ISSN: 1449-1907
Int J Med Sci 2021; 18(9):1946-1952. doi:10.7150/ijms.55421 This issue Cite
Research Paper
Bullous Pemphigoid and Diabetes medications: A disproportionality analysis based on the FDA Adverse Event Reporting System
1. Department of Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, 215123, China.
2. Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, 215123, China.
3. National Institute for Food and Drug Control, Beijing, 102629, China.
4. Department of pharmacy, The Affiliated Suzhou Science & Technology Town Hospital of Nanjing Medical University, Suzhou, 215153, China.
#These authors contributed equally to this work.
Abstract
Background: The world's first Diabetes Medications (Insulin) was marketed in October 1923. Some studies suggested the association of diabetes medications with Bullous Pemphigoid (BP), especially the Dipeptidyl Peptidase 4 (DPP-4) inhibitors. The study aims to detect an association between diabetes medications (focusing on DPP-4 inhibitors) and bullous pemphigoid based on FDA Adverse Event Reporting System (FAERS).
Methods: All spontaneous reports of diabetes medications inhibitors-related BP recorded in the FAERS between March 2004 and August 2020 were included in the present study. Disproportionality analysis was performed to find the signal between diabetes medications and BP. The Chi-Squared with Yates' correction (χ2Yates), proportional reporting ratio (PRR) and the lower limit of the 95% confidence interval of the Reporting Odds Ratio (ROR025) were calculated as a measure. A signal was detected when ROR025 > 1, PRR > 2, χ2Yates > 4 and at least 3 cases.
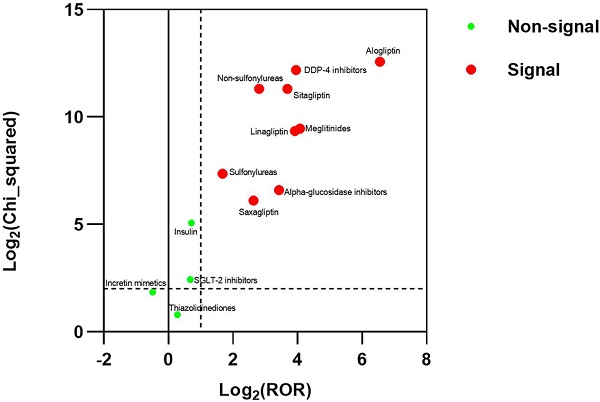
Results: There were 3770 reports for BP in FAERS. The strongest signal for diabetes medications-BP association were DDP-4 inhibitors (ROR025: 13.700, PRR: 15.408), followed by Meglitinides (ROR025: 12.708, PRR: 16.777), Non-sulfonylureas (ROR025: 6.434, PRR: 7.016), Alpha-glucosidase inhibitors (ROR025: 6.105, PRR: 10.738), Sulfonylureas (ROR025:2.655, PRR: 3.200).
Conclusions: This study detected a strong signal between BP and DDP-4 inhibitors, alpha-glucosidase inhibitors, meglitinides, non-sulfonylureas, and sulfonylureas in FAERS. The signal was significantly higher with alogliptin than with the other DPP-4 inhibitors. The study doesn't suggest the association between the incretin mimetics, insulin, SGLT-2 inhibitors, thiazolidinediones and BP in FAERS.
Keywords: diabetes medications, dipeptidyl peptidase 4 inhibitors, Bullous Pemphigoid, FAERS, drug safety

 Global reach, higher impact
Global reach, higher impact