3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(5):1159-1166. doi:10.7150/ijms.51799 This issue Cite
Research Paper
INR-to-platelet ratio (INPR) as a novel noninvasive index for predicting liver fibrosis in chronic hepatitis B
1. Department of Hepatobiliary Medicine, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China.
2. Department of Integrative Medicine, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China.
3. Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai 200040, China.
#These authors have contributed equally to this work.
Abstract
Objective: We aimed to investigate whether a novel noninvasive index, i.e., the international normalized ratio-to-platelet ratio (INPR), was a variable in determining liver fibrosis stage in patients with chronic hepatitis B (CHB).
Methods: A total of 543 treatment-naïve CHB patients were retrospectively enrolled. Liver histology was assessed according to the Metavir scoring scheme. All common demographic and clinical parameters were analyzed.
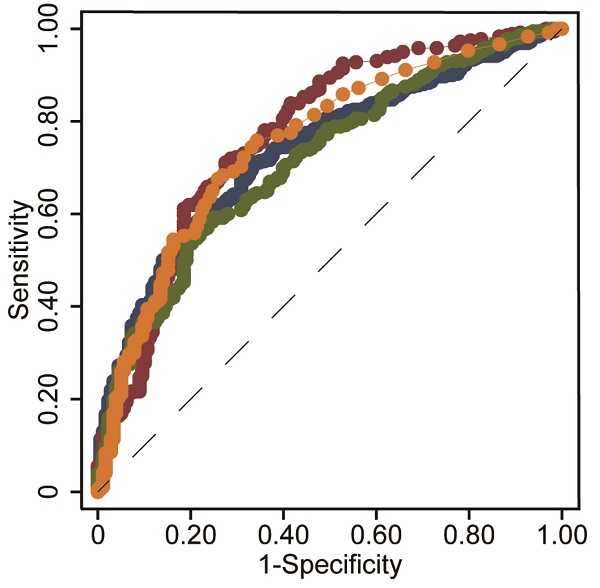
Results: Based on routine clinical parameters (age, sex, HBeAg status, HBV DNA, hematological parameters, coagulation index, and liver biochemical indicators), a novel index, i.e., the INR-to-platelet ratio (INPR), was developed to magnify the unfavorable effects of liver fibrosis on INR and platelets. The AUCs of INPR for predicting significant fibrosis, advanced fibrosis, and cirrhosis were 0.74, 0.76 and 0.86, respectively. Compared with APRI, FIB-4, and GPR, the INPR had comparable predictive efficacy for significant fibrosis and better predictive performance for advanced fibrosis and cirrhosis.
Conclusion: INPR could be an accurate, easily calculated and inexpensive index to assess liver fibrosis in patients with CHB. Further studies are needed to verify this indicator and compare it with other noninvasive methods for predicting liver fibrosis in CHB patients.
Keywords: international normalized ratio, INR, platelet, liver fibrosis, chronic hepatitis B

 Global reach, higher impact
Global reach, higher impact