. Ahadi M, Tehranian S, Memar B. et al. Diagnostic value of carcinoembryonic antigen in malignancy-related ascites: systematic review and meta-analysis. Acta gastro-enterologica Belgica. 2014;77:418-24
40. Chen SJ, Wang SS, Lu CW. et al. Clinical value of tumour markers and serum-ascites albumin gradient in the diagnosis of malignancy-related ascites. J Gastroenterol Hepatol. 1994;9:396-400
41. Felder M, Kapur A, Gonzalez-Bosquet J. et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129
42. Gao Y, Wang J, Zhou Y. et al. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci Rep. 2018;8:2732
43. Yilmaz MB, Zorlu A, Tandogan I. Plasma CA-125 level is related to both sides of the heart: a retrospective analysis. International journal of cardiology. 2011;149:80-2
44. Ozsahin SL, Turgut B, Nur N. et al. Validity of the CA125 level in the differential diagnosis of pulmonary tuberculosis. Jpn J Infect Dis. 2008;61:68-9
45. Ataseven H, Ozturk ZA, Arhan M. et al. Cancer antigen 125 levels in inflammatory bowel diseases. J Clin Lab Anal. 2009;23:244-8
46. Kalantri Y, Naik G, Joshi SP. et al. Role of cancer antigen-125 from pleural & ascitic fluid samples in non malignant conditions. Indian J Med Res. 2007;125:25-30
47. Boyer TD, Kahn AM, Reynolds TB. Diagnostic value of ascitic fluid lactic dehydrogenase, protein, and WBC levels. Archives of internal medicine. 1978;138:1103-5
48. Tian S, Cheng S-B, Guo Y-Y. et al. High Efficient Isolation of Tumor Cells by a Three Dimensional Scaffold Chip for Diagnosis of Malignant Effusions. ACS Applied Bio Materials. 2020;3:2177-84
49. Uzunkoy A, Harma M, Harma M. Diagnosis of abdominal tuberculosis: experience from 11 cases and review of the literature. World J Gastroenterol. 2004;10:3647-9
Author contact
![]() Corresponding author: Wei-Guo Dong, E-mail: dongweiguoedu.cn.
Corresponding author: Wei-Guo Dong, E-mail: dongweiguoedu.cn.

 Global reach, higher impact
Global reach, higher impact